Abstract
Objectives
The objectives of this study were to evaluate differences in intrarenal oxygenation as assessed by blood oxygen level–dependent (BOLD) magnetic resonance imaging in contrast-induced acute kidney injury (CIAKI)–susceptible rats when using 4 contrast media with different physicochemical properties and to demonstrate the feasibility of acquiring urinary neutrophil gelatinase–associated lipocalin (NGAL) levels as a marker of CIAKI in this model.
Materials and Methods
Our institutional animal care and use committee approved the study. Sixty-six Sprague-Dawley rats were divided into CIAKI-susceptible groups (received nitric oxide synthase inhibitor N-nitro-L-arginine methyl ester [10 mg/kg] and cycloxygenase inhibitor indomethacin [10mg/kg]) and control groups (received saline instead). One of the 4 iodinated contrast agents (iothalamate, iohexol, ioxaglate, or iodixanol) was then administered (1600-mg organic iodine per kilogram of body weight). Multiple blood oxygen level–dependent magnetic resonance images were acquired on a Siemens 3.0-T scanner using a multiple gradient recalled echo sequence at baseline, after N-nitro-L-arginine methyl ester (or saline), indomethacin (or saline), and iodinated contrast agent (or placebo). R2* (R2* = 1/T2*) maps were generated inline on the scanner. A mixed-effects growth curve model with first-order autoregressive variance-covariance was used to analyze the temporal data. Urinary NGAL, a marker of kidney injury (unlike serum creatinine), was measured 4 hours after contrast injection in the 2 subgroups.
Results
Differences in blood oxygen level–dependent magnetic resonance imaging results between the contrast media were observed in all 4 renal regions. However, the inner stripe of the outer medulla (ISOM) showed the most pronounced changes in the CIAKI-susceptible group and R2* increased significantly (P < 0.01) over time with all 4 contrast media. In the control groups, only iodixanol showed an increase in R2* (P < 0.05) over time. There was an agreement between increases in NGAL and R2* values in ISOM.
Conclusions
In rats susceptible to CIAKI, those receiving contrast media had significant increases in R2* in renal ISOM compared with those receiving placebo. The agreement between NGAL and R2* values in the ISOM suggests that the observed immediate increase in R2* after contrast injection may be the earliest biomarker of renal injury. Further studies are necessary to establish threshold values of R2* associated with acute kidney injury and address the specificity of R2* to renal oxygenation status.
Keywords: kidney, oxygenation, BOLD, MRI, NGAL, iodinated contrast, acute kidney injury, rats, nitric oxide, cyclooxygenase
The number of interventional cardiac procedures has been steadily increasing over time. This, in turn, has caused contrast-induced nephropathy to become the third leading cause of hospital-acquired acute kidney injury (AKI).1 The pathogenesis of iodinated contrast-induced AKI (CIAKI) is not yet fully understood, but the relevance of renal medullary hypoxia in the pathophysiology of all forms of AKI is well accepted.2–4 Early studies with invasive microprobes have shown iodinated contrast media (CM) adversely affect renal medullary oxygenation status.5 Although quite efficacious, invasive microprobe measurements are too highly complex to be adopted for widespread use. A need for a noninvasive approach to follow renal oxygenation status in animal models is desirable. Noninvasive blood oxygen level–dependent (BOLD) magnetic resonance imaging (MRI) technique has gained acceptance in evaluating relative renal oxygenation status in animal models6,7 and has translated well to human studies.8–10 The feasibility of using BOLD MRI to monitor intrarenal oxygenation changes after administration of CM has been previously reported.11,12
In clinical practice, plasma creatinine levels are used as a surrogate measure of glomerular filtration rate (GFR) to define CIAKI. However, creatinine has major drawbacks as a biomarker for CIAKI. First, the accumulation of creatinine is slow; it requires 48 to 72 hours to rise after contrast exposure, making it insensitive to rapid GFR changes induced by iodinated contrast.1 Second, creatinine is insensitive in patients with normal preexisting renal function because of the exponential relationship between creatinine and GFR.1 However, owing to the simplicity of the test, it is still widely used in the clinic. Scientific studies to better understand the deleterious effects of CM and interventions to mitigate these effects require early markers, preferably based on pathophysiologic pathways rather than as simple outcome measures. This becomes especially important after the recent reports that have questioned the validity of increased serum creatinine as a marker of CIAKI, given that similar increases can be found in patients after undergoing non–contrast-enhanced radiographic studies.13 Owing to these limitations of serum creatinine, novel biomarkers that reflect acute injury to the kidney are being sought. Urinary (and serum) neutrophil gelatinase–associated lipocalin (NGAL) levels have shown promise in recent studies as a viable biomarker for AKI14 and specifically for CIAKI.15 Blood oxygen level–dependent MRI has shown feasibility to monitor changes in tissue hypoxia immediately after CM administration.11,12,16 If shown that these 2 markers of pathophysiologic processes can be correlated, it would have a significant impact on our understanding of the hemodynamic consequences of CM and their relationship to developing CIAKI.
The primary objective of this study was to compare the effects of 4 different CM on the intrarenal oxygenation as evaluated by BOLD MRI in rats susceptible to CIAKI.5 A secondary objective was to evaluate the changes in urinary NGAL levels, as a marker of AKI, in this model and correlate those changes with the BOLD MRI findings.
MATERIALS AND METHODS
Animal
The study protocol was approved by the institutional animal care and use committee. Male Sprague-Dawley rats (Harlan Laboratories, Madison, WI) were anesthetized using inactin (100 mg/kg intraperitoneal injection, Sigma_Aldrich, St Louis, MO). A femoral vein was catheterized for the administration of drugs or saline.
