Light locally induces the accumulation of flavonols to promote cell elongation and asymmetric growth in the root transition zone, suggesting that flavonols serve as positional signals.
Abstract
Roots normally grow in darkness, but they may be exposed to light. After perceiving light, roots bend to escape from light (root light avoidance) and reduce their growth. How root light avoidance responses are regulated is not well understood. Here, we show that illumination induces the accumulation of flavonols in Arabidopsis thaliana roots. During root illumination, flavonols rapidly accumulate at the side closer to light in the transition zone. This accumulation promotes asymmetrical cell elongation and causes differential growth between the two sides, leading to root bending. Furthermore, roots illuminated for a long period of time accumulate high levels of flavonols. This high flavonol content decreases both auxin signaling and PLETHORA gradient as well as superoxide radical content, resulting in reduction of cell proliferation. In addition, cytokinin and hydrogen peroxide, which promote root differentiation, induce flavonol accumulation in the root transition zone. As an outcome of prolonged light exposure and flavonol accumulation, root growth is reduced and a different root developmental zonation is established. Finally, we observed that these differentiation-related pathways are required for root light avoidance. We propose that flavonols function as positional signals, integrating hormonal and reactive oxygen species pathways to regulate root growth direction and rate in response to light.
INTRODUCTION
Plant growth is influenced by environmental cues (Sablowski and Carnier Dornelas, 2014; Sánchez Alvarado and Yamanaka, 2014). Among these cues, light is an important signal regulating many developmental and physiological processes in plants (Kami et al., 2010). Roots are underground organs that normally grow downwards into the soil following the gravity vector. However, roots in nature can be exposed to sunlight in the upper layers of the soil (Mandoli et al., 1990). In this situation, roots tend to respond growing away from the light source, thereby avoiding a suboptimal environment. This process represents a negative phototropism called root light avoidance and has been described for several plant species (Kutschera and Briggs, 2012). Genetic and physiological experiments showed that root negative phototropism is mainly induced by blue light signaling and the activity of PHOT blue-light receptors (Liscum and Briggs, 1995; Briggs and Christie, 2002; Boccalandro et al., 2008; Wan et al., 2012). In roots, PHOT1 is mainly expressed in the transition zone (Sakamoto and Briggs, 2002; Wan et al., 2012), where dividing cells lose the mitotic potential and start to elongate and mature. Tropic responses, which involve organ curvature, occur due to asymmetric growth between sides of the root and normally correlate with an asymmetric distribution of the plant hormone auxin (Mähönen et al., 2014; Wang et al., 2015). Reorientation of root growth during the light avoidance response might require a local response in the transition zone mediated by PHOT1 (Zhang et al., 2014). The role of auxin in phototropism has also been studied (Esmon et al., 2006; Stone et al., 2008; Haga and Sakai, 2012). Light seems to affect auxin carrier distribution in cells to establish an asymmetric auxin accumulation, favoring differential growth during root negative phototropism (Zhang et al., 2013, 2014). In roots of etiolated seedlings, PIN2 accumulates in vacuole compartments, and after unilateral blue light illumination, PIN2 is redistributed to the plasma membrane (Wan et al., 2012). Another auxin carrier, PIN3, is polarized to the outer lateral membrane in root columella cells of etiolated plants when illuminated with blue light (Zhang et al., 2013). Despite this information, the process by which root growth is reoriented to avoid light is not completely understood.
Light also induces secondary metabolism in roots, including the production of flavonols (Buer and Muday, 2004). Flavonols are natural compounds with a polyphenolic structure, and their accumulation is directly regulated by light (Brown et al., 2001; Azari et al., 2010). Indeed, flavonols have been proposed to be UV-B light photoprotectors (Agati et al., 2013). Flavonols inhibit auxin transport, as shown by genetic analyses of mutants that do not synthesize them or by exogenous treatment of wild-type plants with flavonols (Brown et al., 2001; Peer et al., 2001, 2004; Peer and Murphy, 2014). Moreover, flavonols can act as in vivo reactive oxygen species (ROS) scavengers in Arabidopsis thaliana and in tomato (Solanum lycopersicum) plants (Maloney et al., 2014; Watkins et al., 2014) and interfere with indole-3-acetic acid (IAA) catabolism (Peer et al., 2004, 2013). Previous studies have shown that in vitro-grown roots, which are routinely exposed to illumination, accumulate high levels of flavonols (Buer and Muday, 2004).
The development of multicellular organisms requires maintenance of a balance between cell division and differentiation processes. This balance is tightly controlled by specific signal transduction pathways and can be influenced by environmental cues to modulate growth (Sablowski and Carnier Dornelas, 2014; Sánchez Alvarado and Yamanaka, 2014). In Arabidopsis root meristems, cells undergo several mitotic cycles. Cells then enter the transition zone, where they undertake an irreversible expansion program by which cells elongate slowly. Afterwards, cells start a fast and continuous anisotropic expansion in the elongation zone that increases growth of the root (Baluška et al., 2001; Ivanov and Dubrovsky, 2013; Takatsuka and Umeda, 2014). Subsequently, cells acquire tissue-specific features in the differentiation/maturation zone of the root. As a whole, differentiation involves a transition from proliferation, through elongation and growth cessation, to the ultimate development of mature fully functional tissues. The spatial organization of the proliferation/elongation/differentiation processes is referred to as root zonation (Ivanov and Dubrovsky, 2013).
The antagonistic interaction between auxin and cytokinin hormones regulates root zonation (Dello Ioio et al., 2007; Benjamins and Scheres, 2008; Dello Ioio et al., 2008; Su et al., 2011; Mähönen et al., 2014). Auxin and cytokinin signaling converge in the activity of SHY2 (Tian and Reed, 1999), an Aux/IAA repressor. SHY2 function establishes the boundary between cell proliferation, characterized by high auxin and low cytokinin signaling, and cell differentiation, which requires low auxin and high cytokinin signaling (Dello Ioio et al., 2008; Mähönen et al., 2014). In addition, auxin feeds into PLETHORA (PLT) accumulation. PLT proteins are expressed as a longitudinal gradient that determines root zonation in a dose-dependent manner (Aida et al., 2004; Mähönen et al., 2014). Root zonation is also regulated by differential accumulation of ROS. The transcription factor UPBEAT1 (UPB1), a basic helix-loop-helix protein 151, transcriptionally represses a set of peroxidases, regulating the distribution of H2O2 and superoxide anion (O2−) in the differentiation and proliferation zones of the root, respectively (Tsukagoshi et al., 2010). Based on genetic and molecular data, it seems that UPB1 signaling does not interact with auxin or cytokinin pathways (Tsukagoshi et al., 2010).
In roots, light also influences the production of ROS (Yokawa et al., 2011, 2014; Silva-Navas et al., 2015), shoot-to-root auxin transport (Sassi et al., 2012), and root development (Novák et al., 2015), among other processes. ROS play an important role in shaping the root system architecture through regulation of root growth and lateral root formation (Tsukagoshi et al., 2010; Manzano et al., 2014; Silva-Navas et al., 2015). To better study root development and responses, our group engineered a system called D-Root that allows in vitro cultivation of plants with the roots growing in darkness while the shoot is exposed to photoperiodic conditions (Silva-Navas et al., 2015). Results using this system highlighted the significant effect of light on root growth, development, and responses to different stimuli (Silva-Navas et al., 2015). Root growth analyses also showed that blue light was primarily responsible for inhibition of root growth in the presence of light (Silva-Navas et al., 2015).
Here, we aimed to investigate the mechanism by which light influences two interconnected processes: light avoidance and root growth. Genomic and metabolomic studies identified flavonols as metabolites that regulate root light avoidance and root growth. Analyses of the flavonol-deficient mutant tt4 and treatment of dark-grown roots with exogenous flavonol showed that these metabolites are required for the root phototropic response and root growth. We showed that hydrogen peroxide and cytokinin induce flavonol accumulation in the proliferation-differentiation boundary, accelerating the elongation of cell in the transition zone process and subsequently reducing the proliferation zone. Our study provides insight into how flavonols might regulate root growth in response to light, contributing to generate an appropriate negative phototropic response to light. In addition, flavonols seem to regulate root growth by integrating the auxin-cytokinin and ROS signaling pathways.
