Abstract
Elucidating the age-dependent alterations in transgenic (Tg) mice overexpressing amyloid-β protein precursor (AβPP) is important for understanding the pathogenesis of Alzheimer’s disease (AD) and designing experimental therapies. Cross-studies have previously characterized some time-dependent behavioral and pathological alterations in AβPP Tg mice, however, a more comprehensive longitudinal study is needed to fully examine the progressive nature of behavioral deficits in these mice. In order to better understand the age- and gender-dependent progression of behavioral alterations, we performed a longitudinal study wherein Tg mice overexpressing human AβPP751 with the London (V717I) and Swedish (K670M/N671L) mutations under the regulatory control of the neuron specific murine (m)Thy-1 promoter (mThy1-hAβPP751) were behaviorally analyzed at 3 months and then re-tested at 6 and 9 months of age. The results show that there was an age-associated impairment in learning in the water maze task and habituation in the hole-board task. Motor coordination of the mThy1-hAβPP751 Tg mice was well-preserved throughout the investigated life span however, gender-specific deficits were observed in spontaneous activity and thigmotaxis. Neuropathologically, mThy1-hAβPP751 Tg mice displayed a progressive increase in the number of Aβ plaques and mean plaque size in the cortex and hippocampus from 3 to 6 and from 6 to 9 months of age. Taken together, these results indicate that the mThy1-hAβPP751 Tg mice model AD from the early onset of the disease through to later stages, allowing them to be utilized at numerous points during the timeline for drug test designs.
Keywords: Alzheimer’s disease, amyloid-β, amyloid-β protein precursor, hole board task, memory, motor coordination, rodent, transgenic, water maze
INTRODUCTION
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder of the aging population associated with the deposition of amyloid-β (Aβ) protein [1–3], the formation of plaques and tangles [4, 5], and loss of synaptic connectivity among selected neuronal circuits [6–12]. Based on the sequence and regional distribution of Aβ pathology, transgenic (Tg) animal models overexpressing wild type or mutant amyloid-β protein precursor (AβPP) have been developed that mimic some of the behavioral deficits, amyloid deposition patterns, and neurodegenerative aspects of AD [13–18]. These models support the notion that accumulation of oligomeric forms of Aβ at synaptic sites might play a role in the pathogenesis of the neurodegenerative process and memory loss in AD [19–22].
The development of effective therapies for AD is likely to require the use of compounds that are able to prevent the accumulation of Aβ at synaptic sites in the early stages of the disease. The discovery of such disease-modifying therapies has in part relied in preclinical testing in validated animal models [23]. For example, initial studies in AβPP Tg mice that demonstrated that immunotherapy promotes clearance of Aβ from the central nervous system have resulted in a number of such anti-Aβ antibodies going into human clinical trials [24–31]. Subsequent post-mortem examination in a small group of AD cases and more recently PET studies with Pittsburgh compound B showed that in vaccinated patients the levels of Aβ deposits were decreased [32] attesting to the value of some of the AβPP Tg models.
Given the importance of having a validated animal model to aid drug discovery and development, previous cross-sectional studies have characterized some time-dependent behavioral and pathological alterations in AβPP Tg mice [33]. However these studies do not address the progressive nature of these alterations. In this context, this study performed a comprehensive longitudinal analysis at 3, 6, and 9 months of age in mThy1-hAβPP751 Tg mice. The mThy1-hAβPP751 Tg mice, also known as TASD41 or Line 41 mice, are a well-documented model of AD and have been extensively characterized at various ages [34], however, this study is the first to provide a comprehensive longitudinal analysis age- and gender-dependent progression of behavioral alterations in these mice. We report an age-associated and gender dependent impairment in memory and learning in a water maze task and habituation in a hole-board task whilst motor coordination was well-preserved.
The progressive nature of the behavioral deficits in the mThy1-hAβPP751 Tg mice mimic AD from the early onset of the disease through to later stages, making them a suitable animal model in which to test drugs aimed at altering disease progression at a number of different stages of the disease.
MATERIALS AND METHODS
Animals
For this study Tg mice overexpressing human AβPP751 with the London (V717I) and Swedish (K670M/N671L) mutations under the regulatory control of the neuron specific murine (m)Thy-1 promoter (hereafter, mThy1-hAβPP751) were used [34, 35]. The mThy1-hAβPP751 Tg mice originated from the TASD41 (Line 41 mice) mice developed in 2001 by Rockenstein et al. [34]. Age-matched non-transgenic littermates (non-Tg) were used for control studies. A total of 100 mice were used for this study mThy1-hAβPP751 Tg females (n=25), mThy1-hAβPP751 Tg males (n=25), non-Tg females (n=25), and non-Tg males (n=25). The genotype of each animal was confirmed by real time PCR of tail snips using specific primers as previously described [34].
Animals were housed according to standard care protocols, fed ad libitum with regular animal diet (Altromin® standard rodent chow), bedded on ABEDD® standard rodent litter and maintained in a pathogen-free environment in single ventilated cages at the GLP-certified animal facility of JSW-Research. Mice lived at a constant 12 h light / dark cycle with light from 7.a.m to 7 p.m. with air humidity between 40 and 70%.
Behavioral Testing
General testing protocol
All groups of mice underwent a comparable battery of tests with Rotarod on the first day, Open Field (OF) on the second day and hole-board activity on the third day. Mice were allowed four days of rest before undergoing water-maze testing. This two-week procedure started with an age of 3 months and was repeated at 6 and 9 months. All behavioral tests were performed between 1 p.m. and 4 p.m. All steps of this study were conducted according to the appropriate guidelines for animal welfare and testing of the Austrian Government.
RotaRod
To evaluate general motor function mice were put on the treadmill rod (Five-lane RotaRod, TSE-Systems, Germany) at an initial speed of 5 revolutions per min and within 2 min speed was accelerated to 60 revolutions per min. Trial time was elapsed after 5 min.
