Characterization of the steps leading to maize natural resistance against insect pests.
Abstract
The century-old maize (Zea mays) salmon silks mutation has been linked to the absence of maysin. Maysin is a C-glycosyl flavone that, when present in silks, confers natural resistance to the maize earworm (Helicoverpa zea), which is one of the most damaging pests of maize in America. Previous genetic analyses predicted Pericarp Color1 (P1; R2R3-MYB transcription factor) to be epistatic to the sm mutation. Subsequent studies identified two loci as being capable of conferring salmon silks phenotypes, salmon silks1 (sm1) and sm2. Benefitting from available sm1 and sm2 mapping information and from knowledge of the genes regulated by P1, we describe here the molecular identification of the Sm1 and Sm2 gene products. Sm2 encodes a rhamnosyl transferase (UGT91L1) that uses isoorientin and UDP-rhamnose as substrates and converts them to rhamnosylisoorientin. Sm1 encodes a multidomain UDP-rhamnose synthase (RHS1) that converts UDP-glucose into UDP-l-rhamnose. Here, we demonstrate that RHS1 shows unexpected substrate plasticity in converting the glucose moiety in rhamnosylisoorientin to 4-keto-6-deoxy glucose, resulting in maysin. Both Sm1 and Sm2 are direct targets of P1, as demonstrated by chromatin immunoprecipitation experiments. The molecular characterization of Sm1 and Sm2 described here completes the maysin biosynthetic pathway, providing powerful tools for engineering tolerance to maize earworm in maize and other plants.
INTRODUCTION
Maize (Zea mays) is one of the most cultivated cereals worldwide, covering ∼40% of the global arable land, a consequence of its nutritional value as a feeding crop and feedstock. In addition, it is utilized for biofuels, plastic, fabric production, and by the food industry (Morris and Sands, 2006). The process of selective breeding applied to increased productivity, high kernel quality for better edibility, increased starch content, thin pericarps surrounding kernels, and loose husks to allow easier drying of the cob has resulted in the increased susceptibility of maize to several pests (Bailey and Bailey, 1938). Chief among them is damage by the lepidopteran maize earworm (CEW, Helicoverpa zea Boddie), responsible for significant grain losses in the US. CEW destruction starts when the newly hatched larvae start feeding on silks, the elongated stigmas on the female flower, until they reach the ear and continue to feed on kernels (Waiss et al., 1979). Antibiosis to CEW is primarily provided by maysin [2′′-O-α-rhamnosyl-6-C-(6-deoxy-xylo-hexos-4-ulosyl)luteolin] (Waiss et al., 1979), a C-glycosyl flavone with strong feeding deterrent effects, first characterized from the Mexican maize landrace Zapalote Chico (Elliger, 1980; Wiseman et al., 1992; Widstrom and Snook, 1998a, 1998b).
Given the devastating effects of CEW, efforts involving quantitative trait locus mapping were performed to identify specific genomic regions responsible for regulating the accumulation of maysin and therefore increasing natural resistance to CEW. A region in the short arm of chromosome 1 accounted for more than half of the phenotypic variance (Byrne et al., 1996). This genomic region harbors the pericarp color1 (P1) locus, encoding an R2R3-MYB transcription factor (Grotewold et al., 1991) that, when expressed in maize Black Mexican Sweet cells, was sufficient to induce the formation of C-glycosyl flavones (Grotewold et al., 1998). P1 had been known for over a century for its ability to control the accumulation of the brick-red phlobaphene pigments in pericarp and cob glume tissues (Emerson, 1917; Anderson and Emerson, 1923), which are a landmark of ‘Indian Maize’. Phlobaphenes result from the polymerization of luteoforol (or apiforol), a flavan-4-ol derived from eriodictyol (or naringenin) by the action of dihydroflavonol-4-reductase, encoded by the A1 gene (Figure 1). Alternatively, luteoforol can be converted to the 3-deoxy anthocyanidin luteolinidn by an unknown enzyme, probably with anthocyanidin synthase-like activity (Figure 1). P1 alleles can cause red pigment to accumulate in the pericarp and the cob glumes (P1-rr, for red pericarp and red cob), only the cob glumes (P1-wr, for white pericarp and red cob), only the pericarp (P1-rw, for red pericarp and white cob), or neither the cob glumes nor the pericarp (P1-ww, for white pericarp and white cob).
Figure 1.
Proposed 3-Deoxyflavonoid Biosynthetic Pathway.
Condensation of p-coumaroyl-CoA and malonyl-CoA by chalcone synthase (CHS; C2) results in naringenin chalcone, which is then converted to naringenin by chalcone isomerase (CHI; encoded by CHI1). Naringenin is converted to eriodictyol by a flavanone-3′-hydroxylase (F3′H1; encoded by PR1), which could then be converted to the flavone luteolin by a flavone synthase (FNS; FNSI-1) (Falcone Ferreyra et al., 2015). Eriodictyol is converted to isoorientin (C-glycosyl flavone) by a flavanone-2-hydroxylase (F2H; CYP93G5) (Morohashi et al., 2012) and a C-glucosyl transferase (CGT; UGT708A6) (Falcone Ferreyra et al., 2013). The proposed steps for conversion of isoorientin to maysin involve at least two enzymatic conversions by as yet uncharacterized enzymes. Eriodictyol is also converted to luteoforol (flavan-4-ol) by a dihydroflavonol reductase (DFR; A1) and subsequently to luteolinidin (3-deoxy anthocyanidin) by what is thought to be an ANS-like enzyme (Liu et al., 2010) or is polymerized into phlobaphenes. Enzymes identified are shown in black, and proposed steps are in gray. Phlobaphenes are shown in red, and 3-deoxyanthocyanidins are shown in pink.
Maysin biosynthesis involves the conversion of eriodictyol to 2-hydroxyeriodictyol by the P1-regulated flavanone 2-hydroxylase (F2H1, CYP93G5; Morohashi et al., 2012). Subsequently, 2-hydroxyeriodictyol is C-glycosylated by the C-glucosyl transferase UGT708A6 (Falcone Ferreyra et al., 2013), and following a likely spontaneous dehydration reaction (Brazier-Hicks et al., 2009), isoorientin (luteolin 6-C-glucoside) is formed (Figure 1). From the structures determined by McMullen et al. (2004) using NMR, the conversion of isoorientin to maysin requires at least two additional enzymatic steps: the first one involving the incorporation of a rhamnose to the glucose moiety of isoorientin to form rhamnosylisoorientin (isoorientin 2′′-O-rhamnoside) and the second involving the dehydration of glucose to 4-keto-6-deoxy glucose to convert rhamnosylisoorientin into maysin (Figure 1). The enzymes responsible for these last steps in the maysin biosynthetic pathway remain unknown, but they were proposed to correspond to the salmon silks loci (McMullen et al., 2004). The original salmon silks mutant (currently known as sm1) was originally identified at a Nebraska fair in 1909 (Anderson, 1921); their name describes the characteristically strong salmon-colored silks of these lines, compared with the usual green or purple color of silks. So far, two salmon silks loci have been identified, sm1 (on the long arm of chromosome 6) and sm2, on the long arm of chromosome 2 (Anderson, 1921; McMullen et al., 2004).
Here, we describe the cloning and biochemical characterization of Sm1 and Sm2, resulting in the completion of the maysin biosynthetic pathway. Selecting P1-regulated genes in the sm1 and sm2 respective mapping intervals identified candidate genes, which were further tapered by testing whether mRNA accumulation was affected in the respective mutants. To unequivocally demonstrate the identity of the identified genes as Sm1 and Sm2, we developed a maize biochemical complementation system consisting of sm1 or sm2 protoplasts, which upon transformation with P1, accumulate metabolic intermediates corresponding to where the pathway is blocked in each mutant. Complementation by transformation with the candidate genes hence results in flux through the pathway being restored and maysin accumulation. Using this approach, we show here that Sm2 encodes a rhamnosyl transferase (UGT91L1) that uses UDP-rhamnose (UDP-Rha) and isoorientin as substrates, producing rhamnosylisoorientin. In turn, Sm1 encodes an UDP-rhamnose synthase (RHS1), which in a very unusual reaction converts the glucose moiety in rhamnosylisoorientin to 4-keto-6-deoxy glucose, resulting in maysin formation. Thus, an elegant combination of genetics, high-throughput sequencing, and biochemistry permitted us to identify elusive genes responsible for the last biosynthetic steps of an agronomically important antibiosis compound.