RESULTS
Root Light Avoidance Is Mediated by PHOT Photoreceptors in Response to Blue Light
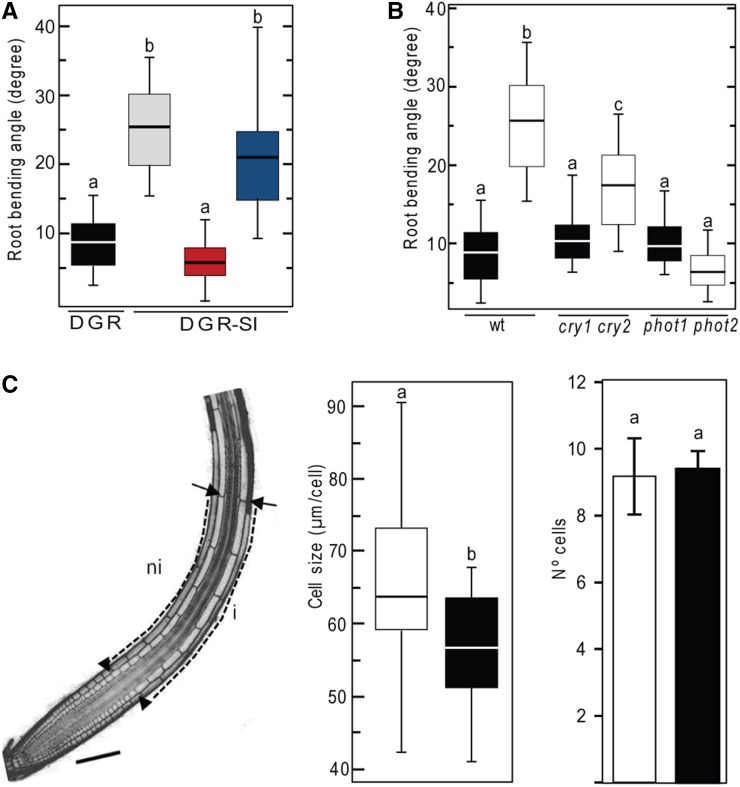
Plant roots have the ability to adapt their growth to escape from light, which is known as light avoidance or negative phototropism (Kutschera and Briggs, 2012). Some reports, using etiolated seedlings followed by unidirectional illumination of the entire plant, have suggested that blue light and phototropins control root negative responses (Christie and Briggs, 2001; Lin, 2002). Recently, our group engineered a growing device, called D-Root, to grow the root system in darkness and the shoot in the light. Briefly, this system consists of a black-methacrylate box in which a square Petri plate tightly fits. Inside the plate, a methacrylate insert is positioned between the root and the shoot to block light coming from top fluorescent lamps (Silva-Navas et al., 2015). We adapted the D-Root device to grow roots in darkness and, at a specified moment, to illuminate only one side of the box (DGR-SI) with white light or with specific wavelengths of light (Supplemental Figure 1). Using this device, we found that dark-grown roots (DGRs) of wild-type seedlings, whose shoots were grown in the presence of light, were able to turn away from the white light with an average angle of 25 degrees (Figure 1A). We found that if DGRs were not exposed to one-sided illumination, they did not curve (Figure 1A). When red or blue light was used, the average angle was 6 or 20, respectively (Figure 1A), suggesting that DGRs preferentially respond to blue wavelengths. Next, we analyzed the root phototropism response in mutants of the blue light photoreceptors CRY and PHOT. We found that DGR-LI roots of the cry1 cry2 double mutant escaped from light with a slightly lower angle than wild-type plants (Figure 1B). However, DGR-SI phot1 phot2 roots did not grow away from the light source (Figure 1B), suggesting that, in our conditions, PHOT receptors can also mediate root light avoidance when DGRs are exposed to a side light source. To understand the nature of the root bending at the cellular level during the light avoidance response, we measured the number and length of cells located at the illuminated and shaded sides. Cells at the illuminated side were longer than those at the shaded side, while the numbers of cells were similar on both sides (Figure 1C). Thus, our data suggest that roots have a rapid or short-term response to light (3 to 8 h), which primarily affects cell elongation, resulting in differential root side growth (more on the illuminated side). This differential growth causes root bending to avoid the light source. During this short-term response, we did not observe any significant changes in cell proliferation between the two sides.
Figure 1.
Root Negative Phototropism Is Induced by Blue Light Perceived by Roots.
(A) Box plot showing the root bending angle of 8-d-old wild-type DGRs in plants after side illumination (DGR-SI) with white light (white box), red light (red box), or blue light (blue box) or without illumination (DGR, black) for 16 h. n ≥ 20.
(B) Box plot showing the root bending angle of 8-d-old wild-type, cry1 cry2, or phot1 phot2 DGRs (black) or DGRs illuminated on one side with white light (slashed lines). n ≥ 20.
(C) Light stimulates cell elongation during the light avoidance response of wild-type roots. The distances from the last meristematic cell (arrowheads) to the cell where the turn initiates (arrows) in the illuminated (i) and nonilluminated (ni) sides were divided by the number of cells contained in such distances. Right panels show the average cell length and number of cells in illuminated (white box and bar) and nonilluminated (back box and bar) sides. n = 20.
Different letters indicate significant differences analyzed by ANOVA and Tukey HSD post-test. P < 0.01.
Long-Term Exposition to Light Reduces Root Meristem Size
We observed that prolonged exposure of roots to light significantly reduced their growth (Silva-Navas et al., 2015). To better analyze the effect of light on root growth, DGRs were transferred to white light and root growth measured. After 24 h of illumination, root growth rate was reduced by 26 and 38% in Columbia (Col-0) and Landsberg erecta (Ler) ecotypes, respectively (Figure 2A). Accordingly, Arabidopsis roots that were grown for 8 or 12 d in the presence of light (light-grown roots [LGRs]) were ∼20 to 25% shorter than DGRs (Figure 2B; Silva-Navas et al., 2015). Thus, a long duration of root illumination causes root growth reduction (long-term response), while side illumination for 3 to 8 h causes root light avoidance (short-term response).
Figure 2.
Light Affects Root Growth and Reduces Meristem Size.
(A) Root growth of DGRs maintained in DGR conditions (black bars) or transferred to illumination under white light for 24 h (LGR; white bars). mm/h, millimeters per hour.
(B) Root length of wild-type DGRs and LGRs at 8 or 12 d postimbibition (dpi). n ≥ 50.
(C) Confocal images of wild-type DGRs and LGRs at 12 dpi root meristems. Arrowheads: quiescent center. Arrows: end of the meristem.
(D) Cortical cell number and length of wild-type DGR and LGR meristems. n ≥ 20.
Values are means ± sd. Significance was analyzed by ANOVA and Tukey HSD post-test . P < 0.01. Bar = 100 µm. Black bars, DGRs; white bars, LGRs.
Root growth mostly relies on cell division and elongation. To study the effect of light on cell proliferation and elongation, we analyzed the meristem size and number of cortical cells in LGRs and DGRs. Confocal analyses revealed that LGRs had significantly shorter meristems with fewer cortical cells than DGRs (Figures 2C and 2D). These results suggest that light may reduce growth through reduction of meristem size.
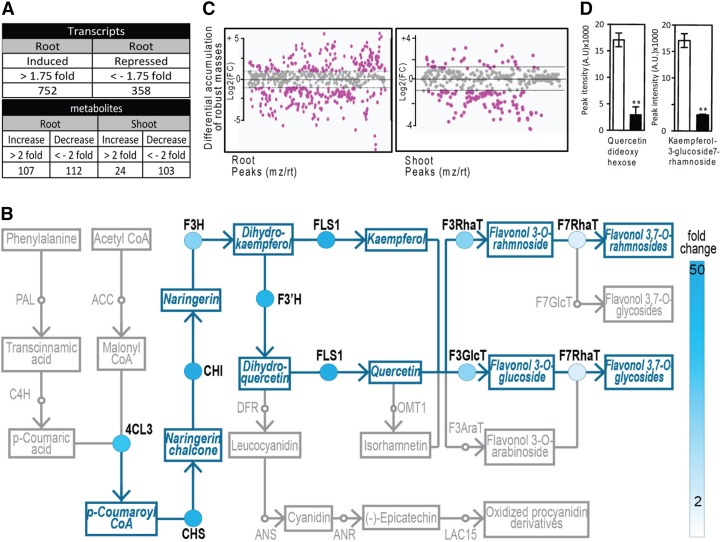
Root Illumination Alters Transcriptomic and Metabolomic Profiles
To gain further information on the effect of light on root development, we compared the genome-wide transcript levels and metabolomic profiles of 12-d-old DGRs with those of LGRs. About one thousand transcripts (725 upregulated; 358 downregulated) differentially accumulated in response to root exposure to light (Figure 3A; Supplemental Data Set 1). A significant proportion of the upregulated transcripts was associated with UV, photosynthesis, and light responses. In addition, other significantly overrepresented categories were related to oxidative stress, hormonal signaling, secondary metabolism, and stress responses (Supplemental Data Set 1). The majority of transcripts related to the flavonoid biosynthetic pathway were upregulated, in particular genes encoding enzymes that converge in the production of quercetin, kaempferol, and their glycosylated forms (Figure 3B; Supplemental Data Set 1). This is in agreement with previous data that showed that flavonol biosynthetic genes are light regulated (Kubasek et al., 1992, 1998).
Figure 3.
Root Illumination Affects Gene Expression and Metabolite Accumulation.
(A) Number of transcripts and metabolites showing levels altered by light exposure to the root.
(B) Root illumination activates the flavonoid biosynthesis pathway. Circles represent the flavonol biosynthesis genes and the color intensity their relative expression level in light versus dark. The metabolites are labeled with rectangular boxes.
(C) Differential accumulation of all robust masses (see Methods) in roots or shoots of LGR or DGR plants. Fold change plot of metabolites with differential (pink dots) and nondifferential (gray dots) accumulation in roots and shoots.
(D) Relative accumulation (peak intensity, arbitrary units) of two flavonol glycosides identified in LGRs (white bars) or DGRs (black bars).
Values are means ± sd. Asterisks indicate statistically significant changes compared with the wild type calculated by a two-tailed Student’s t test for P < 0.01.
