Abstract
We investigated the phenomena of antimicrobial peptides (AMPs) directly attacking the cytoplasmic membranes of Escherichia coli spheroplasts. We developed a procedure for fluorescence recovery after photobleaching to examine dye leakage through bacterial membranes as AMPs in solution bound to the membranes. We found that the AMP binding did not increase the apparent membrane area of a spheroplast, contrary to the response of a lipid-bilayer vesicle, which always showed a membrane area expansion by AMP binding. The permeability through the bacterial membrane increased in a sigmoidal fashion as the AMP binding increased in time, exhibiting a cooperative behavior of AMPs. The analysis of fluorescence recovery after photobleaching showed that the fluxes of dye molecules into and out of the cell were consistent with diffusion of molecules through a number of pores that increased with binding of AMPs and then saturated to a steady level. We discovered a new, to our knowledge, experimental parameter called the flux rate that characterizes the AMP-induced permeability of dye molecules through bacterial membranes. The phenomena observed in bacterial membranes are consistent with the pore-forming activities of AMPs previously observed in lipid bilayers. The experimental value of the flux rate per pore is much smaller than a theoretical value that assumes no friction for the dye molecule’s permeation through the pore. We believe that experimental studies of the flux rate will be useful for further analysis of AMPs’ permeabilization mechanisms.
Introduction
Host-defense antimicrobial peptides (AMPs) were first discovered in the early 1980s and subsequently found in almost every organism (1, 2, 3, 4, 5). Initially, the discoverers and pioneers of the field had to wrestle with questions about the molecular targets of these antibiotics (1, 2, 3, 4, 5). By the late 1980s, accumulated evidence, especially an experimental result showing that the natural all-L peptides and their D enantiomers were equally active (4), convincingly showed that the main targets of AMPs are bacterial cytoplasmic membranes, rather than stereospecific molecular receptors (5). Since then, investigators have sought to answer the major question regarding AMPs: what are their molecular mechanisms in bacterial cytoplasmic membranes? Some studies attempted to address this issue using live bacteria (3, 6, 7, 8, 9). However, bacterial studies are limited by the methods that can be used, complicated by both the presence of outer membranes (6) and the fact that the action of antimicrobials can induce secondary effects, including activation of autodigestive enzymes resulting in cell membrane lysis (10, 11).
Perhaps for this reason, studies of AMPs have mostly been performed with model membranes or lipid bilayers. Thus, there are persistent questions as to whether the action of AMPs on artificial membranes can be reproduced with bacterial membranes, and whether one can extend the results of model membranes to bacterial membranes (12). The mechanism of AMPs is the basis for understanding their efficacy, toxicity, and bacterial resistance. A well-understood membrane permeabilization mechanism is also potentially useful for drug and gene delivery.
In this study, we investigated the action of AMPs on Escherichia coli spheroplasts from which the outer membranes had been removed. The removal of the outer membrane made it possible to observe the action of AMPs on the cytoplasmic membranes. In preparation for this study, we previously investigated the physical properties of spheroplast membranes by using the micropipette aspiration method (13). We found that the properties of bacterial cell membranes are dominated by the existence of a membrane reservoir (e.g., membrane folds), and thus are significantly different from those of a lipid-bilayer vesicle made of bacterial lipid extract (13). Furthermore, we found that these characteristic properties of bacterial membranes are metabolically maintained. Therefore, we were very curious to know how the actions of AMPs on spheroplast membranes would compare with the actions on lipid bilayers.
We studied the actions of human AMP LL-37 (14) and bee venom toxin melittin (15) on E. coli spheroplasts by using the micropipette aspiration method. It is very important to note that we studied the effect of AMPs in the micromolar range of concentration. This is the range of concentration in which the antibacterial activities of AMPs, including the minimal inhibitory concentrations, were previously measured (5, 16). The interaction of AMPs with lipid bilayers is strongly concentration dependent, and the phenomena of AMPs may look different at much lower or much higher concentrations (17). We developed a procedure for fluorescence recovery after photobleaching (FRAP) to examine dye leakage through bacterial membranes as AMPs in solution bound to the membranes. We found a new, to our knowledge, experimental parameter called the flux rate that characterizes the AMP-induced permeability of dye molecules through a membrane. We believe that this parameter is potentially useful for in-depth studies of AMP mechanisms.
Materials and Methods
Bacterial strains and culture
The E. coli K-12 strain MG1655 (ATCC 700926) was purchased from ATCC (Manassas, VA). Luria-Bertani medium (5 g/L yeast extract, 10 g/L peptone from casein, and 10 g/L sodium chloride; EMD Millipore, Billerica, MA) containing 15 g/L agar (EMD) was used for the growth of colonies of E. coli. To ensure sterility, the medium was autoclaved before use.