A total of 66 rats (weighing 325.3 ± 4.9 g) were used. Table 1 shows the group descriptions. Initially, 48 animals were split into 8 groups of 6 animals each. The animals were assigned to different groups in a random order, and data acquisition and analysis were performed in a blinded fashion. There were 2 major categories of the animals on the basis of the specific pretreatments received. The first category was the CIAKI-susceptible rats, receiving pretreatments of nitric oxide (NO) synthase inhibitor, N-nitro-L-arginine methyl ester5 (L-NAME, 10 mg/kg; Sigma_Aldrich, St Louis, MO), and cyclooxygenase (COX) inhibitor indomethacin5 (10 mg/kg, Sigma-Aldrich, St Louis, MO) before CM administration. The second category consisted of control animals, receiving inactive pretreatments, saline (1 mL/kg), and saline (1 mL/kg) before CM. Each category was further divided into 4 subgroups on the basis of the type of CM (iothalamate, iohexol, ioxaglate, iodixanol) used.
TABLE 1.
Group Descriptions
| Group No. | Name of Group | No. Animals | Sequence of Administrations | Measurements | Note |
|---|---|---|---|---|---|
| 1 | Iothalamate | 6 | Baseline → L-NAME → indomethacin → iothalamate | BOLD | Randomized and blinded |
| 2 | Iothalamate_ctrl | 6 | Baseline → saline → saline → iothalamate | BOLD | |
| 3 | Iohexol | 6 | Baseline → L-NAME → indomethacin → iohexol | BOLD | |
| 4 | Iohexol_ctrl | 6 | Baseline → saline → saline → iohexol | BOLD | |
| 5 | Iodixanol | 6 | Baseline → L-NAME → indomethacin → iodixanol | BOLD | |
| 6 | Iodixanol_ctrl | 6 | Baseline → saline → saline → iodixanol | BOLD | |
| 7 | Ioxaglate | 6 | Baseline → L-NAME → indomethacin → ioxaglate | BOLD | |
| 8 | Ioxaglate_ctrl | 6 | Baseline → saline → saline → ioxaglate | BOLD | |
| 9 | Placebo | 6 | Baseline → L-NAME → indomethacin → saline | BOLD + NGAL | |
| 10 | Placebo_ctrl | 6 | Baseline → saline → saline → saline | BOLD | |
| 11 | Iodixanol | 6 | Baseline → L-NAME → indomethacin → iodixanol | BOLD + NGAL | |
BOLD indicates blood oxygen level–dependent; ctrl, control; L-NAME, N-nitro-L-arginine methyl ester; NGAL, neutrophil gelatinase-associated lipocalin.
Three additional groups were later included for further scientific rigor. Groups 9 and 10 served as placebo groups for the CIAKI-susceptible and control animals, respectively. We used saline (1 mL/kg) as placebo in place of the CM. Furthermore, we added group 11 that was identical to group 5 (ie, CIAKI-susceptible animals receiving iodixanol). To test preliminary feasibility, urinary NGAL levels were evaluated in the animals in groups 9 and 11.
Drugs
The L-NAME solution was prepared on the same day as that of the experiment. Indomethacin was dissolved in saline using potassium hydroxide and hydrochloric acid to balance the pH from 7.4 to 8.0. Detailed information of CM is listed in Table 2. One of the 4 CM, iothalamate (Conray; Mallinckrodt, St Louis, MO), iohexol (Omnipaque; GE Healthcare, Princeton, NJ), ioxaglate (Hexabrix; Guerbet, LLC, Bloomington, IN), or iodixanol (Visipaque; GE Healthcare, Princeton, NJ), was administered at 1600 mg of organic iodine per kilogram of body weight (mgI/kg) as a bolus over 2 minutes on the basis of a previous report.17 Saline of 0.2 mL was injected to flush the intravenous line. For reference, a contrast-enhanced CT delivers approximately 500 mgI/kg, whereas cardiac angiography and percutaneous coronary intervention could deliver 750 to 1500 mgI/kg (contrast volumes of 150–00 mL).
TABLE 2.
Physicochemical Properties of Radiocontrast Agents
| Systemic Name | Iothalamate | Iohexol | Ioxaglate | Iodixanol |
|---|---|---|---|---|
| Brand name | Conray | Omnipaque | Hexabrix | Visipaque |
| Ionic | Ionic | Nonionic | Ionic | Nonionic |
| Monomer/dimer | Monomeric | Monomeric | Dimeric | Dimeric |
| Osmolality (mOsmol/L) | 1000 | 672 | 600 | 290 |
| Iodine concentration (mg/mL) | 282 | 300 | 320 | 320 |
| Viscosity at 37 °C (cP) | 4.0 | 6.3 | 7.5 | 11.8 |
cP indicates centipoise.
MRI Scan
Blood oxygen level–dependent MRI was performed on a 3.0-T scanner (MAGNETOM Verio; Siemens, Erlangen, Germany) using a multiple gradient recalled echo sequence (echo time, 3.6–41.3 milliseconds; field of view, 12 × 6 cm; repetition time, 69 milliseconds; bandwidth, 320 hertz per pixel; field angle, 30 degrees; matrix, 256 × 256; slice thickness, 2 mm; number of excitations, 20) to acquire 12 T2*-weighted images by one of the investigators who remained blinded to the different CM administrations. The rats were placed in a right decubitus position to minimize susceptibility artifacts from bowel loops. The kidneys were placed in the middle of an 8-channel standard knee coil. On the basis of our experience, the kidney at the bottom had the least bulk susceptibility-induced artifacts, so we performed all of our measurements in that kidney. One transverse slice was selected in the middle of the kidney.
Each rat underwent 4 imaging phases: baseline; pretreatment 1 (rats received either L-NAME or saline), pretreatment 2 (rats received either indomethacin or saline), and CM (rats received either one of the 4 contrast agents or placebo). Five sets of BOLD images were acquired for each of the first 3 phases. Twenty sets of BOLD images were acquired after CM or placebo administration. Each BOLD image took 3 minutes to acquire. Figure 1 shows the imaging timing diagram.
FIGURE 1.