To gain further insight into the effect of light on roots, we also performed metabolite profiling. Comparisons between LGR and DGR seedlings (whose aerial and underground parts were processed separately) showed that the levels of 219 metabolites were significantly different in roots, while 127 metabolites were significantly different in shoots (Figures 3A and 3C; Supplemental Data Set 2). Only 10 metabolites were decreased in both roots and shoots (Supplemental Data Set 2), indicating that illumination of roots also induced important metabolic changes in the shoot. Next, we analyzed the differential accumulation of all robust masses, defined as those masses that occur in at least 75% of the sample series, between LGRs and DGRs using a database that contained the Arabidopsis elemental compositions. We experimentally determined retention times of known metabolites from a number of previous articles (Zhang et al., 2005; Moussaieff et al., 2013; Morreel et al., 2014). This targeted screening approach revealed that a large number of flavonol-related metabolites changed in response to light (Supplemental Figure 2). Differential accumulation of quercetin and kaempferol between LGRs and DGRs was confirmed in vivo through targeted metabolic analyses (Figure 3D).
Flavonols Regulate Root Responses to Light
To analyze the possible effects of flavonols in light responses of roots, we examined the spatial accumulation of quercetin and kaempferol in LGRs and DGRs using the fluorescent dye DPBA (diphenylboric acid-2-aminoethyl ester; see Methods) and confocal microscopy analyses (Supplemental Figure 3). We found that levels of quercetin and kaempferol were significantly higher in the transition zone of LGRs than in that of DGRs (Figure 4A). As this flavonol accumulation was coincident with the reduction in root growth in LGRs, we decided to analyze the role of flavonols in root development. We found that quercetin reduced root growth. Root length of quercetin-treated DGRs was similar to that of LGRs and quercetin-treated LGRs (Figure 4B). TRANSPARENT TESTA4 (TT4) encodes the biosynthetic enzyme chalcone synthase, which catalyzes the production of naringerin chalcone, one of the precursors of quercetin and kaempferol (Figure 3B). To test whether endogenous flavonols can regulate root growth, we measured root length in the tt4 mutant, which lacks flavonols. As shown in Figure 4C, tt4 LGRs were longer than those of control plants, which is in agreement with previous observations (Lewis et al., 2011). In addition, exogenous application of quercetin reduced tt4 root length to similar levels as those of wild-type LGRs.
Figure 4.
Flavonols Mediate Root Growth and Phototropic Responses.
(A) Confocal images showing kaempferol (K) and quercetin (Q) accumulation in wild-type DGRs or LGRs. BF, bright field. Arrows and dashed lines: end of the meristem. Note: DGR brightness is increased by 10-fold.
(B) Root length of 8-d-old wild-type DGRs or LGRs treated with or without quercetin (Q). n ≥ 50.
(C) Root length of 8-d-old wild-type LGRs and tt4 LGRs treated with or without quercetin. n ≥ 50.
(D) Confocal images showing flavonol accumulation in 8 dpi wild-type DGR (T = 0) and after 3 h side light stimulation (T = 3).
(E) GUS staining of 8-d-old TT4:GUS DGR before (T = 0) and after 3 h (T = 3 h) of side illumination.
(F) Box plots showing the root bending angle in the wild type or tt4 DGR after side illumination with white light (white boxes) for 16 h or without illumination (black boxes). n ≥ 25.
(G) Box plots showing the root bending angle in wild-type DGRs grown in presence or absence of quercetin and cop1-4 mutant after side illumination with white light (white boxes) for 16 h or without illumination (black boxes). n ≥ 20.
(H) Representative images of wild-type DGR, and DGR-SI or tt4 DGR-SI illuminated for 8 h. Right panels show the relative cell length between illuminated side and nonilluminated side in wild-type and tt4 roots during light avoidance response and the number of cells in both tt4 illuminated (white box and bar) and nonilluminated (back box and bar) sides. Values are means ± sd. n = 16.
Different letters indicate significantly differences analyzed by ANOVA and Tukey HSD post-test. P < 0.01. Bars = 100 µm.
We also wanted to analyze whether flavonols have a role in the light avoidance response. Using DPBA staining, we analyzed flavonol content at different times after exposure to light from the side. We found differential accumulation in the root, with accumulation in the illuminated side starting 3 h after exposure (Figure 4D). Using the TT4:GUS marker line, we found differential higher expression of TT4 at the illuminated side (Figure 4E). Next, we analyzed root curvature in the tt4 and constitutive photomorphogenesis 1-4 (cop1-4) mutants. tt4 does not accumulate flavonols, while the cop1-4 mutant constitutively accumulates flavonols in roots (Buer and Muday, 2004) (Supplemental Figure 4). As shown in Figures 4F and 4G, the light avoidance angle was significantly reduced in tt4 roots, suggesting that a lack of flavonols reduces the root negative phototropic response. A similar reduction was observed in the cop1-4 mutant or upon quercetin treatment (Figure 4G), likely because of the inability to differentially accumulate flavonols at the illuminated part of the root. As we had observed that differential cell elongation was responsible for root light avoidance (Figure 1C), we decided to investigate if flavonols could affect cell elongation during this response. Examination of cell length and number at the illuminated and shaded sides of tt4 roots showed that both sides had similar numbers of cells, with no significant differential elongation ratio (∼1; Figure 4H). By contrast, a significant differential cell elongation ratio was observed for illuminated wild-type roots (Figure 4H).
Flavonol Levels Regulate Cell Division and Elongation in Root Meristem
Our data show that prolonged exposure of roots to light reduces both their growth and meristem size. Thus, we decided to investigate whether flavonols could affect cell proliferation in the root meristem. Supplementation with quercetin significantly reduced root meristem size of DGRs, which became almost undistinguishable from meristems of LGRs (Figure 5A). We found that quercetin prevented the meristem expansion and cell number increases from day 8 to day 12 that were observed in untreated DGRs (Figure 5A). In addition, tt4 LGR meristem size, which was larger than that of wild-type LGRs, was reduced by the addition of quercetin (Figure 5B). To analyze cell proliferation in the meristem, we used the pCYCB1:CYCB1;1-GUS marker (CYCB1-GUS), which accumulates in the G2/M phase of the cell cycle. Meristems of DGRs showed more cells expressing the mitotic marker CYCB1-GUS than meristems of LGRs. Supplementation of DGRs with quercetin reduced the number of mitotic cells to numbers similar to those found in LGRs (Figure 5C).
Figure 5.
Flavonols Regulate Root Meristem Size and Cell Elongation.
(A) Meristem length and number of cortical cells of 8- or 12-d-old wild-type LGRs or DGRs grown in MS medium with or without 50 µM quercetin (Q). n ≥ 20.
(B) Meristem length and number of cortical cells of 8- or 12-d-old wild-type and tt4 DGRs or LGRs grown in MS medium with or without 50 µM quercetin. n ≥ 18.
(C) Number of cells expressing CYCB1;1:CYCB1;1-GUS marker per root meristem at 8 dpi in DGRs or LGRs treated with our without quercetin. n = 20. Right panel shows representative pictures of 8 dpi LGR and DGR CYCB1-GUS root tips grown in medium with or without quercetin.
(D) Cell length of the six consecutive cells in the root transition zone (just after the last cortex meristematic cell) were measured in 8-d-old DGRs, LGRs, or DGRs supplemented with 50 µM quercetin. n ≥ 30.
(E) Cell length of epidermis (Ep) or cortical (Co) cells in the differentiation zone, corresponding to the area where the root hairs initiated in DGRs or LGRs. n = 19. Right panel shows representative confocal images corresponding to epidermal cells analyzed.
Values shown are means ± sd. Significance was analyzed by ANOVA and Tukey HSD post-test. P < 0.05. Bar = 100 µm.
Next we analyzed whether flavonols affect cells in the transition zone. We quantified the length of cells in the transition zone of DGRs, DGRs treated with quercetin, and LGRs. As shown in Figure 5D, cells in the transition zone of illuminated or flavonol-treated roots elongated faster than those of DGRs. To investigate if this faster cell elongation is maintained in fully differentiated cells, we measured the first epidermal cell (from the root tip) with a root hair and the cortical cell next to it. As shown in Figure 5E, cell size of both maturing cell types was similar for DGRs and LGRs (Figure 5E). Taken together, these results suggest that accumulation of quercetin and kaempferol (or their glycosylated derivatives) likely participates in the regulation of root growth by limiting cell proliferation and accelerating the cell elongation phase. As the final cell length in LGRs and DGRs is indistinguishable, the reduction in root length of LGRs (as compared with DGRs) is likely caused by less cell proliferation in the meristem.
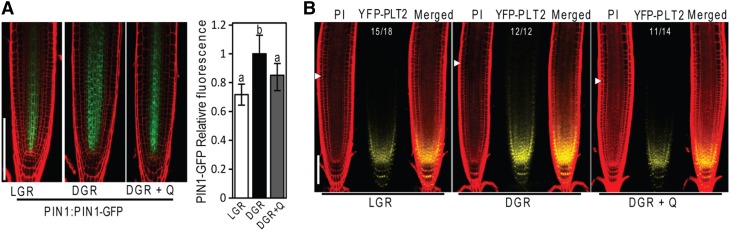
Flavonols Reduce PIN1 Levels and the PLT Gradient
The hormone auxin plays an important role in defining root meristem size through formation of the PLT gradient that regulates cell proliferation. As we found that flavonols repress cell proliferation (Figures 5A and 5C) and it is also known that they inhibit auxin transport (Brown et al., 2001; Peer et al., 2004; Yin et al., 2014; Peer and Murphy, 2014), we decided to investigate if flavonols could function through modification of the formation of auxin maxima and the PLT gradient. We found that protein levels of the auxin exporter PIN1-GFP was lower in LGRs (high flavonol content) than in DGRs (low flavonol content) (Figure 6A), whereas levels of PIN2-GFP (Silva-Navas et al., 2015), PIN3-GFP, and the auxin importer AUX1-YFP did not significantly change in response to light (Supplemental Figure 5). We found that DGRs treated with quercetin showed a significant reduction in PIN1-GFP levels compared with DGRs (Figure 6A), suggesting that a reduction of auxin transport to the root tip in the presence of light is mediated, at least partially, by quercetin or its derivatives. We detected a decrease in the auxin response marker DR5:YFP in LGRs in comparison with DGRs (Supplemental Figure 6). We also analyzed the PLT2 gradient in LGRs and DGRs. We found that the PLT2 gradient increased in DGRs, and supplementation with quercetin reduced this gradient along with meristem size (Figure 6B). Therefore, our data are consistent with flavonol content regulating meristem size through regulation of the auxin-PLT gradient.
