Abstract
Ticks, as vectors of several notorious zoonotic pathogens, represent an important and increasing threat for human and animal health in Europe. Recent applications of new technology revealed the complexity of the tick microbiome, which may affect its vectorial capacity. Appreciation of these complex systems is expanding our understanding of tick-borne pathogens, leading us to evolve a more integrated view that embraces the ‘pathobiome’; the pathogenic agent integrated within its abiotic and biotic environments. In this review, we will explore how this new vision will revolutionize our understanding of tick-borne diseases. We will discuss the implications in terms of future research approaches that will enable us to efficiently prevent and control the threat posed by ticks.
KEYWORDS : co-infections, emerging diseases, Ixodes ricinus, new paradigm, next-generation sequencing, pathobiome, unknown pathogens, vector competence, zoonoses
Recent applications of next-generation sequencing (NGS) technology revealed the complexity of the tick microbiome, which may impact upon its vectorial capacity and consequently affect vector–reservoir host interactions. Appreciation of these complex systems is increasing our understanding of tick-borne pathogens, leading us to evolve a more integrated view that embraces the ‘pathobiome’ representing the pathogenic agent integrated within its abiotic and biotic environments including other pathogens, commensals or mutualists. In this review, we will explore how this emerging vision of tick-borne pathogens will revolutionize our understanding of tick-borne diseases, which are a growing concern given their exponential increase since the discovery of the Lyme disease agent. We will discuss the implications in terms of future research approaches that will enable us to efficiently prevent and control the threat posed by ticks.
Current state-of-the-art knowledge of tick-borne pathogens using ‘conventional vision’
• Expanding horizons of tick-borne pathogens
In Europe, the most prevalent tick-borne disease in humans is Lyme borreliosis (LB), caused by a group of bacteria belonging to the Borrelia burgdorferi sensu Iato group with at least five different species infecting humans in Europe [1]. Recently, B. miyamotoi, belonging to the more distantly related relapsing fever group, has been detected in patients in USA, Japan, Russia and The Netherlands [2–5], and is transmitted by the tick species involved in LB. Ticks can also be infected with other pathogens that might be transmitted to humans [6] (see Table 1). Among them, Anaplasma phagocytophilum is responsible for granulocytic anaplasmosis, Candidatus Neoerhlichia mikurensis has emerged as a cause of severe febrile illness in immunocompromised patients [7,8], while rickettsiae of the spotted fever group are known (R. monacenis, R. conorii) or suspected (R. helvetica) to cause rickettsioses [9,10]. Other bacterial pathogens such as Francisella tularensis, causing tularemia, and the Q fever agent Coxiella burnetii have also been detected in I. ricinus, but the direct role of this tick species in the epidemiology of these diseases is probably not significant [11,12]. Humans may develop babesiosis following tick-borne transmission of protozoans belonging to the genus Babesia, mainly B. divergens, however, the virulence of additional members of this genus, such as B. venatorum, have recently been confirmed [13]. Babesia microti, an emerging human tick-borne pathogen in USA, has also been identified in ticks in Europe, with one single human case to date [14]. Tick species also transmit arboviruses, with the tick-borne encephalitis virus being the most notorious in terms of public health in Europe [15,16]. Beside TBEV, many tick-borne viruses are known to be transmitted by other ticks. Among them, Crimean–Congo hemorrhagic fever virus (CCHFV) is considered to be one of the major emerging disease threats in the European Union following an expanding distribution of its main tick vector, the genus Hyalomma [17]. More anecdotally, Omsk virus, an endemic virus from rural regions in Siberia and transmitted by Dermancentor species, is expanding its range. This virus caused capillary damage responsible for the hemorrhagic manifestations [15]. Other European tick-borne viruses are less well established as causes of disease but case reports are emerging. Among them, Powassan virus, a member of the genus Flavivirus, has been recovered from the brains of patients following fatal infection [15]. Louping ill virus, also member of the genus Flavivirus, causes encephalitis in sheep, whereas exposed humans develop asymptomatic infection [15].
Table 1. . The predominant tick species present in the northern hemisphere, the pathogens they transmit, associated diseases, animal hosts as well as animal reservoirs of the corresponding pathogens.
| Ticks species | Pathogens | Diseases (hosts) | Reservoirs |
|---|---|---|---|
| Ixodes species | Borrelia burgdorferi sensu lato | Lyme disease (human, cattle, dog, horse) | Rodent, bird, reptile |
| Borrelia miyamotoi | Recurrent fever | Rodent, bird | |
| Anaplasma phagocytophilum | Granulocytic anaplasmosis (flu-like symptoms in human, cattle, goat, sheep, horse, dog, cat) | Wild ruminants, rodent | |
| Babesia divergens | Babesiosis (human, cattle) | Deer, cattle | |
| Babesia microti | Babesiosis (human) | rodent | |
| Babesia venatorum | |||
| Babesia capreoli | |||
| Coxiella burnetii | Q fever (human, goat, sheep…) | Rodent | |
| Francisella tularensis | Tularemia (human, rodents, sheep, goat, …) | Hare | |
| Bartonella henselae | Bartonellosis (human) | Cat | |
| Bartonella berkhoffii | Bartonellosis (dog, human) | Dog | |
| Tick-borne encephalitis Virus | TBE (human, dog) | Rodent | |
| Candidatus Neoehrlichia mikurensis | Fever (human, dogs) | Rodent | |
| Rickettsia helvetica (suspected) | Fever (human)? | Unknown | |
| Rickettsia monacensis | Fever (human) | Unknown | |
| Powassan virus | Fever, neurological signs (human) | Rodent | |
| |
Louping hill virus |
Encephalitis (human, sheep) |
Mountain hare, sheep |
| Dermacentor spp. | Anaplasma ovis | Anaplasmosis (goat, sheep) | Unknown |
| Babesia caballi | Babesiosis (horse) | Horse | |
| Theileria/Babesia equi | Theileriosis (horse) | Horse | |
| Babesia canis | Canine Babesiois | Dogs | |
| Rickettsia slovaca | TIBOLA/SENLAT (human) | Unknown | |
| Rickettsia raoultii | TIBOLA/SENLAT (human) | Unknown | |
| Anaplasma marginale | Bovine anaplasmosis (cattle) | Cattle | |