Open field
To test exploratory behavior, mice were placed into the OF box (ActiMot8, TSE-Systems, Germany) after one hour habituation in the testing room. Any movement was detected by interruptions of closely arranged infrared light beams (each 1.4 cm one beam) with a 10 ms sampling rate for 5 min. Testing was done under constant room light conditions deriving from standard cold light halogen bulbs (4×60 Watt).
Hole-board task
Hole-board Activity (ActiMot8 OF equipped with 16-hole poke hole boards TSE-Systems, Germany) was measured like in the OF paradigm, but with a 10 min trial time. Each mouse was placed into the same test box as used in the day before during OF testing, which was equipped with an additional 16-hole board. Mice were tested approximately at the same time of day in the same testing room under the same light conditions. Number and locations of investigations of the holes were detected as well as the time spent on each investigation. The size of the holes allowed mice to explore them with the nose and the whiskers or paws providing olfactory and tactile cues, respectively.
Water Maze Test
The water-maze system consisted of a 100 cm diameter black pool and a software controlled video tracking, outfitted with a 40 ms sampling rate, adapted to the agile swimming characteristics of mice (Kaminsky and Krekule, 1997, Kaminsky Biomedical Systems Inc., Czech Republic). Water temperature was kept at 22±1°C. Mice swam four trials a day lasting maximally 60 s on four consecutive days. On the last day one 60 s probe trial, with platform removed, was performed with a delay of 30 min after the last acquisition trial. As an adaptation to the longitudinal test design the platform position learned three months ago was tested at the first day of the latter two sessions. For the three remaining days the platform was positioned to a new location and again, at the end of each test period, a probe trial was performed. The starting position was changed randomly from trial to trial (north, south, west, or east from the view of the experimenter) without repetition of a position. All experiments were conducted with an inter-trial time of 10 to 15 min. The time to find the hidden platform (i.e., the escape latency) and the length of the trajectory (i.e., the swimming path) were recorded. The average swimming speed, an indicator for swimming ability and/or strategy, was calculated (swimming path / escape latency). For evaluation of the probe trial the number of crossings of the target position was counted. Short-term memory was assessed by the difference of escape latencies between the first and second trial (separated by 10-min period) in all three test sessions when the platform location was new (relocated in the second (six month) and third (nine month) test session).
Tissue preparation
Following behavioral testing mice from each group, gender and age category were deeply anesthetized by Isoflurane “Baxter” (BAXTER®, Austria). The thorax of the mice was opened to excavate the heart. Animals were flush-perfused transcardially with 0.9% saline through the left ventricle. The brain hemispheres were divided sagittally, one hemibrain was immersionfixed in 4% Paraformaldehyde in 0.1 M Phosphate buffer and subsequently embedded into Histosec® (Merck & Co., Germany) for histology, the other hemibrain was shock-frozen in liquid nitrogen and then stored at −80°C for Aβ determination. Tissue was taken from 8 mThy1-hAβPP Tg mice and 8 non-Tg mice per age-group (4 males and 4 females).
Determination of plaque load
Plaque load was evaluated in 10 µm thick sagittal paraffin embedded slices of five different layers in the whole hippocampus and the whole cortex, relying on the principal of uniform, systematic, random sampling. The 6E10 (Signet®) antibody to amino acids (AA) 1–17 of the hAβPP protein was used in a 1:1000 dilution labeled with a fluorescent Cy3 (Jackson®) secondary in a 1:500 dilution. Unspecific binding was reduced with Vector® M.O.M. blocking kit. Tiled images of the complete sagittal slice were recorded with a PixelFly PCO camera mounted on a NikonE800 microscope using StagePro at 100-fold magnification. Brain region area, plaque area and number as well as mean plaque size were determined with ImageProPlus Software (Version 4.5.1.29), for all measurements using the same recording time, contrasting and threshold. Immunoreactive spots above a minimal size of 8 µm2 were defined as plaques, smaller spots were filtered as probable unspecific secondary binding.
Aβ1–40 and Aβ1–42 determination by ELISA
Samples out of the TRIS buffered saline (TBS), the Triton X-100, the sodium dodecyl sulphate (SDS) as well as of the formic acid (FA) brain fraction were analyzed in both, an Aβ1–40 as well as an Aβ1–42 ELISA (The Genetics Company®, Switzerland). Measurements were performed in duplicate. The fractionation method to extract unbound and bound Aβ is described elsewhere [36]. In brief, frozen hemispheres were homogenized in TBS-buffer (5 ml) containing protease inhibitor cocktail. After centrifugation the supernatants were aliquoted and kept at −20°C. The pellets were homogenized in Triton X-100 (5 ml), centrifuged and the supernatants were aliquoted and kept at −20°C. These steps were repeated with SDS (5 ml). The pellets out of the SDS fraction were homogenized in 70% formic acid (1 ml) prior to the following centrifugation. The obtained supernatants were neutralized with 1M TRIS (19 ml) and were aliquoted and kept at −20°C. Samples out of all four extractions were used for ELISA-determination. Undiluted blood plasma was analyzed for Aβ1–40 and Aβ1–42 with the same ELISA kits. Detection limit of the two kits at the time point of testing was at 40 pg/ml for Aβ1–40 and around 60 pg/ml for Aβ1–42 determinations accordingly to the values found in the littermates, intra-assay variability is below 6% according to the manufactures specifications.
Statistics
Statistical evaluations were done using STATISTICA (Version 5.5.99 edition, StatSoft, Inc., Tucsa, USA). If normally distributed (Shapiro-Wilks W-test), an ANOVA post hoc test was used to separate the groups (Tukey’s Honest Significant Difference Test). The α-error level was set to 0.05. If data were not normally distributed, groups were compared with a non-parametric Kruskal-Wallis ANOVA. All behavioral measures were further evaluated by means of a comprehensive correlation matrix. The following discrimination analysis was done in standard mode (for STATISTICA) at tolerance 0.01. To classify groups the three measurement time points were calculated separately first for transgene expression and secondly for both gender and transgenity combined. RotaRod data were excluded from the discrimination analysis, since there were no differences measurable in motor abilities and therefore values were not discriminative. All data shown in graphs are represented as means ± SEM.
