Arabidopsis CASEIN KINASE1-LIKE PROTEIN2 associates with actin filaments and phosphorylates actin depolymerizing factors, and this is required for actin filament reorganization and stomatal closure.
Abstract
The opening and closing of stomata are crucial for plant photosynthesis and transpiration. Actin filaments undergo dynamic reorganization during stomatal closure, but the underlying mechanism for this cytoskeletal reorganization remains largely unclear. In this study, we identified and characterized Arabidopsis thaliana casein kinase 1-like protein 2 (CKL2), which responds to abscisic acid (ABA) treatment and participates in ABA- and drought-induced stomatal closure. Although CKL2 does not bind to actin filaments directly and has no effect on actin assembly in vitro, it colocalizes with and stabilizes actin filaments in guard cells. Further investigation revealed that CKL2 physically interacts with and phosphorylates actin depolymerizing factor 4 (ADF4) and inhibits its activity in actin filament disassembly. During ABA-induced stomatal closure, deletion of CKL2 in Arabidopsis alters actin reorganization in stomata and renders stomatal closure less sensitive to ABA, whereas deletion of ADF4 impairs the disassembly of actin filaments and causes stomatal closure to be more sensitive to ABA. Deletion of ADF4 in the ckl2 mutant partially recues its ABA-insensitive stomatal closure phenotype. Moreover, Arabidopsis ADFs from subclass I are targets of CKL2 in vitro. Thus, our results suggest that CKL2 regulates actin filament reorganization and stomatal closure mainly through phosphorylation of ADF.
INTRODUCTION
Stomata regulate the uptake of CO2 for photosynthesis, water loss through transpiration, and defense responses during pathogen attack (Kim et al., 2010; Du et al., 2014). To cope with changes in environmental conditions, such as light, temperature, humidity, CO2, and salt in soil, plants must tightly regulate the opening and closing of stomata (Roelfsema and Hedrich, 2005; Vavasseur and Raghavendra, 2005; Israelsson et al., 2006). Many cellular signals (e.g., abscisic acid [ABA], H2O2, Ca2+, CO2, and NO) regulate stomata by influencing the activities of H+, K+, Ca2+, and anion transporters and channels (Pei et al., 2000; Schroeder et al., 2001; Hosy et al., 2003; Desikan et al., 2004; Hirayama and Shinozaki, 2007; Wang and Song, 2008; Gayatri et al., 2013; Kollist et al., 2014). Actin filament reorganization occurs during stomatal closure. The actin cytoskeleton in the guard cells changes from well-organized cortical filaments in the guard cells of open stomata, to randomly distributed filaments, and then finally reorganizes into highly bundled long cables in the longitudinal direction in the guard cells of closed stomata (Hwang and Lee, 2001; Zhao et al., 2011). This regulatory process involves actin binding proteins such as SCAB1 and the Arp2/3 complex (Zhao et al., 2011; Jiang et al., 2012; Li et al., 2014). SCAB1 stabilizes actin filaments, and loss of SCAB1 in plants causes defects in stomatal closure (Zhao et al., 2011). The Arp2/3 complex mediates stomatal closure in response to external stimuli and regulates actin reorganization in guard cells (Jiang et al., 2012; Li et al., 2014). However, how such actin filament reorganization in guard cells is regulated remains an open question.
Actin filaments are highly dynamic, undergoing rapid reorganization and turnover regulated by actin binding proteins such as ADF/cofilin, villin, profilin, fimbrin, and capping protein (Wasteneys and Galway, 2003; Hussey et al., 2006; Staiger and Blanchoin, 2006; Higaki et al., 2007; Thomas et al., 2009; Li et al., 2010; Su et al., 2012; Qu et al., 2013; Wang et al., 2015). ADF/cofilin proteins function as key regulators of actin filament dynamics and reorganization through binding to both globular and filamentous actin. ADF/cofilin proteins promote actin filament severing and depolymerization and inhibit nucleotide exchange on actin monomers (Hotulainen et al., 2005; Andrianantoandro and Pollard, 2006; Henty et al., 2011). The Arabidopsis thaliana genome encodes 11 ADF proteins, which play important roles in various biological processes. ADF4 is involved in innate immune signaling (Tian et al., 2009; Henty-Ridilla et al., 2014); ADF7 promotes pollen tube growth (Zheng et al., 2013); and ADF2 is required for cell growth, development, and root-knot nematode infection (Clément et al., 2009). In addition, the 14-3-3 λ protein interacts with phosphorylated ADF1 to regulate actin dynamics during hypocotyl elongation (Zhao et al., 2015). Overexpression of ADF1 causes disruption of F-actin cables in guard cells and results in a stomatal closure-defect phenotype following ABA treatment, suggesting that ADF proteins might function in this process (Dong et al., 2001).
In animals and plants, many factors regulate the F-actin disassembling activity of ADF/cofilin. Two proteins, actin-interacting protein-1 and cyclase-associated protein, enhance the F-actin disassembling activity of ADF/cofilin (Moriyama and Yahara, 2002; Ono, 2003; Ketelaar et al., 2004; Shi et al., 2013). The F-actin disassembling activity of ADF/cofilin can also be enhanced by increased intracellular pH (Bernstein et al., 2000; Allwood et al., 2002). The F-actin disassembling activity of ADF/cofilin is decreased by phosphoinositide and cortactin binding (Yonezawa et al., 1990; Allwood et al., 2002; Maciver and Hussey, 2002) as well as by phosphorylation at the Ser-3 residue of animal cofilin (Agnew et al., 1995). Changes in the Ser-3 phosphorylation level are tightly associated with extracellular stimuli, actin rearrangement, and cell activities, which implies a critical role of such phosphorylation for modulation of cofilin activity (Mizuno, 2013). Ser-6 of plant ADFs has been considered to function analogously to Ser-3 of animal cofilin based on amino acid sequence similarity searches and tertiary structure prediction (Chen et al., 2002). Similar to Ser-3 phosphorylation in cofilin, the phosphorylation of the Ser-6 in ADFs results in a reduction of their F-actin disassembling activity (Agnew et al., 1995; Moriyama et al., 1996; Nagaoka et al., 1996; Smertenko et al., 1998). In yeast, casein kinase 1 (CK1) Hrr25 phosphorylates a number of actin binding proteins, including the cofilin Cof1 (Peng et al., 2015). Two kinase families, LIM (Lin-11/Isl-1/Mec-3) and TES (testicular protein), specifically phosphorylate and deactivate ADF/cofilin (Toshima et al., 2001). However, mammalian LIMK does not phosphorylate plant ADFs, and the plant LIMK homologs do not phosphorylate ADFs (Bernard, 2007). Rapid dephosphorylation of ADF/cofilin occurs in response to various stimuli in animal cells and results in changes of F-actin organization and assembly (Davidson and Haslam, 1994; Samstag et al., 1994; Kanamori et al., 1995). In plants, the phosphorylation of the wheat (Triticum aestivum) ADF is regulated by low temperature (Ouellet et al., 2001). A protein fraction purified from cell suspension cultures of French bean (Phaseolus vulgaris cv Immuna 1.1) and enriched in calmodulin-like domain protein kinase(s) (CDPKs) can phosphorylate maize (Zea mays) ADF3 at Ser-6, and this phosphorylation is inhibited by the addition of anti-CDPK antibodies (Smertenko et al., 1998; Allwood et al., 2001). Recently, Arabidopsis CDPK6 protein has been shown to phosphorylate ADF1 in vitro (Dong and Hong, 2013). However, it remains to be determined whether these plant CDPKs associate with F-actin and what the physiological function of the phosphorylation is.
CK1 is a family of serine/threonine protein kinases highly conserved in eukaryotic organisms. CK1 is involved in biological processes including circadian rhythm establishment, vesicular trafficking, DNA repair, the cell cycle, and morphogenesis (Gross et al., 1995; Akashi et al., 2002; Cheong and Virshup, 2011). A number of cytoskeleton-related proteins have been identified as targets of CK1 kinases; these targets include cofilin, twinfilin, myosin, tropin, spectrin3, dynein, α-/β-tubulin, microtubule-associated protein, and kinesin-like protein (Boesger et al., 2014; Knippschild et al., 2014; Peng et al., 2015). In addition to the other cytoskeleton-related proteins, CK1 directly phosphorylates actin protein in vitro (Shibayama et al., 1986; Knippschild et al., 2005). Arabidopsis casein kinase 1-like protein 6 (CKL6) phosphorylates tubulin and regulates microtubule organization (Ben-Nissan et al., 2008).
In this study, we show that CKL2 and actin depolymerizing factor 4 (ADF4) are involved in stomatal closure in response to drought stress and ABA treatment. Although CKL2 does not bind to and stabilize actin filaments in vitro, it decorates and stabilizes actin filaments in guard cells. Interestingly, we found that CKL2 physically interacts with ADF4 and that phosphorylation of ADF4 decreases its F-actin disassembling activity in vitro. In terms of ABA-induced stomatal closure, CKL2 deletion and ADF4 deletion have opposite effects. Importantly, deletion of ADF4 in the ckl2 mutant background partially rescues its ABA-insensitive stomatal closure phenotype. Considering that CKL2 nonselectively phosphorylates Arabidopsis ADFs from subclass I in vitro, we propose that CKL2 stabilizes actin filaments by phosphorylating ADFs and inhibiting their F-actin disassembling activity in guard cells to promote the reassembly of actin filaments during drought/ABA-induced stomatal closure.
RESULTS
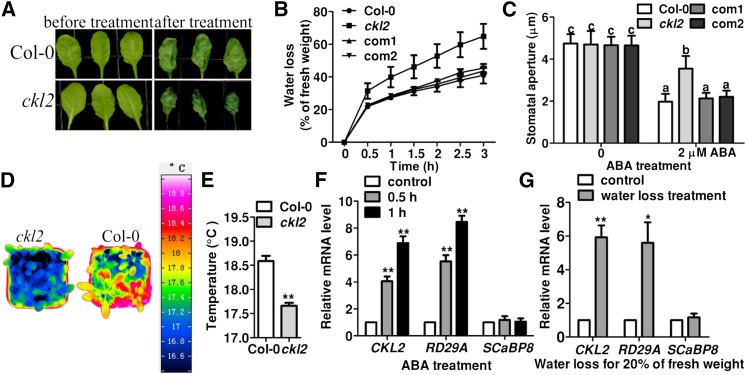
The ckl2 Mutant Exhibits Rapid Water Loss and Impaired ABA-Induced Stomatal Closure
To obtain mutants that lost water faster and wilted earlier than the wild type, we performed a genetic screen of T-DNA insertion lines ordered from the ABRC (Ohio State University). Rosette leaves of 3-week-old plants were detached and the rate of water loss was monitored. After identification of mutants with both more rapid and less rapid water-loss phenotypes, we further performed a stomatal aperture assay with an ABA stimulus as a standard during the screening. We identified a mutant (SALK_104209) that showed faster water loss and was less sensitive to ABA-induced stomatal closure than the wild type (Figures 1A to 1C). The T-DNA insertion in the Arabidopsis gene At1g72710 was confirmed by PCR using the T-DNA left border and the gene-specific primers. Because At1g72710 encodes CKL2, SALK_104209 was designated ckl2. RT-PCR analysis revealed that transcript accumulation of CKL2 was significantly decreased in the ckl2 homozygous mutant (Supplemental Figures 1A and 1B). To determine the transpiration rates of shoots, we used an infrared camera to measure the leaf temperature of 4-week-old Col-0 and ckl2 mutant plants (Merlot et al., 2002). The leaf temperature of the ckl2 mutant was significantly lower than that of Col-0, suggesting that the mutant leaves display a higher transpiration rate and lose more water than the wild type (Figures 1D and 1E).
Figure 1.
The ckl2 Mutant Shows Impaired Stomatal Closure.
(A) Leaves detached from wild-type and ckl2 plants for 0 (left) and 3 h (right) of water loss treatment.
(B) Cumulative leaf transpirational water loss in Col-0, ckl2, and two rescued lines (com1 and com2) at the indicated times after detachment (means ± sd, n = 3).
(C) Stomatal bioassays for ABA-induced closure in Col-0, ckl2, and two rescued lines (com1 and com2). The data represent the means ± sd of three independent experiments; 50 stomata were analyzed per line. The data sets were tested as normal distribution by the Shapiro-Wilk test. Statistical significance was determined by Student’s t test; significant differences are indicated by different lowercase letters. The t test analysis of the data indicates the levels of significance to be P = 0.0034, 0.9986, and 0.9347 for ckl2 and the two rescued lines, respectively, compared with Col-0 after ABA treatment. Before ABA treatment, the levels of significance were P = 0.8075, 0.2705, and 0.6197 for ckl2 and the two rescued lines, respectively, compared with Col-0.
(D) Representative pseudocolored infrared images of leaf temperature of Col-0 and ckl2 mutant plants.
(E) Leaf (surface) temperature of Col-0 and ckl2 mutant plants measured from images obtained by infrared thermography, as in (D), and analyzed by InfraTec reporter software. Twenty leaves were analyzed per line. Data are means ± sd (n = 3). The data sets were tested for normal distribution by Shapiro-Wilk test. Statistical significance (**P < 0.01) was determined by Student’s t test.