Figure 6.
Flavonols Regulate Auxin Transport and the PLT Gradient in the Root Meristem.
(A) Confocal images showing PIN1-GFP signal in LGRs and DGRs treated with or without quercetin. Right panel shows quantification of PIN1-GFP fluorescence. n = 20.
(B) Confocal images of PLT2-YFP LGRs and DGRs treated with or without 50 µM quercetin (Q). Arrows, end of the meristem; PI, stained with propidium iodide; YFP-PLT2, yellow fluorescent signal from YFP-PLT2; Merged, merged signal of PI and YFP. Numbers on the picture indicate the number of meristems showing a phenotype similar to the one shown in the figure.
Values shown are means ± sd. Different letters indicate significant difference analyzed by ANOVA, P < 0.01. Bars = 100 µm.
Flavonols Limit ROS Accumulation in the Root Meristem
Our transcriptomic analyses indicated that the level of ROS-related transcripts also changed in response to light. Higher expression levels of genes for NADPH oxidase were found in DGRs compared with LGRs (Supplemental Data Set 1). NADPH participates in the production of O2− (Foreman et al., 2003). O2− promotes cell proliferation (Dunand et al., 2007; Tsukagoshi et al., 2010), while flavonols may function as ROS scavengers (Pietta, 2000; Maloney et al., 2014; Watkins et al., 2014). Thus, we decided to analyze the interaction of O2− and flavonol accumulation in the regulation of the root meristem size. Using a specific stain for O2− (Nitroblue tetrazolium [NBT]; see Methods), we found that O2− levels were significantly higher in DGR meristems than in LGR meristems (Figure 7A). tt4 LGR meristems also accumulated significantly more O2− than wild-type LGRs, with these levels comparable to those found in meristems of DGRs (Figure 7A). These data suggest that flavonol accumulation might decrease O2− levels, in turn, reducing cell proliferation.
Figure 7.
Flavonols Regulate Superoxide Anion Content in the Root Meristem.
(A) NBT staining of 12 dpi wild-type and tt4 LGRs and DGRs. Right graph shows NTB quantification (arbitrary units) values. Values shown are means ± sd. n = 20.
(B) NBT staining of wild-type and tt4 DGRs treated with or without 0.5 nM IAA for 24 h. Graph shows NTB quantification (arbitrary units) values. n = 20.
(C) Root length of 12-d-old wild-type and tt4 DGRs grown in presence or absence of quercetin (Q). n ≥ 35.
(D) Meristem length of 12-d-old wild-type or tt4 DGRs grown in presence or absence of quercetin. n ≥ 20.
Values are shown means ± sd. Significance was analyzed by ANOVA and Tukey HSD post-test. P < 0.01 for (A) to (C), and P < 0.05 for (D). Bars = 300 µm.
Our results show that flavonols have an effect on both auxin-PLT and O2− content. To analyze whether flavonols might connect auxin and O2− pathways, we treated seedlings grown in the DGR conditions, to avoid light influence, with auxin at a low concentration (0.5 nM) that has been shown to promote cell division (Dello Ioio et al., 2008). Auxin-treated DGRs, which have low flavonol content, showed increased O2− levels in the root meristem (Figure 7B). This increase was significantly higher in the tt4 mutant, indicating that O2− accumulation could be inhibited by flavonols (Figure 7B).
Based on this result and our previous observation that quercetin and kaempferol accumulate in the transition zone of DGRs (Figure 4A), we decided to evaluate whether flavonols could regulate root growth in the absence of light. We analyzed root growth of wild-type and tt4 DGRs treated with or without quercetin. DGRs of tt4 were significantly longer than wild-type DGRs, while addition of quercetin significantly reduced root growth of tt4 (Figure 7C). In addition, we found that that meristems of tt4 DGRs were longer than wild-type DGR meristems and that addition of quercetin eliminated these differences (Figure 7D). Taken together, our results suggest that the low level of flavonols found in DGRs (likely similar to natural conditions) might have an endogenous role in the regulation of root growth.
Cytokinin and the Hydrogen Peroxide Pathway Increase Flavonol Content in the Transition Zone
Cytokinin and H2O2 signaling induce cell differentiation (Dello Ioio et al., 2008; Tsukagoshi et al., 2010). We investigated whether these pathways could regulate flavonol content in roots. We found that H2O2 treatment increased levels of quercetin and kaempferol in the differentiation zone of DGRs (Figures 8A and 8C). In addition, overexpression of UPB1 transcription factor, which increases H2O2 levels in the differentiation zone (Tsukagoshi et al., 2010), also augmented the content of quercetin and kaempferol (Figures 8B and 8C). These data indicate that the UPB1-H2O2 pathway increases flavonol contents in cells undergoing differentiation, particularly during the elongation phase. As UPB1-overexpressing plants accumulate higher amounts of flavonols in the root transition zone, we checked for possible defects in light avoidance. UPB1-overexpressing roots turned away from light with a lower angle than control plants (Figure 8D), suggesting that UPB1-dependent flavonol accumulation may disturb negative phototropism, similarly to what was observed in roots with high flavonol content, such as cop1-4 or quercetin-treated roots (Figure 4G).
Figure 8.
Flavonols Are Activated by H2O2.
(A) Confocal images showing flavonol accumulation in 8-d-old wild-type DGRs treated with or without 100 µM H2O2 for 24 h.
(B) Confocal images showing flavonols in 8-d-old 35S:UPB1-3xGFP DGRs. K, kaempferol; Q, quercetin; -DPBA, DGR not stained; green fluorescent signal corresponds to GFP; BF, bright field. Bars = 100 µm.
(C) Quantification of the fluorescence in (A) and (B). Values are expressed as arbitrary units relative to the maximum value. Green (K) and red (Q) bars correspond to kaempferol and quercetin quantification, respectively.
(D) Box plot showing the root bending angle of 8-d-old 35S:UPB1 DGRs after side illumination (DGR-SI) with white light (white boxes) or without illumination (DGR, black) for 16 h. n = 16.
Significance was analyzed by ANOVA and Tukey HSD post-test. P < 0.01.
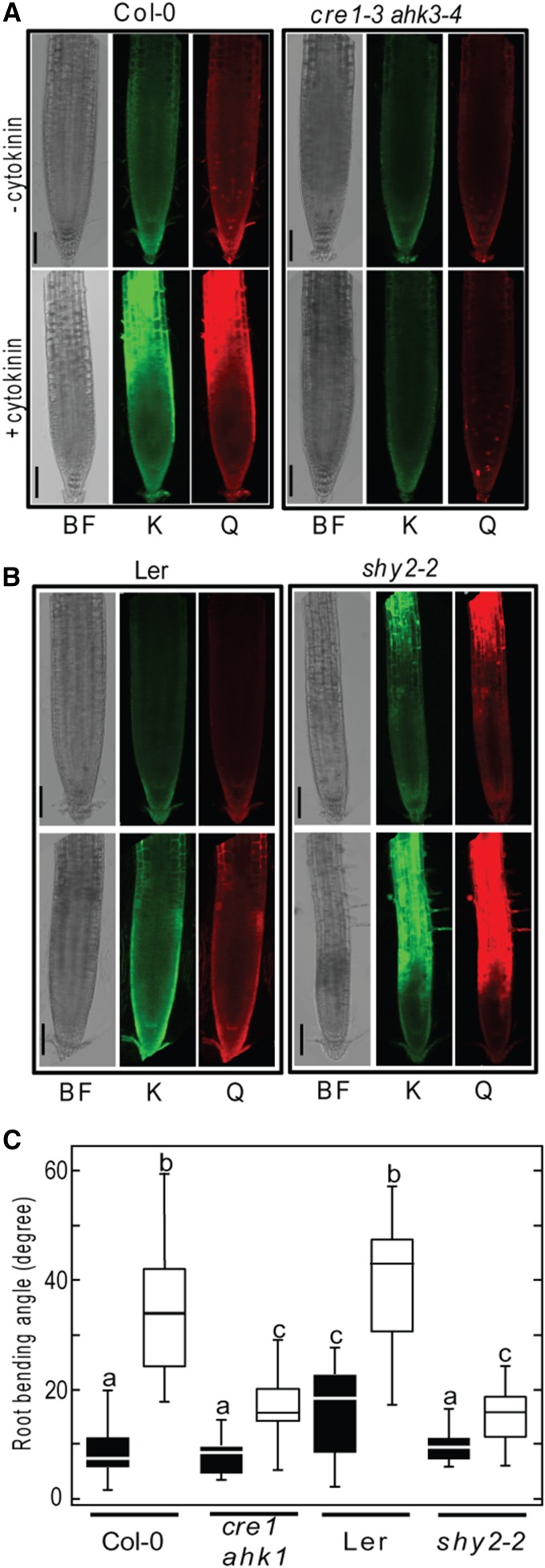
To assess the effect of cytokinin on flavonol content, wild-type DGRs were treated with cytokinin. As shown in Figure 9A, cytokinin-treated roots significantly accumulated quercetin and kaempferol, preferentially in the root differentiation zone. Furthermore, this increment was dependent on cytokinin perception, as double mutants of the cytokinin receptors CYTOKININ RECEPTOR1 (CRE1) and ARABIDOPSIS HISTIDINE KINASE3 (AHK3), cre1 ahk1 (Franco-Zorrilla et al., 2005), lacked accumulation of flavonols in DGRs upon cytokinin treatment. SHORT HYPOCOTYL2 (SHY2) mediates cytokinin regulation of cell differentiation in Arabidopsis roots, limiting meristem size (Dello Ioio et al., 2008). We measured the levels of quercetin and kaempferol in the meristem of the gain-of-function mutant shy2-2, which shows enhanced cell differentiation. We detected a significant increase in quercetin and kaempferol levels at the elongation and differentiation zones of shy2-2 DGRs (Figure 9B). Taken together, our data suggest that cytokinin might induce flavonol biosynthesis through SHY2 as part of the regulatory mechanism by which cytokinin signaling represses root growth and limits meristematic activity.