Experimental timing diagram. The vertical lines represent the timing of administration of pretreatments and iodinated contrast or placebo. Time periods are shown to scale. blood oxygen level–dependent MRI was performed every 3 minutes for the entire study. The first pretreatment was L-NAME or saline and the second pretreatment was indomethacin or saline. Iodinated contrast refers to one of the 4 agents (iothalamate, ioxaglate, iohexol, or iodixanol) and saline was used as placebo.
Urinary Biomarker NGAL
In the animals belonging to groups 9 and 11, urine was collected before BOLD MRI scan and 4 hours after administration of iodixanol or placebo. Urine was collected into a nonsterile 2-mL centrifuge tube and placed into a –80°F freezer for storage. Urinary NGAL concentrations were measured using GAL ELISA kit (046, BioPorto Diagnostics, Gentofte, Denmark) according to manufacturer's instruction. Urinary creatinine was analyzed by the clinical laboratory at our institution. Concentrations levels of NGAL were normalized to urine creatinine concentrations to minimize any confounding effects of urine flow rate.18
Data Processing
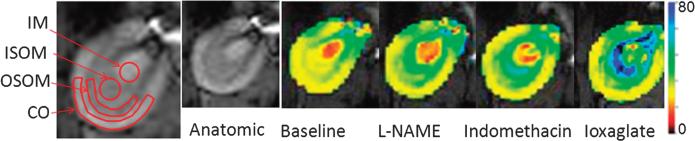
The natural logarithm of signal intensity versus echo time data was fit to a linear function to generate R2* maps inline. Regions of interests (ROIs) were defined by one of the investigators, who also remained blinded to the group assignments, on the anatomic image and copied to R2* maps to obtain mean R2* readings in the inner and outer stripes of outer medulla (ISOM, OSOM), inner medulla (IM), and cortex (CO) as shown in Figure 2. The ROI size was defined to be as large as possible in each renal region. A circular ROI was used in renal IM and ISOM (>20 pixels). Freehand ROIs (>30 pixels) were placed in the renal OSOM and CO area as shown in Figure 2. R2* maps (Fig. 2) were independently generated for color illustration purposes using custom Matlab (Mathworks, Natick, MA) code.
FIGURE 2.
Representative R2* maps were obtained from one rat in the CIAKI group. Maps are from the same slice position and displayed with the same window and level settings. All the maps were scaled from zero (black) to 80 (blue), representing a range of R2* levels. Larger R2* values correspond to higher levels of hypoxia. The anatomic image of a rat kidney with typical ROI definitions is shown on the far left. Note the progressively increasing R2* values (ie, color changes from yellow and red to green and blue) in the renal outer medulla (ISOM and OSOM) after L-NAME, indomethacin, and iodinated contrast. The cortex did not show change in color, suggesting minimal alteration in oxygenation levels.
Statistical Analysis
To fully take advantage of the repeated measurements, a mixed-effect regression model was used to assess BOLD R2* measurements in terms of changes over time (slope). Given this design and data structure, we proposed a model that allows comparison of change over time between the groups. All 35 continuous time points (at 3-minute intervals) were included, spanning a total of 105 minutes. Fixed effects in the model include group, time (continuous), and group by time interactions. The first-order autoregressive variance-covariance structure was specified for the model and random effect accounted for the variation in individual rats. Akaike Information Criterion was used to determine the appropriate variance-covariance structure. The multiple comparisons were adjusted by Hochberg step-up method. Statistical analyses were carried out by SAS 9.2 (SAS, Cary, NC), and P < 0.05 was regarded statistically significant.
For the preliminary evaluation of NGAL, along with the corresponding BOLD MRI data in the same animals, repeated-measure analysis of variance was used to compare the changes from baseline between the 2 groups. Multiple comparisons were adjusted. A single representative R2* value was generated for each animal by averaging all R2* readings at each condition (5 time points at baseline and 20 time points after CM/placebo administration).
RESULTS
Figure 2 shows the images illustrating the typical changes observed in a representative animal. The figure includes an anatomic image (with typical ROI definitions), R2* maps obtained at baseline and after administration of L-NAME, indomethacin, and CM. The colors on the maps represent the relative levels of hypoxia. The medulla shows progressively higher R2* values (especially in ISOM) after each pretreatment and CM administration, suggesting an increased level of hypoxia.