(F) and (G) Real-time PCR analysis revealed the induced expression of CKL2 by ABA treatment or water loss treatment. Seven-day-old seedlings were treated with 20 μM ABA for 0.5 or 1 h (F) or were treated for water loss until leaves had lost 20% of their fresh weight (G). RD29A and SCaBP8 were used as controls. Expression levels of CKL2, RD29A, and SCaBP8 without ABA/water loss treatment were set as 1.0, respectively. The experiments were repeated three times. Data are means ± sd. Statistical significance (**P < 0.01 and *P < 0.05) was determined by Student’s t test. t test analysis of the data shown in (F) indicates the level of significance to be P = 0.0042 and 0.0023 for the data of CKL2 relative mRNA level at 0.5 and 1 h after ABA treatment, respectively, compared with the control. The positive control RD29A also had a higher relative mRNA level when treated with ABA for 0.5 h (P = 0.0033) and 1 h (P = 0.0012) compared with the control. The negative control SCaBP8 showed no obvious difference when treated with ABA for 0.5 h (P = 0.6890) and 1 h (0.7891) compared with the control. As shown in (G), CKL2 was induced by water loss treatment (P = 0.0066). The positive control RD29A also had a higher relative mRNA level when treated with water loss until seedlings lost 20% of their fresh weight (P = 0.0225). As a negative control, the relative mRNA level of SCaBP8 was not significantly different when treated with water loss until seedlings lost 20% of their fresh weight (P = 0.5851) compared with the control.
To rescue the water-loss and stomatal closure phenotypes of the mutant, we transformed the ckl2 mutant with a construct harboring a 5.09-kb CKL2 genomic DNA fragment including 1.06-kb promoter and 0.5-kb 3′ untranslated region. The expression level of CKL2 in the transgenic lines was similar to that in the wild type (Supplemental Figure 1B). The water-loss and stomatal closure phenotypes were fully rescued in the transgenic lines (Figures 1B and 1C), suggesting the phenotype is indeed caused by the loss of function of CKL2.
CKL2 Expression Is Induced by Water Loss and ABA Treatment
To determine whether the expression of CKL2 is regulated by drought stress and ABA, total RNA was extracted from 7-d-old wild-type seedlings treated with 20 μM ABA for 0, 0.5, and 1 h or water loss treatment until the seedlings lost 20% of their fresh weight. Real-time RT-PCR analysis showed that CKL2 expression was induced by both water-loss and ABA treatments (Figures 1F and 1G). As a positive control, the expression of RESPONSIVE TO DESICCATION 29A (RD29A) was confirmed to be induced by both treatments; RD29A is an abiotic stress/ABA-responsive gene (Ishitani et al., 1997). For the negative control, we monitored the expression of a salt-responsive gene, SOS3-LIKE CALCIUM BINDING PROTEIN8 (SCaBP8) (Quan et al., 2007; Lin et al., 2009), which showed no obvious changes under these conditions. The induction of CKL2 under water-loss and ABA treatments is consistent with microarray data from AtGeneExpress (http://jsp.weigelworld.org/expviz/expviz.jsp; Supplemental Figure 1C) and a previous study (Cui et al., 2012).
CKL2 is widely expressed in a variety of tissues throughout Arabidopsis development (Gene Express Map of Arabidopsis, http://jsp.weigelworld.org/expviz/expviz.jsp; Supplemental Figure 2A). To determine the tissue-specific expression pattern of CKL2, real-time RT-PCR was performed using total RNA extracted from various tissues of 4-week-old Col-0 plants. Consistent with results obtained in the TAIR website, expression of CKL2 was detected in roots, stems, cauline leaves, rosette leaves, flowers, and siliques (Supplemental Figure 2B).
CKL2 Colocalizes with Actin Filaments in Cells but Does Not Directly Interact with Actin Filaments in Vitro
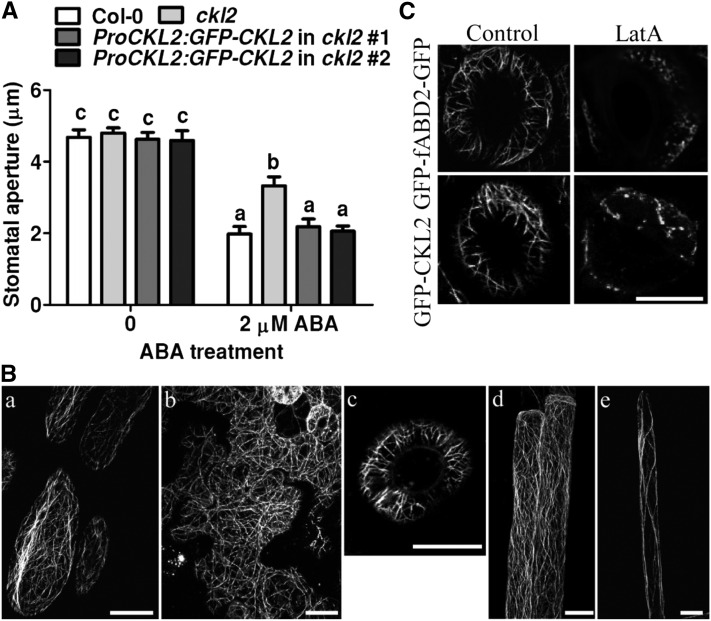
To determine the subcellular localization of CKL2, the GFP-CKL2 construct driven by the CKL2 native promoter that was used in the genetic rescue experiment was transformed into Col-0 and the ckl2 mutant. The stomatal closure phenotype of the ckl2 mutant was rescued by the CKL2pro:GFP-CKL2 transgene (Figure 2A), and the expression level of CKL2 in the transgenic lines was similar to that in the wild type (Supplemental Figure 2C). Although the GFP signal formed filamentous structures in the cells of these transgenic plants, it was very weak, and it was difficult to obtain high-quality images. The GFP-labeled CKL2 expressed from the CKL2 native promoter was observed in filamentous structures in the cytoplasm of hypocotyl epidermal cells, leaf epidermal cells, guard cells, root epidermal cells, and root hairs of the Col-0 transgenic line (Figure 2B). These results suggest that GFP-CKL2 accurately indicates the intracellular localization of CKL2. To further investigate the subcellular localization of CKL2, we fused GFP to the N terminus of CKL2 under the control of the CaMV 35S promoter. This construct was used to transform Col-0 and the ckl2 mutant. The GFP-CKL2 fusion gene rescued the stomatal-closure defect and drought-sensitive phenotypes of the ckl2 mutant. GFP-labeled CKL2 in the transgenic plants formed fine fibrous networks in the cytoplasm of hypocotyl epidermal cells (Supplemental Figure 3A), leaf epidermal cells (Supplemental Figure 3B), guard cells (Supplemental Figure 3C), root epidermal cells (Supplemental Figure 3D), and root hairs (Supplemental Figure 3E).
Figure 2.
Localization of GFP-CKL2 Expressed from the Native CKL2 Promoter.
(A) Stomatal bioassays for ABA-induced closure in Col-0, ckl2, and two ProCKL2:GFP-CKL2 transgenic lines. The data represent the means ± sd of three independent experiments; 50 stomata were analyzed per line. The data sets were tested for normal distribution by the Shapiro-Wilk test. Statistical significance was determined by Student’s t test; significant differences are indicated by different lowercase letters. The t test analysis of the data indicated the levels of significance to be P = 0.0024, 0.8673, and 0.8358 for ckl2 and two rescued lines data, respectively, compared with Col-0 after ABA treatment. Before ABA treatment, the levels of significance were P = 0.9023, 0.3251, and 0.6601 for ckl2 and two rescued lines, respectively, compared with Col-0.
(B) Confocal images were taken of epidermal cells of hypocotyls (A), leaves (B), guard cells (C), roots (D), and root hairs (E) of ProCKL2:GFP-CKL2 transgenic seedlings in the Col-0 background. Bars = 10 μm.
(C) GFP-CKL2 driven by the CKL2 native promoter and GFP-fABD2-GFP transgenic seedlings were treated with 200 nM LatA for 0.5 h. Confocal images were taken of guard cells. Bars = 10 μm.
To determine if CKL2 was associated with actin filaments or microtubules, a suspension cell line was generated from the rosette leaves of the GFP-CKL2 transgenic plants. The GFP-labeled CKL2 colocalized with rhodamine-phalloidin-stained actin filaments in the suspension cells (Supplemental Figure 3F). Colocalization was analyzed by plotting GFP-CKL2 and actin filament signal intensities using ImageJ software. The Pearson’s correlation coefficient value was 0.83 in the indicated regions of interest, suggesting a strong correlation between the spatial localizations of GFP-CKL2 and the actin filaments (Supplemental Figure 3G). To further confirm the association of CKL2 with actin filaments, the GFP-CKL2 transgenic plants were treated with latrunculin A (LatA), an inhibitor of actin polymerization that disrupts actin filaments by binding actin monomers, or oryzalin, a microtubule-disrupting reagent. After 0.5 h treatment with 200 nM LatA, the filamentous network of GFP-CKL2 was disrupted in most of the hypocotyl cells. As a control, GFP-fABD2-GFP-labeled actin filaments were also disrupted (Supplemental Figure 3H). However, after 1 h treatment with 10 μM oryzalin, the filamentous structure of GFP-CKL2 remained intact in most of the hypocotyl cells, whereas MBD-GFP-labeled microtubules were disrupted (Supplemental Figure 3I). Disruption of filamentous structures by LatA was also observed in guard cells of CKL2pro:GFP-CKL2;ckl2 (Figure 2C). These results indicate that CKL2 colocalizes with actin filaments but not microtubules in cells.
To test whether CKL2 binds to actin filaments directly in vitro, we performed an actin cosedimentation assay by high-speed centrifugation. We found that most of the CKL2 remained in the supernatant in both the absence and presence of F-actin; however, a significant amount of the actin binding protein Fim1 (Su et al., 2012) was pulled down by F-actin (Supplemental Figure 4A). These results indicate that CKL2 does not directly bind to actin filaments.
To test if CKL2 has another effect on actin filaments in vitro, we directly visualized actin filaments stained with Alexa-488-phalloidin by epifluorescence light microscopy. The length of F-actin showed no significant differences in the presence or absence of His-CKL2, indicating that CKL2 has no effect on the length distribution of actin filaments in vitro (Supplemental Figures 4B and 4C). To determine whether CKL2 is involved in actin assembly, we determined the effect of CKL2 on spontaneous actin assembly and found that CKL2 had no such activity in vitro (Supplemental Figure 4D). Thus, these results suggest that CKL2 neither interacts with actin filaments nor affects actin assembly and disassembly in vitro.
CKL2 Stabilizes Actin Filaments and Regulates Stomatal Closure
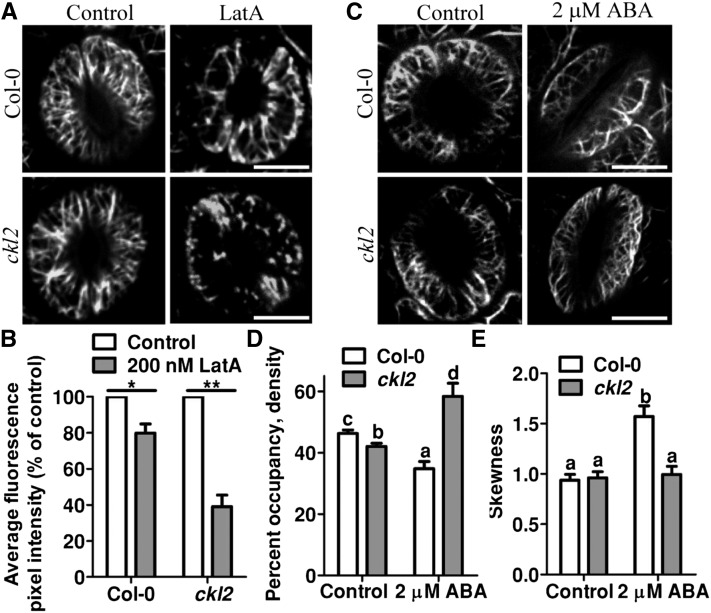
To test if CKL2 affects actin filaments in cells, we measured the F-actin stability in ckl2 and wild-type cells. To visualize the actin filaments, we generated transgenic plants harboring 35S:GFP-fABD2-GFP (containing the second actin binding domain of At-Fim1) in the Col-0 and ckl2 background and examined the stability of actin filaments in the guard cells of the ckl2 mutant and Col-0. After the transgenic lines were treated with 200 nM LatA for 30 min, the actin filaments became more fragmented and less abundant in both Col-0 and ckl2 guard cells compared with the untreated guard cells (Figure 3A). However, the actin filaments were disrupted more rapidly in ckl2 guard cells than in wild-type guard cells. In the ckl2 mutant, more GFP-labeled dot-like structures were detected after LatA treatment (Figure 3A). When the GFP-fABD2-GFP signal was monitored and quantified by measuring average GFP fluorescence pixel intensity, the GFP signal was similar between ckl2 and Col-0 in widely opened stomata. However, the GFP signal in the ckl2 guard cells decreased to a greater extent than in wild-type guard cells after the LatA treatment (Figure 3B). These results indicate that CKL2 plays a role in stabilizing actin filaments in guard cells.
Figure 3.
CKL2 Stabilizes Actin Filaments in Guard Cells.
(A) Actin filament organization in guard cells of Col-0 and ckl2 35Sp:GFP-fABD2-GFP transgenic plants before (right) and after (left) 200 nM LatA treatment for 30 min.