Figure 9.
Cytokinin Signaling Regulates Flavonol Content and Light Avoidance Response.
(A) Flavonol accumulation in 8 dpi control (Col-0) or cre1-3 ahk3-4 DGRs treated with or without 5 µM zeatin (cytokinin) for 24 h.
(B) Flavonol accumulation in 8 dpi control (Ler) and shy2-2 DGRs treated with or without 5 µM zeatin for 24 h. BF, bright field; K, kaempferol; Q, quercetin.
(C) Box plot showing the root bending angle of 8-d-old wild-type (Col-0), cre1 cre2, wild-type (Ler), and shy2-2 DGRs after side illumination with white light (white boxes) for 16 h or without illumination (black boxes).
Significance was analyzed by ANOVA and Tukey HSD post-test. P < 0.01; n ≥ 20. Bars = 100 µm.
As we showed that cytokinin regulates flavonol accumulation, and cytokinin signaling represses root growth (Dello Ioio et al., 2007), we decided to investigate the role of cytokinin signaling in the root negative phototropic responses. To test this, we analyzed the light avoidance angles of cre1 ahk1 and shy2-2 roots. As shown in Figure 9C, light avoidance response was attenuated in the double mutant cre1 ahk1 and almost blocked in shy2-2 roots. These results indicate that cytokinin signaling has an active role in root phototropism.
DISCUSSION
Flavonols Mediate Root Phototropic Responses
In this work, we studied root phototropic responses in conditions imitating those they might experience in nature by growing the roots in darkness and the shoot in the presence of light. For this purpose, we used the recently developed system, D-Root (Silva-Navas et al., 2015). The data presented here are in agreement with previous studies showing that PHOT photoreceptors and blue light as stimulus mediate root light avoidance in dark-grown roots of nonetiolated plants (Liscum and Briggs, 1995; Briggs and Christie, 2002). However, we did not find a positive response to the red light as previously described (Kiss et al., 2003). This could be due to our different growth conditions and approach and suggests that red light might be involved shoot-to-root communication during phototropic responses.
Flavonols are secondary metabolites involved in diverse processes and responses to environmental cues in plants (Taylor and Grotewold, 2005; Roberts and Paul, 2006; Grunewald et al., 2012). Over the last few years, it has been proposed that flavonols might act as developmental regulators or signaling molecules (Pollastri and Tattini, 2011). Flavonols have been implicated in gravitropism; the flavonol-deficient mutant tt4 shows delayed gravitropic response and elevated root basipetal auxin transport (Brown et al., 2001; Buer and Muday, 2004). After gravistimulation, epidermal root cells accumulate higher levels of flavonols, with maximum differences coinciding with the timing of bending. However, differential accumulation of flavonols at the inner or outer side of the bend was not reported (Buer and Muday, 2004). In this work, we show that differential flavonol accumulation in the organ sides regulates root negative phototropism. Physiological studies have shown that negative root phototropism is regulated by an asymmetrical auxin redistribution (Wan et al., 2012; Zhang et al., 2013, 2014). Connected with this regulation, quercetin and kaempferol, the two main Arabidopsis flavonols (Winkel-Shirley, 2002), inhibit auxin transport (Brown et al., 2001; Peer et al., 2004). Peer et al. (2004) also showed that the lack of flavonols in the tt4 mutant significantly reduces PIN1 transcript abundance and affects PIN1 protein accumulation/cycling. We did not find differences in PIN1 transcript levels between DGRs and LGRs, suggesting that light and/or flavonol treatment in DGRs affects PIN1 protein accumulation/cycling.
A role for cytokinin signaling in root phototropism has not been reported previously. Here, we show that flavonol accumulation in roots is regulated by cytokinin-SHY2 signaling. This is in agreement with recent work showing that cytokinin induces the expression of key regulators of the flavonol biosynthetic pathway in roots (Brenner and Schmülling, 2012). We show that the light avoidance response is significantly lower in the cytokinin receptor mutant cre1 ahk1 and almost abolished in shy2-2, harboring a dominant-negative mutation that has been shown to repress auxin signaling (Tian and Reed, 1999) and to constitutively promote cytokinin responses (Dello Ioio et al., 2008). We show that shy2-2 accumulates high levels of flavonols and that it is impaired in root phototropic responses, likely because differential flavonol accumulation cannot be established. Supporting this idea, cop1-4, a mutant that accumulates high levels of flavonols in light- or dark-grown roots also shows similar phototropic defects. Based on our model (Figure 10), differential accumulation of flavonols on the illuminated side of the root, which likely requires cytokinin signaling, may affect the transport of auxin, causing its asymmetric distribution and signaling. Flavonols also acts as ROS scavengers in Arabidopsis and tomato plants (Maloney et al., 2014; Watkins et al., 2014). ROS generation and signaling are involved in root tropic responses (Joo et al., 2001; Clore et al., 2008). We show that ROS regulate root light avoidance. In our model, accumulation of flavonols at the illuminated side might generate an imbalance of auxin and/or specific ROS to promote cell elongation in the transition zone at the illuminated side. Consistent with this idea, our results show that side illumination of roots leads to rapid cell elongation (within hours) on the lighted side of the root. More cell elongation results in asymmetric organ growth, originating its bend away from the light source.
Figure 10.
Model of Transition Between Cell Division and Differentiation in Dark-Grown Root Meristems and in Response to Light.
(A) Flavonols regulate root zonation. Flavonols accumulate in cells undergoing differentiation, repressing proliferation and promoting differentiation. Cytokinin-SHY2 and H2O2-UPB1 induce biosynthesis of flavonols to reduce cell proliferation through inhibition of PIN1, auxin polar transport, and by scavenging superoxide anion (O2−). Auxin can trigger O2− production, which is dependent on flavonol content. These mutual interactions define the boundary between cell proliferation and cell differentiation (dashed line). As light induces flavonol accumulation, the system is perturbed when roots are exposed to light, a stimulus that reduces meristem size along with root growth.
(B) Avoidance of light by roots requires flavonols. Lighting of roots from one side induces flavonol accumulation at that side, promoting differentiation and growth away from the spotlight. Dashed line indicates temporal movement of the boundary between cell proliferation and differentiation responsible for the light tropism.