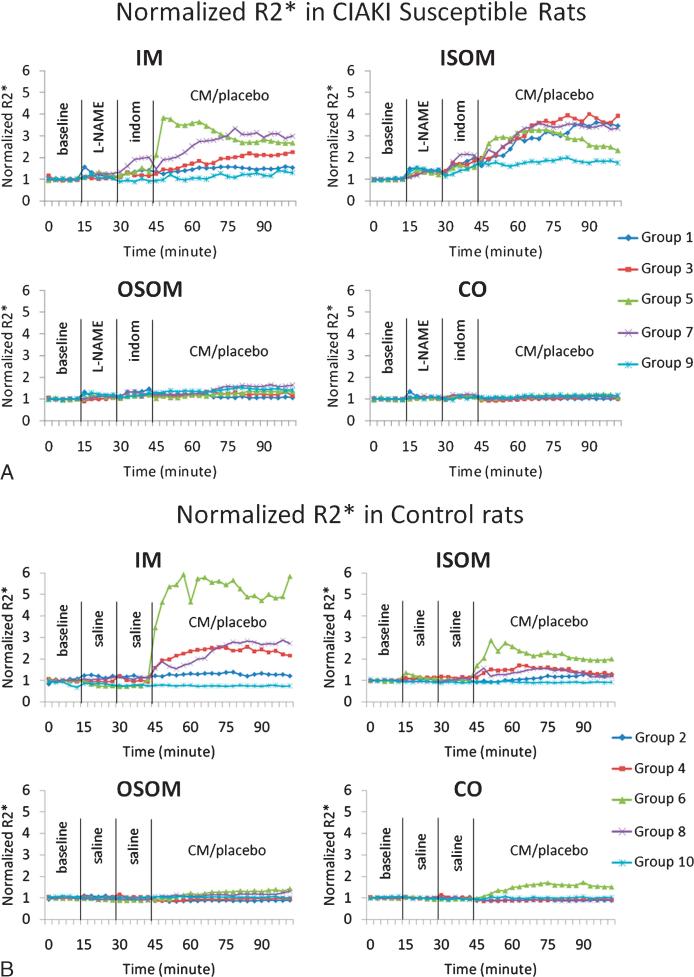
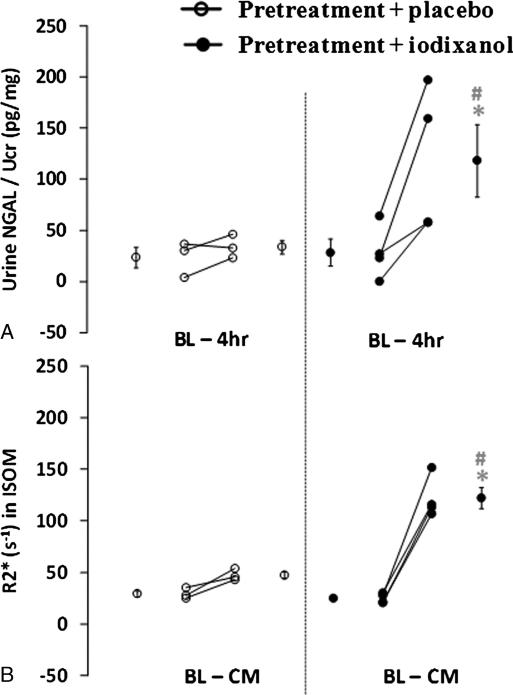
Figure 3 summarizes the mean time course for R2* obtained from groups 1 to 10 evaluated in the 4 renal regions. Figure 3A is the comparison between different CM in the CIAKI-susceptible groups. Figure 3B is the comparison between different CM in the control groups. Figure 4 summarizes the data (mean ± SE) from the individual rats with both urinary NGAL and BOLD MRI (R2* in ISOM) measurements. Although the experiment included 6 rats in each of the groups, only 3 rats in the placebo group and 4 rats in the iodixanol group had NGAL readings available because of technical difficulties. Missing data were caused by either insufficient sample quantity or out-of-range readings on ELISA and/or creatinine tests. Blood oxygen level–dependent R2* images were collected from all 12 rats, but R2* presented here only includes the rats with valid NGAL readings. There is a significant increase in NGAL at 4 hours after CM (28.7 ± 13.3 to 118.3 ± 35.6; P = 0.007). Similarly, there is a significant increase in R2* in ISOM (25.0 ± 2.4 s–1 to 122.1 ± 10.2 s–1; P < 0.001).
FIGURE 3.
Summary of the temporal changes in R2* measurements in 4 renal regions. R2* values were normalized to baseline values to allow combining data from different subgroups. The vertical lines show the time of administration of pretreatments and CM agents or saline. Each time course consisted of 35 time points; each point is the average of R2* value from all 6 rats in each subgroup. A, Time courses in 5 subgroups of the CIAKI-susceptible rats. B, Time courses in the 5 subgroups of the control group. As a reference, R2* values at baseline is 21.2 ± 3.4 s–1 in IM, 37.0 ± 7.1 s–1 in ISOM, 44.6 ± 4.9 s–1 in OSOM, and 31.8 ± 3.7 s–1 in CO in the CIAKI-susceptible group with iodixanol.
FIGURE 4.
Summary of individual rat urinary NGAL and BOLD R2* measurements are from 2 groups, the CIAKI-susceptible group with iodixanol (group 11, n = 4) and the placebo group (group 9, n = 3). The group mean values at baseline and after iodixanol/placebo as well as its standard error are displayed on the corresponding sides. Plot A shows individual NGAL measurements. A urine sample was collected at baseline and 4 hours after iodixanol/placebo for NGAL analysis. Urinary NGAL concentrations were normalized to urinary creatinine concentrations. Plot B is the summary of individual BOLD R2* measurements in the renal ISOM from the same rats depicted in plot A. Each data point is the average of R2* readings in the related time period.
Table 3 is the summary of estimated slope of R2* using a linear mixed-effects growth curve model, along with the P values. There are significant slope differences among the 10 groups (P < 0.0001) across 4 renal regions. The following observations can be made on the basis of these data:
Group 10, which only received saline, showed the least changes over time resulting in an estimated slope of approximately 0.0 over the measurement period. This confirms the stability of the BOLD MRI measurement over the measurement period.
Group 9 received the pretreatments of L-NAME and indomethacin but no CM. Only ISOM (estimated slope, 0.27) reached statistical significance. However, urinary NGAL showed no significant change at the 4-hour time point in this group (Fig. 4). On the basis of these observations, we considered only mean slope values greater than 0.3 that reached statistical significance as a pathophysiologically relevant response related to CM administration (values highlighted in Table 3).
The CO and OSOM showed the least amount of changes (estimated mean slope, <0.3) after any of the pretreatments or CM.
The ISOM demonstrated the most changes, especially in the CIAKI-susceptible groups. All the CM showed a substantial increase in R2* (estimated mean slope of 0.79 from Table 3) in the CIAKI-susceptible group compared with the control group (estimated mean slope of 0.24 from Table 3). Only iodixanol resulted in a substantial increase in R2* in the control group (estimated slope of 0.49)
The IM demonstrated similar levels of change compared with ISOM but only with iodixanol in both groups. Interestingly, the slope in the control group with iodixanol was substantially higher compared with the CIAKI group (1.16 versus 0.45).
TABLE 3.