(B) Quantification of the relative average fluorescence pixel density of GFP-fABD2 signal in guard cells as shown in (A). After LatA treatment, Col-0 and ckl2 mutant had lower relative average fluorescence pixel density of GFP-fABD2 signal compared with control (P = 0.0204, 0.0037).
(C) Actin filament organization in guard cells of Col-0 and ckl2 35Sp:GFP-fABD2-GFP transgenic plants before (right) and after (left) 2 μM ABA treatment for 0.5 h.
(D) The extent of filament bundling (skewness) of guard cells shown in (C). The ckl2 mutant had significantly increased average actin filament density compared with Col-0 after ABA treatment (P = 0.0029). The ckl2 mutant had significantly decreased average actin filament density than Col-0 before ABA treatment (P = 0.0056).
(E) Average filament density of Col-0 and ckl2 guard cells before and after 2 μM ABA treatment as shown in (C). ckl2 mutant had significantly decreased average actin filament skewness values compared with Col-0 after ABA treatment (P = 0.0068). No significant difference of average actin filament skewness values between Col-0 and ckl2 mutant was observed before ABA treatment (P = 0.0993).
Values of (B), (D), and (E) represent the means ± sd of three independent experiments; 50 stomata were analyzed per line. The data sets were tested for normal distribution by the Shapiro-Wilk test. Statistical significance was determined by Student’s t test. Significant differences are denoted with asterisks (**P < 0.01 and *P < 0.05) in (B). Significant differences (P < 0.01) are indicated by different lowercase letters in (D) and (E).
We next determined the physiological relevance of the actin-filament-stabilizing activity of CKL2 during stomatal closure. Since it has been shown that the actin cytoskeleton in guard cells is reorganized during ABA-induced stomatal closure (Lemichez et al., 2001; Zhao et al., 2011) and the ckl2 mutant displayed impaired ABA-induced stomatal closure, we examined whether the reorganization of actin filaments during ABA-induced stomatal closure was altered in ckl2 by the exogenous application of ABA. When the stomata were in an opened state, the actin filaments were present as well-organized cortical filaments in both Col-0 and ckl2 guard cells. After 2 μM ABA treatment for 0.5 h, ∼73% of stomata were closed and the actin filaments were randomly rearranged and then bundled preferentially as long cables in Col-0. However, the stomatal closure was less sensitive to ABA treatment in the ckl2 mutant and 71% of stomata were not closed; the actin filaments failed to form bundled cables and were trapped in a randomly distributed state (Figure 3C). To quantify the actin architecture in the guard cells, skewness and density (Higaki et al., 2010) parameters were measured to determine the extent of actin filament bundling and the percentage of occupancy of actin filaments. In widely opened guard cells, the ckl2 mutant had a lower density value than wild type (Figure 3D); however, the skewness value showed no significant difference in the ckl2 mutant compared with wild type (Figure 3E). After the plants were treated with 2 μM ABA for 0.5 h, 73% of stomata were closed in Col-0 and actin filaments became bundled with higher skewness in these guard cells; however, 71% of stomata were not closed in the ckl2 mutant and actin displayed no obvious change in skewness in these guard cells. The density value decreased in Col-0 and increased in the ckl2 mutant after the treatment (Figure 3D). These results suggest that the actin-filament-stabilizing activity of CKL2 is required for the subsequent construction of longitudinal actin cables and therefore facilitates actin filament reorganization during stomatal closure.
CKL2 Regulates the Activity of Actin Filament Severing in Guard Cells
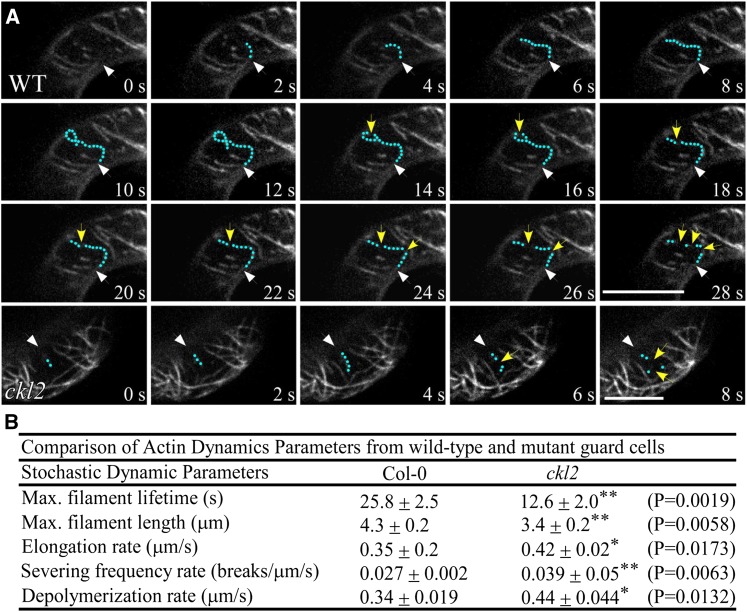
Observation of a single actin filament lifetime, including elongation rate and depolymerizing rate, in living cells has been employed to study actin dynamics in vivo (Smertenko et al., 2010; Henty-Ridilla et al., 2013; Qin et al., 2014; Li et al., 2014; Li et al., 2015). To examine the actin filament dynamics in the guard cells of Col-0 and the ckl2 mutant, we performed time-lapse imaging using spinning disk confocal microscopy to determine single actin filament dynamics (Figure 4A; Supplemental Movies 1 and 2). Several parameters of actin filament dynamics in guard cells were significantly different between wild type and the ckl2 mutant (Figure 4B). The average lifetime of single actin filaments in Col-0 was ∼2-fold longer than that in ckl2 guard cells. Most of the single actin filaments in Col-0 guard cells kept growing for more than 10 s before the first severing event occurred. However, most of the single actin filaments kept growing <8 s in ckl2 guard cells. In Col-0 guard cells, the severing rate was 0.027 ± 0.002 breaks μm−1 s−1 and a single actin filament elongated to an average maximum filament length of 4.3 ± 0.2 μm. In the ckl2 mutant, however, the severing rate was increased to 0.039 ± 0.05 breaks μm−1 s−1, and the average maximum actin filament was 3.4 ± 0.2 μm. There was about a 1.5-fold increase in severing frequency in the ckl2 mutant compared with the wild type. These results demonstrate that loss of CKL2 led to more severing events in the ckl2 guard cells and suggest that CKL2 plays an important role in controlling actin filament severing in guard cells.
Figure 4.
The ckl2 Mutant Shows Different Actin Dynamics from the Wild Type.
(A) Time-lapse images of single actin filaments in guard cells of Col-0 and ckl2 35Sp:GFP-fABD2-GFP transgenic plants. Green dots highlight a representative single actin filament. Yellow arrows indicate actin filament-severing events. White arrows indicate the position at which growth of the single actin filament began. Bars = 5 μm.
(B) Actin filament dynamic parameters in Col-0 and ckl2. The parameters associated with single actin filament dynamics in Col-0 and ckl2 guard cells were quantified from spinning disk confocal micrographs. Values represent means ± sd, n = 30. The data sets were tested for normal distribution by the Shapiro-Wilk test. Statistical significance was determined by Student’s t test. Quantification of *P < 0.05 and **P < 0.01.
CKL2 Interacts with and Phosphorylates ADF4
Our in vitro analyses indicated that CKL2 did not have any effect on the actin cytoskeleton on its own; these results suggest that an actin binding protein (ABP) might mediate the regulatory effect of CKL2 on the actin cytoskeleton in vivo. Furthermore, our findings regarding actin filament dynamics and severing in Col-0 and ckl2 guard cells suggest that an ABP that could sever actin filaments and be regulated by phosphorylation might be relevant, especially considering that CKL2 is a protein kinase. ADF family members function as important regulators of actin dynamics via severing actin filaments and promoting monomer dissociation from the pointed end of actin filaments, and phosphorylation of ADFs is critical for regulating their activity. We reasoned that ADFs with similar expression patterns to CKL2 might act as substrates for CKL2 and mediate the regulation of actin dynamics by CKL2. ADF4 belongs to subclass I, members of which are expressed abundantly in all vegetative tissues and reproductive tissues, and its expression pattern is similar to that of CKL2 (Supplemental Figures 2A to 2C; Ruzicka et al., 2007; Henty et al., 2011). Both CKL2 and ADF4 are expressed in guard cells. However, the expression of CKL2 was induced by ABA treatment, whereas that of ADF4 was not (Supplemental Figure 5). The adf4 mutant displays different F-actin dynamic behavior from that of the wild type, including decreased severing frequency and increased maximum filament length and filament lifetime (Henty et al., 2011). We therefore selected ADF4 for further analysis as a potential target of CKL2 kinase to mediate its regulatory effects on the actin cytoskeleton.
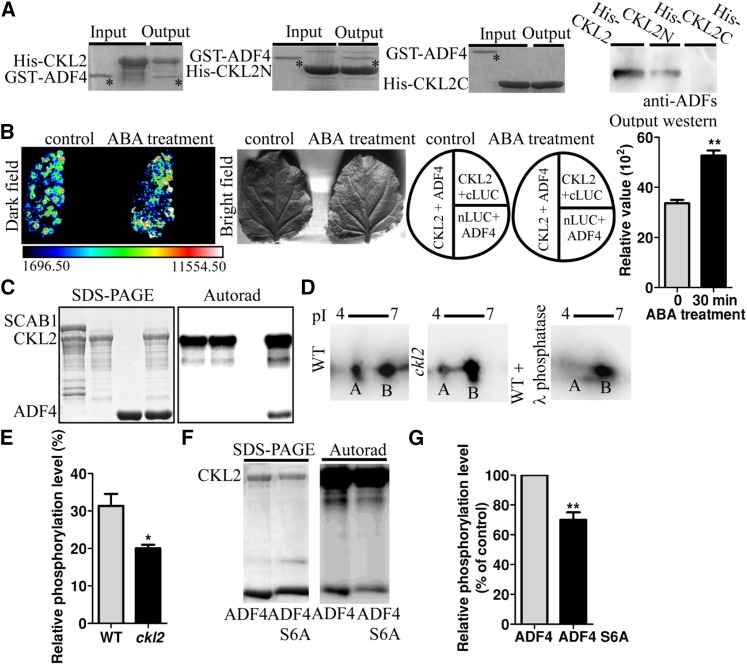
First, we determined the interaction between ADF4 and CKL2 in vitro. His-CKL2 and GST-ADF4 recombinant proteins were therefore purified. Pull-down assays showed that GST-ADF4 coprecipitated with His-CKL2 (Figure 5A), suggesting that CKL2 interacts with ADF4. To determine which domain of CKL2 interacts with ADF4, we fused the CKL2 N-terminal domain (catalytic domain, from 1 to 295 amino acids) and CKL2 C-terminal domain (regulatory domain, including 296 to 464 amino acids) to His tag, respectively, purified both recombinant proteins and performed pull-down assays with ADF4. GST-ADF4 coprecipitated with the His-CKL2 N terminus but not the C terminus. Immunoblotting analyses further confirmed that the full-length CKL2 and the N terminus of CKL2 interacted with ADF4 (Figure 5A).
Figure 5.
CKL2 Interacts with and Phosphorylates ADF4.
(A) CKL2 interacts with ADF4 in pull-down assays. Equal amounts of affinity-purified His-CKL2 (left first panel), His-CKL2N (second panel), or His-CKL2C fusion protein (third panel) were incubated with GST-ADF4. The input and output proteins were stained with Coomassie blue on a SDS-PAGE gel. The output proteins were also subject to immunoblot assay with anti-ADF antibodies (right panel). The asterisks indicate the GST-ADF4 bands.
(B) Split-luciferase complementation imaging assays in N. benthamiana. Quantitative analysis of luminescence intensity was determined. Relative values are mean ± sd, n = 3. Higher luminescence intensity was observed after ABA treatment compared with control (denoted by asterisk, P = 0.0022, t test).
(C) CKL2 phosphorylates ADF4 in vitro. The input proteins His-CKL2 and His-ADF4 were detected by Coomassie blue staining (left). Phosphorylation activity was detected by [γ-32P]ATP autoradiography (right).
(D) Two-dimensional immunoblotting. The Flag-ADF4 protein was immunoprecipitated with anti-Flag agarose from Col-0 or ckl2 mutant plants. Equal amounts of Flag-ADF4 protein immunoprecipitated from Col-0 were treated with λ phosphatase as a control. Anti-Flag antibody was used for immunoblot assays. Arrowheads point to the location of the more acidic ADF4 spot, representing phosphorylated ADF4. Experiments were repeated three times.
(E) Quantification of relative ADF4 phosphorylation level in (D). Values represent mean ± sd, n = 3. Statistical significance was determined by Student’s t test; significant differences (P < 0.05) are indicated with asterisks. The ckl2 mutant had a lower relative ADF4 phosphorylation level compared with control (P = 0.0258).
(F) ADF4 Ser-6 is important for the phosphorylation of CKL2. Purified His-ADF4 and His-ADF4S6A as substrates were phosphorylated in the in vitro kinase assays.
(G) Quantification of relative ADF4 phosphorylation level in (F). Values represent mean ± sd, n = 3. The data sets were tested for normal distribution by the Shapiro-Wilk test. Statistical significance was determined by Student’s t test; significant differences (P < 0.01) are indicated with asterisks. ADF4 Ser-6 mutation led to a lower relative phosphorylation level compared with the wild type (P = 0.0091).