RESULTS
Biochemical Validation of Flavonoids Accumulating in sm Silks
Previous studies had identified the main flavones accumulating in sm1 and sm2 mutants (McMullen et al., 2004). To obtain a baseline flavone profile for the other experiments described in this study, we obtained seeds for different sm alleles from the Maize Genetics Cooperation Stock Center. For simplicity, we named those alleles sm1-1 through sm1-8, and sm2-1 through sm2-5 (Supplemental Table 1). Visual kernel and cob inspection of each mutant line permitted us to establish the most likely P1 allele present in each one (Supplemental Table 1). To determine whether the various sm2 and sm1 lines accumulated the expected pathway intermediates, isoorientin (IO) for sm2 and rhamnosylisoorientin (RIO) for sm1 (McMullen et al., 2004; Figure 2A), we performed HPLC analysis following absorbance at 350 nm on silk methanolic extracts and compared the retention times on each chromatographic profile with those of available authentic standards. Silk extracts from the sm2-1 mutant (Figure 2B) and other sm2 alleles (Supplemental Figure 1) show a peak at 5.2 min, corresponding to IO, and little to no accumulation of RIO or maysin, which are highly accumulated in P1-rr (expressing Sm1 and Sm2) silks (Figure 2B). The peak at ∼7 min may correspond to derhamnosylmaysin (Figure 2B), which was previously identified in sm2 silks and proposed to be a product of sm1 acting on IO instead of RIO (McMullen et al., 2004).
Figure 2.
Biochemical Analysis of sm Lines.
(A) Proposed pathway for maysin formation. The reaction from IO to RIO is proposed to be mediated by sm2 and the dehydration of RIO to maysin (M) by sm1.
(B) HPLC chromatograms of methanolic extracts from salmon silks mutant silks following absorbance at 350 nm. Upper panel: IO (blue), RIO (red), and maysin (green) standards. Second panel: extracts from P1-rr silks, in which sm2 and sm1 are active, accumulate maysin. Third panel: sm2 mutant extracts from sm2-1 silks accumulate IO. Lower panel: sm1 mutant extracts from sm1-3 silks accumulate RIO.
(C) Upper panel: LC-MS/MS profiles following m/z− ratios of 447 for IO, 593 for RIO, and 574 for maysin standards. Second and third panels depict representative LC-MS/MS profiles of sm2 protoplast extracts transformed with the negative control (DP611) and with 35Spro:P1 showing the m/z− 447 molecular ion corresponding to IO. Fourth and fifths panels depict representative LC-MS/MS profiles of sm1 protoplast extracts transformed with the negative control (DP611) or with 35Spro:P1 showing the m/z− 447 and 593 molecular ions corresponding to IO and RIO, respectively.
Silk extracts from the sm1-3 mutant (Figure 2B) and other sm1 alleles (Supplemental Figure 2) show a peak at 4.5 min, corresponding to RIO, which is absent in P1-rr Sm1 silks (Figure 2B, third panel). In addition, silks from sm1-1 mutants show the presence of variable amounts of maysin (Supplemental Figure 2), suggesting that the respective sm1 alleles are not complete loss of function.
To determine whether the ectopic expression of P1 in the respective mutants recapitulates the accumulation of pathway intermediates present in sm1 and sm2 silks, we developed a transformation system using maize protoplasts obtained from sm mutant seedlings. Extracted flavonoids from protoplasts isolated from sm2-1 or sm1-3 seedlings transformed with a negative control were analyzed by liquid chromatography followed by tandem mass spectrometry (LC-MS/MS) and showed trace levels of IO and RIO, respectively (Figure 2C, second and fourth panels). This is consistent with P1 not normally being expressed in maize vegetative tissues (Sidorenko et al., 2000; Cocciolone et al., 2001). Transformation with P1 expressed from the constitutive CaMV 35S promoter (35Spro:P1) resulted in a significant accumulation of IO in sm2-1 and of both IO and RIO in sm1-3 (Figure 2C), indicating that the presence of P1 in seedling protoplasts is sufficient to recapitulate precursor accumulation similarly to what happens in silks. Taken together, these results confirm that sm2 is blocked in the conversion of IO to RIO and sm1 in the conversion of RIO to maysin and that P1 is epistatic to both sm1 and sm2.
Identification and Characterization of sm2 Candidate Genes
The sm2 locus maps between the phi127 and bnlg1721 markers on the long arm of chromosome 2 (T218 × GT119 F2, 1997 map; McMullen et al., 2004). This region corresponds to ∼11 Mb, and the available draft maize genome sequence (Maize B73 RefGen v2) shows 336 putative genes from the working gene set in this interval (Figure 3A). Taking advantage of previous mRNA-sequencing (RNA-seq) information from contrasting P1-rr and P1-ww silks and pericarps at different developmental stages (Morohashi et al., 2012), we investigated which of these 336 genes showed an increase in mRNA accumulation in P1-rr compared with P1-ww, comparable to other flavonoid pathway genes (e.g., C2, encoding chalcone synthase, and A1; Figure 3A). Of the 336 gene models contained in the sm2 mapping interval (Figure 3A; Supplemental Table 2), mRNA levels for nine genes were significantly higher in P1-rr compared with P1-ww silks (Supplemental Table 2). Sequence analyses from proteins encoded by these genes yielded UGT91L1 (GRMZM2G180283) as a putative UDP-glycosyltransferase (UGT) that clustered together with previously characterized UGTs that use flavonoid glycosides as substrate acceptors and catalyze the formation of sugar-O-sugar links, including the rhamnosyl transferases from Petunia hybrida and Citrus maxima (Falcone Ferreyra et al., 2013). Moreover, in phylogenetic analyses of all putative maize UGTs, five additional UGTs clustered together with UGT91L1, but none of the genes encoding these UGTs are upregulated by P1 in silks, further supporting UGT91L1 as an Sm2 candidate (Supplemental Figure 3 and Supplemental Data Set 1).
Figure 3.
UGT91L1 Corresponds to the sm2 Locus.
(A) The sm2 mapping region on chromosome 2 covers an 11-Mb region between markers phi127 and bnlg1721 comprising 336 gene models, out of which a putative glycosyl transferase (UGT91L1, GRMZM2G180283; red arrow) was identified as a candidate. A1 and C2 levels are shown as reference for the expression of other flavonoid genes upregulated by P1. Expression levels are in FPKM (fragments per kilobase of transcript sequence per million base pairs sequenced).
(B) LC-MS/MS analysis of in vitro UGT91L1 activity assayed using UDP-Rha and IO as substrates showing the production of a molecular ion of m/z+ 595 (lower panel) that corresponds to RIO compared with the corresponding standard (upper panel), confirming that UGT91L1 functions as Sm2. E. coli cells transformed with the empty vector (pET28) did not show the production of this compound (middle panel). Upper panel: representative ion chromatograms of true standards IO (blue, m/z+ = 449) and RIO (red, m/z+ = 595).
(C) LC-MS/MS analysis shows that maysin accumulation following m/z− = 574 is reconstituted in sm2-1 protoplasts cotransformed with 35Spro:UGT91L1-GFP and 35Spro:P1 (lower panel) but not with 35Spro:P1 alone (Figure 2C). Upper panel: representative ion chromatograms following the m/z ratios of true standards IO (blue, m/z− = 447), RIO (red, m/z− = 593), and maysin (green, m/z− = 574).
We rationalized that if UGT91L1 corresponds to Sm2, then the UGT91L1 gene could be mutated, and/or UGT91L1 mRNA levels could be reduced in the sm2 mutant alleles. To test this, we sequenced the genomic region corresponding to UGT91L1 to ∼1.5 kb upstream of the predicted transcription start site and identified several nucleotide differences with the B73 reference allele primarily located in the upstream regulatory region, but none resulting in an amino acid change (Supplemental Figure 4A and Supplemental Table 7). Since the genetic background of the sm mutants is not known, these DNA sequence differences with B73 (shared between all sequenced sm2 alleles) could correspond to genetic background differences and not necessarily be responsible for the sm2 phenotypes. To evaluate whether UGT91L1 mRNA levels are reduced, we performed RT-qPCR on cDNA obtained from silk tissues from the various sm2 alleles using three different primers sets and compared mRNA levels to those in P1-rr and P1-ww silks, in which a wild-type Sm2 allele is present (as positive and negative controls, respectively; Supplemental Figure 4 and Supplemental Table 6). Consistent with the flavone profile analyses indicating the absence of maysin in silks from sm2-1, sm2-2, and sm2-5 and a significant reduction in sm2-3 (Figure 2; Supplemental Figure 1), RT-qPCR experiments showed an almost complete absence of UGT91L1 transcripts in silks of these mutant lines (Supplemental Figure 4B). Thus, given the high identity with rhamnosyl transferases, the location in the sm2 interval, the regulation by P1, and the reduced expression in several sm2 mutant alleles, our results strongly suggest that UGT91L1 is a good candidate for Sm2.
UGT91L1 Encodes a Rhamnosyl Transferase That Complements the sm2 Mutation
To determine whether UGT91L1 is able to carry out the rhamnose addition required for the conversion of IO to RIO (Figure 2A), we cloned the full open reading frame of UGT91L1 in the pET28a vector (Figure 3B) and expressed the encoded protein in Escherichia coli as an N-terminal fusion protein with a six-histidine tag (His6-UGT91L1; see Methods).