| Francisella tularensis | Tularemia (human, rodents sheep, goat, …) | Hare | |
| Coxiella burnetii | Q fever (human, goat, sheep, …) | Rodent | |
| Omsk hemorrhagic virus | Hemorrhagic manifestations (human) | Muskrat | |
| |
Powassan virus |
Fever, neurological signs (human) |
Rodent |
| Haemaphysalis spp. | Babesia spp. | Babesiosis (human, possibly cattle and dog) | Unknown |
| |
Theileria spp. |
Theileriosis (cattle) |
Unknown |
| Hyalomma spp. | Theileria annulata | Theileriosis (cattle) | Unknown |
| Theileria equi | |||
| |
Crimean-Congo hemorrhagic fever virus* |
Hemorrhagic fever (human) |
Rodent, bird? |
| Rhipicephalus sanguineus | Rickettsia conorii | Mediterranean spotted fever (human) | Dog? |
| Ehrlichia canis | Ehrlichiosis (dog) | Dog | |
| Anaplasma platys | Cyclic thrombocytopenia | Dog | |
| Babesia vogeli/canis | Canine babesiosis | Dog | |
| Hepatozoon canis | Hepatozoonosis | Dog | |
| Babesia gibsoni | Canine Babesiosis | Dog |
An increasing number of new species, strains or genetic variants of other micro-organisms are being detected in ticks, resulting in an ever-increasing list of (potential) pathogens capable of infecting livestock, companion animals and humans. However, it needs to be taken into account that a significant portion of these ‘new’ species/genotypes are not truly emerging, but rather newly detected. This increasing recognition of pathogen biodiversity is not generating answers, but instead raising rather complex questions regarding ecological cycles of pathogens, their polymicrobial cross-talk and their influence upon infection mechanisms, clinical differential diagnosis and intervention opportunities.
Identification of micro-organisms in ticks has been largely dominated by the use of conventional molecular approaches mostly using specific primers combined with (real-time) PCR, and less frequently by culture-dependent methods. However, pathogen detection in an arthropod is not sufficient to validate its vector competence. This entails use of vector competence studies to establish both the interaction of new or unexpected pathogen with ticks, and to evaluate the risk of exposure for both humans and animals. These types of studies require living ticks raised under controlled conditions. Because of their complex biological cycle and their feeding biology, maintenance of tick colonies and their infection with micro-organisms is not easy. However, several methods have been successfully developed and used to infect hard ticks with pathogens, for example, feeding ticks on infected animals, injecting pathogens through the cuticule, by using thin capillary tubes and feeding ticks on infected blood through artificial or animal-derived membranes [18]. These methods have been successfully employed to validate vector competence for a number of tick-borne pathogens, including Lyme spirochaetes [19], A. phagocytophilum [20], Babesia sp. EU1 (or B. venatorum) [21] Bartonella sp. [22,23] and tick-borne encephalitis virus [24,25]. However, for some established tick-borne pathogens, such as Ca. N. mikurensis or R. helvetica (both of which currently lack any cultivable strain), the tick vector competence remains to be proven. These are consequently considered ‘de facto’ tick-borne pathogens under more or less strong ‘epidemiological evidence’.
• Diagnostic challenges posed by tick-borne pathogens/diseases
Given a clinical history of tick bites, Lyme borreliosis is the primary consideration, but in some this diagnosis remains elusive being unconfirmed by conventional serological tests [26]. People bitten by ticks can also be infected by tick-borne encephalitis virus (TBEV) causing severe encephalitis, which is readily diagnosed by serological tests [15]. TBE can be successfully prevented by active immunization, but no specific treatment is available [27]. As already mentioned, ticks are capable of transmitting the largest variety of pathogens among arthropod vectors, and pathogens other than the Lyme or tick-borne encephalitis agents might be involved in tick-borne diseases (TBDs). Interestingly, the majority of those pathogens have been discovered during the last 20 years. The symptoms induced by those pathogens are often mild and nonspecific (high fever, fatigue, body aches, chills, etc.) and can be confused with symptoms caused by infection with other agents. This is probably the underpinning reason why these infections are poorly recognized in humans by medical practitioners despite their abundance in ticks and/or reservoir animals. A striking example is that of B. miyamotoi. This Borrelia species was first isolated from Japanese Ixodes ticks in 1995 whereby it was considered a nonpathogenic endogenous tick bacterium until the first human cases of B. miyamotoi infection were reported in Russia some 16 years later [2]. Subsequently, human infections have been described in the USA and most recently in The Netherlands [3–5,28]. Circulation of B. miyamotoi between I. ricinus and wild animals has been confirmed in other European countries such as France, Estonia, Poland and Switzerland [29], which has confirmed that the French genotype is identical to an isolate from a Dutch patient [30]. Despite this apparent absence of human cases of B. miyamotoi infections among these countries, this is likely to reflect the absence of serological or molecular tests for B. miyamotoi combined with the lack of knowledge of these bacteria among medical practitioners. Thus, it is likely that the absence of human infections is rather due to missed diagnoses than to an actual absence of infection.
Those patients bitten by ticks are additionally at risk for co-infection by several pathogens. For instance, Horowitz et al. [31] described co-infection rates ranging from 2 to 5% for Borrelia species and A. phagocytophilum among patients with erythema migrans, the diagnostic hallmark for Lyme borreliosis. Co-infections between B. afzelii and R. monacensis were also identified in skin biopsy of erythema migrans patients in The Netherlands [32]. However, co-infections are rarely diagnosed in routine practice, alerting us to the problem that co-infection in humans a relevant, albeit understudied issue, with important implications for public health.
Consequently, individuals infected by pathogens other than Lyme borreliosis spirochaetes or TBEV, are rarely identified. In recent years, unexplained syndromes occurring after tick bites have became an increasingly important issue leading to considerable discord between scientists, patients and institutions of infectious disease.