Chemicals and media
Sucrose, glucose, Tris, hydrochloric acid, lysozyme, DNase, EDTA, magnesium chloride, sodium hydroxide, cephalexin, and carboxyfluorescein were purchased from Sigma Aldrich (St. Louis, MO). Texas Red sulfonyl chloride (TRsc, molecular weight 625) and calcein (molecular weight 623) were purchased from Invitrogen (Grand Island, NY).
Preparation of E. coli spheroplasts
We prepared giant spheroplasts of E. coli (13) by following the detailed procedure described by Renner and Weibel (18). Briefly, cells were grown in Luria-Bertani medium. To grow long filamentous cells, cephalexin (60 μg/mL) was added. After the cells reached an average length of ∼50 μm, they were harvested. Spheroplasts from filamentous cells were formed by adding reagents in the following order: 30 μL 1 M Tris⋅HCl (pH 8.0), 24 μL 0.5 mg/mL lysozyme, 6 μL 5 mg/mL DNase, and 6 μL 125 mM EDTA-NaOH (pH 8.0). After 5–20 min at room temperature, 100 μL of STOP solution (10 mM Tris⋅HCl at pH 8, 0.7 M sucrose, 20 mM MgCl2) was added to stabilize the spheroplasts. The spheroplasts were stored in liquid nitrogen for no longer than 2 weeks. Frozen spheroplasts were thawed slowly on ice before each use. Then, 30 μL of spheroplasts was injected into an observation chamber containing STOP solution at 0.338 osmole/kg osmolality.
Method of micropipette aspiration
The method used for micropipette aspiration was a modification of the original method of Kwok and Evans (19) as described in Sun et al. (20, 21). A micropipette was connected to a water-filled U tube manometer and a negative pressure in the pipette was produced by adjusting the height of the water level with reference to the atmosphere pressure (20). Although the same technique is used to measure spheroplasts and giant unilamellar vesicles (GUVs), the small size of the spheroplasts imposes some restrictions on spheroplast measurements. The technical details of the micropipette aspiration experiment on E. coli spheroplasts were described in (13).
Results
Spheroplasts attacked by LL37 and melittin
In our previous study (13) we established a procedure for producing and maintaining E. coli spheroplasts in a range of osmolalities. In general, the average size of the cells increased with decreasing osmolality, but in all cases the spheroplast membranes were without a surface tension, unless the spheroplast was extremely swollen (13). Nonetheless, if a spheroplast was subjected to an aspiration pressure, a surface tension would develop reversibly with the applied suction pressure (13). For the experiment described here, we let spheroplasts equilibrate in 0.338 osmole/kg (Osm), from which we transferred a small number of cells, ∼5 μm in diameter and tensionless, to an observation chamber that contained calcein at 60 μM. A micropipette equipped with an adjustable sucking pressure via a water column was used to hold a spheroplast at a pressure that produced a very small membrane tension, <0.25 mN/m (13). The purpose of the micropipette aspiration was to hold the cell in position for confocal imaging and for monitoring possible membrane area changes (13).
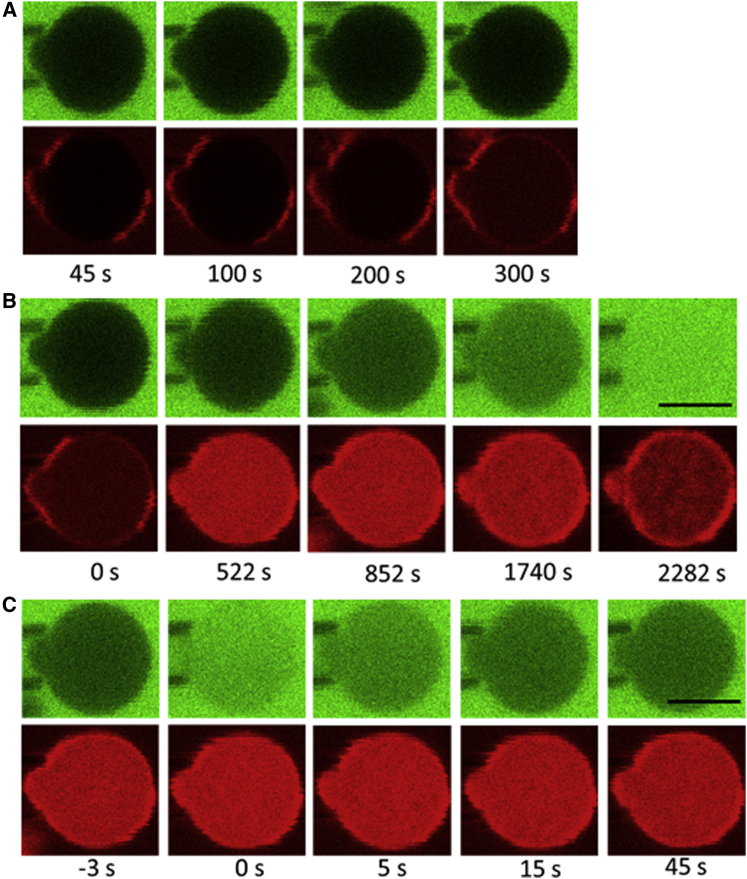
At time zero, an open pipe (120 μm in diameter) containing LL37 at 6 μM was introduced into the observation chamber to let LL37 diffuse to the spheroplast (the distance from the opening of the pipe to spheroplast was ∼30–40 μm). We monitored the protrusion length of the spheroplast into the aspiration micropipette, which would report on the membrane area change of the spheroplast (13), and the fluorescence intensity of calcein in a region including both inside and outside of the spheroplast. A typical run of such an experiment produced the following result (Fig. 1): before the introduction of LL37, there was no leakage of calcein into the spheroplast. After the introduction of LL37, the fluorescence intensity of calcein inside the spheroplast increased in a sigmoidal fashion to a steady value (i.e., a plateau). Then the fluorescence intensity might suddenly jump to the same intensity as observed outside. When that happened, the phase contrast of the cell interior was also lost. The spheroplasts typically lasted ∼40 min or longer without losing their phase contrast while being subjected to the attack of LL37. As such, we generally limited our experiment to ∼40 min. In all cases, no membrane area changes were detected. Fig. 1 shows the confocal images of the spheroplast during one run of such experiments. The protein-labeling dye TRsc (22) at 40 μM in solution was used to mark the boundary of the spheroplast for the purpose of measuring possible membrane area changes. Note that TRsc spontaneously permeated through the membrane before the introduction of LL37, when no leakage of calcein was detected.