To explore the interaction between CKL2 and ADF4 in vivo, we performed coimmunoprecipitation assays in vivo. Anti-ADF4 antibodies were generated. They recognized endogenous ADF1 and ADF4 but were not specific to ADF4 (Supplemental Figure 6A). For coimmunoprecipitation assays in vivo, total protein was isolated from the transgenic plants expressing the construct Pro35S:Flag-HA-CKL2. CKL2 was immunoprecipitated with anti-Flag antibody conjugated agarose, and ADFs were detected in the pull-down products by anti-ADF antibodies. PKS5, a cytoplasmic localized protein kinase (Fuglsang et al., 2007), was used as a control and showed no interaction with ADFs (Supplemental Figure 6B). We further used split-luciferase (split-LUC) complementation assays in Nicotiana benthamiana to determine the interaction of ADF4 and CKL2 (Figure 5B). ADF4 was fused to the C terminus of LUCIFERASE (ADF4-cLUC), and CKL2 was fused to the N terminus of LUCIFERASE (nLUC-CKL2). The split-LUC assays showed that transient coexpression of nLUC-CKL2 and ADF4-cLUC in N. benthamiana yielded strong fluorescence signals, but no fluorescence signal was detected in the control leaves coexpressing nLUC-CKL2 and cLUC or nLUC and ADF4-cLUC, which further confirmed that CKL2 interacts with ADF4 in vivo (Figure 5B). In addition, this interaction was induced by ABA treatment (Figure 5B).
To detect whether ADF4 is a substrate of CKL2, we conducted in vitro kinase assays using the His-CKL2 and His-ADF4 proteins. A phosphorylated ADF4 signal was detected (Figure 5C). However, CKL2 did not phosphorylate the actin binding protein SCAB1 in vitro (Zhao et al., 2011), suggesting that phosphorylation of ADF4 by CKL2 is relatively specific.
To determine if CKL2 phosphorylates ADF4 in vivo, the construct Pro35S:3Flag-ADF4 was used to transform Col-0 and the ckl2 mutant. Flag-ADF4 was immunoprecipitated with anti-Flag antibody-conjugated agarose and eluted using a Flag peptide. Equal amounts of the Flag-ADF4 protein were used for 2D immunoblotting assays with anti-Flag antibody to detect the phosphorylation level of ADF4. Two ADF4 spots with different pI values were detected in the 2D gel from both the wild type and the ckl2 mutant. Spot A, close to the low pI region, was significantly decreased, and spot B, close to the high pI region, was increased in the ckl2 mutant. When the wild-type sample was treated with Lambda Protein Phosphatase, spot A almost disappeared (Figures 5D and 5E), suggesting that spot A represented phosphorylated ADF4. These results suggest that ADF4 is phosphorylated in plant cells and that CKL2 is required for this phosphorylation.
Arabidopsis ADF subclass I family proteins contain a conserved amino acid Ser-6 (Supplemental Figure 6C). Phosphorylation at Ser-6 has been observed in all tested plant ADF subclass I family proteins (Allwood et al., 2001, 2002; Chen et al., 2002). To determine whether Ser-6 is required for the phosphorylation by CKL2, we generated a mutated form of ADF4 with Ser-6 exchanged for Ala (ADF4S6A). Recombinant proteins His-ADF4 and His-ADF4S6A were purified and used for the kinase assay. The phosphorylation level of ADF4 by CKL2 was significantly reduced by the mutation at Ser-6, although this point mutation did not abolish phosphorylation (Figures 5F and 5G). Taken together, our data suggest that CKL2 phosphorylates ADF4 and that Ser-6 is one of the phosphorylation sites.
Given that ADF proteins are highly conserved and the ADF family in Arabidopsis consists of 11 members that are phylogenetically divided into four subclasses (Mun et al., 2000; Ruzicka et al., 2007), we wondered whether CKL2 could phosphorylate other ADFs besides ADF4 in Arabidopsis. To explore this, we selected other ADF proteins from subclass I (ADF1-4) and found that all 4 ADFs in the subclass I were phosphorylated by CKL2 (Supplemental Figure 6D), suggesting that at least the subclass I ADFs are potential targets of CKL2 in Arabidopsis.
Phosphorylation of ADF4 by CKL2 Inhibits the Activities of ADF4
Phosphorylation of ADFs at the N-terminal serine (Ser-3 in animals and Ser-6 in plants) is conserved in both animal and plant cells and plays an important role in regulating ADF actin binding and disassembly activity (Ressad et al., 1998; Chen et al., 2002).
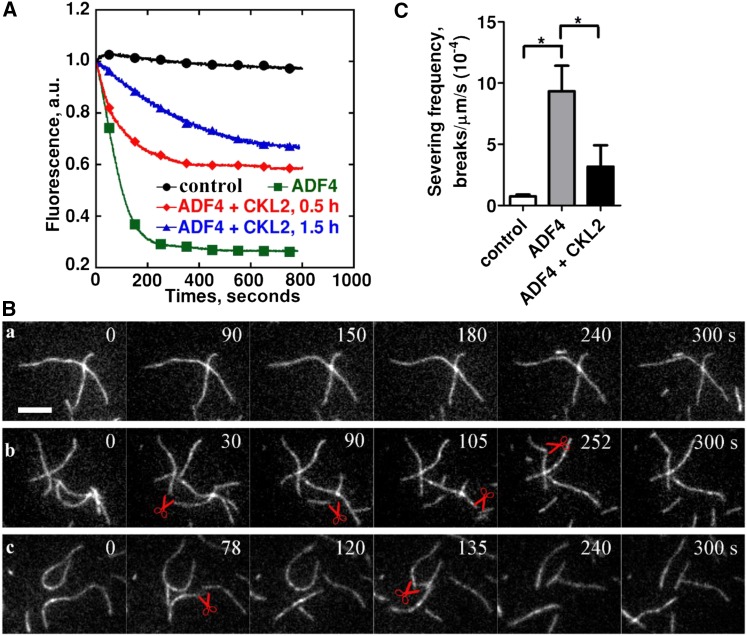
To further detect the effects of CKL2-mediated phosphorylation on the actin disassembling activity of ADF4, we performed bulk actin disassembly assays and found that CKL2-mediated phosphorylation decreased ADF4-induced actin disassembly (Figure 6A). We next monitored the effects of the phosphorylation of ADF4 by CKL2 on the actin filament-severing activity in real time using total internal reflection fluorescence (TIRF) microscopy. In the absence of ADF proteins, few breaks were observed along actin filaments (Figures 6Ba and 6C; Supplemental Movie 3). However, the addition of ADF4 resulted in increased filament breakage (Figures 6Bb and 6C; Supplemental Movie 4). By contrast, the addition of ADF4 phosphorylated by CKL2 resulted in less filament breakage (Figures 6Bc and 6C; Supplemental Movie 5), suggesting that CKL2-mediated phosphorylation inhibited ADF4-induced actin filament severing. In summary, our results suggest that the CKL2-mediated phosphorylation on ADF4 reduces the F-actin disassembling activity of ADF4.
Figure 6.
Phosphorylation of ADF4 by CKL2 Affects ADF4 Activity.
(A) The effect of ADF4 on F-actin disassembly was determined by a pyrene-actin assay. ADF4, after being phosphorylated by CKL2, was able to depolymerize actin filaments but was less potent than nonphosphorylated ADF4. Preassembled actin filaments (from 2 µM G-actin, 10% pyrene-labeled) were incubated with 250 nM ADF4 or 250 nM CKL2-phosphorylated ADF4 to induce actin disassembly at pH 7.0. Black closed circles, 0.5 µM F-actin; green closed squares, 0.5 µM F-actin + 4 µM ADF4; red closed diamonds, 0.5 µM F-actin + 4 µM ADF4 after phosphorylation by CKL2 for 0.5 h; blue closed triangles, 0.5 µM F-actin + 4 µM ADF4 after phosphorylation by CKL2 for 1.5 h. a.u., arbitrary units.
(B) Time-lapse TIRF microscopy analysis of actin filament severing by ADF4 after being phosphorylated by CKL2. Time-lapse images were recorded at 3-s intervals with TIRF microscopy. Actin filaments (from 1 µM G-actin, 50% Oregon-green labeled) were monitored for 300 s without ADF4 (A), in the presence of 500 nM nonphosphorylated ADF4 (B) or 500 nM CKL2-phosphorylated ADF4 (C). The red pairs of scissors indicate severing events. See Supplemental Movie 3 for the entire series.
(C) Quantification of ADF4-mediated actin-filament-severing frequency. Averages for each condition are from at least 30 individual filaments obtained from three independent trials. Error bars represent means ± sd (n = 3). Statistical significance (*P < 0.5) was determined by Student’s t test. The t test analysis of the data indicated the level of significance to be P = 0.0186 and 0.1380 for ADF4 and ADF4 + CKL2 data relative to the control data, respectively. The level of significance between ADF4 and ADF4 + CKL2 data was P = 0.0219.
Regulation of Stomatal Closure by CKL2 Is Partially Dependent on ADF4
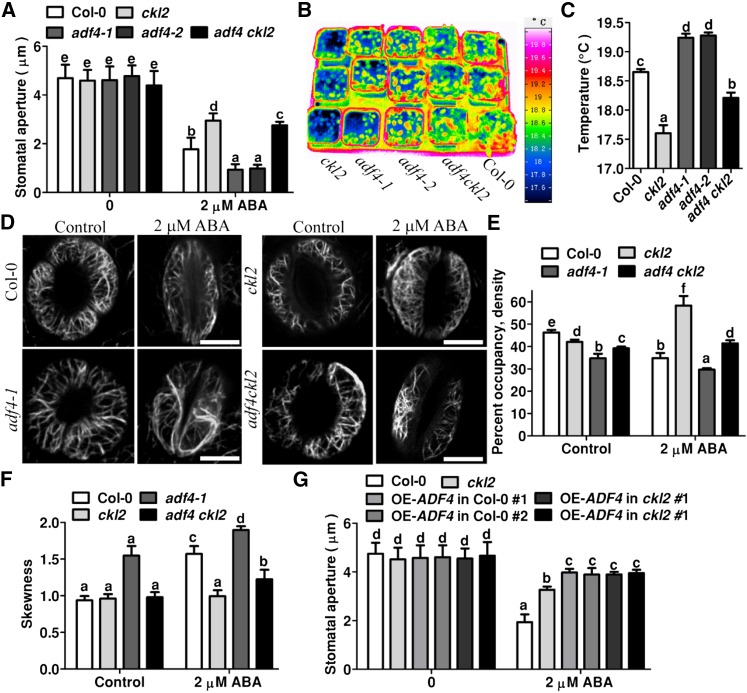
To determine the genetic interaction between CKL2 and ADF4, we generated adf4 ckl2 double mutants by crossing ckl2 with an adf4 T-DNA insertion line, adf4-1 (Tian et al., 2009). The wild type, adf4-1, adf4-2 (Salk_121647, obtained from the ABRC), ckl2, and adf4-1 ckl2 mutants were used to monitor stomatal closure in response to ABA treatment. RT-PCR results showed that the two adf4 homozygous lines are knockout mutants (Supplemental Figure 7A). Stomatal closure in both adf4-1 and adf4-2 was more sensitive to ABA than was the wild type (Figure 7A). Consistent with our previous results (Figure 1C), the stomatal closure in ckl2 was insensitive to ABA compared with the wild type (Figure 7A). The stomatal closure in the adf4-1 ckl2 double mutant in response to ABA was intermediate between that in the respective single mutants (Figure 7A). Consistent with the results of stomatal closure measurement, leaf temperature in the adf4 ckl2 double mutant was higher than that in the ckl2 single mutant but lower than that in the wild type (Figures 7B and 7C). Both adf4-1 and adf4-2 mutant plants displayed higher leaf temperatures than did the wild type (Figures 7B and 7C). These results suggest that loss of function of ADF4 partially rescues the stomatal closure phenotype in ckl2.
Figure 7.
ADF4 Is Required for CKL2-Mediated Stomatal Closure.
(A) Stomatal bioassays for ABA-induced closure in Col-0, ckl2, adf4-1, adf4-2, and adf4 ckl2 plants. The data represent the means ± sd of three independent experiments; at least 50 stomata were analyzed per genetic background. The data sets were tested for normal distribution by the Shapiro-Wilk test. Statistical significance was determined by Student’s t test; significant differences (P < 0.01) are indicated by different lowercase letters. The ckl2 mutants had wider stomatal apertures than Col-0 (P = 0.0052). Both adf4-1 and adf4-2 mutants had smaller stomatal apertures than Col-0 (P = 0.0017 and P = 0.0043). The adf4 ckl2 mutants had smaller apertures than the ckl2 mutants (P = 0.0019) and wider apertures than Col-0 (P = 0.0012). Before ABA treatment, there were no significant differences in the data for ckl2, adf4-1, adf4-2, and adf4 ckl2 compared with Col-0 (P = 0.8216, 0.4420, 0.5870, and 0.2290, respectively).
(B) Representative pseudocolored infrared images of leaf temperature of Col-0, ckl2, adf4-1, adf4-2, and adf4 ckl2 plants were obtained by infrared thermography.