RESULTS
mThy1-hAβPP751 Tg mice display a longitudinal age-related decline in memory and procedural learning in the water maze
The water maze was used in order to assess memory and learning in the mThy1-hAβPP751 Tg mice over time. The design of the water maze protocol over this longitudinal study was devised in order to examine the ability of the mice to perform the task and their long-term ability to remember the location of the platform (retention) to account for repeated exposure and habituation to the apparatus. To this end, the test was designed such that when the mice were tested at 6 months and 9 months old the first day of the four-day test period had the platform in the same place it had been for the previous test 3 months earlier while in subsequent trials (day 2 and day 3) the platform was moved to a new location, on the final day of testing the platform was removed for the probe test.
At 3 months of age, the escape latency (time taken to find the hidden platform) differed significantly between mThy1-hAβPP751 Tg mice and age-matched non-Tg littermates. Both mThy1-hAβPP751 Tg and non-Tg mice were able to find the hidden platform and their escape latencies fell with subsequent trials over the 4-day test period (Figure 1A).
Figure 1. mThy1-hAβPP751 Tg mice display an age-related longitudinal decline in spatial memory and learning in the water maze.
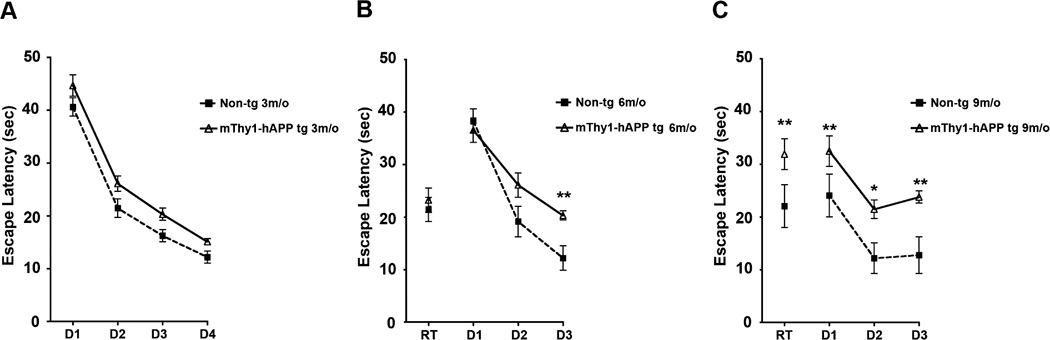
A) Learning curves in the non-Tg and mThy1-hAβPP751 Tg mice at 3 months of age in the water-maze over a four-day test period (D1 to D4). B) Learning curves in the non-Tg and mThy1-hAβPP751 Tg mice at 6 months of age in the water-maze over a four-day test period consisting of a retesting day (RT, where the platform was in the same position as on the last day of testing at 3 months of age) and three days during which the location of the platform was changed from trial to trial (D1 to D3). C) Learning curves in the non-Tg and mThy1-hAβPP751 Tg mice at 9 months of age in the water-maze over a four-day test period consisting of a retesting day (RT, where the platform was in the same position as on the last day of testing at 6 months of age) and three days during which the location of the platform was changed from trial to trial (D1 to D3). Data are represented by means (over four trials, each day) ± SEM.
* Indicates significant difference between non-Tg and mThy1-hAβPP751 Tg mice with p<0.05 by one-way ANOVA; ** Indicates significant difference between non-Tg and mThy1-hAβPP751 Tg mice with p<0.01 by one-way ANOVA.
When tested at 6 months old, both mThy1-hAβPP751 Tg mice and age-matched non-Tg littermates performed comparably in the retention (RT) part of the test, where the platform was placed in the same location it had been when the mice were tested at 3 months old (Figure 1B, Figure 2A). There was no significant difference in the time taken by either cohort to find the platform in this location, indicating that both were equally able to retain this information from 3 months to 6 months. In the subsequent trials (D1-3), where the hidden platform had been moved to a new location (Figure 1B), both mThy1-hAβPP751 Tg and non-Tg mice were able to locate the platform in a comparable time on the first day and though the time taken by the mThy1-hAβPP751 Tg and non-Tg mice to find the platform decreased over the test period, the mThy1-hAβPP751 Tg mice displayed slower learning (as evidenced by longer escape latency times) on the second day and were significantly slower than the non-Tg mice on the third day (Figure 1B).
Figure 2. mThy1-hAβPP751 Tg mice display an age-related longitudinal decline memory retention in the water maze.
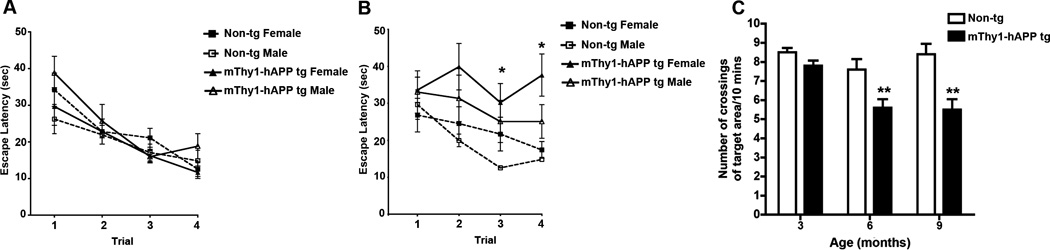
A) Relearning ability of non-Tg and mThy1-hAβPP751 Tg mice at 6 months of age on the retest days (four trials in which the platform was in the same position it had been on the final day of testing at 3 months). B) Relearning ability of non-Tg and mThy1-hAβPP751 Tg mice at 9 months of age on the retest days (four trials in which the platform was in the same position it had been on the final day of testing at 6 months). C) Retention abilities of non-Tg and mThy1-hAβPP751 Tg mice in the water maze indicated by the number of target crossing in the probe trials. Data are represented by means ± SEM. * Indicates significant difference between female and male mThy1-hAβPP751 Tg mice with p<0.05 by one-way ANOVA; ** Indicates significant difference between non-Tg and mThy1-hAβPP751 Tg mice with p<0.01 by one-way ANOVA.