The technology-driven revolution of tick-borne pathogen's vision
• From pathogen to pathobiome
Until now, most studies detecting pathogens in ticks have used assays that are only able to assess a limited number of agents simultaneously [33,34]. This is partly due to technological limitations making complete screens of micro-organisms in their natural vector/reservoir populations using standard laboratory procedures unachievable. Within the last few years, the rapid development of NGS methods has revolutionized the research field of epidemiology and diagnosis of infectious diseases, thus facilitating complete screening of pathogens within their hosts, discovery of new pathogens or the detection of unexpected ones. NGS has recently been successfully used to identify the bacterial communities associated with I. ricinus [35–38] based on the amplification and sequencing of hypervariable regions of the 16S rRNA encoding genes (metagenomic profile), revealing a highly diverse microbial community (108 genera representing all bacterial phyla). As expected, those approaches have allowed detection without a priori established tick-borne pathogens, such as the Borrelia, Anaplasma, Coxiella, Francisella or Rickettsia genus. Those genera, mostly known as pathogenic for vertebrates, while other species are considered as endosymbionts (e.g., the Rickettsia-endosymbiont of I. scapularis) [39], highlight the challenge of differentiating between pathogens and endosymbionts. Adding further complexity, some authors consider Rickettsia species as endosymbionts that are transmitted vertically in arthropods, and only secondarily serve as pathogens of vertebrates [40]. For the Coxiella genus, the species C. burnetii is mostly considered as a vertebrate pathogen while numerous other Coxiella species have been found associated to ticks [41]. Phylogenetic analyses combined with experimental approaches suggested that these might also be considered as endosymbionts of ticks [11,42]. Thus, the pathogenic nature of C. burnetii could be an exception within the genus [43]. Beside the well-known vertebrate pathogenic species, F. tularensis (occasionally found in ticks), Francisella-like-endosymbionts associated with Dermacentor spp. have been described but their potential pathogenic nature remains to be investigated [44]. The Wolbachia and Arsenophonus genera are also bacteria associated with arthropods (mostly insects) and influence reproduction and/or immunity of their hosts [45,46]. They have also been found associated with ticks [47]. However, a recent study revealed that in I. ricinus, the finding of Wolbachia is a consequence of parasitism by a parasitoid wasp (Ixodiphagus hookeri) [48]. The role of Arsenophonus as a tick endosymbiont is still to be demonstrated. Finally, the endosymbiont Midichloria mitochondrii was initially observed in tick cells (especially in ovarian cells of I. ricinus) [49]. Use of molecular probes specific for this alphaproteobacteria have demonstrated their presence in almost 100% of I. ricinus females derived from natural populations [50], but also in other tick species [51]. Furthermore, M. mitochondri has recently been implicated as a potential vertebrate pathogen [52].
Use of NGS technology will undoubtedly shed new light on the intriguing bacterial communities associated with ticks [37]. The clear-cut boundaries between the so-called ‘vertebrate-pathogens’, ‘arthropod-pathogens’ or ‘arthropod-symbionts’ may thus fade into a more dynamic and complex vision of bacterial–vector–vertebrate communities. Better knowledge of the role of these bacteria could even constitute useful resources for developing antivectorial control measures.
Besides the known micro-organisms (either belonging to pathogens, endosymbionts or both), NGS also revealed that the majority of RNA/DNA sequences carried by ticks belonged to unknown micro-organisms. For instance, 80% of the viral nucleic sequences detected from tick extracts represented currently unidentified micro-organisms (Vayssier-Taussat et al., Unpublished Data). Among these new viral sequences, we identified genera transmissible to humans and/or animals via arthropods, including Bunyaviridae (Nairovirus and Phlebovirus), Rhabdoviridae (Vesiculovirus) and Reoviridae (Coltivirus) [vayssier-taussat et al., unpublished data]. In the USA, a similar study, undertaken by Lipkin et al., characterized the virome of different tick species. Powassan virus, a well-known human pathogenic tick-borne virus, and eight novel viruses belonging to nairovirus, phlebovirus and mononegavirus genera were identified among the three ticks assessed [53]. New viruses recently identified in ticks by NGS are listed in Table 2.
Table 2. . New viruses recently identified in ticks by next-generation sequencing.
| Viruses | Diseases | Tick species | Ref. |
|---|---|---|---|
|
Nairovirus (South Bay virus) |
Unknown |
Ixodes scapularis |
[53] |
| Blacklegged tick Phlebovirus (BTPV) |
Unknown |
Ixodes scapularis |
[53] |
| American dog tick Phlebovirus (ADTPV) |
Unknown |
Ixodes scapularis/D. variabilis |
[53] |
| Monongavirales-like virus |
Unknown |
Ixodes scapularis |
[53] |
|