(C) Leaf (surface) temperatures of Col-0, ckl2, adf4-1, adf4-2, and adf4 ckl2 plants were analyzed by InfraTec reporter software from images in (B). Twenty leaves were analyzed per line. Data are means ± sd (n = 3). The data sets were tested for normal distribution by the Shapiro-Wilk test. Statistical significance was determined by Student’s t test; significant differences (P < 0.01) are indicated by different lowercase letters. The ckl2 mutant had lower leaf temperatures than Col-0 (P = 0.0053). Both adf4-1 and adf4-2 had higher leaf temperatures than Col-0 (P = 0.0012 and P = 0.0091). adf4 ckl2 had higher leaf temperatures than ckl2 (P = 0.0036) and lower leaf temperatures than Col-0 (P = 0.0065).
(D) Actin filament organization in guard cells from Col-0, ckl2, adf4-1, adf4-2, and adf4 ckl2 transgenic plants harboring 35Sp:GFP-fABD2-GFP before and after 2 μM ABA treatment for 0.5 h.
(E) Quantitative analysis of actin filament density in guard cells as shown in (D). Before ABA treatment, the levels of significance were P = 0.0059, 0.0063, and 0.0052 for actin filament density in ckl2, adf4-1, and adf4 ckl2 mutants, respectively, relative to Col-0. After ABA treatment, the levels of significance were P = 0.0041, 0.0033, and 0.0045 for ckl2, adf4-1, and adf4 ckl2, respectively, relative to Col-0.
(F) Bundling (skewness) quantitative analysis in guard cells shown in (D). Before ABA treatment, the levels of significance were P = 0.0845, 0.0048, and 0.137 for ckl2, adf4-1, and adf4 ckl2, respectively, relative to the Col-0 skewness value. After ABA treatment the levels of significance were P = 0.0072, 0.0013, and 0.0025 for ckl2, adf4-1, and adf4 ckl2, respectively, relative to Col-0.
(G) Stomatal bioassays for ABA-induced closure in leaves of Col-0, ckl2, and plants overexpressing ADF4 in Col-0 and ckl2 mutant. Before ABA treatment, no significant differences were observed when comparing the stomatal apertures of ckl2 and plants overexpressing ADF4 in Col-0 and ckl2 mutant with Col-0, respectively (P = 0.66, 0.5830, 0.3932, 0.4523, and 0.6488). After ABA treatment, the levels of significance changed significantly (P = 0.0029, 0.0046, 0.0065, 0.0035, and 0.0073, respectively).
Values of (E) to (G) represent the means ± sd of three independent experiments; 50 stomata were analyzed per line. The data sets were tested for normal distribution by Shapiro-Wilk test. Statistical significance was determined by Student’s t test; significant differences (P < 0.01) are indicated by different lowercase letters.
To determine how CKL2 and ADF4 coordinately regulate the ABA-induced actin dynamics in guard cells, we transformed the 35S:GFP-fABD2-GFP construct into the adf4 and adf4 ckl2 double mutant. In widely opened guard cells, although the F-actin in the adf4 mutant also displayed radially arranged filaments, the skewness value increased and actin filament density value decreased significantly compared with these in Col-0, suggesting that the extent of actin filament bundling increased in adf4 guard cells (Figures 7D to 7F). However, in the adf4 ckl2 double mutant, the effect of ADF4 deletion on actin filaments was partially rescued by CKL2 deletion. The double mutant showed an intermediate density between that of the adf4 and ckl2 mutants, which was still lower than that in Col-0. The skewness in the adf4 ckl2 double mutant showed no significant difference from Col-0 (Figures 7D to 7F). After treatment with 2 μM ABA for 0.5 h, the skewness further increased and the density further decreased in closed stomata (69% of cell populations) of the adf4 mutant compared with these before the treatment (Figures 7D to 7F). However, in stomata of the adf4 ckl2 double mutant (75% of cell populations), both skewness and density displayed intermediate values between those of the adf4 and ckl2 mutants after ABA treatment (Figures 7D to 7F). The skewness value of the double mutant was lower than that of Col-0 and the density value was higher than that of Col-0. These results showed that deletion of ADF4 partially rescued the ckl2 mutant F-actin reorganization phenotype and suggest that loss of function of ADF4 at least partially accounts for the F-actin reorganization phenotype in ckl2.
To further investigate the function of ADF4 in CKL2-mediated stomatal closure, we generated ADF4 overexpression lines (35S:ADF4) in the Col-0 and ckl2 mutant background. There was no significant difference in ABA-induced stomatal closure between the Col-0 and ckl2 mutant upon overexpressing ADF4. Moreover, the plants overexpressing ADF4 in both the Col-0 and the ckl2 mutant backgrounds were even less sensitive than the ckl2 mutant plants in terms of ABA-induced stomatal closure (Figure 7G). These results further suggest that ADF4 interacts with CKL2 in regulating stomatal closure in response to ABA.
DISCUSSION
In this study, we found that an ABA-responsive actin filament-associated protein kinase, CKL2, regulates actin dynamics during stomatal closure, presumably via phosphorylating ADF proteins. CKL2 therefore emerges as an important player in regulating ABA-mediated stomatal closure. CKL2 and other factors, including receptor-like kinase, protein kinases (CDPK, SnRK2.2, 2.3, and 2.6), transcription factors (ABI3, 4, and 5), and ion channel SLACs (Desikan et al., 2004; Israelsson et al., 2006; Kim et al., 2010; Hua et al., 2012), constitute a sophisticated regulatory network for ABA-mediated stomatal closure. Our study adds another important component to the ABA signaling network in guard cells and enriches our understanding of the regulation of stomatal closure.
In guard cells, actin filaments undergo dynamic reorganization, which is important for proper stomatal closure (Kim et al., 1995; Eun and Lee, 1997; Hwang and Lee, 2001; MacRobbie and Kurup, 2007; Zhao et al., 2011; Jiang et al., 2012). However, how the reorganization of the actin cytoskeleton is achieved during stomatal closure remains poorly understood. Two stages are involved in this reorganization, with actin filament disassembly followed by filament reassembly when stomata close (Eun and Lee, 1997, 2000). Therefore, actin filament destabilization (for disassembly) and stabilization (for reassembly) are both necessary aspects of this process. This work shows that a lack of CKL2 results in the blockage of actin filament reassembly and the actin filaments remain in a disrupted state. These results suggest that CKL2 has an effect on stabilization of actin filaments and, thus, that CKL2 functions in the reassembly of actin filaments during stomatal closure.
ADFs bind to and sever actin filaments, thereby acting as major regulators of F-actin dynamics (Henty et al., 2011; Zheng et al., 2013) implicated in regulating F-actin reorganization during stomatal closure and opening (Dong et al., 2001). Precise regulation of ADF activity is required for the correct balance between F-actin assembly and disassembly (Ressad et al., 1998; Bamburg, 1999). Exactly how ADFs regulate F-actin reorganization and how the activity of ADFs is fine-tuned to meet the cellular demands during stomatal closure and opening are interesting topics. Based on our experimental results, we propose a brief model to explain how CKL2 stabilizes actin filaments to promote actin reassembly during ABA-induced stomatal closure (Figure 8). When ABA/drought-induced stomatal closure initiates, actin filaments begin to disassemble, which requires higher ADF activity. During this stage, CKL2 is accumulated due to upregulation by drought and ABA. The increased CKL2 level results in more phosphorylated ADF molecules, which in turn reduces their actin filament binding and severing activity, leading to actin arrays being reorganized into highly bundled long cables. The resulting changes in actin filament architecture promote stomatal closure. Furthermore, the stabilization of actin filaments would be maintained in the continued presence of ABA so that the stomata would remain closed.
Figure 8.
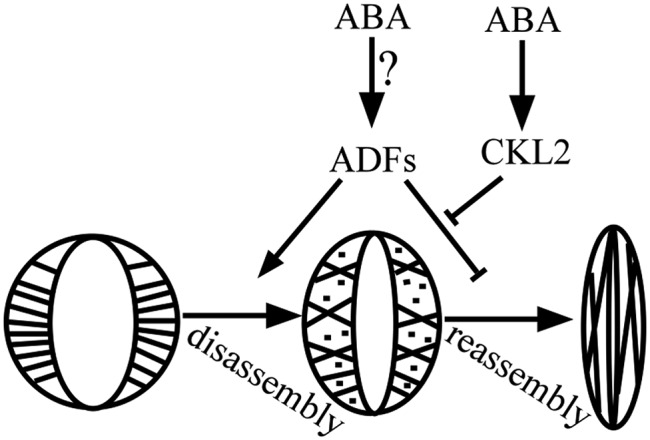
A Simplified Working Model for the Role of CKL2 and ADF4 in Regulating Actin Reorganization during ABA-Induced Stomatal Closure.
During stomatal closure, microfilaments reorganize, first disassembling and then reassembling. ABA/drought-induced CKL2 represses ADF activity to stabilize microfilaments and keep stomata closed.
Given that ADFs play an important role in actin filament disassembly during stomatal closure, how is their activity regulated at the early stage of ABA/drought response and reactivated after the action of CKL2? It is possible that a phosphatase(s) is critical at this stage by either activating ADFs or deactivating CKL2. PP2C is thought to be involved in actin reorganization in guard cells during ABA-induced stomatal closure. The ABA-insensitive mutant abi1-1 (a PP2C mutant) displays disturbed actin filament reorganization in guard cells when treated with ABA (Eun et al., 2001; Hwang and Lee, 2001; Lemichez et al., 2001). How PP2C cooperates with CKL2/ADFs to regulate actin dynamics in guard cells remains an interesting question.
CKL2 associates with actin filaments but does not directly bind to the actin filaments. We found that ABA induces the interaction of CKL2 with ADF4. Perhaps the interaction between CKL2 and ADFs not only protects the actin filaments from severing by ADFs, but also plays a role in recruiting CKL2 to the actin filaments. Our study suggests that Ser-6 in ADF4 is one of the residues phosphorylated by CKL2; the other phosphorylated residues may play roles in regulation at different levels and stages for the full function of ADF in modulating Arabidopsis stomatal closure.
The essential role of actin dynamics in regulating stomatal closure and opening is well appreciated (Kim et al., 1995; Eun and Lee, 1997; Hwang and Lee, 2001; MacRobbie and Kurup, 2007; Zhao et al., 2011; Jiang et al., 2012; Li et al., 2014), but how actin dynamics are functionally coupled to the underlying cellular processes in guard cells remains poorly understood. For instance, actin dynamics have been implicated in mediating Ca2+ concentration changes in the cytosol, K+ channel activity, and vacuole shape determination (Hwang et al., 1997; Gao et al., 2009; Zhao et al., 2013), but the related molecular mechanisms remain to be established. One possibility is regulation of membrane recycling by actin reorganization (Cheung et al., 2002; Lee et al., 2008; Zhao et al., 2010; Bou Daher and Geitmann, 2011; Zhu et al., 2013), which would change the localization of ion channels/transporters and the area of vacuolar membrane. Actin dynamics may also act as an important component in signal transduction to mediate such changes (Gao et al., 2008; Zhao et al., 2011, 2013; Jiang et al., 2012).
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana Col-0 was used as the wild type. The ckl2 mutant (Salk_104209) was obtained from the ABRC.
Primers used to confirm the homozygous mutant lines are listed in Supplemental Table 1. Arabidopsis seedlings were grown in soil under 8 h light (light intensity of 30 µmol m–2 s–1)/16 h darkness (short-day conditions) at 23°C and 60% relative humidity (RH) for 3 to 4 weeks. Nicotiana benthamiana plants were grown in soil under 16 h light/8 h darkness (long-day conditions) at 23°C and 60% relative humidity for 3 to 4 weeks.
RNA Extraction and Real-Time Quantitative RT-PCR Analysis
Total RNA was extracted from 7-d-old seedlings grown on Murashige and Skoog (MS) medium or the roots, stems, cauline leaves, rosettes, flowers, or siliques of 4-week-old plants grown in soil with Trizol reagent (Invitrogen). Total RNA treated with RNase-free DNase I (Invitrogen) was used for reverse transcription with M-MLV reverse transcriptase (Promega). Real-time quantitative PCR was conducted using SYBR Premix Ex Taq (Takara). Elongation factor 1α (EF) was used as an internal control. The primers used for RT-PCR or Real-time quantitative PCR are listed in Supplemental Table 1.
Water-Loss and Stomatal Aperture Assays
To detect the stomatal closure in response to ABA treatment, rosette leaves of 5-week-old plants were detached and incubated in stomata-opening buffer (containing 50 mM KCl, 10 μM CaCl2, and 10 mM MES, pH 6.15) in a growth chamber at 23°C under constant illumination. Stomatal apertures were measured after adding 2 μM ABA for 0.5 h. The apertures of 50 stomata were measured in three independent experiments.
For water-loss measurement, rosette leaves were detached from 5-week-old plants. The weight of the detached leaves was measured every 0.5 h.
Infrared Thermograph Imaging
To monitor the leaf temperature, thermal imaging was performed as described previously with slight modification (Xie et al., 2006). Well-watered 3-week-old plants were transferred from a high humidity growth condition (RH 70%) to a low humidity condition (RH 40%). The leaf temperature was recorded by a thermal imaging camera after 3 d.