When the mice were tested again at 9 months of age the mThy1-hAβPP751 Tg mice took significantly longer than their age-matched non-Tg littermates to find the platform in the retention part of the test, where the platform was in the same location it had been when the mice were tested at 6 months of age (Figure 1C, Figure 2B). This suggests that between 6 months and 9 months of age the mThy1-hAβPP751 Tg mice had lost the ability to retain information for this length of time. The time taken by the mThy1-hAβPP751 Tg mice to find the platform in the RT stage was comparable to the time they took with the platform at a new location (Figure 1C). In the subsequent trials, where the hidden platform was moved to a new location, both mThy1-hAβPP751 Tg and non-Tg mice were able to locate the platform and escape latencies in both groups fell from day 1 to day 2, however mThy1-hAβPP751 Tg mice took significantly longer to find the platform at both day one and day two in comparison to the non-Tg mice (Figure 1C). On the third day of testing the non-Tg continued to improve with their escape latency falling again from day 2, however the time taken by the mThy1-hAβPP751 Tg mice increased from day 2 to day 3 and they again took significantly longer to find the platform in comparison to non-Tg mice of the same age (Figure 1C). Further analysis of the ability of mThy1-hAβPP751 Tg mice to retain the location of the platform from 3 to 6 months and from 6 to 9 months and their ability to relearn the testing procedure was also performed by segregating the trial on the RT day by gender. This demonstrated gender-specific differences between male and female mThy1-hAβPP751 Tg mice at 9 months of age (Figure 2A, B).
The mThy1-hAβPP751 Tg mice had significantly lower probe trial scores at 6 months and 9 months of age (Figure 2C) in comparison to age-matched non-Tg littermates. The performance of mThy1-hAβPP751 Tg mice in the probe trial decreased between 3 months and 6 months of age while non-Tg performed consistently at all ages (Figure 2C). Further analysis segregating the mThy1-hAβPP751 Tg and non-Tg mice by gender revealed no significant effect of age upon performance in the probe trial (data not shown).
mThy1-hAβPP751 Tg mice display a longitudinal decline in hole-board activity
The hole-board test is designed to measure memory and exploratory-based behavior. While the non-Tg mice habituated to the apparatus over time, the mThy1-hAβPP751 Tg mice displayed no decrease in the levels of hole-poke activity from 3, 6, to 9 months of age (Figure 3A). At both 6 and 9 months of age mThy1-hAβPP751 Tg mice were more curious about the holes than their age-matched non-Tg littermates (Figure 3A). No differences were observed between mThy1-hAβPP751 Tg males and females (Figure 3B).
Figure 3. mThy1-hAβPP751 Tg mice display a curiosity deficit in the hole-board task.
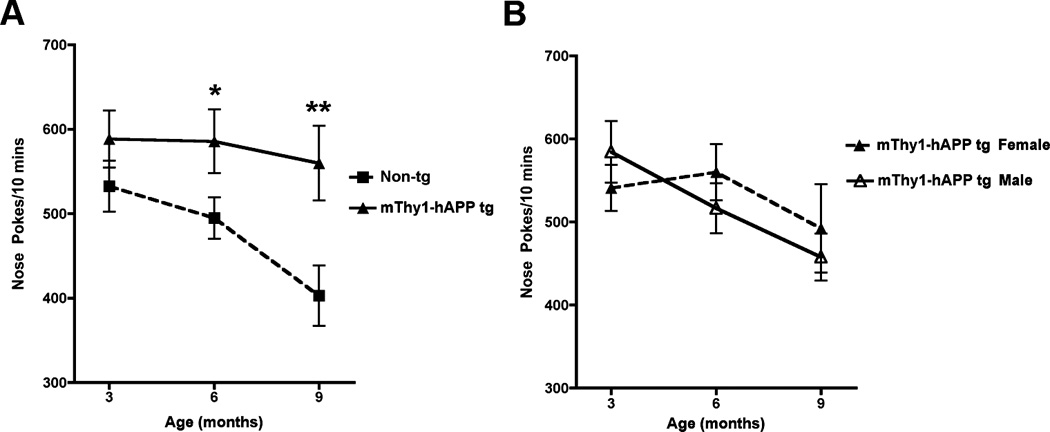
A) Curiosity levels of non-Tg and mThy1-hAβPP751 Tg mice expressed by the number beam breaks by nose or paw poking into the holes during a ten-minutes trial, at 3, 6, and 9 months of age. B) Gender stratification of the mThy1-hAβPP751 Tg mice at 3, 6, and 9 months of age. Data are represented by means ± SEM. * Indicates a significant difference between non-Tg and mThy1-hAβPP751 Tg mice with p<0.05 by one-way ANOVA ** Indicates a significant difference between non-Tg and mThy1-hAβPP751 Tg mice with p<0.01 by one-way ANOVA.
mThy1-hAβPP Tg mice display a longitudinal preservation of spontaneous motor activity
The OF activity assay was conducted in order to assess levels of spontaneous motor activity in the mice over time compared to their age-matched non-Tg littermates. The mThy1-hAβPP751 Tg mice did not display any significant differences in spontaneous activity or thigmotaxis at 3, 6, or 9 months of age in comparison to non-Tg littermates (Figure 4A, C), however in both the mThy1-hAβPP751 and non-Tg mice there was a significant decrease in activity and thigmotaxis from 3 to 6 months of age (Figure 4A,C). At 3 months of age, female mThy1-hAβPP751 Tg mice displayed significantly higher activity levels compared to male mThy1-hAβPP751 Tg mice, but these differences were no longer apparent when these animals reached 6 or 9 months of age (Figure 4B). In contrast, thigmotaxis did not differ between male and female mThy1-hAβPP751 Tg mice at 3 or 6 months but was significantly higher in female mThy1-hAβPP751 Tg mice at 9 months of age (Figure 4D). Motor coordination and balance were also assessed using RotaRod, performance, no significant effect of transgene or gender was noted between the mThy1-hAβPP751 Tg or non-Tg mice in this paradigm at any age (Figure 5).