Figure 1.
Confocal images of a micropipette-aspirated spheroplast during the experiment, in both green (calcein) and red (TRsc) channels. Scale bar, 5 μm. (A) The spheroplast was in a solution containing calcein at 60 μM for detecting leakage into the cell and TRsc at 40 μM for marking the boundary of the spheroplast so as to measure any possible membrane area changes. TRsc is a protein-labeling dye that can spontaneously permeate through the membrane. No leakage of calcein was detected in the absence of AMPs. (B) At time zero, an open pipe containing LL37 at 6 μM was introduced close to the spheroplast. Thereafter, the green fluorescence intensity inside the cell sigmoidally increased to a steady state. At 2282 s, the inside green fluorescence abruptly increased to the same intensity as observed outside. (C) One of many FRAP experiments performed during (B). After a dark period from −3 to 0 s, the intracellular green fluorescence intensity decayed from a high value back to a level close to the intensity right before the dark period (at−3 s). The confocal scanning time for each image was 0.5 s.
Melittin produced effects similar to those observed for LL37. Qualitatively, there was no difference between the results obtained with melittin and LL37.
FRAP experiment on spheroplasts
The light that excited the fluorescent dye calcein also photobleached a fraction of the dye molecules per unit time. A steady state of fluorescence intensity within the spheroplast was possible only if unbleached dye molecules diffused into the cell to replace the bleached dye molecules. If the incident light for fluorescence was blocked for a time, the fluorescence intensity outside the spheroplast did not change when the fluorescence measurement resumed. This was because the dye molecules outside the cell mixed rapidly with unbleached dye molecules beyond the lighted region. As a result, the concentration of bleached dye molecules outside the spheroplast was constantly diluted to a negligible level, so the fluorescence intensity outside the cell was constant in time. The fluorescence intensity inside the cell was different. The exchange of dye molecules between inside and outside of the cell was slower than free diffusion. There was always a significant concentration of bleached dye molecules inside the cell, which is why the fluorescent intensity during the steady state was lower than that of the outside solution. After a dark period, the fluorescence intensity within the spheroplast increased because unbleached dye molecules had entered the spheroplast from outside to replace the bleached dye molecules inside during the dark period. Phenomenologically, we can describe the time (t) dependence of the unbleached calcein concentration inside a spheroplast as follows:
During the fluorescence measurement, the concentration is reduced at the rate of due to photobleaching. Imagine that the spheroplast membrane has pores and the molecules diffuse into and out of the cell through these pores. The influx (in molecules per second) through a circular pore of radius a is given by the Berg-Purcell formula (23), where D is the diffusion constant of the dye molecule and is the unbleached dye concentration outside the cell. Thus, if the total number of pores is N, the total dye influx is . If V is the volume within the spheroplast that is accessible to the dye molecules, the influx will increase the dye concentration per unit time by . Similarly, the outflux of unbleached dye from within the cell is . The kinetics of the unbleached dye concentration inside the spheroplast is then given by
| (1) |
Although there is a theoretical formula for , it should be regarded as an experimental parameter. Thus, this equation contains two unknown quantities: the photobleaching time constant and the flux rate . The value of depends on the instrumentation. The flux rate is characteristic of the permeability induced by the AMP. Both quantities are measurable by the FRAP experiment.