Phlebovirus (Hearltand virus) |
Severe febrile illness |
Amblyomma americanum |
[84] |
| Shibuinji virus (New tick-borne virus phlebovirus) | Unknown | Rhipicephalus spp. | [85] |
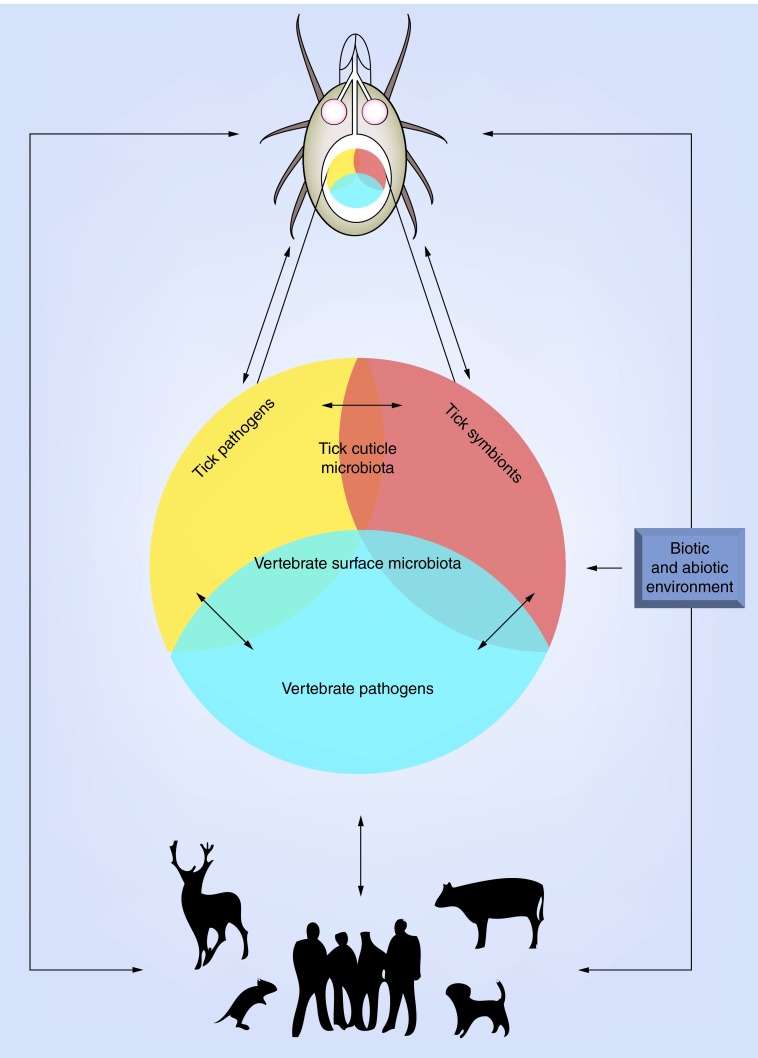
By understanding the entire tick microbial community, we can identify that pathogens are intimately associated to the vast community of micro-organisms (including other pathogens) and by elucidating their influence on tick biology, pathogen persistence, transmission and virulence justifies the need to shift from the study of isolated pathogens to a more integrated approach. Within this context, we define the ‘pathobiome’ as representing the pathogen within its abiotic and biotic environment [54] (See Figure 1). Understanding the multifactorial pathobiome requires comprehensive knowledge of the microbial community comprising the pathobiome, the network of interactions between microbes and the biological relevance of these interactions.
Figure 1. . The tick pathobiome concept.
• Deciphering microbial interactions within the tick ecosystem
Microbial interactions have largely been considered on a one-to-one interaction level, where the infection by one pathogen influences the acquisition of and/or dynamics of infection by a second pathogen. However, interactions between sets of pathogens can be considered when different pathogens interact within a network or through ‘cascade consequence’ [55,56]. In experimental studies, one can investigate how the presence of one pathogen may interfere with infection by another, however, this is not possible using the pathobiome perspective where many pathogens and other micro-organisms are present, including those members that remain poorly understood. In such a scenario, one can use population studies to assess dynamics of change through the probability of finding those pathogens together beyond those which could occur by chance. Seeking microbial congruence initially assesses this, even though this can also result from confounding factors that create statistical associations between pathogens, without true biological interactions. In population studies, longitudinal or time series data are useful for identifying pathogen associations, identifying whether the presence of one pathogen modifies subsequent infection by another [57]. However, such studies are resource intensive. An alternative is to run one-off cross-sectional studies, which are cheaper and less time consuming than longitudinal studies. Cross-sectional studies can easily be used to detect several pathogens and are especially appropriate in the case of emerging or poorly known pathogens or host species. In such cases, numerous approaches are available to detect pathogen associations. Multivariate analyses (e.g., PCA, FCA, DA, CoA) [58] will evaluate which pathogens tend to group together. However, statistical tests associated with these analyses are usually not available [but see, for example, permutation methods, 59,60]. A new modeling approach was to develop ‘the association screening approach’ to detect the overall and more detailed multipathogen associations [61]. This method is quite powerful but would require over 1000 samples if one were to study over ten micro-organisms. However, strong methodological developments on robust network analytical methods have been made [62] and continue to evolve (e.g., in medicine: metabolic pathways [63,64]; in computer science: peer-to-peer networks [65]; or in social science: scientific collaboration [66]). They also offer an attractive representation of assessing dynamics of multiple pathogen relationships. They provide indices of association such as connectance [67], nestedness [68] or betweeness [69]. However, to date, statistical tests regarding the network parameters have rarely been used, although developments in this field are promising.
• Importance of the pathobiome concept to elucidate competence mechanisms
Microbes present with other pathogens in ticks may interfere with pathogen transmission. For instance, Rickettsial endosymbionts are thought to alter transmission of other rickettsial pathogens, as seen by the inverse relationship between the infection prevalence of R. rickettsii (pathogen) and R. peacockii (symbiont) in Dermancentor andersoni [47,70]. Furthermore, the presence of Coxiella-related symbionts in the salivary glands of Amblyomma ticks impairs transmission of Ehrlichia chaffeensis [71]. In addition to symbionts, ticks are also colonized by a natural bacterial microbiota mainly belonging to the Proteobacteria, Firmicutes and Bacteroides phyla [72]. It has also been demonstrated that these tick microbiomes can interfere with pathogens. For example, when ticks were bred in a sterile environment, the absence of microbiota altered gut integrity and the ability of B. burgdorferi to colonize [72]. Microbiome alterations might also result in a modulated immune response which might then interfere with pathogen survival and infection, as shown for other arthropod vectors [73]. Thus taking into account the pathobiome rather than the isolated pathogens, is crucial to understand how pathogens are transmitted and how they survive within ticks.