Figure 4. mThy1-hAβPP751 Tg mice display preserved motor function in the Open Field.
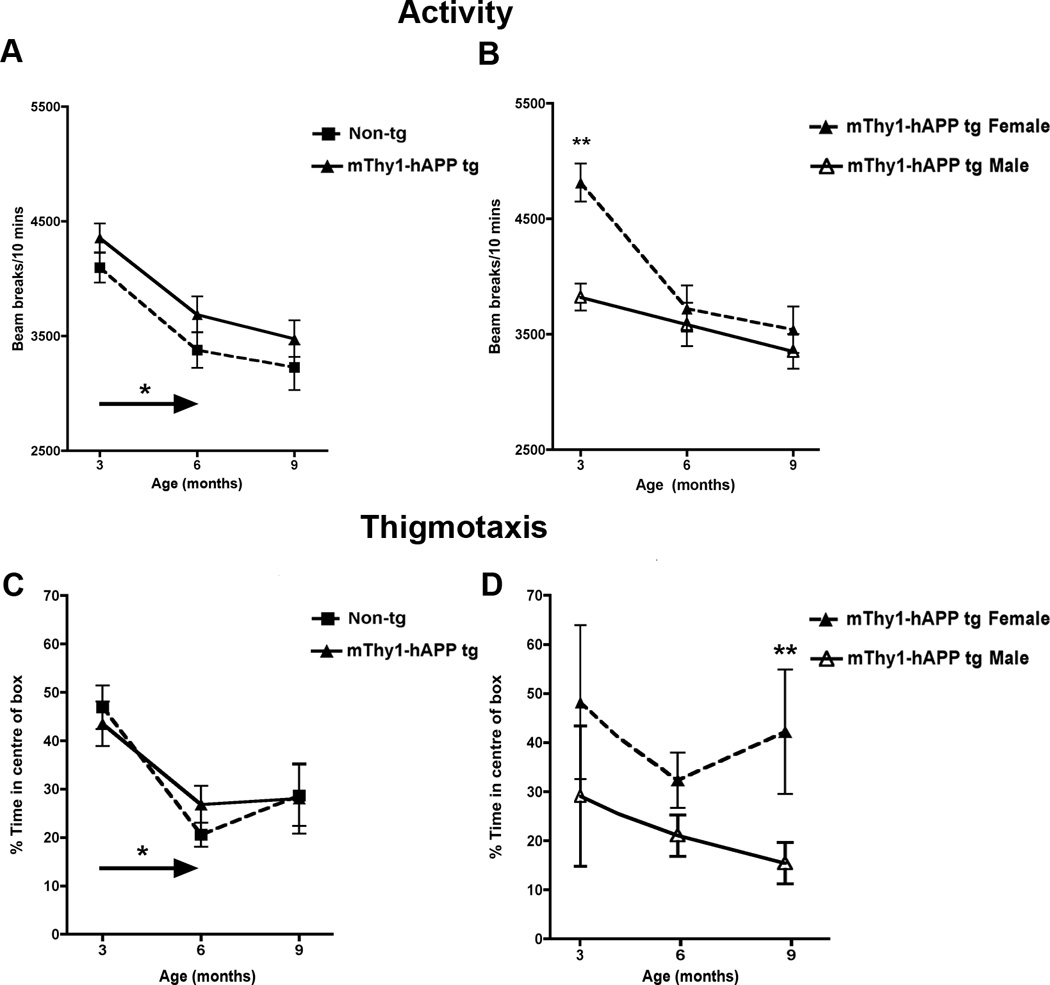
A) Total activity in the Open Field of the non-Tg and mThy1-hAβPP751 Tg mice at 3, 6, and 9 months of age. B) Gender-stratification of the total activity in the mThy1-hAβPP751 Tg mice at 3, 6, and 9 months of age. C) Thigmotactic behavior in the non-Tg and mThy1-hAβPP751 Tg mice at 3, 6, and 9 months of age. D) Gender-stratification of thigmotaxis in the mThy1-hAβPP751 Tg mice at 3, 6, and 9 months of age. Data are presented as means ± SEM. * Indicates a significant difference between 3 and 6 month old non-Tg or 3 and 6 month old mThy1-hAβPP751 Tg mice with p<0.05 by one-way ANOVA. ** Indicates a significant difference between non-Tg and mThy1-hAβPP751 Tg mice with p<0.01 by one-way ANOVA.
Figure 5. mThy1-hAβPP751 Tg mice display preserved motor function in the Rotarod.