R2* Slope Estimates From the Liner Mixed-Effects Growth Model
| Group No | IM | ISOM | OSOM | CO | |
|---|---|---|---|---|---|
| Iothalamate | 1 | 0.10 | 0.91 *†‡ | 0.06 | −0.01 |
| Iothalamate_ctrl | 2 | 0.03 | 0.07 | −0.04 | −0.03 |
| Iohexol | 3 | 0.15 | 0.66 *†‡ | 0.09 | −0.01‡ |
| Iohexol_ctrl | 4 | 0.24† | 0.24† | 0.02 | 0.25† |
| Iodixanol | 5 | 0.45 *†‡ | 0.73 *† | 0.17† | 0.08†‡ |
| Iodixanol_ctrl | 6 | 1.16 † | 0.49 † | 0.16† | 0.19† |
| Ioxalgate | 7 | 0.32† | 0.88 *†‡ | 0.29*†‡ | 0.04 |
| Ioxalgate_ctrl | 8 | 0.26† | 0.04 |
0.05 | −0.05 |
| Placebo | 9 | 0.12 | 0.27† |
0.11† | 0.05 |
| Placebo_ctrl | 10 | −0.01 | −0.04 | −0.06 | 0.01 |
Pretreated animals receiving saline (group 9) showed a significant change in slope in ISOM; however, NGAL showed no increase in this group at 4 hours. We used this as a reference slope and a defined slope of greater than 0.3 and reaching statistical significance to be physiologically relevant. These values are shown in BOLD. In general, the slope estimates are higher in the CIAKI-susceptible group compared with the control group in IOSM.
A significant change in slope compared with group 9 (P < 0.05).
Slope is significantly different from zero (P < 0.05).
A significant change in slope compared with the control group (P < 0.05).
CO indicates cortex; ctrl, control; IM, inner medulla; ISOM, inner stripes of the outer medulla; OSOM, outer stripes of the outer medulla.
DISCUSSION AND CONCLUSIONS
Our results revalidate the model on the basis of the CIAKI-susceptible rats. The BOLD MRI data in the ISOM clearly show differences between the animals pretreated with NOS and COX inhibitors before CM administration compared with the controls that received only saline pretreatment (Table 3; Figs. 3A, B). Only iodixanol showed comparable changes in both the pretreatment and saline groups. The results with iothalamate are in good agreement with those of a previous report using BOLD MRI at 1.5 T using the same animal model.11 It should be noted that the previous study only reported on measurements in the outer medulla and CO. The fact that R2* showed only minimal change in renal medulla with iothalamate in the control rats (Table 3) is consistent with the previous study using invasive probes.5 The relatively smaller rise in R2* seen with other contrast agents (other than iodixanol) in the control group is also in agreement with previous reports on the basis of the evaluation of renal blood flow.17,19 More importantly, the data also show that the pretreatments alone do not cause AKI, although they do result in statistically significant increases in R2*. The data further demonstrate that CM administered after pretreatments lead to much higher R2* (P < 0.01) values compared with those receiving placebo and that these changes are reflected in significantly higher urinary NGAL levels. Neutrophil gelatinase–associated lipocalin is generated in tubule cells of the kidney, including those of the ISOM, in response to ischemic injury and released into the urine within hours.20
Using rats pretreated with L-NAME as well as indomethacin and receiving placebo (group 9) as a reference group, we were able to define pathophysiologically relevant changes observed after CM administration, that is, slope greater than 0.3 and reaching statistical significance. This threshold for a significant level of hypoxia was supported by the urinary NGAL data, which showed no significant change at 4 hours in the animals receiving the placebo (slope, 0.27) compared with a large and statistically significant increase in the group receiving iodixanol (slope of 0.73 in ISOM). With this definition, we were able to draw some general conclusions on the basis of the data in Table 3:
The ISOM demonstrated the most changes after CM, especially in the CIAKI-susceptible group. This is consistent with the literature regarding the relative sensitivity of this region to ischemic injury.20,21 A recent study demonstrated changes in diameter of the descending vasa recta when exposed to CM.22 The descending vasa recta regulates the blood flow to the ISOM. All CM showed comparable change in the CIAKI-susceptible groups (slopes of 0.66 to 0.91). In the control groups, only iodixanol showed a change that was closer in magnitude (slope of 0.49). This could be related to the higher viscosity of iodixanol as shown in several recent reports.23,24 Higher viscosity has been associated with damage to cells and generation of oxygen radicals perhaps through the increased contact time of nephrotoxic CM with the tubule epithelial cells and vascular endothelium.1
Although a few measurements in the OSOM and CO reached statistical significance, by applying our defined reference value, none of the measurements were deemed pathophysiologically relevant (ie, slopes of less than 0.3).
The IM showed comparable changes compared with ISOM but only with iodixanol. These observations are consistent with a recent report in a swine model.25
In summary, we believe the BOLD MRI changes observed in ISOM after CM administration could be useful in determining the subsequent development of AKI. Interestingly, there was not much of a difference in the magnitude of change after CM administration in the CIAKI-susceptible group between different CM (P > 0.05). This may explain some of the recent clinical experience that failed to show differences between agents in terms of risk for developing CIAKI26–29 (probably because, in subjects at risk, the type of CM does not matter). On the other hand, only iodixanol showed a difference in R2* in ISOM in the control groups. This could explain some of the more recent observations that suggest a higher incidence of CIAKI with iodixanol30,31 (probably these studies included subjects at risk and those not at risk). The clinical data are ambiguous probably because of the differences in the demographics, subject preparation, as well as the type and dose of CM between the different studies.30 Animal models may avoid this complication.
The dose used in this study is higher than those typically used in the clinic. We rationalized the dose in the following way. For drugs that are cleared from the body via the kidney and do not undergo metabolism, it is reasonable to scale the dose to GFR. This is particularly relevant when looking at renal injury. In humans undergoing percutaneous coronary intervention, 70 gram of organic iodine (g I) is a usual dose for a patient with a GFR of 70 mL/min or approximately 1 g I per 1 mL/min of GFR. For high-risk patients with estimated GFR less than 60 mL/min, the dose per kilogram would be higher. A number of studies have found that the risk for CIAKI increases when doses greater than 3.7 mL per estimated GFR are used. For a typical low-osmolar contrast media with 370 mg I/mL, this equates to an upper limit of 1.37 g I/L per milliliter per minute of GFR.32 In our rat model, the usual GFR is 0.8 mL/min per 100 g of total body weight. For a 250-g animal, the GFR is approximately 2 mL/min. We gave the animals 1.6 g I per 2 mL/min of GFR or 0.8 g I per 1 mL/min GFR.