We anticipated that, in addition to IO, a reaction involving UGT91L1 would require UDP-Rha as a substrate, which is not commercially available. Thus, to generate UDP-Rha, we cloned a previously characterized rhamnose synthase from Arabidopsis thaliana (At-RHM1) into the pGZ25 vector and transformed it into the T334 yeast strain (Watt et al., 2004; Oka et al., 2007). Crude protein extracts from yeast cells transformed with the empty vector (see Methods) were used as negative control. Total protein extracts from yeast cultures expressing At-RHM1 and supplied with UDP-glucose (UDP-Glu), NAD+, and NADPH (Oka et al., 2007) were used to obtain UDP-Rha. Subsequently, total protein extracts from E. coli cultures expressing UGT91L1 were assayed for rhamnosyl transferase activity by incubation with IO and the UDP-Rha previously generated. LC-MS/MS analyses indicated that UGT91L1 produced a compound (with a retention time of ∼2.5 min), absent when only the empty plasmid was used (compared with the second and third panels in Figure 3B), with an m/z of 595.2 that was identified as RIO, by comparison with the respective standard.
To unequivocally demonstrate that UGT91L1 corresponds to Sm2, we investigated whether UGT91L1 complemented the sm2 mutation, taking advantage of the maize protoplast transformation system described above. To this end, we cloned the full open reading frame of UGT91L1 as a C-terminal translational fusion to enhanced GFP, expressed under the control of the 35S promoter (35Spro:UGT91L1-GFP; Supplemental Figure 5A). We transformed sm2-1 protoplasts with 35Spro:P1, with and without 35Spro:UGT91L1-GFP. After transformation, methanolic extracts were analyzed for RIO accumulation by LC-MS/MS. As shown in Figure 3C, maysin is present in the 35Spro:P1 transformed protoplasts only if 35Spro:UGT91L1-GFP was present as well (Figure 3C, second panel, negative control). Taken together, our results demonstrate that UGT91L1 encodes a functional rhamnosyl transferase that uses IO as substrate and that it biochemically complements the sm2 mutation. Thus, UGT91L1 corresponds to Sm2.
Identification and Characterization of sm1 Candidate Genes
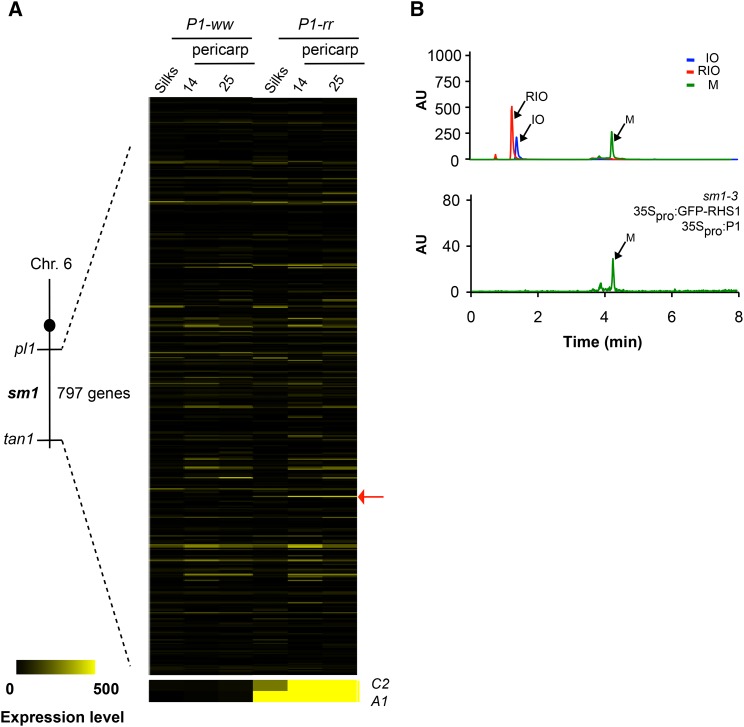
To molecularly characterize sm1, we determined that the genetic interval that contains this locus (Anderson, 1921), and which spans the region between tangled1 (tan1) to purple plant1 (pl1) loci on chromosome 6, corresponds to ∼35 Mb and contains ∼797 gene models (Genetic Map 2008; Wei et al., 2009; Figure 4A).
Figure 4.
RHS1 Is a Strong sm1 Candidate.
(A) The sm1 mapping region on chromosome 6 covers a 35.5-Mb region between pl1 and tan1 comprising 797 gene models, from which a putative UDP-rhamnose synthase (RHS1, GRMZM2G031311; red arrow) was identified as a candidate. A1 and C2 levels are shown as reference for the expression of other flavonoid genes upregulated by P1. Expression levels are in FPKM (fragments per kilobase of transcript sequence per million base pairs sequenced).
(B) LC-MS/MS analysis following m/z− of 574 shows that maysin accumulation is reconstituted in sm1-3 protoplasts cotransformed with 35Spro:GFP-RHS1 and 35Spro:P1 but not with 35Spro:P1 alone (Figure 2C). Upper panel: representative ion chromatograms following the m/z ratios of true standards IO (blue, m/z− = 447), RIO (red, m/z− = 593), and maysin (green, m/z− = 574).
Similarly as we did for sm2, we determined that 27/797 gene models were expressed significantly higher in P1-rr compared with P1-ww silks (Figure 4A; Supplemental Table 3). Within the genes in the sm1 interval, GRMZM2G031311 was particularly attractive as an sm1 candidate because sequence analyses indicated it encoded a putative rhamnose synthase with 79.8% identity and 88.0% similarity at the amino acid level with At-RHM1 (Rice et al., 2000) and which we called RHS1. The conversion of UDP-Glu to UDP-Rha by rhamnose synthases involves a first step that results in UDP-4-keto-6-deoxy glucose catalyzed by the N-terminal domain with dehydratase activity, which is then converted into UDP-Rha by the action of the epimerase/reductase activity of the C-terminal domain (Figures 5A and 5B; Giraud and Naismith, 2000). Because the conversion of RIO into maysin involves the dehydration of the glucose group in RIO to 4-keto-6-deoxy glucose, RHS1 provided a good candidate for sm1 (Figures 2A and 5C). To further support the identity of RHS1 as Sm1, we analyzed all putative rhamnose synthase genes present in the maize genome (Supplemental Table 4). We found three additional genes encoding putative RHMs, but none of them are in the sm1 interval or are as significantly regulated by P1 as RHS1. These results supported RHS1 as a possible Sm1 candidate (Supplemental Table 2). Nevertheless, the remaining genes could potentially provide the UDP-Rha to be used as substrate by UGT91L1, in addition to other metabolic functions.
Figure 5.
Proposed Mechanism for RHS1 as sm1 Candidate.
RHS1 contains two domains characteristic of plant rhamnose synthases.
(A) The N-terminal domain between residues 20 and 262 corresponds to a NAD+-dependent dehydratase and the C-terminal domain corresponds to an epimerase/reductase between residues 389 and 652.
(B) UDP-rhamnose biosynthesis starts with a dehydration step to form the UDP-4-keto-6-deoxy glucose intermediate, which is then converted to UDP-rhamnose by an epimerization reduction step.
(C) Proposed mechanism for RHS1 involvement in maysin biosynthesis by dehydrating the glucose moiety in rhamnosylisoorientin.
To investigate the nature of the sm1 mutations, we sequenced the RHS1 regions in sm1-1, sm1-2, sm1-3, and sm1-6, up to 2 kb upstream of the TSS. While we found no nucleotide differences between sm1-6 and the B73 reference allele in the 5.6 kb of genomic region sequenced, several nucleotide changes were evident in sm1-1, sm1-2, and sm1-3 (Supplemental Figure 6A and Supplemental Table 7). One of those changes in sm1-3 resulted in the replacement of Met-525 to Leu. Met-525 is conserved in all plant RHMs so far characterized, suggesting an important functional role (Yin et al., 2011; Reiter and Vanzin, 2001). The other changes (shared between both alleles) are in the region upstream of the TSS or result in conservative changes that do not affect the protein sequence (Supplemental Figure 6A).
To determine whether RHS1 mRNA accumulation is affected in the sm1 mutants, we performed RT-qPCR experiments using cDNA obtained from silks from sm1-1, sm1-3, and sm1-6 and RHS1-specific primers, normalized to actin1. All experiments were performed in biological triplicate using three sets of primers (Supplemental Figure 6A and Supplemental Table 6). We compared mRNA levels to those in P1-rr and P1-ww, both lines with a wild-type allele of Sm1 (as positive and negative controls, respectively; Supplemental Figure 6). Consistent with the HPLC results that showed that these alleles accumulated reduced levels of maysin (Supplemental Figure 2), sm1-6 accumulated low RHS1 mRNA levels (Supplemental Figure 6). By contrast, sm1-1 accumulated higher levels of RHS1 mRNA than those present in P1-ww, but lower than those in P1-rr (Supplemental Figure 6). These results suggest that RHS1 is a candidate gene for the Sm1 locus.