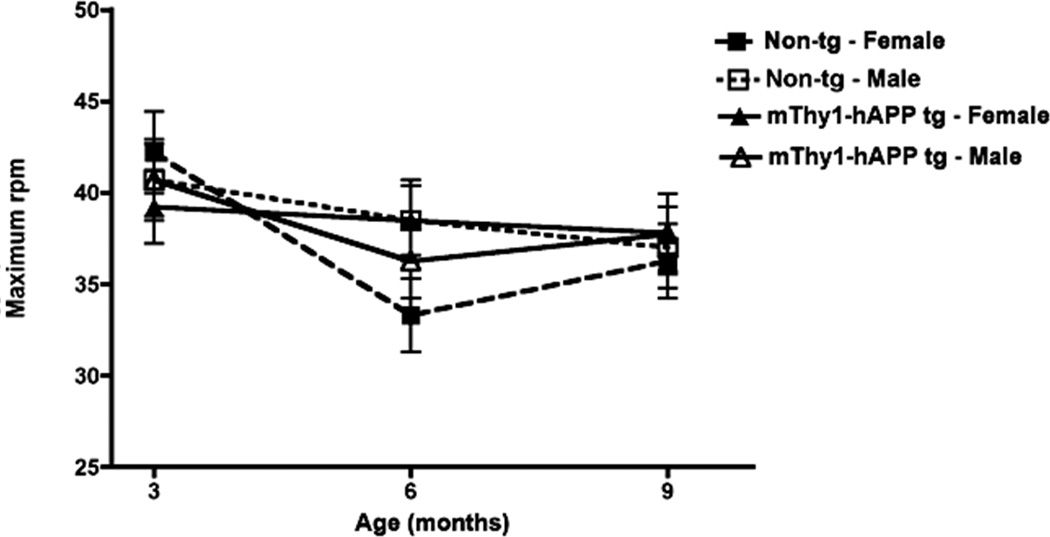
Motor coordination in the non-Tg and mThy1-hAβPP751 Tg mice on the Rotorod, expressed as the revolutions per minute (rpm) at which mice were unable to remain on the rotating rod. Data are presented as means ± SEM.
Discriminative power of tests
Discrimination analysis was performed in order to address the issue of whether the applied behavioral testing battery was selective enough to distinguish between mThy1-hAβPP751 Tg and non-Tg mice. The set of parameters leading to best group division varied slightly on the three tested age points, but were at any time a mixture of factors including body weight and parameters in the OF, hole-board activity, and water-maze paradigms (see Table 1). At the age of three months differences deriving from genotype were rare. In spite of this, the discrimination power between mThy1-hAβPP751 Tg mice and non-Tg mice was at least 78%. Including gender to the analysis resulted in a right group allocation for male mice with a probability of at least 95% and for female mice with a probability of at least 86%, respectively. At 6 months of age the progression of pathology led to significant differences in the behavioral tests, which increased the discrimination power. At 9 months of age differences in the water-maze and in the hole board paradigm were clearly detectable. Therefore, the discrimination analysis assigned 100% of the mThy1-hAβPP751 Tg and non-Tg to the appropriate group by means of behavioral measures only. Using only the parameters of the third test session of the water-maze testing led to a mean selectivity of 84%.
Table 1. Summary of behavioral differences between mThy-1-751 transgenic and non-transgenic mice by age and gender.
Impairment is stated in case of a significantly worse performance of mThy1-hAβPP751 Tg mice compared to their age-matched non-Tg littermates. ‘Progressive’ means a significant poorer performance as compared to an earlier testing time point. ‘ND’ mice not different at that age (left three columns) or between the testing time points (right three columns).
| Task | 3M | 6M | 9 M | 3 vs. 6 M | 3 vs. 9 M | 6 vs. 9 M |
|---|---|---|---|---|---|---|
| RotaRod | ND | ND | ND | ND | ND | ND |
| Open Field | ||||||
| Activity/Hyperactivity | ND* | ND | ND | ND | ND | ND |
| Thigmotaxis | ND | ND | ND | ND | ND | ND |
| Rearings | ND | ND | ND | ND | ND | ND |
| Poke hole activity | ||||||
| Total activity | ND | increased | increased | ND | increased | increased |
| Hole explorations | ND | increased | increased | increased | increased | ND |
| Water Maze | ||||||
| Water-maze task learning | ND | ND | impaired | ND | ND | ND |
| Water-maze acquisition | impaired | impaired | impaired | progressive | progressive | progressive |
| Water-maze retention | ND | ND | impaired | ND | ND | progressive |
| Probe trial performance | ND | impaired | impaired | progressive | progressive | ND |
| Water-maze Short-term memory |
ND | ND | impaired | ND | progressive | progressive |
indicates a gender-specific difference in this parameter, independent from transgenity. “Different” or “increased/decreased” is stated for effects not easily appraisable.
Age-related increase in hAβ1–40, hAβ1–42 levels and plaque formation in the brain of mThy1-hAβPP751 Tg mice
In order to verify the development of AD-related neuropathological alterations in the mThy1-hAβPP751 Tg mice at the various ages examined, levels of Aβ were examined by immunohistochemistry and ELISA.
Immunohistochemical analysis with the 6E10 antibody against amino acids 1–17 of the hAβ protein showed that mThy1-hAβPP751 Tg mice are characterized by an age-dependent increase of plaque load, starting with sparse small plaques in the frontal cortex at 3 months of age, followed by a more than 20-fold increase of the plaque number at 6 months of age, colonizing all the cortex, the hippocampus and the upper part of the thalamus (Figure 6) [34]. During the following three months, from 6 to 9 months of age, the number of plaques increased 3-fold. During this period, plaque size, but not number, increased in females by 30% while it remained constant in the males, leading to a significant gender-specific difference in plaque load at 9 months of age when comparing mThy1-hAβPP751 Tg females and mThy1-hAβPP751 Tg males.
Figure 6. mThy1-hAβPP751 Tg mice display age-related, longitudinal accumulation of Aβ in the cortex and hippocampus.
