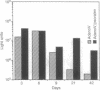
Abstract
We are developing efficient methods for gene transfer into tissue culture cells. We have previously shown that coupling of a chimeric adenovirus with polylysine allowed the construction of an adenovirus-polylysine-reporter-gene complex that transferred the transporter gene with great efficiency into HeLa cells. We have now explored simpler, biochemical means for coupling adenovirus to DNA/polylysine complexes and show that such complexes yield virtually 100% transfection in tissue culture cell lines. In these methods adenovirus is coupled to polylysine, either enzymatically through the action of transglutaminase or biochemically by biotinylating adenovirus and streptavidinylating the polylysine moiety. Combination complexes containing DNA, adenovirus-polylysine, and transferrin-polylysine have the capacity to transfer the reporter gene into adenovirus-receptor- and/or transferrin-receptor-rich cells.
Full text
PDF

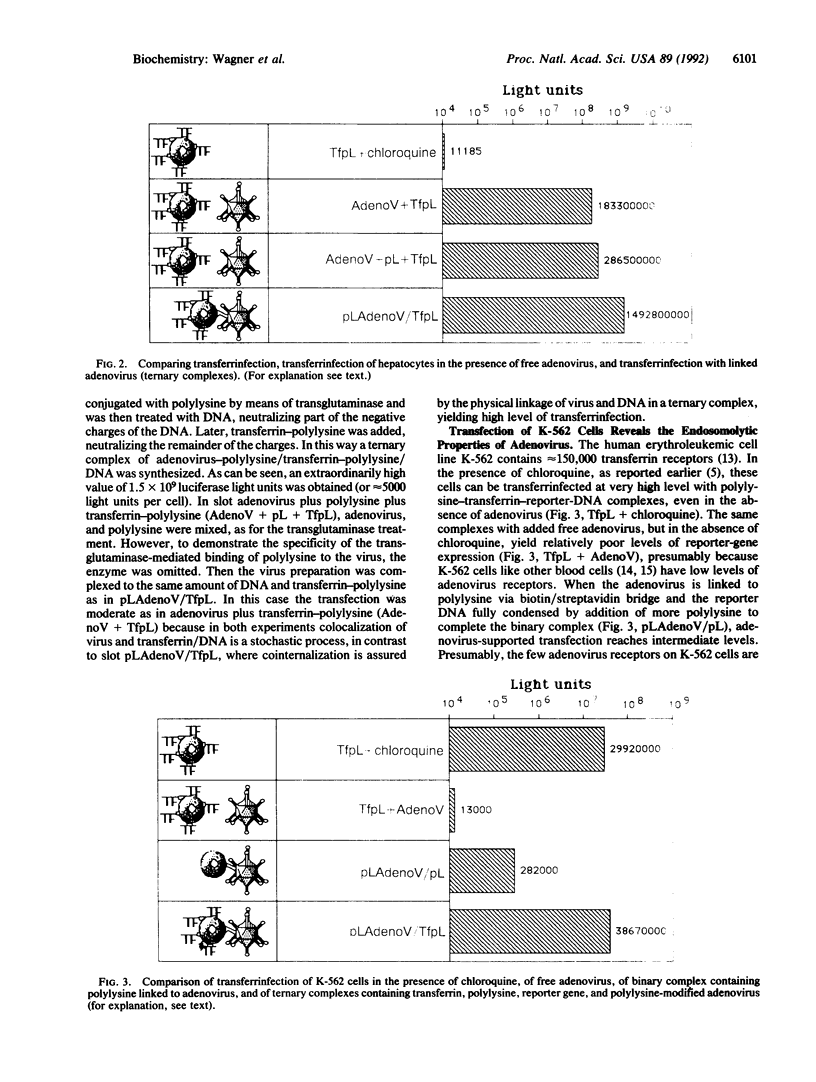
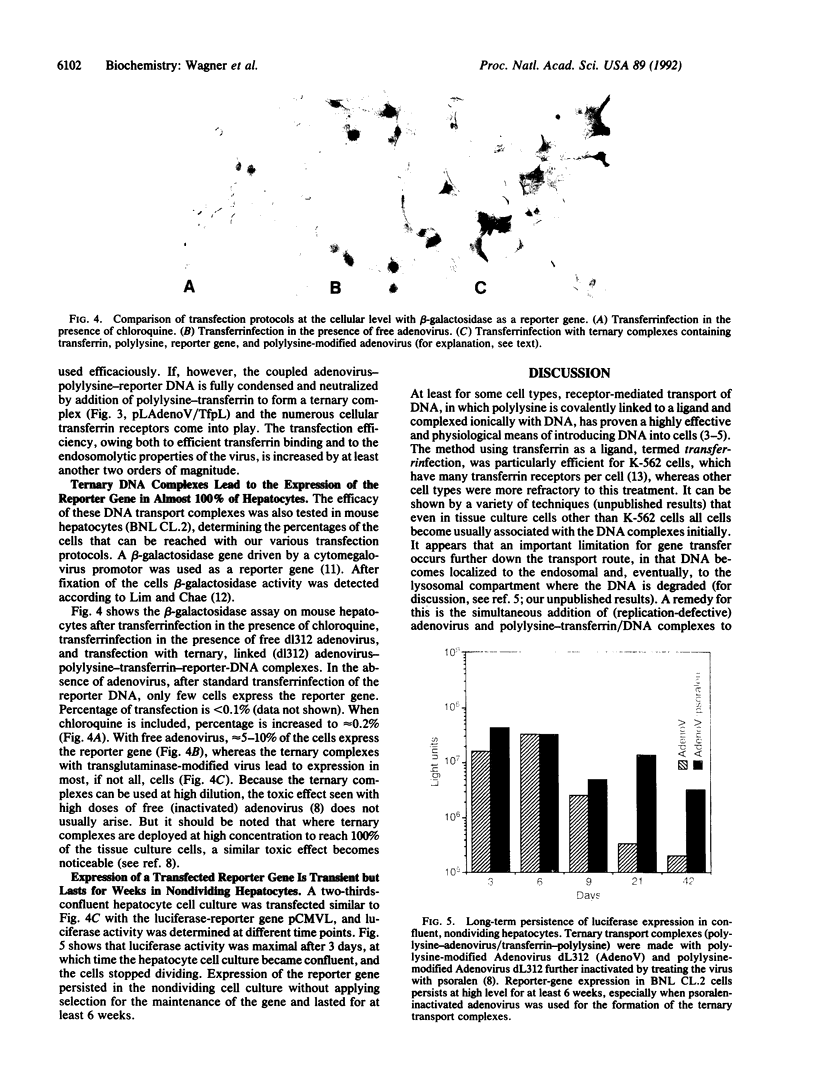

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cotten M., Längle-Rouault F., Kirlappos H., Wagner E., Mechtler K., Zenke M., Beug H., Birnstiel M. L. Transferrin-polycation-mediated introduction of DNA into human leukemic cells: stimulation by agents that affect the survival of transfected DNA or modulate transferrin receptor levels. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4033–4037. doi: 10.1073/pnas.87.11.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M., Wagner E., Zatloukal K., Phillips S., Curiel D. T., Birnstiel M. L. High-efficiency receptor-mediated delivery of small and large (48 kilobase gene constructs using the endosome-disruption activity of defective or chemically inactivated adenovirus particles. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6094–6098. doi: 10.1073/pnas.89.13.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel D. T., Agarwal S., Wagner E., Cotten M. Adenovirus enhancement of transferrin-polylysine-mediated gene delivery. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8850–8854. doi: 10.1073/pnas.88.19.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel D. T., Wagner E., Cotten M., Birnstiel M. L., Agarwal S., Li C. M., Loechel S., Hu P. C. High-efficiency gene transfer mediated by adenovirus coupled to DNA-polylysine complexes. Hum Gene Ther. 1992 Apr;3(2):147–154. doi: 10.1089/hum.1992.3.2-147. [DOI] [PubMed] [Google Scholar]