Pathobiome approach for surveillance, diagnosis & prevention of tick-borne diseases
• Surveillance & diagnosis
Considering the vast number of potential tick-borne pathogens that can result in disease, either alone or in association, there is an urgent need to develop methods that are capable of assessing this diversity, as well as providing insights into the biology of tick-borne pathogens. For instance, many tick-borne pathogens colonize blood (residing within either intra- or extracellular niches) of vertebrate hosts. Thus, it makes sense to detect the presence of their DNA in the blood of infected human patients. However, blood infection does not occur for all tick-borne pathogens. A notable exception is the Lyme spirochete that does not stably infect the blood of human hosts, therefore, detection of DNA in the blood of such a patient bitten by a tick is unhelpful, necessitating the use of more specific samples (such as skin biopsies) or serological tests, although their specificity and sensitivity are not always optimal. Molecular identification of tick-borne pathogens has mostly been based on the use of specific primers combined with real-time PCR, which can only detect a selected and limited number of species simultaneously. To overcome these limitations, new tools enabling high-throughput monitoring of tick-borne pathogens were an urgent priority. Based upon NGS data on presence of tick-borne pathogens in ticks in different European geographical regions, we developed a microfluidic system allowing multiple parallel real-time PCRs for TBD surveillance that might be adapted to diagnostic settings [74]. This has the unique ability to simultaneously analyze multiple pathogens (up to 48 different species) in the same sample. This new tool presents the major advantage and can be easily adapted to new or emerging situations as it is entirely possible to remove primers/probe sets in order to modify the panel of targeted pathogens. If developed by private companies, this approach will represent an important improvement for the diagnosis of TBD.
• Vaccination
Given the vast number of pathogens/potential pathogens that could be transmitted by the same tick species, deployment of tick vaccines would be both a smart and environmentally friendly alternative to protect human and animal populations against tick-borne diseases. This novel approach for control of vector infestations and thus reducing subsequent pathogen transmission necessitates a deep understanding of microbial interactions within the tick. For that purpose, research on molecular interactions between ticks and pathogens, as well as the identification of suitable targets for vaccine development, are major challenges for the implementation of new TBD control strategies [75]. Among these, target molecules playing key roles in vector capacity are particularly promising [76]. To date, the only commercially available antitick vaccine is based on the R. microplus midgut protein BM86 that interferes with tick feeding and subsequent egg production [77]. However, owing to technological advances for tick infection combined with improved resolution of molecular investigative methods, further promising candidates have recently been identified. These include tick proteins derived from I. ricinus [78,79], I. scapularis [80], Rhipicephalus microplus [81,82], as well as candidates common to several hard tick species [83]. Improving our understanding of molecular interactions between ticks and tick-borne pathogens is an essential prerequisite for the conception of future generations of vaccines and for vectors and disease control.
Conclusion
Owing to powerful molecular and technological advancements, the tick pathobiome vision now offers a new vantage point to understand tick-borne pathogens from a more holistic point of view.
Future perspective
Shifting the paradigm from pathogens to pathobiome will have many research consequences; the most important being how to determine the significance of micro-organisms revealed by NGS technology in human and/or animal idiopathic disease following tick bites; and to decipher the impact of complex microbial interactions between pathogens and/or other tick endogenous micro-organisms that might influence pathogen transmission, persistence, virulence and evolution. Based upon this new knowledge, new research avenues will have to be followed to develop adequate strategies to better diagnose and combat tick-borne diseases
EXECUTIVE SUMMARY.
Current knowledge on tick-borne pathogens
An increasing number of ‘new’ species, strains or genetic variants of micro-organisms are being detected in ticks, resulting in an ever-increasing list of potential pathogens.
This increasing recognition of pathogen diversity is raising complex questions regarding ecological cycles of the pathogen, polymicrobial cross-talk, diagnosis and intervention opportunities.
The new vision
Next-generation technology sheds new lights on bacterial communities associated with ticks.
The majority of DNA/RNA sequences carried by ticks belong to unknown micro-organisms.
Pathogens are intimately associated with the tick microbial community.
This justifies the need to shift the research focus from isolated pathogens to a more integrated pathobiome approach.
Future research directions in term of surveillance, diagnosis & prevention of tick-borne diseases
New tools enabling high-throughput monitoring of tick-borne pathogens are an urgent priority.
Given the vast number of pathogens that could be transmitted by the same tick species, deployment of tick vaccines would be a smart and environmentally friendly alternative to protect human and animal populations from tick-borne diseases.
Acknowledgements
The authors thank the community of enthusiastic scientists exploring new insights in ticks and tick-borne pathogens in Europe, especially those participating in the Edenext project and the COST action Eurnegvec.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Rizzoli A, Hauffe H, Carpi G, Vourc HG, Neteler M, Rosa R. Lyme borreliosis in Europe. Euro. Surveill. 2011;16(27):1–8. [PubMed] [Google Scholar]
- 2.Platonov AE, Karan LS, Kolyasnikova NM, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011;17(10):1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chowdri HR, Gugliotta JL, Berardi VP, et al. Borrelia miyamotoi infection presenting as human granulocytic anaplasmosis: a case report. Ann. Intern. Med. 2013;159(1):21–27. doi: 10.7326/0003-4819-159-1-201307020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Krause PJ, Narasimhan S, Wormser GP, et al. Human Borrelia miyamotoi infection in the United States. N. Engl. J. Med. 2013;368(3):291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hovius JW, De Wever B, Sohne M, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382(9892):658. doi: 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First case of Borrelia miyamotoi in Europe.