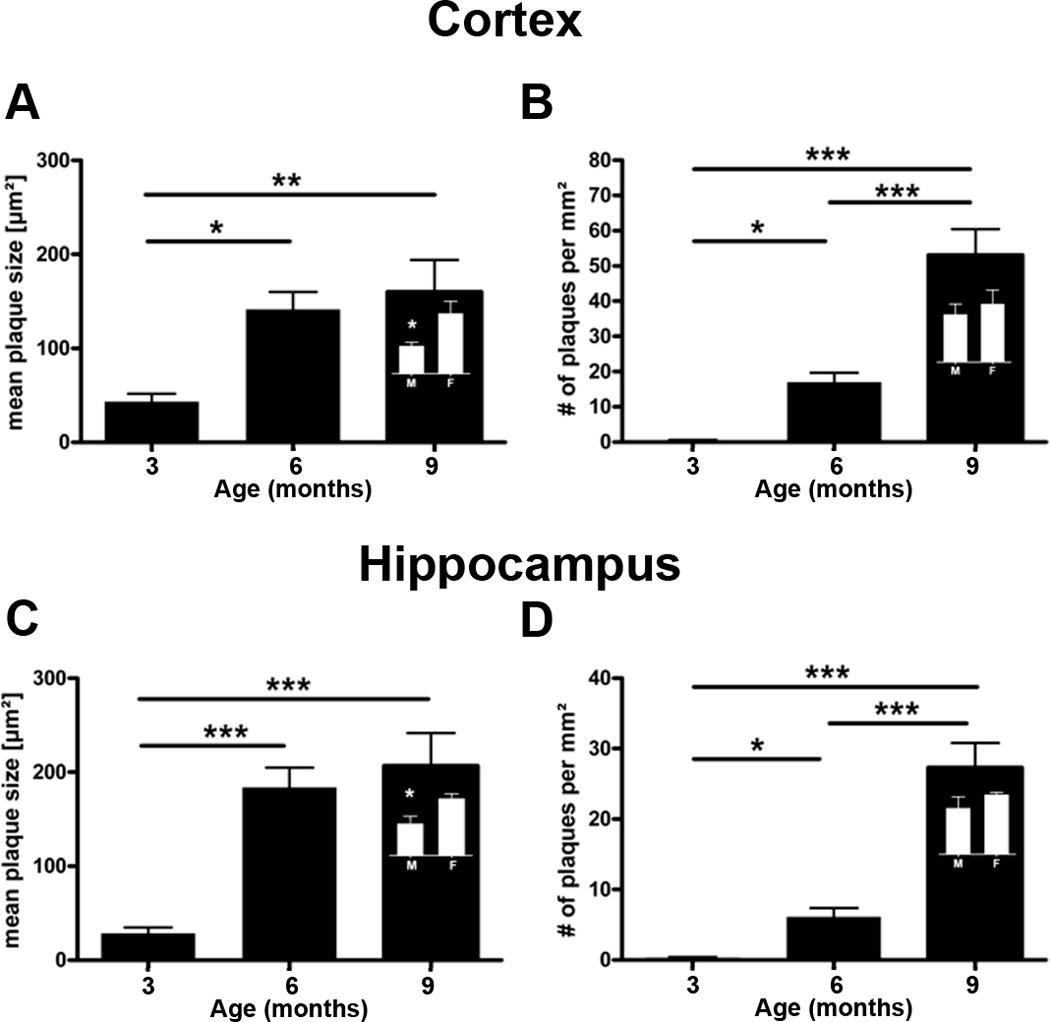
Aβ plaques were visualized using the 6E10 antibody to AA1-17 of the hAβ protein.
A) Plaque size at 3, 6, and 9 months of age in the cortex of mThy1-hAβPP751 Tg mice. B) Plaque number at 3, 6, and 9 months of age in the cortex of mThy1-hAβPP751 Tg mice. C) Plaque size at 3, 6, and 9 months of age in the hippocampus of mThy1-hAβPP751 Tg mice. D) Plaque number at 3, 6, and 9 months of age in the hippocampus of mThy1-hAβPP751 Tg mice. Insets in the bars at 9 months indicate gender-related differences in cortical and hippocampal plaque size and number. Data are presented as means ± SEM.* Indicates a significant difference with p<0.05 by one-way ANOVA. ** Indicates a significant difference with p<0.01 by one-way ANOVA. *** Indicates a significant difference with p<0.001 by one-way ANOVA
Analysis of the levels of formic acid (FA) soluble hAβ1–42 species by ELISA demonstrated an age-dependent increase in the plasma and brain homogenates from mThy1-hAβPP751 Tg mice in comparison to age-matched non-Tg littermates (see Figure 7A, B). Separation by gender demonstrated a gender-specific difference in Aβ levels between 6 months and 9 months of age wherein lower levels of FA soluble hAβ1–42 were detectable in male mThy1-hAβPP751 Tg mice than in their female counterparts (Figure 7B). Similar results were observed for Aβ1–40 levels (data not shown). Soluble Aβ in the TBS and TritonX-100 fractions was generally at relatively low levels (<60 ng/g brain weight), which is consistent with the increase in plaques sequestering this soluble Aβ.
Figure 7. Plasma and brain Aβ1–42 levels in mThy1-hAβPP751 Tg mice with age by ELISA.
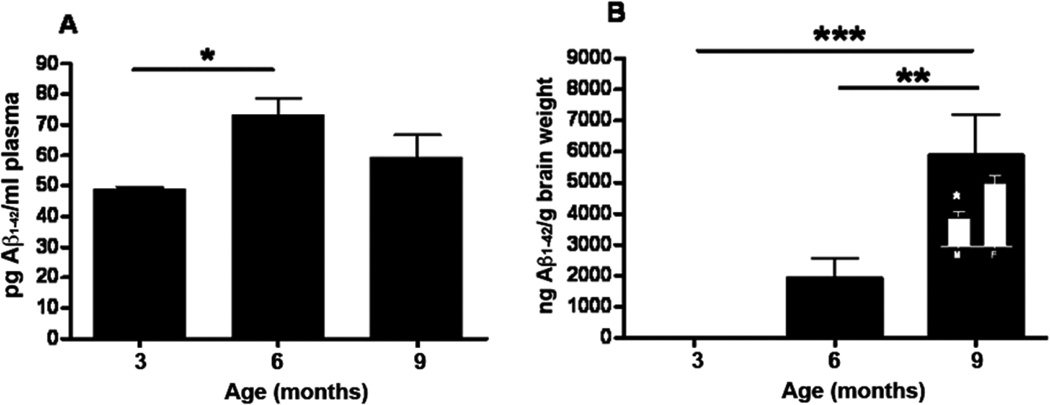
A) Plasma levels of soluble Aβ1–42 in the mThy1-hAβPP751 Tg mice at 3, 6, and 9 months of age. B) Insoluble hAβ1–42 in the FA fraction from the brain of mThy1-hAβPP751 Tg mice at 3, 6, and 9 months of age. Inset in the bar at 9 months indicates a gender-specific difference in FA- soluble hAβ1–42 at this age. Data are represented as means ± SEM * Indicates a significant difference with p<0.05 by one-way ANOVA. ** Indicates a significant difference with p<0.01 by one -way ANOVA. *** Indicates a significant difference with p<0.001 by one-way ANOVA.