- Horvath J., Weber J. M. Nonpermissivity of human peripheral blood lymphocytes to adenovirus type 2 infection. J Virol. 1988 Jan;62(1):341–345. doi: 10.1128/jvi.62.1.341-345.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckett B., Ariatti M., Hawtrey A. O. Evidence for targeted gene transfer by receptor-mediated endocytosis. Stable expression following insulin-directed entry of NEO into HepG2 cells. Biochem Pharmacol. 1990 Jul 15;40(2):253–263. doi: 10.1016/0006-2952(90)90686-f. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K., Chae C. B. A simple assay for DNA transfection by incubation of the cells in culture dishes with substrates for beta-galactosidase. Biotechniques. 1989 Jun;7(6):576–579. [PubMed] [Google Scholar]
- MacGregor G. R., Caskey C. T. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989 Mar 25;17(6):2365–2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver L., Anderson C. W. Interaction of human adenovirus serotype 2 with human lymphoid cells. Virology. 1988 Aug;165(2):377–387. doi: 10.1016/0042-6822(88)90582-x. [DOI] [PubMed] [Google Scholar]
- Sipe D. M., Jesurum A., Murphy R. F. Absence of Na+,K(+)-ATPase regulation of endosomal acidification in K562 erythroleukemia cells. Analysis via inhibition of transferrin recycling by low temperatures. J Biol Chem. 1991 Feb 25;266(6):3469–3474. [PubMed] [Google Scholar]
- Wagner E., Cotten M., Mechtler K., Kirlappos H., Birnstiel M. L. DNA-binding transferrin conjugates as functional gene-delivery agents: synthesis by linkage of polylysine or ethidium homodimer to the transferrin carbohydrate moiety. Bioconjug Chem. 1991 Jul-Aug;2(4):226–231. doi: 10.1021/bc00010a006. [DOI] [PubMed] [Google Scholar]
- Wagner E., Zenke M., Cotten M., Beug H., Birnstiel M. L. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Y., Wu C. H. Receptor-mediated gene delivery and expression in vivo. J Biol Chem. 1988 Oct 15;263(29):14621–14624. [PubMed] [Google Scholar]
- Zenke M., Steinlein P., Wagner E., Cotten M., Beug H., Birnstiel M. L. Receptor-mediated endocytosis of transferrin-polycation conjugates: an efficient way to introduce DNA into hematopoietic cells. Proc Natl Acad Sci U S A. 1990 May;87(10):3655–3659. doi: 10.1073/pnas.87.10.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]