The availability of urinary NGAL data provided direct evidence of AKI associated with contrast administration in the CIAKI-susceptible rats. This, unfortunately, was not available to us at the time of study inception. However, these results support feasibility and future studies should include them as correlative outcome measures. Future studies should be designed to determine the threshold values of R2* in ISOM that lead to AKI as determined by NGAL or other suitable markers.
Limitations
The study did have some limitations, mainly the lack of urinary NGAL data in all groups.
Confounding Effects
The choice of R2* as a BOLD MRI parameter has some inherent limitations. It is not specific to blood oxygenation and potentially depends on blood volume, hemoglobin levels, and R2. Because all these could potentially change with administration of vasoactive drugs (L-NAME, indomethacin, and CM), the interpretation of changes in R2* is not straightforward. However, one could consider the following arguments. If blood volume was the dominant contributor to the observed change in R2*, one would expect R2* to reduce with vasoconstrictors (L-NAME and indomethacin). Because we actually observed significant increases in R2* after administration of these drugs, the contribution of blood volume at least is not a dominant contributor. Similarly, any potential temporal reduction in hemoglobin levels associated with high-viscosity CM should result in reduced R2* values.33 Because the reduction in hematocrit in one region should be associated with aggregation of cells downstream, a temporary increase in R2* is expected. However, we observed a large and persistent increase in R2* after high-viscosity CM such as iodixanol, which cannot be explained by changes in hematocrit alone. On the other hand, any direct physiological consequence of lower hemoglobin level is possible in terms of less oxygen delivery to the tissue. That would be consistent with an increased R2* because of reduced blood oxygenation. We could find no evidence in the literature for an effect of any CM at the doses used in clinical practice to reduce hemoglobin levels. Changes in R2 are potential confounders as demonstrated by recent report34 because R2 could change with compartmental volumes (tubular, vascular, and interstitial compartments), vasoactive drugs such as the ones used in this study could influence R2 and, hence, R2*. Interestingly, a previous report35 indicated that L-NAME, which would be considered a vasoconstrictor, resulted in an increase in tubular volume and, therefore, in a decrease in R2. On the basis of all the previously mentioned scenarios, the expected changes due to blood volume, hemoglobin, and R2 may not only be non–dominant contributors, but their effects may actually be counteracting the large increases observed in R2*. This suggests that our observations may actually be underestimated. Additional future studies should include R2 mapping to allow for better interpretation of changes observed with R2*. R2* may need to be corrected for bulk B0 inhomogeneity contributions.36 Future studies could also implement direct R2, (R2* – R2) mapping techniques.37
There are few things to note with our data in IM. Although our choice of ROIs is consistent with previously published reports,38 one cannot be sure about partial volume effects in IM with the collecting system. It is possible that IM could have contributions from the urine in the pelvis where changes in viscosity have been measured after high-viscosity CM.39 Previous studies with iodixanol have reported decreased apparent diffusion coefficient values in the urine after iodixanol because of the high viscosity.40 Because high viscosity should also decrease T2 and, hence, increase R2, there may be a significant contribution of R2 to the observed R2* values in IM. In 2 additional animals where we acquired additional R2 maps (data not shown), the data did suggest an increase in R2 in IM after iodixanol; however, the magnitude of change was relatively small (approximately 10 s–1) compared with the observed R2* change (approximately 100 s–1). Because it was also previously shown that L-NAME could reduce urine output,41 it may explain why the R2* was lower in the CIAKI-susceptible group after iodixanol. Although ioxalgate did show statistically significant increase in R2* in IM, the values were comparable with the defined threshold (estimated slope of 0.32 versus 0.3).
To evaluate any direct effect of CM on MRI measured parameters, we acquired data in a phantom made of tubes filled with different concentrations of iodixanol (0%, 25%, 50%, 75%, and 100%). We did observe a linear relationship between R2 (and R2*) and concentration (data not shown). However, the slopes with R2 (0.12) and R2* (0.16) were comparable, suggesting that the observed changes are predominantly caused by R2 in phantom studies. On the other hand, R1 showed much smaller change (slope, 0.007). These suggest that the observed changes are not caused by paramagnetic effect (ΔR1 > ΔR2). The observed changes in R2 most probably reflect the differences in physical properties of the fluid compared with saline. The R2* value for 100% iodixanol was 12 s–1, which is much smaller than the observed change in R2* in vivo after CM (approximately 100 s–1) especially in the CIAKI-susceptible animals.
In conclusion, our data support the use of BOLD MRI in CIAKI-susceptible rats (ie, pretreated with NO synthase and COX inhibitors) as a useful platform to test different CM and evaluate the efficacy of potential interventions to mitigate the risk for AKI. Our results lend support to the argument that viscosity of the contrast agent may play an equal or greater role than osmolality does in determining the degree of hypoxia in the ISOM. The agreement between NGAL and R2* values in ISOM (in groups 9 and 11) suggests that the observed immediate increase in R2* was pathophysiologic resulting in kidney injury and, hence, may be the earliest indicator of renal injury. Further studies are necessary to establish threshold values of R2* associated with AKI. Further studies should also address the specificity of R2* to renal oxygenation status. Given the noninvasive nature of BOLD MRI and previously demonstrated feasibility of using NGAL to document CIAKI in humans,18,42 the methodology can be immediately translated to humans.
ACKNOWLEDGMENTS
The authors thank Dr. Ioannis Koktzoglou for his assistance during the preparation of this article and Claire Feczko for her assistance in editing this article.