Flavonols Connect Auxin/Cytokinin Functions with ROS Signaling in the Root Meristem
As part of the long-term negative root phototropic responses (occurring in days), we observed that root growth was reduced. The equilibrium between cell division and differentiation involves the function of several regulatory pathways, including the auxin/cytokinin balance and ROS signaling. An antagonistic interaction between auxin and cytokinin is required for establishing the root meristem and the different boundaries (Dello Ioio et al., 2008; Chapman and Estelle, 2009). The PLT proteins have been proposed as the molecular effectors of auxin required to maintain cell proliferation activity in the root meristem (Aida et al., 2004; Mähönen et al., 2014). Conversely, cytokinin promotes cell differentiation and antagonizes the function of auxin through SHY2 (Dello Ioio et al., 2008). The dominant shy2 mutants have shorter root meristems, similar to cytokinin-treated seedlings (Dello Ioio et al., 2007, 2008). Our data support a model wherein the increase of cytokinin levels or SHY2 function, which is light regulated (Tian et al., 2002), might increase flavonol content. In turn, flavonols would reduce auxin transport (Brown et al., 2001; Peer et al., 2001, 2004; Peer and Murphy, 2014) and the PLT gradient (this work), reducing proliferation and meristem size and subsequently root growth (Figure 10). The effect of the reduction of auxin transport on the PLT gradient is not immediate, but rather occurs within days, constituting a specific temporal dose response (Mähönen et al., 2014). Consistent with this, we observed that reduction in root growth upon prolonged light exposure required at least 24 h and was accompanied by the establishment of new root zonation. Root zonation is also regulated by active accumulation of O2− and H2O2, which contribute to regulate the transition from cell proliferation to differentiation (Tsukagoshi et al., 2010). UPB1 represses a set of peroxidases in the transition zone of the root meristem, generating a zone-dependent accumulation of H2O2 that is opposite to O2− content. In addition, UPB1 may activate the expression of genes of the flavonol biosynthetic pathway (Tsukagoshi et al., 2010). According to our model, high flavonol content might diminish O2− levels, reducing the cell division/differentiation rate. Reduction of the cell/division differentiation rate would result in shorter meristems. Previously, it has been suggested that auxin/cytokinin and O2−/H2O2 pathways might function independently in light-grown roots (Tsukagoshi et al., 2010). As light induces flavonol biosynthesis, it is likely that root illumination masks the connection between hormonal and ROS pathways. We found that flavonol content in dark-grown roots regulates the interaction between auxin and O2− levels. Although endogenous levels of flavonols in dark-grown roots are low, our data suggest that this low flavonol content might dynamically regulate root growth in response to endogenous signals. Thus, the total lack of flavonols might explain why the tt4 mutant has longer roots in either light (Lewis et al., 2011) or darkness (Figures 4 and 6) than wild-type roots. In this regard, we show that a low concentration of auxin can trigger O2− production that might promote cell division in dark-grown roots. However, this auxin-O2− mechanism might be counteracted by the ROS-scavenger potential of flavonols (Maloney et al., 2014; Watkins et al., 2014). An additional aspect of the flavonol-dependent connection between auxin-cytokinin and ROS pathways is that as cytokinin and H2O2 regulate the accumulation of flavonols in darkness, both can restrict O2− levels and affect auxin transport, creating a complex regulatory mechanism. Taken together, the outcome of all these interactions could be the establishment of tightly controlled root boundaries.
In conclusion, we propose that flavonols act as intrinsic and light-responsive growth cues contributing to the regulation of the slow (zonation) or fast (light tropism) root growth responses (Figures 10A and 10B).
METHODS
Material
The tt4 (SALK_661740) mutant and cre1-3 ahk3-4 (Franco-Zorrilla et al., 2005) double mutant were in the Col-0 ecotype, whereas shy2-2 (Kim et al., 1996) was in Ler. Marker lines 35S:UPB1-3xYFP (Tsukagoshi et al., 2010), AUX1:AUX1-GFP (Swarup et al., 2004), DR5:venusYFP (Heisler et al., 2005), CYCB1:CYCB1-GUS (Colón-Carmona et al., 1999), PIN1:PIN1-GFP, PIN3:PIN3-GFP (Blilou et al., 2005), and PLT2:PLT2-YFP (Galinha et al., 2007) were also in the Col-0 ecotype. All seedlings were sown under sterile conditions on vertically oriented 12-cm-square plates containing half-strength Murashige and Skoog (MS1/2) with 0.05% MES, 1% sucrose, and 1% plant-agar (Duchefa Biochemie) plates.
Light Conditions
Arabidopsis seedlings were grown in controlled chambers under a 16-h-light/8-h-dark photoperiod at 21 to 23°C. Light fluence rate was measured with a photometer/quantum/radiometer (LI-COR Bioscience LI-250A light meter). The fluorescent lights over the seedlings provided white light with an intensity of 90 to 110 µmol m−1 s−1. For the side illumination, we used an LED system: white light (75 µmol m−1 s−1) and blue light (35 µmol m−1 s−1).
Root Growth Assays and Microscopy Analysis
To analyze the root avoidance response, we modified the D-Root system (Silva-Navas et al., 2015). LGRs and DGRs were cultivated in the D-Root system and primary root length was determined as described previously (Silva-Navas et al., 2015). Root meristem size was calculated based on the number of meristematic cortex cells and/or the distance from the quiescent center to the last meristematic cell using confocal images. The end of the meristem was considered to be where a meristematic cortical cell was double the size of the previous one. To quantify the size of fully differentiated epidermis and cortical cells, confocal micrographs were taken in similar differentiation zones, which corresponded to the first visualized trichoblast formed (i.e., closest to the root meristem) and the adjacent cortical cell.
Leica AF lite software was used to quantify GFP and YFP levels.
Quantification of Mitotic Cells
The number of mitotic cells was quantified using CYCB1:CYCB1-GUS marker plants (Colón-Carmona et al., 1999). These plants were grown in MS1/2 with or without 50 µM quercetin for 8 d. Afterwards, the seedlings were stained for GUS activity as described previously (Manzano et al., 2012) and the number of spots was quantified (Jurado et al., 2008).
ROS Determination
NBT staining was used to visualize superoxide anions (O2−). Roots were stained for 2 min in a solution of 1 mM NBT in 20 mM phosphate buffer, pH 6.1. The reaction was stopped by transferring roots to distilled water. Pictures were taken with a Zeiss Axiophot equipped with a Leica DFC 300FX camera with identical settings for the entire experiment. The ImageJ histogram function (Schneider et al., 2012), reading gray intensity in inverted pictures where black has the maximum value, was used to determine mean staining intensity. The area measured covered from the quiescent center to 550 µm shootward in the root.
RNA Isolation and Microarray Hybridizations
We compared the transcriptome of roots grown in the presence of light (using top fluorescent lights, 100 µmol m−1 s−1) for 12 d (with a 16-h-light and 8-h-dark cycle) with that of roots grown in darkness. For these analyses, we used the D-Root system (Silva-Navas et al., 2015). Roots were harvested on day 12, 6 h after initiation of the light cycle. Total RNA (three independent biological samples) was isolated from roots using Trizol (Life Technologies) following the manufacturer’s instructions and purified using a Qiagen RNA isolation kit. cDNA was synthesized from 10 μg RNA using the double-stranded cDNA synthesis kit (Invitrogen) and Cy3-labeled with the NimbleGen one-color DNA labeling kit (Roche). Labeled cDNA for each condition was hybridized to the 100718:Athal_TAIR9_exp_H12 genome array (NimbleGen Gene Expression 12x135K platform; Roche) using the NimbleGen hybridization and wash buffer kit. Microarrays were scanned at 2-μm double-pass at 16-bit resolution, and data were analyzed with NimbleGen Scan software (Roche) through differential expression analysis using the Bayes t-statistics from linear models for Microarray data (Limma). P values were corrected for multiple testing using the q-value method (Storey and Tibshirani, 2003)
Metabolite Isolation and Analyses
One milliliter of chloroform:methanol:water (20:60:20) was added to 100 mg seedling samples, and the samples were incubated at 70°C with slight agitation for 1 h. After centrifugation (at 20,350g at room temperature for 30 min), the supernatant was collected and taken to dryness using a vacuum centrifuge. The pellet was dissolved in 100 μL methanol containing 1% acetic acid (v/v). Then, 5 μL was separated using a Dionex UltiMate3000 RSLC system and a 50 mm × 2.1 mm i.d., 1.7-µm, Acquity UPLC BEH C18 column maintained at 30°C. The following binary gradient was applied: 0 to 2 min isocratic 98% A (deionized water, 0.1% formic acid [v/v]), 2% B (acetonitrile, 0.1% formic acid [v/v]; Fluka; liquid chromatography/mass spectrometry grade]; 2 to 30 min linear gradient to 95% B; then, immediate step to 100% B isocratic for 2 min; reconditioning of the column, 3 min isocratic 2% B. The flow rate was 400 µL/min. Eluted compounds were detected by a micrOTOF-Q II mass spectrometer (Bruker Daltonik) equipped with an electrospray ionization source in positive ion mode. Typical instrument settings were as follows: capillary voltage, 4500 V; capillary exit, 130 V; dry gas temperature, 200°C; dry gas flow, 10 L/min. Ions were detected from m/z 50 to 1000 at a repetition rate of 2 Hz. Mass calibration was performed using sodium formate clusters (10 mM solution of NaOH in 50/50% [v/v] isopropanol/water containing 0.2% formic acid).
After the calculation of accurate m/z values for the pseudomolecular ions, [M+H]+, on the bases of the molecular formulae, extracted ion chromatograms were generated in the data sets included in this study, allowing a tolerance of 3 mD. Using the peak-finder algorithm, chromatographic peaks were detected and the mass spectra found within were averaged. The deviation of both the mass positions and the intensity ratios of the isotopic peaks were evaluated according to the SigmaFit approach (Ojanperä et al., 2006). Robust mass refers to the masses that occurred in at least 75% of the sample series of a given experiment (DGRs or LGRs). With respect to the experiment presented here, which consisted of five biologically independent replicates per genotype and condition, it refers to masses that occurred in at least four of the five measurements.
Flavonol Staining
Flavonol accumulation was analyzed using DPBA dye followed by confocal microscopy in a Leica SP8 confocal microscope. Quercetin-DPBA emission was collected at 570 to 650 nm and kaempferol-DPBA at 495- to 545-nm ranges, after excitation with 488-nm (argon) and 561-nm (HeNe) lasers. In addition, when indicated, kaempferol and quercetin were analyzed by epifluorescence microscopy using a Leica DM2000 microscope. The specificity of the signal was tested using the tt4 mutant, which lacks these flavonols, with and without narigenin, the precursor that tt4 is unable to not synthesize, in the medium (Supplemental Figure 3).