Subcellular Localization Assays
For subcellular localization analysis of GFP-CKL2, the CKL2 cDNA coding region was amplified and cloned into the pCambia1205 vector (Zhao et al., 2011) between the BamHI and EcoRI sites to generate Pro35S:GFP-CKL2. For the ProCKL2:GFP-CKL2 construct, the 1.06-kb CKL2 promoter fragment was amplified from BAC plasmid F18A22 (ABRC) and cloned into the pCAMBIA1305 vector (Zhao et al., 2011) between the HindIII and PstI sites, and then the CKL2 cDNA coding region was amplified and cloned between the SalI and BamHI sites. The resulting Pro35S:GFP-CKL2 and ProCKL2:GFP-CKL2 vectors were used to transform Col-0 and the ckl2 mutant, respectively. The confocal images were taken from 6-d-old seedlings of the transgenic plants with a Zeiss LSM 510 Meta confocal microscope using a Plan-Apochromat 63×/1.4 oil immersion differential interference contrast lens. GFP was excited at 488 nm.
Preparation of Suspension Cells
For suspension cell preparation, seedlings were grown on MS medium (4.43 g/L MS salt, 30 g/L sucrose, and 0.3% agar) for 7 d. The leaves were then cut from the seedlings and cultured on MS medium with 1 mg/L 2,4-D and 0.1 mg/L 6-benzylaminopurine to induce calli. The calli were cultured in the MS liquid medium with 1 mg/L 2,4-D on a rotor set to 120 rpm in the dark at 23°C to induce suspension cells. The suspension cells were subcultured in darkness for 3 weeks and then used for actin staining assays.
Colocalization Analysis
For colocalization analysis, suspension cells generated from the 35Sp:GFP-CKL2 transgenic plants were incubated in actin staining buffer (0.18 μM rhodamine-phalloidin, 100 mM PIPES, 10 mM EGTA, 5 mM MgSO4, 5% DMSO, and 0.05% Nonidet P-40, pH 6.8) for 15 min at room temperature. The samples were observed by confocal microscopy. The images were collected through visualizing the red/green fluorescence signals from the GFP-CKL2 and the rhodamine-phalloidin-labeled actin filaments. The colocalization between GFP-CKL2 and the actin cytoskeleton was analyzed by calculating Pearson’s correlation coefficient (Dunn et al., 2011; Wu et al., 2012; McDonald and Dunn, 2013). Relative pixel intensity in the indicated regions of interest was measured using Image J software. Thresholds were set manually to account for background, and Pearson’s correlation coefficient was calculated to determine the correlation degree.
Confocal Laser Scanning Microscopy to Visualize Actin Filaments in Vivo
To visualize actin filaments in plant cells, the Pro35S:GFP-fABD2-GFP construct was transformed into the wild type, the ckl2 mutant, the adf4-1 mutant, and the adf4 ckl2 double mutant. Six-day-old seedlings of the transgenic lines grown on MS medium were used for actin filament visualization in hypocotyl cells and roots. Three-week-old plants were used for actin filament visualization in guard cells. To detect the effect of LatA or ABA on actin filaments, the transgenic plants were treated with 200 nm LatA or 20 μM ABA for 0.5 h or 1 h. Then the actin cytoskeleton was visualized by detecting the GFP fluorescence signal in hypocotyl cells or guard cells with a Zeiss LSM 510 META confocal microscope using a Plan-Apochromat 63×/1.4 oil immersion differential interference contrast lens. GFP was excited at 488 nm.
To observe actin filament dynamics in guard cells, time-lapse images were captured every 2 s with an Andor iXon charge-coupled device camera. To observe actin filament dynamic behavior, parameters including maximum filament lifetime, maximum filament length, elongation rate, severing frequency, and depolymerization rate with single actin filament turnover were calculated using Image J software as described previously (Henty et al., 2011; Qin et al., 2014).
Antibody Preparation and Immunoblotting
Anti-ADF4 antibodies were generated by immunizing mice with Escherichia coli-expressed ADF4. To determine the specificity of the antibodies in plants, total protein was isolated from the 7-d-old Col-0, the adf4 mutant (SALK_121647), the adf1 mutant (SALK_144459), and the transgenic plants harboring the construct Pro35S:3×Flag-ADF4 or Pro35S:3×Flag-ADF1 and subjected to immunoblot analysis with anti-ADF4 antibodies. The blots were probed with primary mouse anti-ADF4 (diluted 1:1000).
Protein Purification and Pull-Down Assays
The coding regions of CKL2 and ADF4 were amplified and cloned into the vectors of PET28a and pGEX-6p-1, respectively. The N terminus of CKL2 (295 amino acids, CKL2N) and the C terminus of CKL2 (170 amino acids, CKL2C) were cloned into the PET28a vector. The resulting vectors were transformed into E. coli (strain BL21). The recombinant proteins were purified with Ni-NTA agarose or glutathione sepharose.
For the pull-down assay, 5 μg His-CKL2, -CKL2N, or -CKL2C on Ni-NTA agarose was incubated with 1 μg GST-ADF4 for 30 min at 4°C in 100 μL binding buffer (20 mM Tris-HCl, pH 7.2, 10 mM MgCl2, and 2 mM DTT). After washing three times with the binding buffer, protein on the agarose was separated on 15% SDS-PAGE, followed by Coomassie Brilliant Blue R 250 staining. One microliter of agarose was analyzed by immunoblot using anti-ADF antibodies.
For in vivo coimmunoprecipitation assays, the CKL2 coding region was amplified and cloned into the pCM1307-N-Flag-HA vector between the XbaI and SalI sites. The resulting plasmid was transformed into wild-type plants. Total protein was extracted from 7-d-old transgenic plants using 2 mL immunoprecipitation buffer (10 mM Tris, pH 7.5, 0.5% Nonidet P-40, 2 mM EDTA, 150 mM NaCl, 1 mM PMSF, and 1% protease inhibitor cocktail [Sigma-Aldrich]). Flag-HA-CKL2 was purified with 30 μL anti-FLAG agarose (Sigma-Aldrich) from the total protein. After washing three times with 2 mL immunoprecipitation buffer, the agarose was used for immunoblot assays with anti-ADFs antibodies.
Split-Luciferase Complementation Assays
For the split luciferase assays, the CKL2 and ADF4 cDNA coding regions were amplified and cloned into the KpnI and SalI sites of the pCM1307-nLUC and pCM1307-cLUC vector, respectively. The plasmids were introduced into Agrobacterium tumefaciens GV3101 and coinfiltrated into the leaves of N. benthamiana. After a 3-d incubation, the LUC activity was measured using a cooled CCD imaging camera (1300B; Roper). Then, 1 mM luciferin was sprayed onto the leaves. Relative LUC activity per cm2 infiltrated leaf area was calculated using Winview32 software. Each data column contains at least 10 replicates, and three independent experiments were performed.
Kinase Activity Assays
In vitro kinase activity assays were performed as described previously (Quan et al., 2007). Recombinant protein His-CKL2, His-ADF4, and His-ADF4S6A were purified with Ni-NTA agarose. One microgram of His-ADF4 or His-ADF4S6A was incubated with 1 μg His-CKL2 in a kinase reaction buffer (20 mM Tris-HCl, pH 8.0, 5 mM MgCl2, 10 μM ATP, 1 mM DTT, and 2 μCi [γ-32P]ATP) in 15 μL total volume at 30°C for 30 min. The reaction was terminated with 2× SDS loading buffer. After incubation at 100°C for 5 min, the reaction products were separated on 15% SDS-PAGE and stained using Coomassie Brilliant Blue R 250. Then the SDS-PAGE gels were exposed to a phosphor screen. The phosphor signals were detected by a Typhoon 9410 phosphor imager (Amersham Biosciences). Phosphorylation signals were quantified using Image Quant 5.0 software.
Two-dimensional SDS-PAGE was performed as described (Minamide et al., 1997; Dong et al., 2001). The Pro35S:3× Flag-ADF4 construct was transformed into the wild type and ckl2 mutant, respectively. Total protein was isolated from the resulting transgenic seedlings in extraction buffer (10 mM Tris, pH 7.5, 0.5% Nonidet P-40, 2 mM EDTA, 150 mM NaCl, 1 mM PMSF, and 1% protease inhibitor cocktail [Sigma-Aldrich]). ADF4 protein was immunoprecipitated from the total proteins by incubation with anti-Flag agarose. For phosphatase treatment, the ADF4 protein was incubated with phosphatase (λ phosphatase; New England Biolabs) at 30°C for 15 min. Immunoblotting of ADF4 was performed with an anti-Flag antibody.
Quantification of GFP-fABD2-GFP Fluorescence Pixel Intensity, Density, and Skewness of the Actin Filaments in Guard Cells
Measurement of relative fluorescence pixel intensity levels of GFP-fABD2-GFP-labeled actin filaments in guard cells and hypocotyl epidermal cells was performed as described previously (Huang et al., 2005). The average fluorescence pixel intensity associated with GFP-fABD2-GFP was measured using ImageJ software (http://rsb.info.nih.gov/ij/) to determine the relative amount of actin filaments. The skewness and density of actin filaments were quantified by ImageJ according to Higaki et al. (2010) with slight modification. Individual cells were segmented manually and actin filaments at the cell border were eliminated. More than 50 guard cells were used for the analysis.
Biochemical Assays to Determine the Effect of CKL2 on Actin Polymerization
High-speed cosedimentation assays were performed to examine the binding of CKL2 to actin filaments as previously described (Kovar et al., 2000; Huang et al., 2005). Direct visualization of actin filaments by epifluorescence light microscopy was performed as described previously (Okada et al., 2002). A spontaneous actin nucleation assay was performed according to previously published methods (Amann and Pollard, 2001; Huang et al., 2003; Andrianantoandro and Pollard, 2006). Various concentrations of CKL2 were incubated with G-actin (2 μM 10% pyrene-labeled) for 5 min at room temperature. Actin polymerization was initiated after the addition of 1/10 volume of 10× KMEI (500 mM KCl, 10 mM MgCl2, 10 mM EGTA, and 100 mM imidazole-HCl, pH 7.0). Pyrene fluorescence was monitored using a QuantaMaster Luminescence QM 3 PH Fluorometer (Photo Technology International) with the excitation and emission wavelength set at 365 and 407 nm, respectively.
Determination of the Effect of CKL2 Phosphorylation on ADF4-Mediated Actin Disassembly in Vitro
All proteins and buffers were preclarified by centrifugation at 200,000g for 1 h at 4°C. Equal molar amounts of ADF4 and CKL2 were used for the phosphorylation reaction as described above. G-actin (2 μM, 10% pyrene labeled) was polymerized at 25°C for 2 h in polymerization buffer (described above). The resulting assembled actin filaments from 500 nM G-actin were then incubated with 500 nM nonphosphorylated ADF4 or 500 nM CKL2-phosphorylated ADF4 in 1× KMEI to induce actin filament disassembly. Actin disassembly was traced by monitoring the decrease in pyrene fluorescence for 30 min using a QuantaMaster Luminescence QM 3 PH Fluorometer (Photon Technology International) with the excitation and emission wavelengths set at 365 and 407 nm, respectively.
Direct Visualization of Actin Filament Severing in Vitro by TIRF Microscopy
Single actin filament severing was directly visualized by TIRF microscopy as described previously (Amann and Pollard, 2001; Andrianantoandro and Pollard, 2006; Jansen et al., 2014). The flow chamber was prepared as described previously (Amann and Pollard, 2001; Jansen et al., 2014). For actin-filament-severing assays, the assembled flow chamber was incubated with 25 nM N-ethylmaleimide-myosin for 5 min followed by washing with 1% BSA for 3 min. Next, TIRF microscopy buffer (10 mM imidazole, pH 7.0, 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 50 mM DTT, 0.2 mM ATP, 50 mM CaCl2, 15 mM glucose, 20 mg/mL catalase, 100 mg/mL glucose oxidase, and 0.5% methylcellulose) was injected into the prepared flow chamber. Actin filaments (from 1 μM G-actin, 50% Oregon-Green labeled) in TIRF buffer were injected into the chamber and incubated in darkness for 5 min. Finally, TIRF buffer was injected into the chamber to remove attached actin filaments. After injection of ADF4 or CKL2-phosphorylated ADF4 into the flow chamber, single actin filament severing events were observed by time-lapse TIRF microscopy using an Olympus IX81 microscope equipped with a ×100 oil objective (1.49 numerical aperture). Time-lapse images were recorded every 3 s for 5 min. Actin filament severing frequency was calculated as the maximum filament length divided by the number of breaks per filament length over time (breaks/μm/s). More than 30 actin filaments with length >10 μm were selected for quantification under each condition.
Statistical Analysis
To test the data normality of continuous variables, statistical analysis was performed using the SPSS for Windows software package (version 11.5; IBM), and the Shapiro-Wilk test was applied. Relative values are means ± sd or means ± se. All statistical analysis was performed using two-tailed Student’s t test to determine group differences in means using GraphPad Prism 6.0 software or Kaleida Graph 4.1 (Synergy Software). Significant differences were indicated by different lowercase letters and the threshold was set at 0.01.
Accession Numbers
Sequence data in this study can be found in the Arabidopsis Genome Initiative database under the following accession numbers: CKL2, At1g72710; ADF1, At3g46010; ADF4, At5g59890; EF1α, At5g60390; RD29A, At5g52310; and SCaBP8, At4g33000.
Supplemental Data
Supplemental Figure 1. Identification of the T-DNA Insertion in the ckl2 Mutant.