During a steady state, , . The flux rate is a constant that satisfies the relation , with . The photobleaching is balanced by the net influx of unbleached dye molecules. When we blocked the incident light for a short time, the absence of photobleaching increased to a value greater than . When we turned on the light again for measurements, would decay from back to following the relation . Thus, we could use the measured decay constant (Supporting Materials and Methods in the Supporting Material) and the ratio to calculate the flux rate .
Because the intracellular fluorescence intensity F is from the dye molecules in a cell, its ratio to the outside fluorescence intensity is not equal to , which would be the case if the cell were empty like a lipid vesicle. The preexisting biological structures within the spheroplast make the accessible volume V smaller than the apparent cell volume Vcell. To estimate the ratio , we measured the maximum intracellular fluorescence intensity after a long dark period, and found it to be 0.67–0.80 of the external intensity (this ratio is ). Thus, the intracellular/extracellular concentration ratio should be the corresponding fluorescence intensity ratio divided by : (Supporting Materials and Methods). Thus, we obtain the value of , which is independent of . From the experimental value of , we used the formula to calculate the number N of the hypothetical Berg-Purcell pores (see Discussion below). We used a = 25Å for the size of LL37 pores measured by neutron in-plane scattering (24) and for the diffusion constant of calcein in cells measured by the fluorescence correlation spectroscopy (25).
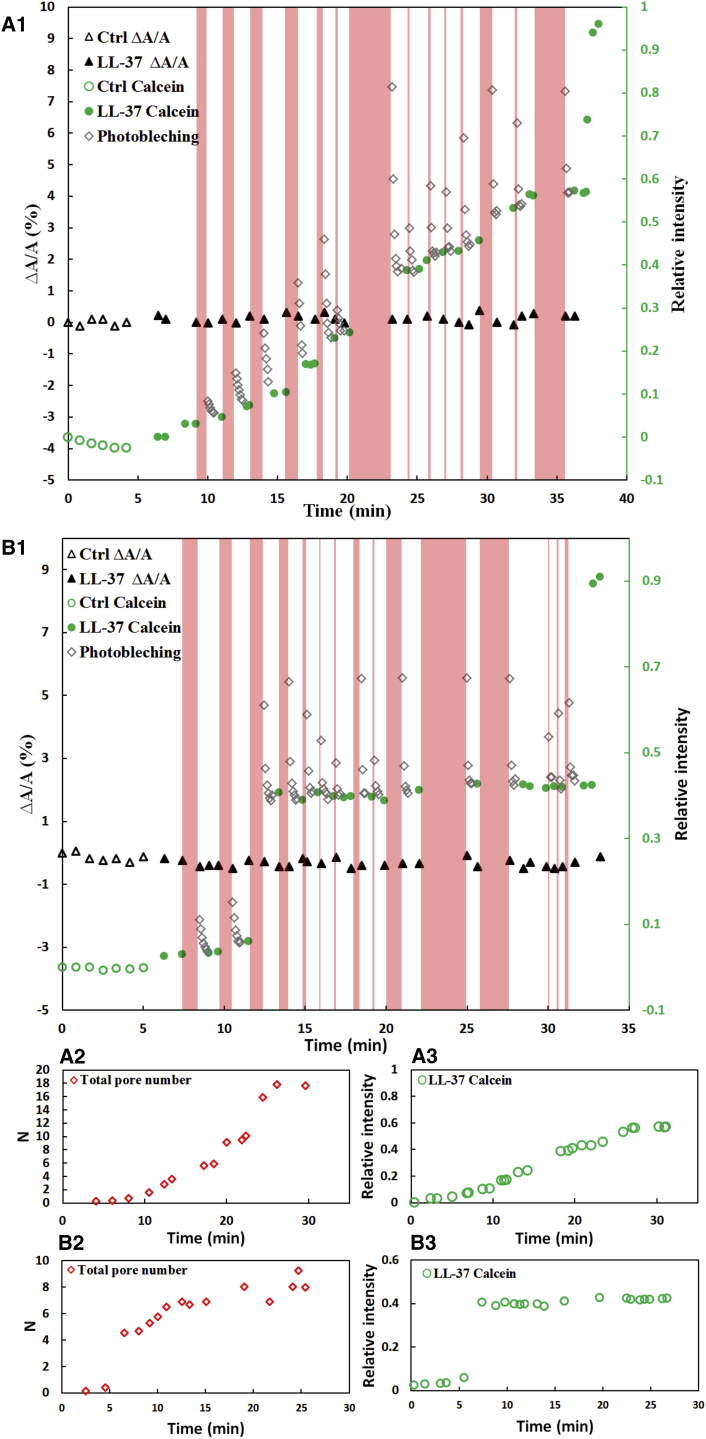
Strictly speaking, the method described above is applicable only after has reached a steady state. However, since the decay rate of FRAP is faster than the changing rate of the fluorescence intensity , except for rare sharp transitions (such as in Fig. 2, B1), we also applied the same method to the presteady-state region to obtain the transient values of throughout the spheroplast experiment. Fig. 2 shows the results of the LL37 experiments. We performed more than 10 runs of LL37 experiments. All were consistent with the qualitative features described above, but there were quantitative variations, as shown by the two examples in Fig. 2. The