RHS1 Complements sm1 Mutants
To unequivocally determine whether RHS1 corresponds to Sm1, we investigated whether RHS1 complements sm1 using the maize protoplast system described for sm2. Cotransformation with 35Spro:P1 and 35Spro:RHS1-GFP (Supplemental Figure 5B), but not with 35Spro:P1 (Figure 2C, third panel) or 35Spro:RHS1-GFP alone, resulted in sm1-3 protoplasts that accumulate maysin (Figure 4B), as determined by LC-MS/MS analyses following the m/z 577 molecular ion corresponding to the M standard. These results confirm that RHS1 corresponds to Sm1.
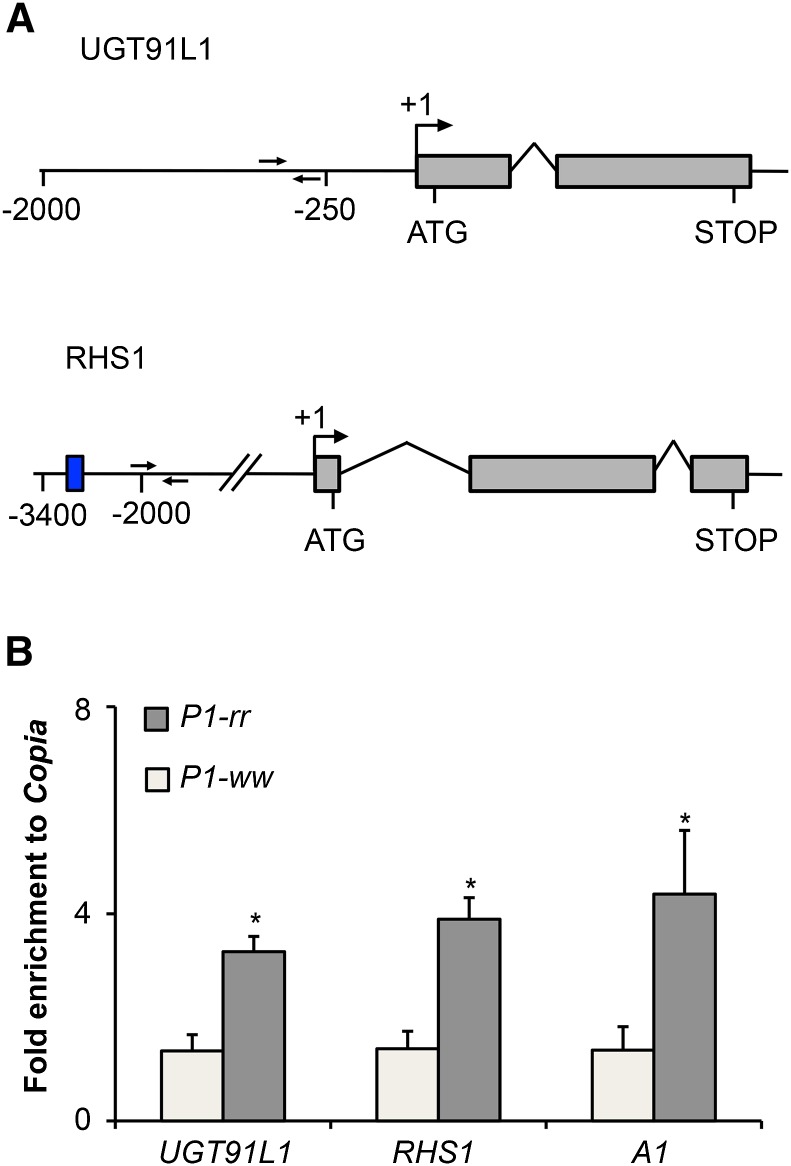
Sm2 and Sm1 Are Direct Targets of P1
UGT91L1 and RHS1 were selected as Sm2 and Sm1 gene candidates, respectively, because RNA-seq experiments showed the respective mRNAs to be significantly higher in P1-rr compared with P1-ww (Figures 3A and 4A). In addition, the mRNA levels from these genes were reduced in the respective sm mutants as confirmed by the RT-qPCR results described above (Supplemental Figures 4 and 6). Finally, complementation experiments further confirmed the identities of these two genes. To investigate whether UGT91L1 and RHS1 are P1 direct targets as is the case for other genes involved in 3-deoxyflavonoid and flavone biosynthesis (Morohashi et al., 2012), we conducted chromatin immunoprecipitation (ChIP) experiments using a polyclonal antibody specific for P1 (Falcone Ferreyra et al., 2010; Morohashi et al., 2012), followed by quantitative PCR (ChIP-qPCR) using primers close to or flanking P1 binding sites (Morohashi et al., 2012). Our results show a specific enrichment of the upstream regulatory sequences of UGT91L1 and RHS1 (Figure 6) in ChIP experiments conducted on P1-rr, but not P1-ww, pericarps (Figure 6). Taken together, our results demonstrate that, similarly to other genes involved in flavonoid biosynthesis so far identified, P1 directly regulates the expression of UGT91L1 (Sm2) and RHS1 (Sm1) in pericarps and silks of maize, resulting in the accumulation of maysin.
Figure 6.
UGT91L1 and RHS1 Are Direct Targets of P1.
(A) UGT91L1 and RHS1 gene models. Exons are represented as gray boxes, introns as arched lines, and genomic regions as straight lines. Transcriptional start sites are depicted by perpendicular arrows at the +1 position; start (ATG) and stop (STOP) codon positions are also shown. MACS peaks estimated locations are indicated as a blue box. ChIP-qPCR primer locations are shown as head to head black arrows. Positions are given in base pairs from +1 transcriptional start sites.
(B) The bar plot corresponds to results from ChIP-qPCR experiments showing fold enrichment to Copia in P1-rr and P1-ww pericarps at 14 d after pollination for UGT91L1 and RHS1, using A1 as a positive control. Error bars correspond to se of the mean, using six biological replicates (n = 6). Asterisks denote statistically significant differences between P1-rr and P1-ww at P < 0.05 (two-tailed t test).
DISCUSSION
As has been the case for many other maize flavonoid genes (Coe, 2001), the distinctive color of 3-deoxyanthocyanidins provided by the sm mutations (Levings, 1972) permitted us to unequivocally characterize the last two enzymatic steps in the maysin biosynthetic pathway, corresponding to Sm2 and Sm1. Sm2 encodes a rhamnosyl transferase (UGT91L1) that forms rhamnosylisoorientin from UDP-Rha and isoorientin. In turn, rhamnosylisoorientin is the substrate for the rhamnose synthase (RHS1) encoded by Sm1, which converts it into maysin. To demonstrate the molecular identity of UGT91L1 as Sm2 and RHS1 as Sm1, we developed a maize protoplast complementation system that circumvents the need for transgenic plants. This approach could be easily adapted to probe the activity of other metabolic enzymes. These studies, together with our previous characterization of CYP93G5 that converts flavanones (including naringenin and eriodictyol) to the respective 2-hydroxyflavanones (Morohashi et al., 2012) and UGT708A6 that C-glycosylates 2-hydroxyflavanones to the respective flavone-6-C-glucosides (Falcone Ferreyra et al., 2013), complete the maize maysin biosynthetic pathway.
Specialized metabolites, such as flavonoids, frequently provide a first level of defense against pathogens and herbivores (Field et al., 2006), and biological control often furnishes a more durable check against plant diseases than chemical pesticides (Bardin et al., 2015). The Bacillus thuringiensis (Bt) protein is only partially toxic to the CEW, and the development of resistance has been reported (Reisig and Reay-Jones, 2015), suggesting a need to develop other methods of resistance. Moreover, recent analyses suggest a significant future threat of maize and other host crops by Helicoverpa armigera (Kriticos et al., 2015), which, similarly to H. zea (CEW) (Rector et al., 2002), is also sensitive to maysin. Thus, establishing the entire maysin biosynthetic pathway provides an opportunity to improve elite maize lines by marker-assisted breeding and also to transfer the pathway to other Helicoverpa susceptible plants. However, maysin and other C-glycosylflavones are also important in vegetative maize tissues for protection to UV-B exposure, characteristic of high-altitude maize landraces (Casati and Walbot, 2005). It is not yet known whether Sm2 and Sm1 participate in maysin accumulation in leaves in response to UV-B or whether other UGTs and rhamnose synthases are expressed in vegetative tissues. The maize B73 genome encodes ∼160 UGTs, from which six are putative rhamnosyl transferases, based on phylogenetic analysis (Supplemental Figure 3). From these six rhamnosyl transferases, only UGT91L1 was included in the appropriate Sm2 mapping interval and regulated by P1 (Supplemental Table 5).
The identity of Sm2 as a rhamnosyl transferase was adequately predicted by the characterization of the pathway intermediates that accumulate in the sm2 mutant (McMullen et al., 2004). The accumulation of derhamnosylmaysin, in addition to isoorientin, in the sm2 mutant (Figure 2B) was proposed to reflect the ability of Sm1 to also function on isoorientin (McMullen et al., 2004). However, this activity remains to be demonstrated.