Supplemental Figure 2. Expression of CKL2.
Supplemental Figure 3. CKL2 Colocalizes with Actin Filaments.
Supplemental Figure 4. CKL2 Does Not Bind to F-Actin Directly and Has No Effect on Actin Polymerization in Vitro.
Supplemental Figure 5. CKL2 and ADF4 Expression in Guard Cells.
Supplemental Figure 6. CKL2 Interacts with ADF4.
Supplemental Figure 7. Analysis of ADF Protein Level in Transgenic Seedlings.
Supplemental Table 1. Primers Used in This Study.
Supplemental Movie 1. Time-Series Movie Displaying the Severing Events of Single Filaments in Col-0 Guard Cell.
Supplemental Movie 2. Time-Series Movie Displaying the Severing Events of Single Filaments in the ckl2 Mutant Guard Cell.
Supplemental Movie 3. Time-Series Movie of Actin Filament Severing in the Absence of ADF4.
Supplemental Movie 4. Time-Series Movie of Actin Filament Severing in the Presence of Nonphosphorylated ADF4.
Supplemental Movie 5. Time-Series Movie of Actin Filament Severing in the Presence of CKL2-Phosphorylated ADF4.
Supplemental Movie Legends.
Supplementary Material
Acknowledgments
We thank Christopher J. Staiger (Department of Biological Sciences, Purdue University) for kindly providing the Arabidopsis seeds of adf4-1 (GARLIC_823_A11.b.1b.Lb3Fa), 35Sp:ADF4; adf4, and ABRC for ckl2 (Salk_104209) and adf4-2 seeds. We thank Nancy Hofmann at Plant Editors for English editing. This work was supported by the National Basic Research Program of China (Grant 2015CB910202 to Y.G.), the National Natural Science Foundation of China (Grant 31430012 to Y.G.), and Foundation for Innovative Research Group of the National Natural Science Foundation of China (Grant 31121002).
AUTHOR CONTRIBUTIONS
S.Z., Y.J., and Y.Z. performed the research. S.Z., Y.X.Z., and Y.G. designed the research and analyzed the data. Y.Z. contributed to the purification of recombinant proteins. Y.J. and S.H. performed the research on analysis of single actin filament severing. S.Z., Y.G., S.H., M.Y., and Y.X.Z. contributed to the discussion and wrote the article.
Glossary
- ABA
abscisic acid
- LatA
latrunculin A
- TIRF
total internal reflection fluorescence
References
- Agnew B.J., Minamide L.S., Bamburg J.R. (1995). Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J. Biol. Chem. 270: 17582–17587. [DOI] [PubMed] [Google Scholar]
- Akashi M., Tsuchiya Y., Yoshino T., Nishida E. (2002). Control of intracellular dynamics of mammalian period proteins by casein kinase I epsilon (CKIepsilon) and CKIdelta in cultured cells. Mol. Cell. Biol. 22: 1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwood E.G., Anthony R.G., Smertenko A.P., Reichelt S., Drobak B.K., Doonan J.H., Weeds A.G., Hussey P.J. (2002). Regulation of the pollen-specific actin-depolymerizing factor LlADF1. Plant Cell 14: 2915–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwood E.G., Smertenko A.P., Hussey P.J. (2001). Phosphorylation of plant actin-depolymerising factor by calmodulin-like domain protein kinase. FEBS Lett. 499: 97–100. [DOI] [PubMed] [Google Scholar]
- Amann K.J., Pollard T.D. (2001). Direct real-time observation of actin filament branching mediated by Arp2/3 complex using total internal reflection fluorescence microscopy. Proc. Natl. Acad. Sci. USA 98: 15009–15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianantoandro E., Pollard T.D. (2006). Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 24: 13–23. [DOI] [PubMed] [Google Scholar]
- Bamburg J.R. (1999). Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 15: 185–230. [DOI] [PubMed] [Google Scholar]
- Ben-Nissan G., Cui W., Kim D.J., Yang Y., Yoo B.C., Lee J.Y. (2008). Arabidopsis casein kinase 1-like 6 contains a microtubule-binding domain and affects the organization of cortical microtubules. Plant Physiol. 148: 1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O. (2007). Lim kinases, regulators of actin dynamics. Int. J. Biochem. Cell Biol. 39: 1071–1076. [DOI] [PubMed] [Google Scholar]
- Bernstein B.W., Painter W.B., Chen H., Minamide L.S., Abe H., Bamburg J.R. (2000). Intracellular pH modulation of ADF/cofilin proteins. Cell Motil. Cytoskeleton 47: 319–336. [DOI] [PubMed] [Google Scholar]
- Boesger J., Wagner V., Weisheit W., Mittag M. (2014). Comparative phosphoproteomics to identify targets of the clock-relevant casein kinase 1 in C. reinhardtii flagella. Methods Mol. Biol. 1158: 187–202. [DOI] [PubMed] [Google Scholar]
- Bou Daher F., Geitmann A. (2011). Actin is involved in pollen tube tropism through redefining the spatial targeting of secretory vesicles. Traffic 12: 1537–1551. [DOI] [PubMed] [Google Scholar]
- Chen C.Y., Wong E.I., Vidali L., Estavillo A., Hepler P.K., Wu H.M., Cheung A.Y. (2002). The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes. Plant Cell 14: 2175–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong J.K., Virshup D.M. (2011). Casein kinase 1: Complexity in the family. Int. J. Biochem. Cell Biol. 43: 465–469. [DOI] [PubMed] [Google Scholar]
- Cheung A.Y., Chen C.Y., Glaven R.H., de Graaf B.H., Vidali L., Hepler P.K., Wu H.M. (2002). Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and the Golgi bodies and is important to pollen tube growth. Plant Cell 14: 945–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément M., Ketelaar T., Rodiuc N., Banora M.Y., Smertenko A., Engler G., Abad P., Hussey P.J., de Almeida Engler J. (2009). Actin-depolymerizing factor2-mediated actin dynamics are essential for root-knot nematode infection of Arabidopsis. Plant Cell 21: 2963–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Ye J.Z., Guo X.H., Chang H.P., Yuan C.Y., Wang Y., Hu S., Liu X.M., Li X.S. (2012). Arabidopsis casein kinase 1-like 2 involved in abscisic acid signal transduction pathways. J. Plant Interact. 9: 19–25. [Google Scholar]
- Davidson M.M., Haslam R.J. (1994). Dephosphorylation of cofilin in stimulated platelets: roles for a GTP-binding protein and Ca2+. Biochem. J. 301: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R., Cheung M.K., Bright J., Henson D., Hancock J.T., Neill S.J. (2004). ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J. Exp. Bot. 55: 205–212. [DOI] [PubMed] [Google Scholar]
- Dong C.H., Xia G.X., Hong Y., Ramachandran S., Kost B., Chua N.H. (2001). ADF proteins are involved in the control of flowering and regulate F-actin organization, cell expansion, and organ growth in Arabidopsis. Plant Cell 13: 1333–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C.H., Hong Y. (2013). Arabidopsis CDPK6 phosphorylates ADF1 at N-terminal serine 6 predominantly. Plant Cell Rep. 32: 1715–1728. [DOI] [PubMed] [Google Scholar]
- Du M., et al. (2014). Closely related NAC transcription factors of tomato differentially regulate stomatal closure and reopening during pathogen attack. Plant Cell 26: 3167–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K.W., Kamocka M.M., McDonald J.H. (2011). A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 300: C723–C742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun S.O., Lee Y. (1997). Actin filaments of guard cells are reorganized in response to light and abscisic acid. Plant Physiol. 115: 1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun S.O., Lee Y. (2000). Stomatal opening by fusicoccin is accompanied by depolymerization of actin filaments in guard cells. Planta 210: 1014–1017. [DOI] [PubMed] [Google Scholar]
- Eun S.O., Bae S.H., Lee Y. (2001). Cortical actin filaments in guard cells respond differently to abscisic acid in wild-type and abi1-1 mutant Arabidopsis. Planta 212: 466–469. [DOI] [PubMed] [Google Scholar]
- Fuglsang A.T., Guo Y., Cuin T.A., Qiu Q., Song C., Kristiansen K.A., Bych K., Schulz A., Shabala S., Schumaker K.S., Palmgren M.G., Zhu J.K. (2007). Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. Plant Cell 19: 1617–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X.Q., Chen J., Wei P.C., Ren F., Chen J., Wang X.C. (2008). Array and distribution of actin filaments in guard cells contribute to the determination of stomatal aperture. Plant Cell Rep. 27: 1655–1665. [DOI] [PubMed] [Google Scholar]
- Gao X.Q., Wang X.L., Ren F., Chen J., Wang X.C. (2009). Dynamics of vacuoles and actin filaments in guard cells and their roles in stomatal movement. Plant Cell Environ. 32: 1108–1116. [DOI] [PubMed] [Google Scholar]
- Gayatri G., Agurla S., Raghavendra A.S. (2013). Nitric oxide in guard cells as an important secondary messenger during stomatal closure. Front. Plant Sci. 4: 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S.D., Hoffman D.P., Fisette P.L., Baas P., Anderson R.A. (1995). A phosphatidylinositol 4,5-bisphosphate-sensitive casein kinase I alpha associates with synaptic vesicles and phosphorylates a subset of vesicle proteins. J. Cell Biol. 130: 711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henty-Ridilla J.L., Li J., Blanchoin L., Staiger C.J. (2013). Actin dynamics in the cortical array of plant cells. Curr. Opin. Plant Biol. 16: 678–687. [DOI] [PubMed] [Google Scholar]
- Henty-Ridilla J.L., Li J., Day B., Staiger C.J. (2014). ACTIN DEPOLYMERIZING FACTOR4 regulates actin dynamics during innate immune signaling in Arabidopsis. Plant Cell 26: 340–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henty J.L., Bledsoe S.W., Khurana P., Meagher R.B., Day B., Blanchoin L., Staiger C.J. (2011). Arabidopsis actin depolymerizing factor4 modulates the stochastic dynamic behavior of actin filaments in the cortical array of epidermal cells. Plant Cell 23: 3711–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki T., Sano T., Hasezawa S. (2007). Actin microfilament dynamics and actin side-binding proteins in plants. Curr. Opin. Plant Biol. 10: 549–556. [DOI] [PubMed] [Google Scholar]
- Higaki T., Kutsuna N., Sano T., Kondo N., Hasezawa S. (2010). Quantification and cluster analysis of actin cytoskeletal structures in plant cells: role of actin bundling in stomatal movement during diurnal cycles in Arabidopsis guard cells. Plant J. 61: 156–165. [DOI] [PubMed] [Google Scholar]
- Hirayama T., Shinozaki K. (2007). Perception and transduction of abscisic acid signals: keys to the function of the versatile plant hormone ABA. Trends Plant Sci. 12: 343–351. [DOI] [PubMed] [Google Scholar]
- Hosy E., et al. (2003). The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA 100: 5549–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P., Paunola E., Vartiainen M.K., Lappalainen P. (2005). Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol. Biol. Cell 16: 649–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua D., Wang C., He J., Liao H., Duan Y., Zhu Z., Guo Y., Chen Z., Gong Z. (2012). A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Robinson R.C., Gao L.Y., Matsumoto T., Brunet A., Blanchoin L., Staiger C.J. (2005). Arabidopsis VILLIN1 generates actin filament cables that are resistant to depolymerization. Plant Cell 17: 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Anvari B., Torres J.H., LeBaron R.G., Athanasiou K.A. (2003). Temporal effects of cell adhesion on mechanical characteristics of the single chondrocyte. J. Orthop. Res. 21: 88–95. [DOI] [PubMed] [Google Scholar]
- Hussey P.J., Ketelaar T., Deeks M.J. (2006). Control of the actin cytoskeleton in plant cell growth. Annu. Rev. Plant Biol. 57: 109–125. [DOI] [PubMed] [Google Scholar]
- Hwang J.U., Lee Y. (2001). Abscisic acid-induced actin reorganization in guard cells of dayflower is mediated by cytosolic calcium levels and by protein kinase and protein phosphatase activities. Plant Physiol. 125: 2120–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.U., Suh S., Yi H., Kim J., Lee Y. (1997). Actin filaments modulate both stomatal opening and inward K+-channel activities in guard cells of Vicia faba L. Plant Physiol. 115: 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani M., Xiong L., Stevenson B., Zhu J.K. (1997). Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: interactions and convergence of abscisic acid-dependent and abscisic acid-independent pathways. Plant Cell 9: 1935–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelsson M., Siegel R.S., Young J., Hashimoto M., Iba K., Schroeder J.I. (2006). Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr. Opin. Plant Biol. 9: 654–663. [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Szumlanski A., Nielsen E., Yang Z. (2008). Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J. Cell Biol. 181: 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S., Collins A., Golden L., Sokolova O., Goode B.L. (2014). Structure and mechanism of mouse cyclase-associated protein (CAP1) in regulating actin dynamics. J. Biol. Chem. 289: 30732–30742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K., Sorefan K., Deeks M.J., Bevan M.W., Hussey P.J., Hetherington A.M. (2012). The ARP2/3 complex mediates guard cell actin reorganization and stomatal movement in Arabidopsis. Plant Cell 24: 2031–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori T., Hayakawa T., Suzuki M., Titani K. (1995). Identification of two 17-kDa rat parotid gland phosphoproteins, subjects for dephosphorylation upon beta-adrenergic stimulation, as destrin- and cofilin-like proteins. J. Biol. Chem. 270: 8061–8067. [DOI] [PubMed] [Google Scholar]