sigmoidal transition from an initial low level of intracellular fluorescence intensity to a final higher level of steady state is characteristic of all of the experiments, but the slope of the sigmoidal transition and the steady-state level varied from cell to cell (within ∼). The duration of a spheroplast attacked by LL37 before it lost its interior phase contrast also varied (Fig. 1). Such cell-to-cell variations have also been seen in investigations of whole cells (6, 9).
Figure 2.
(A and B) Two complete experiments with LL37 are shown as examples. (A1 and B1) The dark triangles are the fractional membrane area changes ΔA/A, which are close to zero throughout. Green symbols indicate the green fluorescence intensity inside the cell relative to outside the cell (open green circles, before the introduction of LL37 (control (Ctrl) period); solid green dots, after the introduction of LL37 at time zero), and open diamond symbols are the FRAP data. The pink stripes are the dark periods for the FRAP experiments. (A1) The spheroplast abruptly lost its phase contrast at ∼37 min when the inside fluorescence intensity jumped to the same level as observed outside. (A2) A high concentration LL37 was introduced at ∼33 min to cause the spheroplast to lose its phase contrast. (A2 and B2) Number N of hypothetical Berg-Purcell pores, calculated as explained in the text. (A3 and B3) The same green data as in (A1) and (B1) plotted to the same timescale as in (A2) and (B2) for comparison, showing how N varied as the fluorescence intensity varied.
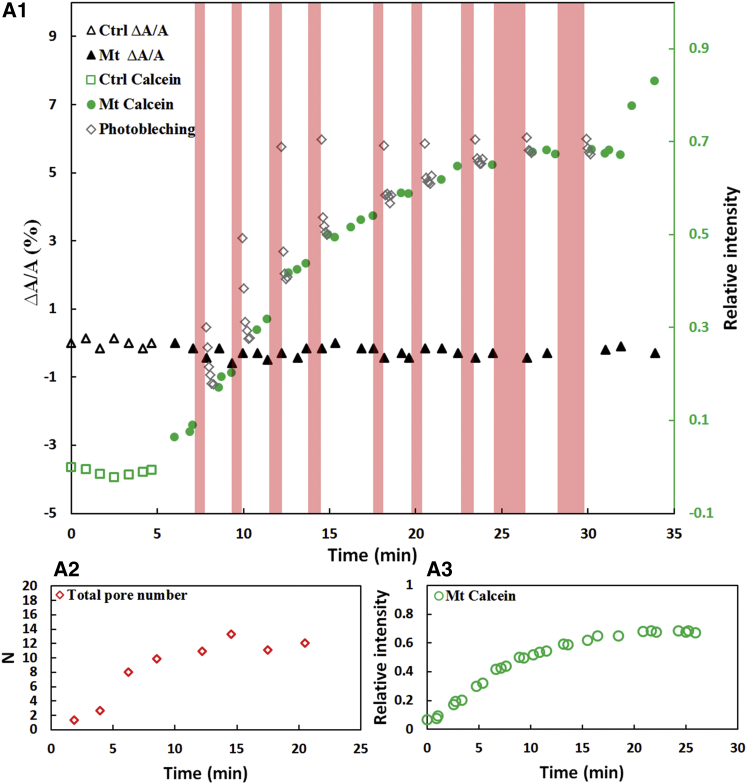
The same setup was used to study the effect of melittin (Fig. 3). The only difference was that the concentration of melittin delivered by the open pipe was 2 μΜ instead of the 6 μM used for LL37. However, the rate of calcein leakage caused by melittin was higher than that caused by LL37, since the steady-state intracellular fluorescence intensity due to melittin was 1.5–2 times that due to LL37. Superficially, this implies that melittin is the more active antimicrobial of the two. The higher activity could mean a lower threshold of the peptide/lipid ratio for pore formation (17) or a higher membrane binding affinity, or both. Otherwise the effects of both peptides are similar, indicating that they operate by the same mechanism.