While Sm1 was also previously predicted to encode a glucose modification enzyme (McMullen et al., 2004), its identity as a rhamnose synthase (RHS1) came initially as a surprise. Rhamnose synthases are best known for the conversion of UDP-glucose to UDP-Rha, which is primarily used in the biosynthesis of cell wall components and for rhamnose-containing specialized metabolites (Kuhn et al., 2011; Lan et al., 2015). Our results demonstrate that rhamnose synthases can also function on glucose residues conjugated to a flavonoid instead of UDP, such as found in rhamnosylisoorientin (Figure 5). Similar to Arabidopsis RHM enzymes (Oka et al., 2007), RHS1 is predicted to encode a multidomain protein (Figure 5A) with an N-terminal-encoded dehydratase domain capable of converting the glucose residue in rhamnosylisoorientin to 4-keto-6-deoxy glucose, as found in maysin (Figure 5C). Interestingly, the conversion stops after the dehydration, suggesting perhaps that RHS1 lacks functional epimerase and keto-reductase activities characteristic of the C-terminal domain (Oka et al., 2007). However, we were able to show that RHS1 is capable of converting UDP-glucose to UDP-Rha by bioconversion assays in yeast (Supplemental Figure 7), indicating that there must be another reason why the reaction stops at maysin (Figure 5C). Whether this is a consequence of the additional rhamnose residue attached to position 2′ or whether this reflects that instead of a nucleotide-sugar the substrate is a flavonoid-sugar remains to be established. The fact that sm1 mutants accumulate rhamnosylisorientin suggests that RHS1 is not the rhamnose synthase enzyme that provides UDP-Rha to UGT91L1 (Sm2) to generate rhamnosylisorientin. Thus, another rhamnose synthase encoded by the maize genome is responsible for the biosynthesis of UDP-Rha that is to be used by UGT91L1 or for cell wall biosynthesis (Supplemental Table 4). Nucleotide-sugar interconversions are part of central metabolism, yet RHS1 has clearly been co-opted for specialized metabolism, as our results indicate. This emergence of reshaped catalytic activities is at the core of the dramatic chemodiversity that plants display (Weng et al., 2012; Moghe and Last, 2015). It is currently unclear whether the ability to use flavonoid-glucose as a substrate is a general ability of plant multidomain rhamnose synthase enzymes or whether this is a unique activity of RHS1.
In conclusion, the results from these studies not only add novel enzymatic activities to one of the best-known plant specialized metabolism biosynthetic pathways, but also provide insight into how enzymes from primary metabolism are used to form important insecticidal specialized compounds. The challenge ahead is to use the knowledge acquired here in maize breeding programs aiming to increase resistance to emerging pests and to transfer the pathway to other plants that are victims of CEW.
METHODS
Maize Stocks and Plant Materials
sm1 and sm2 stocks were obtained from the Maize Cooperation Stock Center. sm1 stocks are as follows: 611A (sm1-1; P1-rr, Pl1, sm1); 611I (sm1-2; P1-rr, tan1, py1, sm1); 5809A (sm1-3; sm1, rau*-6522); M641G (sm1-4; sm1-Brawn184, sm2-Brawn184); M941F (sm1-5; sm1, wx1); and 607J, 607K, and 607L, which contained the alleles sm1-Brawn168, sm1-Brawn178, and sm1-Brawn184, respectively. sm2 stocks are as follows: 201G (sm2-1; sm2-Brawn180); 201H (sm2-2; sm2-Brawn189); 201I (sm2-3; sm2-Brawn190); 201J (sm2-4; sm2-Brawn191), and 201K (sm2-5; sm2-Brawn188) (McMullen et al., 2004). P1-rr stock 65-CFS-305 and P1-ww stock 4Co43 were crossed into line A619 (P1-ww) as previously described (Morohashi et al., 2012). Briefly, the P1-rr CFS-305 allele in the 4Co43 background was introgressed into A619, which has no P1 or P2 expression, followed by six backcrosses and three selfing generations. The P1-ww allele in 4Co43 expressed the P2 allele and was also introgressed into A619 (Morohashi et al., 2012). In all cases, silks were collected 2 to 3 d after emergence.
Chemicals
Isoorientin (luteolin-6-C-glucoside), rhamnosylisoorientin, and maysin were kindly provided by Michael McMullen (USDA-ARS) and Maurice Snook (Iowa State University). UDP-glucose, NAD+, and NADPH were obtained from Sigma-Aldrich. The solvents methanol, acetonitrile, and water were obtained from Sigma-Aldrich.
Flavone Extraction
Fresh silks were freeze-dried overnight and ground in 100% (v/v) methanol (Sigma-Aldrich) to a final concentration of 50 mg dry tissue per mL and incubated overnight at −20°C. Silk extracts were filtered through a 0.2-μm membrane (Pall Nanosep MF) and stored at −20°C until further use (Casas et al., 2014).
HPLC Analysis
HPLC followed by photodiode array detection (HPLC-PDA) was performed on silks extracts as follows. Fresh silks were lyophilized overnight and ground in 100% (v/v) methanol (Sigma-Aldrich) to a final concentration of 50 mg of dry weight per mL and incubated overnight at −20°C. Silk extracts were filtered through a 0.2-μm membrane (Pall Nanosep MF), and 20 μL of the filtrate was injected into a Waters separation module W2690-5 and a W2996 PDA module following absorbance at 350 nm using a Symmetry C18 column (3.5 μm, 4.9 mm, 75 mm; Waters). The separation program started at an initial gradient of 80% solvent A–20% solvent B (solvent A: formic acid 0.1% [v/v] in water; solvent B: formic acid 0.1% [v/v] in acetonitrile) with a 0.75 mL/min flow to 100% solvent B in 15:33 min and kept for 1:20 min to finally return to initial conditions at 18:00 min (Casas et al., 2014).
Cloning of UGT91L1, RHS1, and At-RHM1
The T7pro:His6-UGT91L1 construct in pET28a was previously described (Falcone Ferreyra et al., 2013). The RHS1 (GRMZM2G031311) coding sequence was amplified from P1-rr silks using the OneStep RT-PCR kit (Qiagen). Briefly, 1 μg of RNA was incubated with 1× OneStep buffer, 1 mM of each dNTP, 0.5 μM of each primer, 0.5 μL RNase inhibitor, and 2 μL OneStep enzyme mix in a 50 μL final volume. The conditions for reverse transcription followed by PCR were as follow: 50°C for 30 min, 95°C for 15 min, 30 cycles of 94°C for 1 min, 53°C for 1 min, and 72°C for 2 min, followed by a final extension of 72°C for 10 min. The PCR product was purified, cloned into pENTR-D-TOPO vector (Invitrogen), sequenced, and used as template to clone full-length RHM1 into pET28a vector. PCR reactions were performed as described above for UGT91L1 using primers listed in Supplemental Table 6; extension times were 150 s for the full-length gene. To clone RHS1 and At-RHM1 in the pGZ25 yeast expression vector, the respective coding regions were reamplified by PCR using the pENTR-D-TOPO clones previously generated as templates. In all cases, primers for coding sequences were designed based on the 5b.60b version of the maize genome (www.maizegdb.org).
The U15542 clone containing the At-RHM1 coding sequence in pENTR-SD-D-TOPO was obtained from the ABRC.
UGT91L1 and RHS1 were subcloned into protoplast expression vectors for transient expression fused to enhanced GFP, either at the N-terminal or C-terminal end, p1511BS and p1510BS, respectively, using a recombination cassette derived from the pK7FWG2 vector (Gateway compatible) and transferred into the pBluescript KS+ multicloning site. In all cases, vectors were sequenced to confirm that the fusions were in frame.
Yeast Strains and Transformation
Saccharomyces cerevisiae strain T334 (ura3-52, leu2-3,112, △trp1::hisG, reg1-501, gal1, pep4-3, prb1-1122) obtained from Claudia Spampinato at CEFOBI was used for expressing At-RHM1 and RHS1 (Lewis et al., 1998). Yeast strains were grown in YPD (1% [w/v] yeast extract, 2% [w/v] peptone, and 2% [w/v] glucose). Transformation was performed following the Trafo method (Gietz and Woods, 2002), and transformants were selected on synthetic complete media agar plates containing 2% (w/v) glucose without tryptophan (SCD-W). Transformed colonies appeared within 3 to 4 d of incubation at 30°C.