- Ketelaar T., Allwood E.G., Anthony R., Voigt B., Menzel D., Hussey P.J. (2004). The actin-interacting protein AIP1 is essential for actin organization and plant development. Curr. Biol. 14: 145–149. [DOI] [PubMed] [Google Scholar]
- Kim M., Hepler P.K., Eun S.O., Ha K.S., Lee Y. (1995). Actin filaments in mature guard cells are radially distributed and involved in stomatal movement. Plant Physiol. 109: 1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.H., Böhmer M., Hu H., Nishimura N., Schroeder J.I. (2010). Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61: 561–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippschild U., Gocht A., Wolff S., Huber N., Löhler J., Stöter M. (2005). The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell. Signal. 17: 675–689. [DOI] [PubMed] [Google Scholar]
- Knippschild U., Krüger M., Richter J., Xu P., García-Reyes B., Peifer C., Halekotte J., Bakulev V., Bischof J. (2014). The CK1 Family: Contribution to cellular stress response and its role in carcinogenesis. Front. Oncol. 4: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H., Nuhkat M., Roelfsema M.R. (2014). Closing gaps: linking elements that control stomatal movement. New Phytol. 203: 44–62. [DOI] [PubMed] [Google Scholar]
- Kovar D.R., Staiger C.J., Weaver E.A., McCurdy D.W. (2000). AtFim1 is an actin filament crosslinking protein from Arabidopsis thaliana. Plant J. 24: 625–636. [DOI] [PubMed] [Google Scholar]
- Lemichez E., Wu Y., Sanchez J.P., Mettouchi A., Mathur J., Chua N.H. (2001). Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev. 15: 1808–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Blanchoin L., Staiger C.J. (2015). Signaling to actin stochastic dynamics. Annu. Rev. Plant Biol. 66: 415–440. [DOI] [PubMed] [Google Scholar]
- Li X., Li J.H., Wang W., Chen N.Z., Ma T.S., Xi Y.N., Zhang X.L., Lin H.F., Bai Y., Huang S.J., Chen Y.L. (2014). ARP2/3 complex-mediated actin dynamics is required for hydrogen peroxide-induced stomatal closure in Arabidopsis. Plant Cell Environ. 37: 1548–1560. [DOI] [PubMed] [Google Scholar]
- Li Y., Shen Y., Cai C., Zhong C., Zhu L., Yuan M., Ren H. (2010). The type II Arabidopsis formin14 interacts with microtubules and microfilaments to regulate cell division. Plant Cell 22: 2710–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Yang Y., Quan R., Mendoza I., Wu Y., Du W., Zhao S., Schumaker K.S., Pardo J.M., Guo Y. (2009). Phosphorylation of SOS3-LIKE CALCIUM BINDING PROTEIN8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis. Plant Cell 21: 1607–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciver S.K., Hussey P.J. (2002). The ADF/cofilin family: actin-remodeling proteins. Genome Biol. 3: s3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie E.A., Kurup S. (2007). Signalling mechanisms in the regulation of vacuolar ion release in guard cells. New Phytol. 175: 630–640. [DOI] [PubMed] [Google Scholar]
- McDonald J.H., Dunn K.W. (2013). Statistical tests for measures of colocalization in biological microscopy. J. Microsc. 252: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S., Mustilli A.-C., Genty B., North H., Lefebvre V., Sotta B., Vavasseur A., Giraudat J. (2002). Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 30: 601–609. [DOI] [PubMed] [Google Scholar]
- Minamide L.S., Painter W.B., Schevzov G., Gunning P., Bamburg J.R. (1997). Differential regulation of actin depolymerizing factor and cofilin in response to alterations in the actin monomer pool. J. Biol. Chem. 272: 8303–8309. [DOI] [PubMed] [Google Scholar]
- Mizuno K. (2013). Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell. Signal. 25: 457–469. [DOI] [PubMed] [Google Scholar]
- Moriyama K., Yahara I. (2002). The actin-severing activity of cofilin is exerted by the interplay of three distinct sites on cofilin and essential for cell viability. Biochem. J. 365: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K., Iida K., Yahara I. (1996). Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells 1: 73–86. [DOI] [PubMed] [Google Scholar]
- Mun J.H., Yu H.J., Lee H.S., Kwon Y.M., Lee J.S., Lee I., Kim S.G. (2000). Two closely related cDNAs encoding actin-depolymerizing factors of petunia are mainly expressed in vegetative tissues. Gene 257: 167–176. [DOI] [PubMed] [Google Scholar]
- Nagaoka R., Abe H., Obinata T. (1996). Site-directed mutagenesis of the phosphorylation site of cofilin: its role in cofilin-actin interaction and cytoplasmic localization. Cell Motil. Cytoskeleton 35: 200–209. [DOI] [PubMed] [Google Scholar]
- Okada K., Blanchoin L., Abe H., Chen H., Pollard T.D., Bamburg J.R. (2002). Xenopus actin-interacting protein 1 (XAip1) enhances cofilin fragmentation of filaments by capping filament ends. J. Biol. Chem. 277: 43011–43016. [DOI] [PubMed] [Google Scholar]
- Ono S. (2003). Regulation of actin filament dynamics by actin depolymerizing factor/cofilin and actin-interacting protein 1: new blades for twisted filaments. Biochemistry 42: 13363–13370. [DOI] [PubMed] [Google Scholar]
- Ouellet F., Carpentier E., Cope M.J., Monroy A.F., Sarhan F. (2001). Regulation of a wheat actin-depolymerizing factor during cold acclimation. Plant Physiol. 125: 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z.M., Murata Y., Benning G., Thomine S., Klüsener B., Allen G.J., Grill E., Schroeder J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734. [DOI] [PubMed] [Google Scholar]
- Peng Y., Grassart A., Lu R., Wong C.C.L., Yates J. III, Barnes G., Drubin D.G. (2015). Casein kinase 1 promotes initiation of clathrin-mediated endocytosis. Dev. Cell 32: 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin T., Liu X., Li J., Sun J., Song L., Mao T. (2014). Arabidopsis microtubule-destabilizing protein 25 functions in pollen tube growth by severing actin filaments. Plant Cell 26: 325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X., Zhang H., Xie Y., Wang J., Chen N., Huang S. (2013). Arabidopsis villins promote actin turnover at pollen tube tips and facilitate the construction of actin collars. Plant Cell 25: 1803–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan R., Lin H., Mendoza I., Zhang Y., Cao W., Yang Y., Shang M., Chen S., Pardo J.M., Guo Y. (2007). SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 19: 1415–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressad F., Didry D., Xia G.X., Hong Y., Chua N.H., Pantaloni D., Carlier M.F. (1998). Kinetic analysis of the interaction of actin-depolymerizing factor (ADF)/cofilin with G- and F-actins. Comparison of plant and human ADFs and effect of phosphorylation. J. Biol. Chem. 273: 20894–20902. [DOI] [PubMed] [Google Scholar]
- Roelfsema M.R., Hedrich R. (2005). In the light of stomatal opening: new insights into 'the Watergate'. New Phytol. 167: 665–691. [DOI] [PubMed] [Google Scholar]
- Ruzicka D.R., Kandasamy M.K., McKinney E.C., Burgos-Rivera B., Meagher R.B. (2007). The ancient subclasses of Arabidopsis Actin Depolymerizing Factor genes exhibit novel and differential expression. Plant J. 52: 460–472. [DOI] [PubMed] [Google Scholar]
- Samstag Y., Eckerskorn C., Wesselborg S., Henning S., Wallich R., Meuer S.C. (1994). Costimulatory signals for human T-cell activation induce nuclear translocation of pp19/cofilin. Proc. Natl. Acad. Sci. USA 91: 4494–4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J.I., Kwak J.M., Allen G.J. (2001). Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410: 327–330. [DOI] [PubMed] [Google Scholar]
- Shi M., Xie Y., Zheng Y., Wang J., Su Y., Yang Q., Huang S. (2013). Oryza sativa actin-interacting protein 1 is required for rice growth by promoting actin turnover. Plant J. 73: 747–760. [DOI] [PubMed] [Google Scholar]
- Shibayama T., Shinkawa K., Nakajo S., Nakaya K., Nakamura Y. (1986). Phosphorylation of muscle and non-muscle actins by casein kinase 1 in vitro. Biochem. Int. 13: 367–373. [PubMed] [Google Scholar]
- Smertenko A.P., Deeks M.J., Hussey P.J. (2010). Strategies of actin reorganisation in plant cells. J. Cell Sci. 123: 3019–3028. [DOI] [PubMed] [Google Scholar]
- Smertenko A.P., Jiang C.J., Simmons N.J., Weeds A.G., Davies D.R., Hussey P.J. (1998). Ser6 in the maize actin-depolymerizing factor, ZmADF3, is phosphorylated by a calcium-stimulated protein kinase and is essential for the control of functional activity. Plant J. 14: 187–193. [DOI] [PubMed] [Google Scholar]
- Staiger C.J., Blanchoin L. (2006). Actin dynamics: old friends with new stories. Curr. Opin. Plant Biol. 9: 554–562. [DOI] [PubMed] [Google Scholar]
- Su H., Zhu J., Cai C., Pei W., Wang J., Dong H., Ren H. (2012). FIMBRIN1 is involved in lily pollen tube growth by stabilizing the actin fringe. Plant Cell 24: 4539–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Tholl S., Moes D., Dieterle M., Papuga J., Moreau F., Steinmetz A. (2009). Actin bundling in plants. Cell Motil. Cytoskeleton 66: 940–957. [DOI] [PubMed] [Google Scholar]
- Tian M., Chaudhry F., Ruzicka D.R., Meagher R.B., Staiger C.J., Day B. (2009). Arabidopsis actin-depolymerizing factor AtADF4 mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB. Plant Physiol. 150: 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima J., Toshima J.Y., Takeuchi K., Mori R., Mizuno K. (2001). Cofilin phosphorylation and actin reorganization activities of testicular protein kinase 2 and its predominant expression in testicular Sertoli cells. J. Biol. Chem. 276: 31449–31458. [DOI] [PubMed] [Google Scholar]
- Vavasseur A., Raghavendra A.S. (2005). Guard cell metabolism and CO2 sensing. New Phytol. 165: 665–682. [DOI] [PubMed] [Google Scholar]
- Wang P., Song C.P. (2008). Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol. 178: 703–718. [DOI] [PubMed] [Google Scholar]
- Wang C., Zheng Y., Zhao Y., Zhao Y., Li J., Guo Y. (2015). SCAB3 is required for reorganization of actin filaments during light quality changes. J. Genet. Genomics 42: 161–168. [DOI] [PubMed] [Google Scholar]
- Wasteneys G.O., Galway M.E. (2003). Remodeling the cytoskeleton for growth and form: an overview with some new views. Annu. Rev. Plant Biol. 54: 691–722. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zinchuk V., Grossenbacher-Zinchuk O., Stefani E. (2012). Critical evaluation of quantitative colocalization analysis in confocal fluorescence microscopy. Interdiscip. Sci. 4: 27–37. [DOI] [PubMed] [Google Scholar]
- Xie X., Wang Y., Williamson L., Holroyd G.H., Tagliavia C., Murchie E., Theobald J., Knight M.R., Davies W.J., Leyser H.M.O., Hetherington A.M. (2006). The identification of genes involved in the stomatal response to reduced atmospheric relative humidity. Curr. Biol. 16: 882–887. [DOI] [PubMed] [Google Scholar]
- Yonezawa N., Nishida E., Iida K., Yahara I., Sakai H. (1990). Inhibition of the interactions of cofilin, destrin, and deoxyribonuclease I with actin by phosphoinositides. J. Biol. Chem. 265: 8382–8386. [PubMed] [Google Scholar]
- Zhao S., Zhao Y., Guo Y. (2015). 14-3-3 λ protein interacts with ADF1 to regulate actin cytoskeleton dynamics in Arabidopsis. Sci. China Life Sci. 58: 1142–1150. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Yan A., Feijó J.A., Furutani M., Takenawa T., Hwang I., Fu Y., Yang Z. (2010). Phosphoinositides regulate clathrin-dependent endocytosis at the tip of pollen tubes in Arabidopsis and tobacco. Plant Cell 22: 4031–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Pan Z., Zhang Y., Qu X., Zhang Y., Yang Y., Jiang X., Huang S., Yuan M., Schumaker K.S., Guo Y. (2013). The actin-related Protein2/3 complex regulates mitochondrial-associated calcium signaling during salt stress in Arabidopsis. Plant Cell 25: 4544–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., et al. (2011). The plant-specific actin binding protein SCAB1 stabilizes actin filaments and regulates stomatal movement in Arabidopsis. Plant Cell 23: 2314–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Xie Y., Jiang Y., Qu X., Huang S. (2013). Arabidopsis actin-depolymerizing factor7 severs actin filaments and regulates actin cable turnover to promote normal pollen tube growth. Plant Cell 25: 3405–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Zhang Y., Kang E., Xu Q., Wang M., Rui Y., Liu B., Yuan M., Fu Y. (2013). MAP18 regulates the direction of pollen tube growth in Arabidopsis by modulating F-actin organization. Plant Cell 25: 851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.