Figure 3.
One experiment with melittin is shown as an example. (A1) The dark triangles are the fractional membrane area changes ΔA/A, which are close to zero throughout. Green symbols indicate the green fluorescence intensity inside the cell relative to outside the cell (open green circles, before the introduction of melittin (control (Ctrl) period); solid green dots, after the introduction of melittin at time zero), and open diamond symbols are the FRAP data. The pink stripes indicate the dark periods for the FRAP experiments. At ∼33 min, the spheroplast lost its phase contrast and the fluorescence intensity began to rise to the level observed outside. At ∼34 min, the spheroplast ruptured. (A2) Number N of hypothetical Berg-Purcell pores, calculated as explained in the text. (A3) The same green data as in (A1) plotted to the same timescale as in (A2) for comparison, showing how N varied as the fluorescence intensity varied.
Discussion
The attack on whole E. coli cells by dye-labeled LL37 was studied by Sochacki et al. (6) using fluorescence microscopy. They observed the interruption of cell growth and the spatial distribution of the peptide while it entered the cells in real time, and found that both effects differed between septating and nonseptating cells. The attack was described as consisting of three phases. In phase 1, LL37 bound to the outer membrane. In phase 2, it spread across the periplasm to the cytoplasmic membrane. In septating cells, LL37 appeared to first concentrate at the septum region. Phase 3 involved penetration of the cytoplasmic membrane by LL37 and subsequent permeabilization to the DNA stain SYTOX Green. In our study, the spheroplasts were spherical in shape, without septa, and exhibited no cell growth in a STOP solution. Since we did not use dye-labeled AMPs, the spatial distribution of the AMPs around the spheroplast was not visible. Instead, we measured the time-dependent intracellular and extracellular distributions of the dye molecule calcein and performed FRAP measurements. All spheroplast membranes appeared to be uniform around the cell in our experiments.
The simplest explanation for what we observed is that LL37 and melittin induced pores in the cytoplasmic membranes, and therefore we will discuss our data based on that assumption. In its original form, the Berg-Purcell formula was derived for chemoreception on the surface of a cell (23). If there is a circular receptor-binding site of radius to which certain molecules adsorb, and assume that the site is a perfect sink for the molecules, then the diffusive flux of molecules to the site is (23). If we replace the binding site by a transmembrane pore and disregard the possible friction between the pore wall and the molecular movement, then the diffusive flux through such a hypothetical Berg-Purcell pore would be per pore. In other words, this would represent the diffusion flux to the mouth of the pore, which is assumed to be a perfect sink. In reality, membrane pores are as long as the membrane thickness, are not rigid, and probably are dynamical. Also, the dye molecules have a physical size. Therefore, there must be friction between the pore wall and the molecular movement. The flux through a pore must be slower than the free-diffusion flux. The fact that, if we use the theoretical formula , the measured value of gave a low value (∼0.1) for N (the number of pores) soon after the leakage occurred indicates that the flux through a pore is at least one order of magnitude smaller than the hypothetical Berg-Purcell flux. This reduction from the free-diffusion flux rate to the experimental flux rate represents a slowdown due to the friction of the dye molecule’s permeation through the pore.
Since we do not know the rate of flux through a real pore, we can only deduce a hypothetical number N that is proportional to the real number of pores in the spheroplast membrane induced by LL37 or melittin. The FRAP experiment proved that the dye concentration inside the cell was indeed maintained by diffusive fluxes through the cell membrane. The finding that N was proportional to the instantaneous intracellular fluorescence intensity under steady illumination (Figs. 2, A2–B3, and 3, A2 and A3) shows the self-consistency of the pore model described by Eq. 1.
LL37 and melittin are both α-helical but of different lengths (37 and 26 amino acids long, respectively). Although melittin is a bee venom toxin, it has been studied as a model AMP because its general design is similar to that of AMPs and it is active against bacteria (1, 26). In studies with model membranes, melittin was shown to form toroidal pores in lipid bilayers (17, 27, 28, 29). The human antimicrobial peptide LL37 has been somewhat controversial. In multilamellar samples, many investigators found LL37 in a state with its helical axis lying parallel to the plane of the bilayer (30, 31). Thus, for a long time it was widely believed that LL-37 could not form pores, and therefore it was assumed to permeabilize or disintegrate membranes by a nonpore carpet mechanism (30, 32, 33). However, a later study found that if the multilamellar samples were overhydrated and if the peptide/lipid ratio exceeded a threshold, LL37 would turn its helical orientation to perpendicular to the bilayers, and it indeed formed pores in lipid bilayers as shown by neutron in-plane scattering (24).