Statistical Analyses
Statistical analyses of the data were performed using SPSS 20 software. One-way or two-way ANOVA with Tukey’s post hoc test was used for testing differences between multiple samples. Different letters are used to indicate means that differ significantly.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: TT4 (AT5G13930), COP1 (AT2G32950), SHY2 (AT1G04240), CRE1 (AT2G01830), AHK30 (AT1G27320), UPB1 (AT2G47270), CYCB1;1 (AT4G37490), PLT2 (AT1G51190), and PIN1 (AT1G73590). Array data are deposited in the GEO Data Bank (GSE68093).
Supplemental Data
Supplemental Figure 1. Modified D-Root system to study root light avoidance in dark-grown roots.
Supplemental Figure 2. Root illumination affects metabolite accumulation.
Supplemental Figure 3. Analysis of emission spectrum of kaempferol- and quercetin-DPBA (diphenylboric acid-2-amino ester) complexes in root meristems.
Supplemental Figure 4. Mutations in COP1 induce flavonoid accumulation in the root independently of illumination.
Supplemental Figure 5. Effect of light on auxin transport protein levels.
Supplemental Figure 6. Effect of light on auxin signaling.
Supplemental Data Set 1. Gene expression in root is affected by light.
Supplemental Data Set 2. Metabolite profiles in root and shoot are affected by light.
Supplementary Material
Acknowledgments
Research was supported by grants from the Spanish Government-MICINN (BIO2011-28184-C02-01, CSD-00057, and BIO2014-52091-R) to J.C.d.P. and from MINECO and ERDF/FEDER (BFU2013-41160-P) and FP7 (PCIG11-GA-2012-322082) to M.A.M.-R. J.S.-N. was supported by a predoctoral fellowship from CCAA-Madrid and M.A.M.-R by a Ramon y Cajal contract from MICINN. We thank B. Scheres for PLT2:PLT2-YFP, P. Benfey for 35S:UPB1-3xGFP, O. Leyser for shy2-2, J.M. Franco-Zorrilla for cre1 ahk, and G. Muday for pTT4:GUS. We also thank P. Benfey, C. Gutierrez, and M.A. Blazquez for critical reading of the manuscript.
AUTHOR CONTRIBUTIONS
J.S.-N. isolated RNA for microarray analysis. J.S.-N., M.A.M.-R., F.J.G., and J.C.d.P. designed the experiments and analyzed the transcript expression data. J.S.-N., M.A.M.-R., and J.C.d.P. performed confocal analyses and flavonol quantification and measured root growth and meristem sizes. C.M., B.T.-R., and S.N.-N. performed light phototropism analyses in the wild type and different mutants and quantified dividing cells. J.S.-N., J.C.d.P., V.C., and S.P. performed the metabolic profiling and analyzed the data. J.C.d.P. and M.A.M.-R. wrote the manuscript. All authors approved the manuscript.
Glossary
- IAA
indole-3-acetic acid
- ROS
reactive oxygen species
- DGR
dark-grown root
- LGR
light-grown root
- DPBA
(diphenylboric acid-2-aminoethyl ester
- NBT
nitroblue tetrazolium
- dpi
days postimbibition
References
- Agati G., Brunetti C., Di Ferdinando M., Ferrini F., Pollastri S., Tattini M. (2013). Functional roles of flavonoids in photoprotection: new evidence, lessons from the past. Plant Physiol. Biochem. 72: 35–45. [DOI] [PubMed] [Google Scholar]
- Aida M., Beis D., Heidstra R., Willemsen V., Blilou I., Galinha C., Nussaume L., Noh Y.-S., Amasino R., Scheres B. (2004). The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119: 109–120. [DOI] [PubMed] [Google Scholar]
- Azari R., Tadmor Y., Meir A., Reuveni M., Evenor D., Nahon S., Shlomo H., Chen L., Levin I. (2010). Light signaling genes and their manipulation towards modulation of phytonutrient content in tomato fruits. Biotechnol. Adv. 28: 108–118. [DOI] [PubMed] [Google Scholar]
- Baluška F., Volkmann D., Barlow W.P. (2001). A polarity crossroad in the transition growth zone of maize root apices: cytoskeletal and developmental implications. J. Plant Growth Regul. 20: 170–181. [Google Scholar]
- Benjamins R., Scheres B. (2008). Auxin: the looping star in plant development. Annu. Rev. Plant Biol. 59: 443–465. [DOI] [PubMed] [Google Scholar]
- Blilou I., Xu J., Wildwater M., Willemsen V., Paponov I., Friml J., Heidstra R., Aida M., Palme K., Scheres B. (2005). The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44. [DOI] [PubMed] [Google Scholar]
- Boccalandro H.E., De Simone S.N., Bergmann-Honsberger A., Schepens I., Fankhauser C., Casal J.J. (2008). PHYTOCHROME KINASE SUBSTRATE1 regulates root phototropism and gravitropism. Plant Physiol. 146: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner W.G., Schmülling T. (2012). Transcript profiling of cytokinin action in Arabidopsis roots and shoots discovers largely similar but also organ-specific responses. BMC Plant Biol. 12: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs W.R., Christie J.M. (2002). Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci. 7: 204–210. [DOI] [PubMed] [Google Scholar]
- Brown D.E., Rashotte A.M., Murphy A.S., Normanly J., Tague B.W., Peer W.A., Taiz L., Muday G.K. (2001). Flavonoids act as negative regulators of auxin transport in vivo in arabidopsis. Plant Physiol. 126: 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer C.S., Muday G.K. (2004). The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16: 1191–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E., Estelle M. (2009). Cytokinin and auxin intersection in root meristems. Genome Biol. 10: 210. [Google Scholar]
- Christie J.M., Briggs W.R. (2001). Blue light sensing in higher plants. J. Biol. Chem. 276: 11457–11460. [DOI] [PubMed] [Google Scholar]
- Clore A.M., Doore S.M., Tinnirello S.M. (2008). Increased levels of reactive oxygen species and expression of a cytoplasmic aconitase/iron regulatory protein 1 homolog during the early response of maize pulvini to gravistimulation. Plant Cell Environ. 31: 144–158. [DOI] [PubMed] [Google Scholar]
- Colón-Carmona A., You R., Haimovitch-Gal T., Doerner P. (1999). Technical advance: spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 20: 503–508. [DOI] [PubMed] [Google Scholar]
- Dello Ioio R., Linhares F.S., Scacchi E., Casamitjana-Martinez E., Heidstra R., Costantino P., Sabatini S. (2007). Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 17: 678–682. [DOI] [PubMed] [Google Scholar]
- Dello Ioio R., Nakamura K., Moubayidin L., Perilli S., Taniguchi M., Morita M.T., Aoyama T., Costantino P., Sabatini S. (2008). A genetic framework for the control of cell division and differentiation in the root meristem. Science 322: 1380–1384. [DOI] [PubMed] [Google Scholar]
- Dunand C., Crèvecoeur M., Penel C. (2007). Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol. 174: 332–341. [DOI] [PubMed] [Google Scholar]
- Esmon C.A., Tinsley A.G., Ljung K., Sandberg G., Hearne L.B., Liscum E. (2006). A gradient of auxin and auxin-dependent transcription precedes tropic growth responses. Proc. Natl. Acad. Sci. USA 103: 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J., Demidchik V., Bothwell J.H., Mylona P., Miedema H., Torres M.A., Linstead P., Costa S., Brownlee C., Jones J.D., Davies J.M., Dolan L. (2003). Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446. [DOI] [PubMed] [Google Scholar]
- Franco-Zorrilla J.M., Martín A.C., Leyva A., Paz-Ares J. (2005). Interaction between phosphate-starvation, sugar, and cytokinin signaling in Arabidopsis and the roles of cytokinin receptors CRE1/AHK4 and AHK3. Plant Physiol. 138: 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinha C., Hofhuis H., Luijten M., Willemsen V., Blilou I., Heidstra R., Scheres B. (2007). PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449: 1053–1057. [DOI] [PubMed] [Google Scholar]
- Grunewald W., et al. (2012). Transcription factor WRKY23 assists auxin distribution patterns during Arabidopsis root development through local control on flavonol biosynthesis. Proc. Natl. Acad. Sci. USA 109: 1554–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K., Sakai T. (2012). PIN auxin efflux carriers are necessary for pulse-induced but not continuous light-induced phototropism in Arabidopsis. Plant Physiol. 160: 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler M.G., Ohno C., Das P., Sieber P., Reddy G.V., Long J.A., Meyerowitz E.M. (2005). Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 15: 1899–1911. [DOI] [PubMed] [Google Scholar]
- Ivanov V.B., Dubrovsky J.G. (2013). Longitudinal zonation pattern in plant roots: conflicts and solutions. Trends Plant Sci. 18: 237–243. [DOI] [PubMed] [Google Scholar]
- Joo J.H., Bae Y.S., Lee J.S. (2001). Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 126: 1055–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado S., Díaz-Triviño S., Abraham Z., Manzano C., Gutierrez C., del Pozo C. (2008). SKP2A, an F-box protein that regulates cell division, is degraded via the ubiquitin pathway. Plant J. 53: 828–841. [DOI] [PubMed] [Google Scholar]
- Kami C., Lorrain S., Hornitschek P., Fankhauser C. (2010). Light-regulated plant growth and development. Curr. Top. Dev. Biol. 91: 29–66. [DOI] [PubMed] [Google Scholar]
- Kim B.C., Soh M.C., Kang B.J., Furuya M., Nam H.G. (1996). Two dominant photomorphogenic mutations of Arabidopsis thaliana identified as suppressor mutations of hy2. Plant J. 9: 441–456. [DOI] [PubMed] [Google Scholar]
- Kiss J.Z., Mullen J.L., Correll M.J., Hangarter R.P. (2003). Phytochromes A and B mediate red-light-induced positive phototropism in roots. Plant Physiol. 131: 1411–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasek W.L., Ausubel F.M., Shirley B.W. (1998). A light-independent developmental mechanism potentiates flavonoid gene expression in Arabidopsis seedlings. Plant Mol. Biol. 37: 217–223. [DOI] [PubMed] [Google Scholar]