- 6.Heyman P, Cochez C, Hofhuis A, et al. A clear and present danger: tick-borne diseases in Europe. Expert Rev. Anti Infect. Ther. 2010;8(1):33–50. doi: 10.1586/eri.09.118. [DOI] [PubMed] [Google Scholar]
- 7.Welinder-Olsson C, Kjellin E, Vaht K, Jacobsson S, Wenneras C. First case of human “Candidatus Neoehrlichia mikurensis” infection in a febrile patient with chronic lymphocytic leukemia. J. Clin. Microbiol. 2010;48(5):1956–1959. doi: 10.1128/JCM.02423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Loewenich FD, Geissdorfer W, Disque C, et al. Detection of “Candidatus Neoehrlichia mikurensis” in two patients with severe febrile illnesses: evidence for a European sequence variant. J. Clin. Microbiol. 2010;48(7):2630–2635. doi: 10.1128/JCM.00588-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parola P, Raoult D. Tick-borne bacterial diseases emerging in Europe. Clin. Microbiol. Infect. 2001;7(2):80–83. doi: 10.1046/j.1469-0691.2001.00200.x. [DOI] [PubMed] [Google Scholar]
- 10.Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin. Infect. Dis. 2001;32(6):897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 11.Duron O, Jourdain E, McCoy KD. Diversity and global distribution of the Coxiella intracellular bacterium in seabird ticks. Ticks Tick Borne Dis. 2014;5(5):557–563. doi: 10.1016/j.ttbdis.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Angelakis E, Raoult D. Q Fever. Vet. Microbiol. 2010;140(3–4):297–309. doi: 10.1016/j.vetmic.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Hildebrandt A, Hunfeld KP. [Human babesiosis – a rare but potentially dangerous zoonosis] Dtsch Med. Wochenschr. 2014;139(18):957–962. doi: 10.1055/s-0034-1369936. [DOI] [PubMed] [Google Scholar]
- 14.Hildebrandt A, Hunfeld KP, Baier M, et al. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur J. Clin. Microbiol. Infect Dis. 2007;26(8):595–601. doi: 10.1007/s10096-007-0333-1. [DOI] [PubMed] [Google Scholar]
- 15.Charrel RN, Attoui H, Butenko AM, et al. Tick-borne virus diseases of human interest in Europe. Clin. Microbiol. Infect. 2004;10(12):1040–1055. doi: 10.1111/j.1469-0691.2004.01022.x. [DOI] [PubMed] [Google Scholar]
- 16.Hubalek Z, Rudolf I. Tick-borne viruses in Europe. Parasitol. Res. 2012;111(1):9–36. doi: 10.1007/s00436-012-2910-1. [DOI] [PubMed] [Google Scholar]
- 17.Mertens M, Schmidt K, Ozkul A, Groschup MH. The impact of Crimean-Congo hemorrhagic fever virus on public health. Antiviral Res. 2013;98(2):248–260. doi: 10.1016/j.antiviral.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Liu XY, Bonnet SI. Hard tick factors implicated in pathogen transmission. PLoS Negl. Trop. Dis. 2014;8(1):e2566. doi: 10.1371/journal.pntd.0002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgdorfer W. Discovery of the Lyme disease spirochete and its relation to tick vectors. Yale J. Biol. Med. 1984;57(4):515–520. [PMC free article] [PubMed] [Google Scholar]
- 20.Massung RF, Mather TN, Priestley RA, Levin ML. Transmission efficiency of the AP-variant 1 strain of Anaplasma phagocytophila . Ann. NY Acad. Sci. 2003;990:75–79. doi: 10.1111/j.1749-6632.2003.tb07340.x. [DOI] [PubMed] [Google Scholar]
- 21.Bonnet S, Brisseau N, Hermouet A, Jouglin M, Chauvin A. Experimental in vitro transmission of Babesia sp. (EU1) by Ixodes ricinus . Vet. Res. 2009;40(3):21. doi: 10.1051/vetres/2009004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotte V, Bonnet S, Le Rhun D, et al. Transmission of Bartonella henselae by Ixodes ricinus . Emerg. Infect. Dis. 2008;14(7):1074–1080. doi: 10.3201/eid1407.071110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reis C, Cote M, Le Rhun D, et al. Vector competence of the tick Ixodes ricinus for transmission of Bartonella birtlesii . PLoS Negl. Trop. Dis. 2011;5(5):e1186. doi: 10.1371/journal.pntd.0001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alekseev AN, Chunikhin SP. [The experimental transmission of the tick-borne encephalitis virus by ixodid ticks (the mechanisms, time periods, species and sex differences)] Parazitologiia. 1990;24(3):177–185. [PubMed] [Google Scholar]
- 25.Alekseev AN, Chunikhin SP. [The exchange of the tick-borne encephalitis virus between ixodid ticks feeding jointly on animals with a subthreshold level of viremia] Meditsinskaia parazitologiia i parazitarnye bolezni. 1990;2:48–50. [PubMed] [Google Scholar]
- 26.Perronne C. Lyme and associated tick-borne diseases: global challenges in the context of a public health threat. Front. Cell. Infect. Microbiol. 2014;4:74. doi: 10.3389/fcimb.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaiser R. Tick-borne encephalitis: clinical findings and prognosis in adults. Wien. Med. Wochenschr. 2012;162(11–12):239–243. doi: 10.1007/s10354-012-0105-0. [DOI] [PubMed] [Google Scholar]
- 28.Gugliotta JL, Goethert HK, Berardi VP, Telford SR., 3rd Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N. Engl. J. Med. 2013;368(3):240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vayssier-Taussat M, Moutailler S, Michelet L, et al. Next generation sequencing uncovers unexpected bacterial pathogens in ticks in western Europe. PLoS ONE. 2013;8(11):e81439. doi: 10.1371/journal.pone.0081439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosson Jf ML, Chotte J, Le Naour E, et al. Genetic characterization of the human relapsing fever spirochete Borrelia miyamotoi in vectors and animal reservoirs of Lyme disease spirochetes in France. Vectors Parasites. 2015 doi: 10.1186/1756-3305-7-233. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horowitz HW, Aguero-Rosenfeld ME, Holmgren D, et al. Lyme disease and human granulocytic anaplasmosis coinfection: impact of case definition on coinfection rates and illness severity. Clin. Infect. Dis. 2013;56(1):93–99. doi: 10.1093/cid/cis852. [DOI] [PubMed] [Google Scholar]; • Illustrates the possible co-infection of humans by tick-borne pathogens and their impact on illness severity.