Sources of funding: Supported in part by grant RO1 DK-053221 from the National Institutes of Health.
Footnotes
Conflicts of interest:
The authors report no conflicts of interest.
REFERENCES
- 1.Seeliger E, Sendeski M, Rihal CS, et al. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J. 2012;33:2007–2015. doi: 10.1093/eurheartj/ehr494. [DOI] [PubMed] [Google Scholar]
- 2.Heyman SN, Reichman J, Brezis M. Pathophysiology of radiocontrast nephropathy: a role for medullary hypoxia. Invest Radiol. 1999;34:685–691. doi: 10.1097/00004424-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Heyman SN, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia adaptation, and the pathogenesis of radiocontrast nephropathy. Clin J Am Soc Nephrol. 2008;3:288–296. doi: 10.2215/CJN.02600607. [DOI] [PubMed] [Google Scholar]
- 4.Wong PC, Li Z, Guo J, et al. Pathophysiology of contrast-induced nephropathy. Int J Cardiol. 2012;158:186–192. doi: 10.1016/j.ijcard.2011.06.115. [DOI] [PubMed] [Google Scholar]
- 5.Agmon Y, Peleg H, Greenfeld Z, et al. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J Clin Invest. 1994;94:1069–1075. doi: 10.1172/JCI117421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.dos Santos EA, Li LP, Ji L, et al. Early changes with diabetes in renal medullary hemodynamics as evaluated by fiberoptic probes and BOLD magnetic resonance imaging. Invest Radiol. 2007;42:157–162. doi: 10.1097/01.rli.0000252492.96709.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen M, Dissing TH, Morkenborg J, et al. Validation of quantitative BOLD MRI measurements in kidney: application to unilateral ureteral obstruction. Kidney Int. 2005;67:2305–2312. doi: 10.1111/j.1523-1755.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 8.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94:3271–3275. doi: 10.1161/01.cir.94.12.3271. [DOI] [PubMed] [Google Scholar]
- 9.Gloviczki ML, Lerman LO, Textor SC. Blood oxygen level-dependent (BOLD) MRI in renovascular hypertension. Curr Hypertens Rep. 2011;13:370–377. doi: 10.1007/s11906-011-0218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermathen P, Binser T, Boesch C, et al. Three-year follow-up of human transplanted kidneys by diffusion-weighted MRI and blood oxygenation level-dependent imaging. J Magn Reson Imaging. 2012;35:1133–1138. doi: 10.1002/jmri.23537. [DOI] [PubMed] [Google Scholar]
- 11.Prasad PV, Priatna A, Spokes K, et al. Changes in intrarenal oxygenation as evaluated by BOLD MRI in a rat kidney model for radiocontrast nephropathy. J Magn Reson Imaging. 2001;13:744–747. doi: 10.1002/jmri.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li LP, Franklin T, Du H, et al. Intrarenal oxygenation by blood oxygenation level-dependent MRI in contrast nephropathy model: Effect of the viscosity and dose. J Magn Reson Imaging. 2012;36:1162–1167. doi: 10.1002/jmri.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald RJ, McDonald JS, Bida JP, et al. Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology. 2013;267:106–118. doi: 10.1148/radiol.12121823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peco-Antic A, Ivanisevic I, Vulicevic I, et al. Biomarkers of acute kidney injury in pediatric cardiac surgery. Clin Biochem. 2013;46:1244–1251. doi: 10.1016/j.clinbiochem.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Lacquaniti A, Buemi F, Lupica R, et al. Can neutrophil gelatinase-associated lipocalin help depict early contrast material-induced nephropathy? Radiology. 2013;267:86–93. doi: 10.1148/radiol.12120578. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann L, Simon-Zoula S, Nowak A, et al. BOLD-MRI for the assessment of renal oxygenation in humans: acute effect of nephrotoxic xenobiotics. Kidney Int. 2006;70:144–150. doi: 10.1038/sj.ki.5000418. [DOI] [PubMed] [Google Scholar]
- 17.Lancelot E, Idee JM, Couturier V, et al. Influence of the viscosity of iodixanol on medullary and cortical blood flow in the rat kidney: a potential cause of nephrotoxicity. J Appl Toxicol. 1999;19:341–346. doi: 10.1002/(sici)1099-1263(199909/10)19:5<341::aid-jat584>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 18.Xin C, Yulong X, Yu C, et al. Urine neutrophil gelatinase-associated lipocalin and interleukin-18 predict acute kidney injury after cardiac surgery. Ren Fail. 2008;30:904–913. doi: 10.1080/08860220802359089. [DOI] [PubMed] [Google Scholar]
- 19.Lancelot E, Idee JM, Lacledere C, et al. Effects of two dimeric iodinated contrast media on renal medullary blood perfusion and oxygenation in dogs. Invest Radiol. 2002;37:368–375. doi: 10.1097/00004424-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 20.McMahon GM, Waikar SS. Biomarkers in nephrology: Core Curriculum 2013. Am J Kidney Dis. 2013;62:165–178. doi: 10.1053/j.ajkd.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyman SN, Brezis M, Reubinoff CA, et al. Acute renal failure with selective medullary injury in the rat. J Clin Invest. 1988;82:401–412. doi: 10.1172/JCI113612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sendeski M, Patzak A, Persson PB. Constriction of the vasa recta, the vessels supplying the area at risk for acute kidney injury, by four different iodinated contrast media, evaluating ionic, nonionic, monomeric and dimeric agents. Invest Radiol. 2010;45:453–457. doi: 10.1097/RLI.0b013e3181d77eed. [DOI] [PubMed] [Google Scholar]