Rhamnosyl Transferase Activity Assay
To test for rhamnosyl transferase activity of UGT91L1, At-RHM1 cloned into pGZ25 was transformed into yeast strain T334. Yeast cells were grown in SCD-W at 30°C for 48 h. Cells were harvested by centrifugation at 5000g for 5 min at room temperature, resuspended in 10 mM Tris-HCl (pH 7.5) with 1× of protease inhibitor (Thermo), and lysed with glass beads (425 to 600 μm; Sigma-Aldrich). Six series of 1-min periods of vortex-mixing alternating with 1 min incubation on ice were performed. The supernatants were collected after centrifugation and used as an enzymatic source. Total protein extracts from yeast expressing At-RHM1 or empty pGZ25 vector and total protein extracts from Escherichia coli expressing UGT91L1 or empty pET28a vector were prepared. To obtain E. coli crude extracts, E. coli Rosetta 2 (DE3) cells expressing UGT91L1 were grown as previously described (Falcone Ferreyra et al., 2013). Cells were harvested by centrifugation, resuspended in 50 mM KPi, pH 7.5, with 1 mM PMSF, sonicated, and centrifuged at 12,000g for 15 min at 4°C. Supernatants were used as an enzymatic source. In vitro RHM activity assays were performed in a reaction mix of 100 μL final volume containing 250 mM MOPS-NaOH, pH 7.5, 3 mM NAD+, 3 mM NADPH, 3 mM UDP-glucose, and S. cerevisiae crude extract (absorbance at 600 nm of 5.0 to 7.5, harboring At-RHM1 or the pGZ25 empty vector; Oka et al., 2007). The reaction mixtures were incubated for 60 min at 30°C. The soluble fraction was extracted with chloroform and used as a UDP-Rha source for the following reaction of rhamnosyl transferase. Then, the following was added: 30 μL of E. coli protein extract (containing 85 μg of total protein, expressing UGT91L1 or empty pET28a), 0.15 mM isorientin in 50 mM KPi, pH 7.5, in a final volume of 200 μL. The reaction mixtures were incubated for 60 min at 30°C. Flavonoids were extracted with ethylacetate, vacuum dried, and resuspended in 100 μL 100% (v/v) methanol for LC-MS/MS analysis.
Maize Protoplast Preparation and Transformation
Second and third leaves from 14-d-old sm1 and sm2 etiolated seedlings were chopped to ∼1-mm-thick pieces and digested in 3% (w/v) Cellulase RS, 0.6% (w/v) macerozyme R10 (Yakult Honsha Co.), 0.6 M mannitol, 10 mM KCl, 10 mM MES (pH 5.7), 5 mM CaCl2, and 0.1% (w/v) BSA for 20 min under vacuum followed by 3 h gentle shaking at 30 g and 25°C in darkness. Protoplasts were released by shaking at 80g and 25°C in darkness and filtered through a 35-μm nylon mesh followed by centrifugation at 150g for 1 min at room temperature. Protoplasts were washed in ES buffer (0.6 M mannitol, 5 mM MES, pH 5.7, and 10 mM KCl) and counted using a hemocytometer under a microscope (Nikon SMZ1500, C-DSS115). Individual electroporations were performed on 105 protoplasts with 20 μg of total DNA per transformation for complementation assays, using 100 V/cm, 10 ms, and two pulses with a BTX Electro-Square-Porator T820. After electroporation, protoplasts were incubated for 18 to 22 h in the dark at room temperature before further analyses. Transformation efficiency was estimated as percentage of transformed cells following GFP expression using a fluorescence microscope (Nikon).
Complementation Assays of salmon silks Protoplasts and LC-MS/MS Analysis
Protoplasts from sm1 and sm2 lines were centrifuged for 2 min at low speed (∼270g) at room temperature, the supernatant was removed, and protoplasts were resuspended in 100% (v/v) methanol (Sigma-Aldrich) and incubated for 10 min at 65°C (∼106 initial cell number/mL corresponding to a pool of ten independent transformations). Following incubation, protoplasts were centrifuged for 5 min at 12,000g before filtering through a 0.2-μm membrane (Pall Nanosep MF) for further analysis by LC-MS/MS.
LC-MS/MS was performed in the Center for Applied Plant Sciences Targeted Metabolomics Laboratory on a linear ion trap quadrupole LC-MS/MS with a Q-Trap 5500 mass spectrometer (ABSciEX) in negative mode using multiple reaction monitoring following true standards. A Symmetry C18 column (3.5 μm, 4.9 mm, 75 mm; Waters) was used for a 15-μL injection volume with a flow of 1 mL/min (solvent A, water; solvent B, acetonitrile; Sigma-Aldrich). The program steps were 0.00 to 2.00 min 20% A; 2.00 to 9.00 min from 20 to 60% A; 9.10 to 11.00 min, 90% A; 11.00 to 11.10 min, 90 to 20% A; and 11.10 to 14.00 min, 20% A (Casas et al., 2014).
Gene Expression Analyses by RT-qPCR
Three independent biological replicates (i.e., obtained from three different individual plants) from sm mutants and P1-rr and P1-ww silk samples were snap-frozen in N2 (l) and stored at −80°C. Total RNA was extracted using the RNeasy Plant Mini Kit followed by DNaseI treatment (Qiagen). cDNA was synthesized from 1 μg of total RNA using SuperScript Reverse Transcriptase II (Invitrogen) with oligo-dT12-18 as primer. The resulting cDNA was used as template for PCR amplification in an iCycler iQ detection system with Optical System Software (v3.0a; Bio-Rad) using the intercalation dye SYBR Green I (Invitrogen) as a fluorescent reporter and Platinum Taq Polymerase (Invitrogen). Primers were designed to generate fragments of between 80 and 150 bp using Primer3 (Rozen and Skaletsky, 2000) (Supplemental Table 6). Transcript accumulated values were normalized to expression of actin1 (GRMZM2G126010). Amplification conditions included 2 min initial denaturation at 94°C, 40 to 45 cycles of 94°C for 15 s, 57°C for 20 s, 72°C for 20 s, and a final extension step at 72°C for 5 min (Morohashi et al., 2012). Melting curves for each PCR product were determined by measuring the decrease of fluorescence with increasing temperature (from 65 to 95°C). To confirm the size of the PCR products and to check that they corresponded to a unique and expected PCR product, the final PCR products were separated on a 2% (w/v) agarose gel, stained with SYBR green (Invitrogen), and sequenced. Primers used for UGT91L1 and RHS1 are listed in Supplemental Table 6.
ChIP-qPCR
ChIP was performed by methods previously described (Morohashi and Grotewold, 2009; Morohashi et al., 2009; Falcone Ferreyra et al., 2010). Approximately 0.3 g of pericarps was fixed in Buffer A (0.4 M sucrose, 10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 1 mM PMSF, and 0.1% [v/v] formaldehyde) for 10 min in vacuum. The cross-linking reaction was stopped by addition of 0.1 M glycine followed by an additional 5 min vacuum incubation. Fixed pericarps were washed and ground in liquid N2. Nuclei were isolated using the plant nuclei isolation/extraction kit according to manufacturer’s instructions (Sigma-Aldrich). Nuclear-enriched extracts were resuspended in 100 μL lysis buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% [v/v] Triton X-100, 0.1% [v/v] deoxycholate, 0.1% [w/v] SDS, 1 mM PMSF, and 10 mM sodium butyrate) and plant proteinase inhibitor cocktail (Sigma-Aldrich), followed by sonication with a Bioruptor (Diangenode) to ∼300 bp of average fragment size.
For ChIP-qPCR analyses, and to correct for PCR bias, 1 pg of pUC19 plasmid was spiked into input and immunoprecipitated (ChIP) DNA. qPCR was performed using 0.1 to 1 μL of input or ChIPed DNA with Buffer J of the FailSafe PCR system (Epicentre Biotechnologies). ChIP-qPCR assays were performed using six biological replicates.
Phylogenetic Analysis of Maize Glycosyl Transferases
The phylogenetic tree was constructed using MEGA 6.0 software with the neighbor-joining method based on ClustalW multiple alignments (Larkin et al., 2007; Tamura et al., 2007). The bootstrap consensus tree was inferred from 1000 replicates and the branches corresponding to partitions corresponding to less than 50% bootstrap replicates are collapsed. Distances were computed using p-distance method and are in the units of the number of amino acid differences per site.
Sequencing of sm1 and sm2 Candidates
With the goal of identifying sequence variations that could potentially account for the salmon silk phenotype, each gene was divided in overlapping parts to cover the whole gene including the promoter region. The UGT91L1 genomic region covers 2.72 kb and was divided it into five overlapping regions (Supplemental Figure 4B). The RHS1 genomic region covers 3.94 kb, which was divided into nine overlapping regions (Supplemental Figure 6B).
We isolated genomic DNA from each line and performed PCR using the primer combinations described in Supplemental Table 7.
PCR Conditions
Briefly, 0.3 μg of genomic DNA from each salmon silks line was incubated with 1× buffer, 0.2 mM of each dNTP, 0.4 μM of each primer, and 1.5 units of Taq Polymerase in a 50 μL final volume. The amplification conditions were as follows: initial denaturation at 94°C for 3 min, followed by 30 cycles of 94°C for 1 min, 54°C for 45 s, 72°C for 90 s, and a final extension of 72°C for 5 min. The PCR products were purified and sent for sequencing at the Plant Microbe Genomics Facility at Ohio State University. Sequences were compared with version 2 of the maize genome (5b.60).
Statistical Analysis
All experiments performed in this study were at least done in biological triplicates. Data presented were analyzed using two-sided type 3 Student’s t test, indicating statistically significant difference with letters at a P value of < 0.001, using Microsoft Excel.