There are, however, interesting differences between the membranes of spheroplasts and lipid-bilayer vesicles. In a previous study, we compared E. coli spheroplasts with GUVs made of E. coli total lipid extract (13). When a GUV was exposed to pore-forming peptides, the initial binding of peptides always expanded the membrane area of the GUV, resulting in an increased protrusion length in the aspiration micropipette (17, 20, 24, 34, 35). A simple explanation for this is that the lipid bilayer had added molecules. The subsequent pore formation caused a net water influx if, for example, sucrose and glucose were used as the solutes inside and outside the GUV, respectively, resulting in a decreased protrusion length (17, 20, 24, 34, 35). If the sucrose and glucose were exchanged, the pore formation caused a net water outflux resulting in an increased protrusion length (20). In contrast, we detected no protrusion length increase or decrease in our spheroplast experiments. On the other hand, we found that spheroplast membranes increased or decreased in area (resulting in an increased or a decreased protrusion length) in response to an aspiration pressure exactly like a GUV, albeit with a much smaller elastic area-stretching modulus compared with that of a lipid bilayer (13). Another important difference is that whereas the membrane tension of a GUV increased with decreasing external osmolality, the membrane tension of a spheroplast remained zero with varying external osmolalities (13). One possible explanation for the small area-stretching moduli and the zero membrane tension is that the bacterial membranes possess membrane reservoirs (e.g., membrane folds) (13). We do not know whether the absence of an apparent membrane area change in spheroplasts during peptide binding is due to the existence of membrane reservoirs. In view of these significant differences between spheroplast membranes and lipid bilayers, it is not clear a priori whether the pore formation by AMPs previously observed in lipid bilayers would be reproduced in spheroplasts.
This study shows that the activities of LL37 and melittin on bacterial membranes are consistent with pore formation. However, it is certainly possible to interpret the data presented here in terms of other types of peptide-induced membrane perturbations. More significantly, we discovered a new, to our knowledge, experimental parameter called the flux rate, , that characterizes the AMP-induced permeability of the cytoplasmic membranes to dye molecules. This parameter has an explicit dependence on the cell volume and an implicit dependence on the AMP concentration and the size of the dye molecule. We believe that it potentially could be used for more detailed analyses of AMP mechanisms.
Author Contributions
Y.S. and H.W.H. designed the research. Y.S. and T.-L.S. performed the experiments. Y.S. and H.W.H. analyzed and interpreted the results. H.W.H. wrote the article.
Acknowledgments
This work was supported by the National Institutes of Health (grant GM55203) and the Robert A. Welch Foundation (grant C-0991).
Editor: Paulo Almeida.
Footnotes
Supporting Materials and Methods and three figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30362-9.
Supporting Material
References
- 1.Boman H.G., Hultmark D. Cell-free immunity in insects. Annu. Rev. Microbiol. 1987;41:103–126. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- 2.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steiner H., Andreu D., Merrifield R.B. Binding and action of cecropin and cecropin analogues: antibacterial peptides from insects. Biochim. Biophys. Acta. 1988;939:260–266. doi: 10.1016/0005-2736(88)90069-7. [DOI] [PubMed] [Google Scholar]
- 4.Wade D., Boman A., Merrifield R.B. All-D amino acid-containing channel-forming antibiotic peptides. Proc. Natl. Acad. Sci. USA. 1990;87:4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boman H.G., Marsh J., Goode J.A. John Wiley and Sons; Chichester: 1994. Antimicrobial Peptides. [Google Scholar]
- 6.Sochacki K.A., Barns K.J., Weisshaar J.C. Real-time attack on single Escherichia coli cells by the human antimicrobial peptide LL-37. Proc. Natl. Acad. Sci. USA. 2011;108:E77–E81. doi: 10.1073/pnas.1101130108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rangarajan N., Bakshi S., Weisshaar J.C. Localized permeabilization of E. coli membranes by the antimicrobial peptide Cecropin A. Biochemistry. 2013;52:6584–6594. doi: 10.1021/bi400785j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barns K.J., Weisshaar J.C. Real-time attack of LL-37 on single Bacillus subtilis cells. Biochim. Biophys. Acta. 2013;1828:1511–1520. doi: 10.1016/j.bbamem.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fantner G.E., Barbero R.J., Belcher A.M. Kinetics of antimicrobial peptide activity measured on individual bacterial cells using high-speed atomic force microscopy. Nat. Nanotechnol. 2010;5:280–285. doi: 10.1038/nnano.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bierbaum G., Sahl H.G. Autolytic system of Staphylococcus simulans 22: influence of cationic peptides on activity of N-acetylmuramoyl-L-alanine amidase. J. Bacteriol. 1987;169:5452–5458. doi: 10.1128/jb.169.12.5452-5458.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsbach P., Weiss J. Bactericidal/permeability increasing protein and host defense against gram-negative bacteria and endotoxin. Curr. Opin. Immunol. 1993;5:103–107. doi: 10.1016/0952-7915(93)90088-a. [DOI] [PubMed] [Google Scholar]