- Kubasek W.L., Shirley B.W., McKillop A., Goodman H.M., Briggs W., Ausubel F.M. (1992). Regulation of flavonoid biosynthetic genes in germinating Arabidopsis seedlings. Plant Cell 4: 1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera U., Briggs W.R. (2012). Root phototropism: from dogma to the mechanism of blue light perception. Planta 235: 443–452. [DOI] [PubMed] [Google Scholar]
- Lewis D.R., Ramirez M.V., Miller N.D., Vallabhaneni P., Ray W.K., Helm R.F., Winkel B.S.J., Muday G.K. (2011). Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiol. 156: 144–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. (2002). Blue light receptors and signal transduction. Plant Cell 14 (suppl.): S207–S225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E., Briggs W.R. (1995). Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7: 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mähönen A.P., ten Tusscher K., Siligato R., Smetana O., Díaz-Triviño S., Salojärvi J., Wachsman G., Prasad K., Heidstra R., Scheres B. (2014). PLETHORA gradient formation mechanism separates auxin responses. Nature 515: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney G.S., DiNapoli K.T., Muday G.K. (2014). The anthocyanin reduced tomato mutant demonstrates the role of flavonols in tomato lateral root and root hair development. Plant Physiol. 166: 614–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandoli D.F., Ford G.A., Waldron L.J., Nemson J.A., Briggs W.R. (1990). Some spectral properties of several soil types: implications for photomorphogenesis*. Plant Cell Environ. 13: 287–294. [Google Scholar]
- Manzano C., Ramirez-Parra E., Casimiro I., Otero S., Desvoyes B., De Rybel B., Beeckman T., Casero P., Gutierrez C., Del Pozo J.C. (2012). Auxin and epigenetic regulation of SKP2B, an F-box that represses lateral root formation. Plant Physiol. 160: 749–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano C., Pallero-Baena M., Casimiro I., De Rybel B., Orman-Ligeza B., Van Isterdael G., Beeckman T., Draye X., Casero P., Del Pozo J.C. (2014). The emerging role of reactive oxygen species signaling during lateral root development. Plant Physiol. 165: 1105–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreel K., Saeys Y., Dima O., Lu F., Van de Peer Y., Vanholme R., Ralph J., Vanholme B., Boerjan W. (2014). Systematic structural characterization of metabolites in Arabidopsis via candidate substrate-product pair networks. Plant Cell 26: 929–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaieff A., Rogachev I., Brodsky L., Malitsky S., Toal T.W., Belcher H., Yativ M., Brady S.M., Benfey P.N., Aharoni A. (2013). High-resolution metabolic mapping of cell types in plant roots. Proc. Natl. Acad. Sci. USA 110: E1232–E1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák J., Černý M., Pavlů J., Zemánková J., Skalák J., Plačková L., Brzobohatý B. (2015). Roles of proteome dynamics and cytokinin signaling in root to hypocotyl ratio changes induced by shading roots of Arabidopsis seedlings. Plant Cell Physiol. 56: 1006–1018. [DOI] [PubMed] [Google Scholar]
- Ojanperä S., Pelander A., Pelzing M., Krebs I., Vuori E., Ojanperä I. (2006). Isotopic pattern and accurate mass determination in urine drug screening by liquid chromatography/time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 20: 1161–1167. [DOI] [PubMed] [Google Scholar]
- Peer W.A., Murphy A.S. (2014). Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci. 12: 556–563. [DOI] [PubMed] [Google Scholar]
- Peer W.A., Brown D.E., Tague B.W., Muday G.K., Taiz L., Murphy A.S. (2001). Flavonoid accumulation patterns of transparent testa mutants of Arabidopsis. Plant Physiol. 126: 536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer W.A., Cheng Y., Murphy A.S. (2013). Evidence of oxidative attenuation of auxin signalling. J. Exp. Bot. 64: 2629–2639. [DOI] [PubMed] [Google Scholar]
- Peer W.A., Bandyopadhyay A., Blakeslee J.J., Makam S.N., Chen R.J., Masson P.H., Murphy A.S. (2004). Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16: 1898–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietta P.-G. (2000). Flavonoids as antioxidants. J. Nat. Prod. 63: 1035–1042. [DOI] [PubMed] [Google Scholar]
- Pollastri S., Tattini M. (2011). Flavonols: old compounds for old roles. Ann. Bot. (Lond.) 108: 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M.R., Paul N.D. (2006). Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytol. 170: 677–699. [DOI] [PubMed] [Google Scholar]
- Sablowski R., Carnier Dornelas M. (2014). Interplay between cell growth and cell cycle in plants. J. Exp. Bot. 65: 2703–2714. [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Briggs W.R. (2002). Cellular and subcellular localization of phototropin 1. Plant Cell 14: 1723–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez Alvarado A., Yamanaka S. (2014). Rethinking differentiation: stem cells, regeneration, and plasticity. Cell 157: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi M., et al. (2012). COP1 mediates the coordination of root and shoot growth by light through modulation of PIN1- and PIN2-dependent auxin transport in Arabidopsis. Development 139: 3402–3412. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Navas J., Moreno-Risueno M.A., Manzano C., Pallero-Baena M., Navarro-Neila S., Téllez-Robledo B., Garcia-Mina J.M., Baigorri R., Gallego F.J., del Pozo J.C. (2015). D-Root: a system for cultivating plants with the roots in darkness or under different light conditions. Plant J. 84: 244–255. [DOI] [PubMed] [Google Scholar]
- Stone B.B., Stowe-Evans E.L., Harper R.M., Celaya R.B., Ljung K., Sandberg G., Liscum E. (2008). Disruptions in AUX1-dependent auxin influx alter hypocotyl phototropism in Arabidopsis. Mol. Plant 1: 129–144. [DOI] [PubMed] [Google Scholar]
- Storey J.D., Tibshirani R. (2003). Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 100: 9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y.H., Liu Y.B., Zhang X.S. (2011). Auxin-cytokinin interaction regulates meristem development. Mol. Plant 4: 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R., et al. (2004). Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 16: 3069–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuka H., Umeda M. (2014). Hormonal control of cell division and elongation along differentiation trajectories in roots. J. Exp. Bot. 65: 2633–2643. [DOI] [PubMed] [Google Scholar]
- Taylor L.P., Grotewold E. (2005). Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 8: 317–323. [DOI] [PubMed] [Google Scholar]
- Tian Q., Reed J.W. (1999). Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 126: 711–721. [DOI] [PubMed] [Google Scholar]
- Tian Q., Uhlir N.J., Reed J.W. (2002). Arabidopsis SHY2/IAA3 inhibits auxin-regulated gene expression. Plant Cell 14: 301–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi H., Busch W., Benfey P.N. (2010). Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143: 606–616. [DOI] [PubMed] [Google Scholar]
- Wan Y., Jasik J., Wang L., Hao H., Volkmann D., Menzel D., Mancuso S., Baluška F., Lin J. (2012). The signal transducer NPH3 integrates the Phototropin1 photosensor with PIN2-based polar auxin transport in Arabidopsis root phototropism. Plant Cell 24: 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-Z., et al. (2015). Transcriptional regulation of PIN genes by FOUR LIPS and MYB88 during Arabidopsis root gravitropism. Nat. Commun. 6: 8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J.M., Hechler P.J., Muday G.K. (2014). Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol. 164: 1707–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel-Shirley B. (2002). Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 5: 218–223. [DOI] [PubMed] [Google Scholar]
- Yin R., Han K., Heller W., Albert A., Dobrev P.I., Zažímalová E., Schäffner A.R. (2014). Kaempferol 3-O-rhamnoside-7-O-rhamnoside is an endogenous flavonol inhibitor of polar auxin transport in Arabidopsis shoots. New Phytol. 201: 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokawa K., Fasano R., Kagenishi T., Baluška F. (2014). Light as stress factor to plant roots - case of root halotropism. Front. Plant Sci. 5: 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokawa K., Kagenishi T., Kawano T., Mancuso S., Baluška F. (2011). Illumination of Arabidopsis roots induces immediate burst of ROS production. Plant Signal. Behav. 6: 1460–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K.-X., Xu H.-H., Gong W., Jin Y., Shi Y.-Y., Yuan T.-T., Li J., Lu Y.-T. (2014). Proper PIN1 distribution is needed for root negative phototropism in Arabidopsis. PLoS One 9: e85720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K.X., Xu H.H., Yuan T.T., Zhang L., Lu Y.T. (2013). Blue-light-induced PIN3 polarization for root negative phototropic response in Arabidopsis. Plant J. 76: 308–321. [DOI] [PubMed] [Google Scholar]
- Zhang P., Foerster H., Tissier C.P., Mueller L., Paley S., Karp P.D., Rhee S.Y. (2005). MetaCyc and AraCyc. Metabolic pathway databases for plant research. Plant Physiol. 138: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.