Accession Numbers
Sequence data from this article can be found in in the Arabidopsis Genome Initiative under accession number At1g78570 (At-RHM1) and in the maize genome sequence (version 3b.60 at http://ensembl.gramene.org/Zea_mays/Info/Index) under the following accession numbers: UGT91L1 (GRMZM2G180283 corresponding to accession number ZEAMMB73_290687), RHS1 (GRMZM2G031311 corresponding to accession number ZEAMMB73_283149), and actin1 (GRMZM2G126010 corresponding to accession number ZEAMMB73_109222). Accession numbers used to generate phylogenetic tree in Supplemental Figure 3 are as follows: UGT708A6 (GRMZM2G162783); UGT708A5 (GRMZM2G162755); Bronze1 (GRMZM2G165390); UGT707A8 (GRMZM2G063550); GRMZM2G039129; AC199541.4_FG004; AC199541.4_FG007; AC199541.4_FG001; AC199541.4_FG002; GRMZM2G161335; GRMZM2G373124; GRMZM2G179063; GRMZM2G173315; GRMZM2G066067; GRMZM2G024131; GRMZM2G074395; GRMZM2G031138; GRMZM2G328224; GRMZM2G098892; GRMZM2G098890; GRMZM2G363545; GRMZM2G160523; GRMZM2G416045; GRMZM2G168474; GRMZM2G120016; GRMZM2G021786; GRMZM2G035755; GRMZM2G082249; GRMZM2G009125; GRMZM5G870067; GRMZM2G086925; GRMZM2G154558; GRMZM2G083935; GRMZM2G334336; GRMZM2G463996; GRMZM2G173926; GRMZM2G047910; GRMZM2G110816; GRMZM2G006565; GRMZM2G306328; GRMZM2G171548; GRMZM2G022212; GRMZM2G170228; GRMZM5G834303; GRMZM2G113794; GRMZM2G415973; GRMZM2G022242; GRMZM2G022266; GRMZM2G343426; GRMZM2G075387; GRMZM2G163519; GRMZM2G056335; GRMZM2G372068; GRMZM2G156127; GRMZM2G324466; GRMZM2G131928; GRMZM2G363554; GRMZM2G178209; GRMZM2G479038; GRMZM2G338465; GRMZM2G055446; GRMZM2G105991; GRMZM2G083130; GRMZM2G135722; GRMZM2G061289; GRMZM2G061321; GRMZM2G073376; GRMZM2G004858; GRMZM2G381025; GRMZM2G122072; GRMZM2G051683; GRMZM2G073244; GRMZM5G888620; GRMZM2G319965; GRMZM2G106443; GRMZM2G085054; GRMZM2G085854; GRMZM2G426415; GRMZM2G007012; GRMZM2G389944; GRMZM2G128504; GRMZM2G046994; GRMZM2G041699; GRMZM2G169628; GRMZM2G470524; GRMZM5G832805; GRMZM5G896260; GRMZM2G301148; GRMZM2G130256; GRMZM2G156026; GRMZM2G007795; GRMZM2G304712; GRMZM2G030821; GRMZM2G337048; GRMZM2G417945; GRMZM2G449019; GRMZM2G148277; GRMZM2G325014; GRMZM2G325023; GRMZM2G008935; GRMZM2G151332; GRMZM2G049798; GRMZM2G476049; GRMZM2G132706; GRMZM2G091176; GRMZM2G159918; GRMZM2G432291; GRMZM2G118657; GRMZM2G067361; GRMZM2G050748; GRMZM2G383404; GRMZM2G458776; GRMZM2G316030; GRMZM2G051474; GRMZM2G145670; GRMZM2G440902; GRMZM2G037545; GRMZM2G082037; AC206788.3_FG015; GRMZM2G058314; GRMZM2G159404; GRMZM5G815665; GRMZM5G892627; GRMZM2G097030; GRMZM2G117878; GRMZM2G175910; GRMZM2G078465; GRMZM2G089241; GRMZM2G395508; GRMZM2G095261; GRMZM2G155911; GRMZM2G455075; GRMZM2G096412; GRMZM2G060993; GRMZM2G174192; GRMZM2G457929; GRMZM2G095280; GRMZM2G022101; GRMZM2G042865; GRMZM2G095807; GRMZM2G010987; GRMZM2G110511; GRMZM2G130119; GRMZM2G036409; GRMZM2G111344; GRMZM2G403740; GRMZM2G008539; GRMZM2G099740; GRMZM2G344993; GRMZM2G475884; AC234524.1_FG005; GRMZM2G043295; GRMZM2G113653; GRMZM2G035282; GRMZM2G063042; GRMZM5G854655; GRMZM2G426242; and GRMZM2G067424.
Supplemental Data
Supplemental Figure 1. Biochemical characterization of sm2 mutants.
Supplemental Figure 2. Biochemical characterization of sm1 mutants.
Supplemental Figure 3. Phylogenetic analysis of putative UGT proteins from maize.
Supplemental Figure 4. Molecular characterization of UGT91L1 in sm2 lines.
Supplemental Figure 5. Constructs for mutant protoplast complementation.
Supplemental Figure 6. Molecular characterization of RHS1 in sm1 lines.
Supplemental Figure 7. RHS1 encodes UDP-rhamnose synthase.
Supplemental Table 1. Characteristics of available salmon silks alleles.
Supplemental Table 2. P1-activated genes in the sm2 interval.
Supplemental Table 3. P1-activated genes in the sm1 interval.
Supplemental Table 4. Putative rhamnose synthases present in the maize genome.
Supplemental Table 5. Putative rhamnosyl transferases clustering together with UGT91L1.
Supplemental Table 6. Primers used in this study.
Supplemental Table 7. Primers used for sequencing candidate genes.
Supplemental Data Set 1. Alignment used to generate the phylogeny presented in Supplemental Figure 3.
Supplementary Material
Acknowledgments
This project was supported by the Agriculture and Food Research Initiative competitive grant 2015-67013-22810 of the USDA National Institute of Food and Agriculture and FONCyT Grant PICT 2013-0082 to M.L.F.-F. and by a grant from the National Science Foundation (IOS-1125620) to E.G. We thank Mike McMullen and the Maize Genetics Cooperation Stock Center for providing the seed stocks used in this study and the ABRC for the At RHM1 construct. We thank the Center for Applied Plant Sciences (CAPS) Targeted Metabolomics Laboratory, especially Jean-Christophe Cocuron, for his contributions in the LC-MS/MS method development and Ana P. Alonso for her expertise in sugar and sugar-nucleotide analysis support and discussions. We thank Claudia Spampinato (CEFOBI-CONICET) for providing the T344 yeast strain. We also thank Rob Last, Venkat Gopalan, Keith Slotkin, and David Mackey for their valuable feedback to this study. Lastly, we thank Mike McMullen for numerous discussions and ideas over the years, the three anonymous reviewers of this study for their insightful comments, and the CAPS Computational Biology Laboratory for RNA-seq analysis discussions. M.L.F.-F., E.R., and P.C. are members of the Researcher Career of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
AUTHOR CONTRIBUTIONS
M.I.C., M.L.F.-F., P.C., and E.G. designed research. M.I.C., M.L.F.-F., N.J., E.R., J.E., and T.W. performed research. M.I.C., M.L.F.-F., and M.K.M.-G analyzed data. M.I.C. and E.G. wrote the article. All authors read and approved the final manuscript.
Glossary
- RIO
rhamnosylisoorientin
- IO
isoorientin
- LC-MS/MS
liquid chromatography followed by tandem mass spectrometry
- UGT
UDP-glycosyltransferase
- ChIP
chromatin immunoprecipitation
- ChIP-qPCR
ChIP followed by quantitative PCR
Footnotes
Articles can be viewed without a subscription.