- 32.Tijsse-Klasen E, Sprong H, Pandak N. Co-infection of Borrelia burgdorferi sensu lato and Rickettsia species in ticks and in an erythema migrans patient. Parasit. Vectors. 2013;6:347. doi: 10.1186/1756-3305-6-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halos L, Bord S, Cotte V, et al. Ecological factors characterizing the prevalence of bacterial tick-borne pathogens in Ixodes ricinus ticks in pastures and woodlands. Appl. Environ. Microbiol. 2010;76(13):4413–4420. doi: 10.1128/AEM.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reis C, Cote M, Paul RE, Bonnet S. Questing ticks in suburban forest are infected by at least six tick-borne pathogens. Vector Borne Zoonotic Dis. 2011;11(7):907–916. doi: 10.1089/vbz.2010.0103. [DOI] [PubMed] [Google Scholar]
- 35.Carpi G, Cagnacci F, Wittekindt NE, et al. Metagenomic profile of the bacterial communities associated with Ixodes ricinus ticks. PLoS ONE. 2011;6(10):e25604. doi: 10.1371/journal.pone.0025604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakao R, Abe T, Nijhof AM, et al. A novel approach, based on BLSOMs (Batch Learning Self-Organizing Maps), to the microbiome analysis of ticks. ISME J. 2013;7(5):1003–1015. doi: 10.1038/ismej.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawlena H, Rynkiewicz E, Toh E, et al. The arthropod, but not the vertebrate host or its environment, dictates bacterial community composition of fleas and ticks. ISME J. 2013;7(1):221–223. doi: 10.1038/ismej.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams-Newkirk AJ, Rowe LA, Mixson-Hayden TR, Dasch GA. Characterization of the bacterial communities of life stages of free living lone star ticks (Amblyomma americanum) PLoS ONE. 2014;9(7):e102130. doi: 10.1371/journal.pone.0102130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillespie JJ, Joardar V, Williams KP, et al. A Rickettsia genome overrun by mobile genetic elements provides insight into the acquisition of genes characteristic of an obligate intracellular lifestyle. J. Bacteriol. 2012;194(2):376–394. doi: 10.1128/JB.06244-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perlman SJ, Hunter MS, Zchori-Fein E. The emerging diversity of Rickettsia. Proc. Biol. Sci. 2006;273(1598):2097–2106. doi: 10.1098/rspb.2006.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duron O, Noel V, McCoy KD, et al. The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q fever pathogen, Coxiella burnetii . PLoS Pathog. 2015;11(5):e1004892. doi: 10.1371/journal.ppat.1004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almeida AP, Marcili A, Leite RC, et al. Coxiella symbiont in the tick Ornithodoros rostratus (Acari: Argasidae) Ticks Tick Borne Dis. 2012;3(4):203–206. doi: 10.1016/j.ttbdis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Machado-Ferreira E, Dietrich G, Hojgaard A, et al. Coxiella symbionts in the Cayenne tick Amblyomma cajennense . Microb. Ecol. 2011;62(1):134–142. doi: 10.1007/s00248-011-9868-x. [DOI] [PubMed] [Google Scholar]
- 44.Kreizinger Z, Hornok S, Dan A, et al. Prevalence of Francisella tularensis and Francisella-like endosymbionts in the tick population of Hungary and the genetic variability of Francisella-like agents. Vector Borne Zoonotic Dis. 2013;13(3):160–163. doi: 10.1089/vbz.2012.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudolf I, Mendel J, Sikutova S, et al. 16S rRNA gene-based identification of cultured bacterial flora from host-seeking Ixodes ricinus, Dermacentor reticulatus and Haemaphysalis concinna ticks, vectors of vertebrate pathogens. Folia Microbiol. (Praha) 2009;54(5):419–428. doi: 10.1007/s12223-009-0059-9. [DOI] [PubMed] [Google Scholar]; • First identification of tick microflora using a 16S approach.
- 46.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008;6(10):741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 47.Ahantarig A, Trinachartvanit W, Baimai V, Grubhoffer L. Hard ticks and their bacterial endosymbionts (or would be pathogens) Folia Microbiol. (Praha) 2013;58(5):419–428. doi: 10.1007/s12223-013-0222-1. [DOI] [PubMed] [Google Scholar]
- 48.Plantard O, Bouju-Albert A, Malard MA, Hermouet A, Capron G, Verheyden H. Detection of Wolbachia in the tick Ixodes ricinus is due to the presence of the hymenoptera endoparasitoid Ixodiphagus hookeri . PLoS ONE. 2012;7(1):e30692. doi: 10.1371/journal.pone.0030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis D. The detection of Rickettsia-like micro-organisms within the ovaries of female Ixodes ricinus ticks. Z. Parasitenkd. 1979;59(3):295–298. doi: 10.1007/BF00927523. [DOI] [PubMed] [Google Scholar]
- 50.Sassera D, Beninati T, Bandi C, et al. ‘Candidatus Midichloria mitochondrii’ an endosymbiont of the tick Ixodes ricinus with a unique intramitochondrial lifestyle. Int. J. Syst. Evol. Microbiol. 2006;56(Pt 11):2535–2540. doi: 10.1099/ijs.0.64386-0. [DOI] [PubMed] [Google Scholar]
- 51.Epis S, Sassera D, Beninati T, et al. Midichloria mitochondrii is widespread in hard ticks (Ixodidae) and resides in the mitochondria of phylogenetically diverse species. Parasitology. 2008;135(4):485–494. doi: 10.1017/S0031182007004052. [DOI] [PubMed] [Google Scholar]
- 52.Bazzocchi C, Mariconti M, Sassera D, et al. Molecular and serological evidence for the circulation of the tick symbiont Midichloria (Rickettsiales: Midichloriaceae) in different mammalian species. Parasit. Vectors. 2013;6:350. doi: 10.1186/1756-3305-6-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tokarz R, Williams SH, Sameroff S, Sanchez Leon M, Jain K, Lipkin WI. Virome ana-lysis of Amblyomma americanum, Dermacentor variabilis and Ixodes scapularis ticks reveals novel highly divergent vertebrate and invertebrate viruses. J. Virol. 2014;88(19):11480–11492. doi: 10.1128/JVI.01858-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vayssier-Taussat M, Albina E, Citti C, et al. Shifting the paradigm from pathogens to pathobiome: new concepts in the light of meta-omics. Front. Cell. Infect. Microbiol. 2014;4:29. doi: 10.3389/fcimb.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rigaud T, Perrot-Minnot MJ, Brown MJ. Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. J. Royal Stat. Soc. B. 2010;277(1701):3693–3702. doi: 10.1098/rspb.2010.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bordes F, Morand S. The impact of multiple infections on wild animal hosts: a review. Infect. Ecol. Epidemiol. 2011;1:1–10. doi: 10.3402/iee.v1i0.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Telfer S, Lambin X, Birtles R, et al. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330(6001):243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gauch HG. Multivariate Analysis in Community Ecology. Cambridge University Press; UK: 1982. [Google Scholar]