- 12.Last N.B., Miranker A.D. Common mechanism unites membrane poration by amyloid and antimicrobial peptides. Proc. Natl. Acad. Sci. USA. 2013;110:6382–6387. doi: 10.1073/pnas.1219059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y., Sun T.L., Huang H.W. Physical properties of Escherichia coli spheroplast membranes. Biophys. J. 2014;107:2082–2090. doi: 10.1016/j.bpj.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner J., Cho Y., Lehrer R.I. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habermann E. Bee and wasp venoms. Science. 1972;177:314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 16.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 17.Lee M.T., Sun T.L., Huang H.W. Process of inducing pores in membranes by melittin. Proc. Natl. Acad. Sci. USA. 2013;110:14243–14248. doi: 10.1073/pnas.1307010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renner L.D., Weibel D.B. Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc. Natl. Acad. Sci. USA. 2011;108:6264–6269. doi: 10.1073/pnas.1015757108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys. J. 1981;35:637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y., Hung W.C., Huang H.W. Interaction of tea catechin (-)-epigallocatechin gallate with lipid bilayers. Biophys. J. 2009;96:1026–1035. doi: 10.1016/j.bpj.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y., Lee C.C., Huang H.W. Adhesion and merging of lipid bilayers: a method for measuring the free energy of adhesion and hemifusion. Biophys. J. 2011;100:987–995. doi: 10.1016/j.bpj.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watts T.H., McConnell H.M. High-affinity fluorescent peptide binding to I-Ad in lipid membranes. Proc. Natl. Acad. Sci. USA. 1986;83:9660–9664. doi: 10.1073/pnas.83.24.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg H.C., Purcell E.M. Physics of chemoreception. Biophys. J. 1977;20:193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee C.C., Sun Y., Huang H.W. Transmembrane pores formed by human antimicrobial peptide LL-37. Biophys. J. 2011;100:1688–1696. doi: 10.1016/j.bpj.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida N., Tamura M., Kinjo M. Fluorescence correlation spectroscopy: a new tool for probing the microenvironment of the internal space of organelles. Single Molecules. 2000;1:279–283. [Google Scholar]
- 26.Bucki R., Pastore J.J., Janmey P.A. Antibacterial activities of rhodamine B-conjugated gelsolin-derived peptides compared to those of the antimicrobial peptides cathelicidin LL37, magainin II, and melittin. Antimicrob. Agents Chemother. 2004;48:1526–1533. doi: 10.1128/AAC.48.5.1526-1533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allende D., Simon S.A., McIntosh T.J. Melittin-induced bilayer leakage depends on lipid material properties: evidence for toroidal pores. Biophys. J. 2005;88:1828–1837. doi: 10.1529/biophysj.104.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuzaki K., Yoneyama S., Miyajima K. Pore formation and translocation of melittin. Biophys. J. 1997;73:831–838. doi: 10.1016/S0006-3495(97)78115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L., Harroun T.A., Huang H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 2001;81:1475–1485. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oren Z., Lerman J.C., Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 1999;341:501–513. [PMC free article] [PubMed] [Google Scholar]
- 31.Henzler Wildman K.A., Lee D.K., Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42:6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 32.Sood R., Domanov Y., Kinnunen P.K. Binding of LL-37 to model biomembranes: insight into target vs host cell recognition. Biochim. Biophys. Acta. 2008;1778:983–996. doi: 10.1016/j.bbamem.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Porcelli F., Verardi R., Veglia G. NMR structure of the cathelicidin-derived human antimicrobial peptide LL-37 in dodecylphosphocholine micelles. Biochemistry. 2008;47:5565–5572. doi: 10.1021/bi702036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longo M.L., Waring A.J., Hammer D.A. Interaction of the influenza hemagglutinin fusion peptide with lipid bilayers: area expansion and permeation. Biophys. J. 1997;73:1430–1439. doi: 10.1016/S0006-3495(97)78175-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longo M.L., Waring A.J., Hammer D.A. Area expansion and permeation of phospholipid membrane bilayer by influenza fusion peptides and melittin. Langmuir. 1998;14:2385–2395. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.