References
- Anderson E.G. (1921). The Inheritance of Salmon Color in Maize. Master’s thesis (Ithaca, NY: Cornell University). [Google Scholar]
- Anderson E.G., Emerson R.A. (1923). Pericarp studies in maize I. The inheritance of pericarp colors. Genetics 8: 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D.M., Bailey R.M. (1938). The relation of the pericarp to tenderness in sweet corn. P. Am. Soc. Hortic. Sci. 36: 555–559. [Google Scholar]
- Bardin M., Ajouz S., Comby M., Lopez-Ferber M., Graillot B., Siegwart M., Nicot P.C. (2015). Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Front. Plant Sci. 6: 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazier-Hicks M., Evans K.M., Gershater M.C., Puschmann H., Steel P.G., Edwards R. (2009). The C-glycosylation of flavonoids in cereals. J. Biol. Chem. 284: 17926–17934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne P.F., McMullen M.D., Snook M.E., Musket T.A., Theuri J.M., Widstrom N.W., Wiseman B.R., Coe E.H. (1996). Quantitative trait loci and metabolic pathways: genetic control of the concentration of maysin, a corn earworm resistance factor, in maize silks. Proc. Natl. Acad. Sci. USA 93: 8820–8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas M.I., Duarte S., Doseff A.I., Grotewold E. (2014). Flavone-rich maize: an opportunity to improve the nutritional value of an important commodity crop. Front. Plant Sci. 5: 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casati P., Walbot V. (2005). Differential accumulation of maysin and rhamnosylisoorientin in leaves of high altitude landraces of maize after UV-exposure. Plant Cell Environ. 28: 788–799. [Google Scholar]
- Cocciolone S.M., Chopra S., Flint-Garcia S.A., McMullen M.D., Peterson T. (2001). Tissue-specific patterns of a maize Myb transcription factor are epigenetically regulated. Plant J. 27: 467–478. [DOI] [PubMed] [Google Scholar]
- Coe E.H., Jr (2001). The origins of maize genetics. Nat. Rev. Genet. 2: 898–905. [DOI] [PubMed] [Google Scholar]
- Elliger C.A., Chan B.C., Waiss A.C., Jr. (1980). Flavonoids as larval growth inhibitors. Naturwissenshaften 67: 358–360. [Google Scholar]
- Emerson R.A. (1917). Genetical studies of variegated pericarp in maize. Genetics 2: 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone Ferreyra M.L., Rius S., Emiliani J., Pourcel L., Feller A., Morohashi K., Casati P., Grotewold E. (2010). Cloning and characterization of a UV-B-inducible maize flavonol synthase. Plant J. 62: 77–91. [DOI] [PubMed] [Google Scholar]
- Falcone Ferreyra M.L., Rodriguez E., Casas M.I., Labadie G., Grotewold E., Casati P. (2013). Identification of a bifunctional maize C- and O-glucosyltransferase. J. Biol. Chem. 288: 31678–31688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone Ferreyra M.L., Emiliani J., Rodriguez E.J., Campos-Bermudez V.A., Grotewold E., Casati P. (2015). The identification of maize and Arabidopsis type I FLAVONE SYNTHASEs links flavones with hormones and biotic interactions. Plant Physiol. 169: 1090–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field B., Jordán F., Osbourn A. (2006). First encounters--deployment of defence-related natural products by plants. New Phytol. 172: 193–207. [DOI] [PubMed] [Google Scholar]
- Gietz R.D., Woods R.A. (2002). Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350: 87–96. [DOI] [PubMed] [Google Scholar]
- Giraud M.F., Naismith J.H. (2000). The rhamnose pathway. Curr. Opin. Struct. Biol. 10: 687–696. [DOI] [PubMed] [Google Scholar]
- Grotewold E., Athma P., Peterson T. (1991). Alternatively spliced products of the maize P gene encode proteins with homology to the DNA-binding domain of myb-like transcription factors. Proc. Natl. Acad. Sci. USA 88: 4587–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold E., Chamberlin M., Snook M., Siame B., Butler L., Swenson J., Maddock S., St Clair G., Bowen B. (1998). Engineering secondary metabolism in maize cells by ectopic expression of transcription factors. Plant Cell 10: 721–740. [PMC free article] [PubMed] [Google Scholar]
- Kriticos D.J., Ota N., Hutchinson W.D., Beddow J., Walsh T., Tay W.T., Borchert D.M., Paula-Moreas S.P., Czepak C., Zalucki M.P. (2015). The potential distribution of invading Helicoverpa armigera in North America: Is it just a matter of time? PLoS One 10: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn B.M., Geisler M., Bigler L., Ringli C. (2011). Flavonols accumulate asymmetrically and affect auxin transport in Arabidopsis. Plant Physiol. 156: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W., Lu F., Regner M., Zhu Y., Rencoret J., Ralph S.A., Zakai U.I., Morreel K., Boerjan W., Ralph J. (2015). Tricin, a flavonoid monomer in monocot lignification. Plant Physiol. 167: 1284–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M.A., et al. (2007). ClustalIW and ClustalIX version 2. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- Levings C.S. (1972). A new anthocyanidin in maize: luteolinidin. Maize Cooperation Newsletters 46: 149–151. [Google Scholar]
- Lewis L.K., Kirchner J.M., Resnick M.A. (1998). Requirement for end-joining and checkpoint functions, but not RAD52-mediated recombination, after EcoRI endonuclease cleavage of Saccharomyces cerevisiae DNA. Mol. Cell. Biol. 18: 1891–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Du Y., Chu H., Shih C.H., Wong Y.W., Wang M., Chu I.K., Tao Y., Lo C. (2010). Molecular dissection of the pathogen-inducible 3-deoxyanthocyanidin biosynthesis pathway in sorghum. Plant Cell Physiol. 51: 1173–1185. [DOI] [PubMed] [Google Scholar]
- McMullen M.D., Kross H., Snook M.E., Cortés-Cruz M., Houchins K.E., Musket T.A., Coe E.H. Jr (2004). Salmon silk genes contribute to the elucidation of the flavone pathway in maize (Zea mays L.). J. Hered. 95: 225–233. [DOI] [PubMed] [Google Scholar]
- Moghe G.D., Last R.L. (2015). Something old, something new. Conserved enzymes and the evolution of novelty in plant specialized metabolism. Plant Physiol. 169: 1512–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K., et al. (2012). A genome-wide regulatory framework identifies maize pericarp color1 controlled genes. Plant Cell 24: 2745–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K., Grotewold E. (2009). A systems approach reveals regulatory circuitry for Arabidopsis trichome initiation by the GL3 and GL1 selectors. PLoS Genet. 5: e1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K., Xie Z., Grotewold E. (2009). Gene-specific and genome-wide ChIP approaches to study plant transcriptional networks. Methods Mol. Biol. 553: 3–12. [DOI] [PubMed] [Google Scholar]
- Morris C.E., Sands D.C. (2006). The breeder’s dilemma--yield or nutrition? Nat. Biotechnol. 24: 1078–1080. [DOI] [PubMed] [Google Scholar]
- Oka T., Nemoto T., Jigami Y. (2007). Functional analysis of Arabidopsis thaliana RHM2/MUM4, a multidomain protein involved in UDP-D-glucose to UDP-L-rhamnose conversion. J. Biol. Chem. 282: 5389–5403. [DOI] [PubMed] [Google Scholar]
- Rector B.G., Snook M.E., Widstrom N.W. (2002). Effect of husk characters on resistance to corn earworm (Lepidoptera: Noctuidae) in high-maysin maize populations. J. Econ. Entomol. 95: 1303–1307. [DOI] [PubMed] [Google Scholar]
- Reisig D.D., Reay-Jones F.P. (2015). Inhibition of Helicoverpa zea (Lepidoptera: Noctuidae) growth by transgenic corn expressing Bt toxins and development of resistance to Cry1Ab. Environ. Entomol. 44: 1275–1285. [DOI] [PubMed] [Google Scholar]
- Reiter W.D., Vanzin G.F. (2001). Molecular genetics of nucleotide sugar interconversion pathways in plants. Plant Mol. Biol. 47: 95–113. [PubMed] [Google Scholar]
- Rice P., Longden I., Bleasby A. (2000). EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 16: 276–277. [DOI] [PubMed] [Google Scholar]
- Rozen S., Skaletsky H. (2000). Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132: 365–386. [DOI] [PubMed] [Google Scholar]
- Sidorenko L.V., Li X., Cocciolone S.M., Chopra S., Tagliani L., Bowen B., Daniels M., Peterson T. (2000). Complex structure of a maize Myb gene promoter: functional analysis in transgenic plants. Plant J. 22: 471–482. [DOI] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- Waiss A.C. Jr., Chan B.G., Elliger C.A., Wiseman B.R., McMillian W.W., Widstrom N.W., Zuber M.S., Keaster A.J. (1979). Maysin, a flavone glycoside from corn silks with antibiotic activity toward corn earworm. J. Econ. Entomol. 72: 256–258. [Google Scholar]
- Watt G., Leoff C., Harper A.D., Bar-Peled M. (2004). A bifunctional 3,5-epimerase/4-keto reductase for nucleotide-rhamnose synthesis in Arabidopsis. Plant Physiol. 134: 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F., et al. (2009). The physical and genetic framework of the maize B73 genome. PLoS Genet. 5: e1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J.K., Philippe R.N., Noel J.P. (2012). The rise of chemodiversity in plants. Science 336: 1667–1670. [DOI] [PubMed] [Google Scholar]
- Widstrom N.W., Snook M.E. (1998a). Genetic variation for maysin and its analogs in crosses among corn inbreds. Crop Sci. 38: 372–375. [Google Scholar]
- Widstrom N.W., Snook M.E. (1998b). A gene controlling biosynthesis of isoorientin, a compound in corn silks antibiotic to the corn earworm. Entomol. Exp. Appl. 89: 119–124. [Google Scholar]
- Wiseman B.R., Snook M.E., Isenhour D.J., Mihm J.A., Widstrom N.W. (1992). Relationship between growth of corn earworm and fall armyworm larvae (Lepidoptera: Noctuidae) and maysin concentration in corn silks. J. Econ. Entomol. 85: 2473–2477. [Google Scholar]
- Yin Y., Huang J., Gu X., Bar-Peled M., Xu Y. (2011). Evolution of plant nucleotide-sugar interconversion enzymes. PLoS One 6: e27995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.