- 23.Jost G, Pietsch H, Sommer J, et al. Retention of iodine and expression of biomarkers for renal damage in the kidney after application of iodinated contrast media in rats. Invest Radiol. 2009;44:114–123. doi: 10.1097/RLI.0b013e318190fbd2. [DOI] [PubMed] [Google Scholar]
- 24.Jost G, Pietsch H, Lengsfeld P, et al. The impact of the viscosity and osmolality of iodine contrast agents on renal elimination. Invest Radiol. 2010;45:255–261. doi: 10.1097/RLI.0b013e3181d4a036. [DOI] [PubMed] [Google Scholar]
- 25.Haneder S, Augustin J, Jost G, et al. Impact of iso- and low-osmolar iodinated contrast agents on BOLD and diffusion MRI in swine kidneys. Invest Radiol. 2012;47:299–305. doi: 10.1097/RLI.0b013e318240a8ac. [DOI] [PubMed] [Google Scholar]
- 26.Wessely R, Koppara T, Bradaric C, et al. Choice of contrast medium in patients with impaired renal function undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2009;2:430–437. doi: 10.1161/CIRCINTERVENTIONS.109.874933. [DOI] [PubMed] [Google Scholar]
- 27.Alexopoulos E, Spargias K, Kyrzopoulos S, et al. Contrast-induced acute kidney injury in patients with renal dysfunction undergoing a coronary procedure and receiving non-ionic low-osmolar versus iso-osmolar contrast media. Am J Med Sci. 2010;339:25–30. doi: 10.1097/MAJ.0b013e3181c06e70. [DOI] [PubMed] [Google Scholar]
- 28.Heinrich MC, Haberle L, Muller V, et al. Nephrotoxicity of iso-osmolar iodixanol compared with nonionic low-osmolar contrast media: meta-analysis of randomized controlled trials. Radiology. 2009;250:68–86. doi: 10.1148/radiol.2501080833. [DOI] [PubMed] [Google Scholar]
- 29.Reed MC, Moscucci M, Smith DE, et al. The relative renal safety of iodixanol and low-osmolar contrast media in patients undergoing percutaneous coronary intervention. Insights from Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). J Invasive Cardiol. 2010;22:467–472. [PubMed] [Google Scholar]
- 30.Mehran R, Nikolsky E, Kirtane AJ, et al. Ionic low-osmolar versus nonionic iso-osmolar contrast media to obviate worsening nephropathy after angioplasty in chronic renal failure patients: the ICON (ionic versus non-ionic contrast to obviate worsening nephropathy after angioplasty in chronic renal failure patients) study. JACC Cardiovasc Interv. 2009;2:415–421. doi: 10.1016/j.jcin.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Seeliger E, Flemming B, Wronski T, et al. Viscosity of contrast media perturbs renal hemodynamics. J Am Soc Nephrol. 2007;18:2912–2920. doi: 10.1681/ASN.2006111216. [DOI] [PubMed] [Google Scholar]
- 32.Mager A, Vaknin Assa H, Lev EI, et al. The ratio of contrast volume to glomerular filtration rate predicts outcomes after percutaneous coronary intervention for ST-segment elevation acute myocardial infarction. Catheter Cardiovasc Interv. 2011;78:198–201. doi: 10.1002/ccd.22828. [DOI] [PubMed] [Google Scholar]
- 33.Lin W, Paczynski RP, Celik A, et al. Effects of acute normovolemic hemodilution on T2*-weighted images of rat brain. Magn Reson Med. 1998;40:857–864. doi: 10.1002/mrm.1910400611. [DOI] [PubMed] [Google Scholar]
- 34.Vivier PH, Storey P, Chandarana H, et al. Renal blood oxygenation level-dependent imaging: contribution of R2 to R2* values. Invest Radiol. 2013;48:501–508. doi: 10.1097/RLI.0b013e3182823591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Storey P, Ji L, Li LP, et al. Sensitivity of USPIO-enhanced R2 imaging to dynamic blood volume changes in the rat kidney. J Magn Reson Imaging. 2011;33:1091–1099. doi: 10.1002/jmri.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christen T, Lemasson B, Pannetier N, et al. Is T2* enough to assess oxygenation? Quantitative blood oxygen level-dependent analysis in brain tumor. Radiology. 2012;262:495–502. doi: 10.1148/radiol.11110518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Zhang Y, Yang X, et al. Feasibility of noninvasive quantitative measurements of intrarenal R(2) ’ in humans using an asymmetric spin echo echo planar imaging sequence. NMR Biomed. 2012;26:91–97. doi: 10.1002/nbm.2823. [DOI] [PubMed] [Google Scholar]
- 38.Pohlmann A, Hentschel J, Fechner M, et al. High temporal resolution parametric MRI monitoring of the initial ischemia/reperfusion phase in experimental acute kidney injury. PloS One. 2013;8:e57411. doi: 10.1371/journal.pone.0057411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ueda J, Nygren A, Sjoquist M, et al. Iodine concentrations in the rat kidney measured by x-ray microanalysis. Comparison of concentrations and viscosi-ties in the proximal tubules and renal pelvis after intravenous injections of contrast media. Acta Radiol. 1998;39:90–95. doi: 10.1080/02841859809172157. [DOI] [PubMed] [Google Scholar]
- 40.Jost G, Lenhard DC, Sieber MA, et al. Changes of renal water diffusion coefficient after application of iodinated contrast agents: effect of viscosity. Invest Radiol. 2011;46:796–800. doi: 10.1097/RLI.0b013e31822b1272. [DOI] [PubMed] [Google Scholar]
- 41.Reis WL, Saad WA, Camargo LA, et al. Central nitrergic system regulation of neuroendocrine secretion, fluid intake and blood pressure induced by angiotensin-II. Behav Brain Funct. 2010;6:64. doi: 10.1186/1744-9081-6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu XL, Wang ZJ, Yang Q, et al. Plasma neutrophil-gelatinase-associated lipocalin and cystatin C could early diagnose contrast-induced acute kidney injury in patients with renal insufficiency undergoing an elective percutaneous coronary intervention. Chin Med J (Engl) 2012;125:1051–1056. [PubMed] [Google Scholar]