- 59.Tollenaere C, Bryja J, Galan M, et al. Multiple parasites mediate balancing selection at two MHC class II genes in the fossorial water vole: insights from multivariate analyses and population genetics. J. Evolut. Biol. 2008;21(5):1307–1320. doi: 10.1111/j.1420-9101.2008.01563.x. [DOI] [PubMed] [Google Scholar]
- 60.Salvador AR, Guivier E, Xuereb A, et al. Concomitant influence of helminth infection and landscape on the distribution of Puumala hantavirus in its reservoir, Myodes glareolus . BMC Microbiol. 2011;11(1):30. doi: 10.1186/1471-2180-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaumourin E, Vourc'h G, Telfer S, et al. To be or not to be associated: power study of four statistical modeling approaches to identify parasite associations in cross-sectional studies. Front. Cell. Infect. Microbiol. 2014;4:62. doi: 10.3389/fcimb.2014.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bascompte J. Networks in ecology. Basic Appl. Ecol. 2007;8(6):485–490. [Google Scholar]
- 63.Ravasz E, Somera AL, Mongru DA, Oltvai ZN, Barabasi AL. Hierarchical organization of modularity in metabolic networks. Science. 2002;297(5586):1551–1555. doi: 10.1126/science.1073374. [DOI] [PubMed] [Google Scholar]
- 64.Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in Type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 65.Fox G. Peer to peer networks. Comput. Sci. Eng. 2001;3(3):75–77. [Google Scholar]
- 66.Newman ME. Coauthorship networks and patterns of scientific collaboration. Proc. Natl Acad. Sci. USA. 2004;101(Suppl. 1):5200–5205. doi: 10.1073/pnas.0307545100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yodzis P. The connectance of real ecosystems. Nature. 1980;284(5756):544–545. [Google Scholar]
- 68.Bascompte J, Jordano P, Melian CJ, Olesen JM. The nested assembly of plant-animal mutualistic networks. Proc. Natl Acad. Sci. USA. 2003;100(16):9383–9387. doi: 10.1073/pnas.1633576100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Freeman LC. A set of measures of centrality based on betweenness. Sociometry. 1977;40(1):35–41. [Google Scholar]
- 70.Childs JE, Paddock CD. Passive surveillance as an instrument to identify risk factors for fatal Rocky Mountain spotted fever: is there more to learn? Am. J. Trop. Med. Hyg. 2002;66(5):450–457. doi: 10.4269/ajtmh.2002.66.450. [DOI] [PubMed] [Google Scholar]
- 71.Klyachko O, Stein BD, Grindle N, Clay K, Fuqua C. Localization and visualization of a coxiella-type symbiont within the lone star tick, Amblyomma americanum . Appl. Environ. Microbiol. 2007;73(20):6584–6594. doi: 10.1128/AEM.00537-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Narasimhan S, Rajeevan N, Liu L, et al. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe. 2014;15(1):58–71. doi: 10.1016/j.chom.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cirimotich CM, Dong Y, Clayton AM, et al. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae . Science. 2011;332(6031):855–858. doi: 10.1126/science.1201618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michelet L, Delannoy S, Devillers E, et al. High-throughput screening of tick-borne pathogens in Europe. Front. Cell. Infect. Microbiol. 2014;4:103. doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu XY, Bonnet SI. Hard tick factors implicated in pathogen transmission. PLoS Negl. Trop. Dis. 2014;8(1):e2566. doi: 10.1371/journal.pntd.0002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De La Fuente J, Merino O. Vaccinomics, the new road to tick vaccines. Vaccine. 2013;31(50):5923–5929. doi: 10.1016/j.vaccine.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 77.De La Fuente J, Almazan C, Canales M, Perez De La Lastra JM, Kocan KM, Willadsen P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Animal Health Res. Rev. 2007;8(1):23–28. doi: 10.1017/S1466252307001193. [DOI] [PubMed] [Google Scholar]
- 78.Decrem Y, Mariller M, Lahaye K, et al. The impact of gene knock-down and vaccination against salivary metalloproteases on blood feeding and egg laying by Ixodes ricinus. Int. J. Parasitol. 2008;38(5):549–560. doi: 10.1016/j.ijpara.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 79.Liu XY, De La Fuente J, Cote M, et al. IrSPI, a tick serine protease inhibitor involved in tick feeding and Bartonella henselae infection. PLoS Negl. Trop. Dis. 2014;8(7):e2993. doi: 10.1371/journal.pntd.0002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dai J, Wang P, Adusumilli S, et al. Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell Host Microbe. 2009;6(5):482–492. doi: 10.1016/j.chom.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Merino O, Antunes S, Mosqueda J, et al. Vaccination with proteins involved in tick-pathogen interactions reduces vector infestations and pathogen infection. Vaccine. 2013;31(49):5889–5896. doi: 10.1016/j.vaccine.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 82.Labuda M, Trimnell AR, Lickova M, et al. An antivector vaccine protects against a lethal vector-borne pathogen. PLoS Pathog. 2006;2(4):e27. doi: 10.1371/journal.ppat.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De La Fuente J, Almazan C, Blouin EF, Naranjo V, Kocan KM. Reduction of tick infections with Anaplasma marginale and A. phagocytophilum by targeting the tick protective antigen subolesin. Parasitol. Res. 2006;100(1):85–91. doi: 10.1007/s00436-006-0244-6. [DOI] [PubMed] [Google Scholar]
- 84.Mcmullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 2012;367(9):834–841. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- 85.Matsuno K, Weisend C, Kajihara M, et al. Comprehensive molecular detection of tick-borne phleboviruses leads to the retrospective identification of taxonomically unassigned bunyaviruses and the discovery of a novel member of the genus phlebovirus. J. Virol. 2015;89(1):594–604. doi: 10.1128/JVI.02704-14. [DOI] [PMC free article] [PubMed] [Google Scholar]