DISCUSSION
The present study is the first to investigate longitudinal changes in spatial learning and memory the mThy1-hAβPP751 Tg line and to examine the age-related and gender-specific appearance of behavioral and neuropathological features associated with AD in these mice. Previous studies have analyzed the appearance of behavioral deficit and neuropathology over time, however these studies have been cross-sectional, analyzing different groups of mice at different ages [33]. For the present study, we took a group of mice and followed their development of behavioral alterations and neuropathology. Behavioral analysis in these mice demonstrates that deficits in memory and learning emerge at an early age and performance in the water maze task is affected at the first time point (3 months of age) tested, whereas the recall and re-learning the previous platform position and especially the learning of relocated position of a submerged platform are progressively and markedly affected as the mice age (6 and 9 months of age). In contrast, motor coordination in the mThy1-hAβPP751 Tg mice was well preserved throughout the investigated life span.
Although our model shows early behavioral deficits in the water maze, this disturbed activity is consistent with previous reports in mouse models of AD. Deficits in water maze acquisition and retention have been previously described for a number of transgenic models AD (Tg2576 [37, 38], Tg2576+PS1, [33] and the AβPP23 model [39]. Furthermore, Chen and colleagues (2000) have presented learning deficits in their PDAβPP mice, but as the water-maze setup in this study had been modified with up to forty swimming trials this data was not comparable to standard procedures [40].
A number of studies characterizing motor coordination in AβPP Tg mice have reported deficits in, for example, the string agility of the Tg2576 model [38] or beam-walking in Tg2576+ PS1 mice [33]. In contrast the present study demonstrates a longitudinal preservation of motor function in mThy1-hAβPP751 Tg mice as assessed by open field activity and performance on the RotaRod. These results highlight the specificity of behavioral deficits in these mice to tasks associated with memory and learning such as the water maze.
The present study also investigated the performance of the mThy1-hAβPP751 Tg mice in the hole-board task, while this is an established test to assess neophilic behavior in rodents [41, 42] it has not previously been used to phenotype Tg AD mouse models. The results from this study demonstrate that while non-Tg mice seemed to adapt to the fact that these holes are empty and reduced their exploration activity to about 30% over time, mThy1-hAβPP751 Tg mice continued to explore the holes to more or less the same extent at each testing session as they age. Given that the mThy1-hAβPP751 Tg mice were able to retain information from 3 months to 6 months of age in the water maze (retention) and that they did not show any deficits in OF activity or thigmotaxis until 9 months of age, the results in the hole-board from 3 to 6 months may indicate a difference in ‘curiosity’ behavior in the mThy1-hAβPP751 Tg mice in comparison to the non-Tg mice, rather than the mThy1-hAβPP751 Tg mice just not remembering having been on the board before. At 9 months of age the increased hole poke behavior in the mThy1-hAβPP751 Tg mice in comparison to the non-Tg mice may be a combination of this altered curiosity behavior and the emerging deficits in memory in the mice at this age, as indicated by the loss of retention in the water maze test. This reduction in habituation may be due to disturbances in either acquisition during habituation to novel, specific cues in an environment, reflecting a problem in the long-term storage of olfactory and/or tactile sensations, or earlier during the processing of incoming sensory information. Since the mThy1-hAβPP751 Tg mice also exhibit plaques in the olfactory relevant brain regions, a pathology induced disturbance in the judgment of olfactory stimuli in the olfactory bulb could explain their higher exploration rate. Pathological changes in the olfactory bulb have also been reported in humans as one of the earliest events in the degenerative process in AD [43].
We also demonstrate that plasma levels of soluble hAβ1–42 increase from 3 months to 6 month of age, but at 9 months remained at the level seen at 6 months. Plaque deposition and growth were also reflected by the increase of hAβ1–42 in the FA- soluble fraction, comprising the diminutively soluble fragments like oligomeric and protofibrillary amyloid, which are commonly regarded as the seed for plaque building by β-sheet formation [36, 44, 45]. Both the ELISA and immunohistochemical results showed a gender-specific development of hAβ load in the period between 6 and 9 months of age. The increase of plaque size and of FA-soluble hAβ1–42 levels were more pronounced in female mThy1-hAβPP751 Tg mice. Such gender differences in plaque load are consistent with studies in Tg mice with a single Arctic AβPP mutation [46], AβPP/PS1 mice [47] and triple Tg mice [48], which report that female mice develop a significantly more severe plaque pathology than males, which may parallel the disease and diminution of neurotrophic effects by hormones [49–51].
In conclusion, the results from this study demonstrate that the mThy1-hAβPP751 Tg mouse model incorporates robust and progressive cognitive impairments related to hAβPP overexpression. The relatively rapid time course of the cognitive decline coupled with a progressive human AD-like pathology and well-preserved motor skills and physical state of the mThy1-hAβPP751 Tg mice makes them a suitable model in which to test emerging experimental therapies comparing early versus late disease time-points.
Acknowledgments
Many thanks to the excellent team of JSW-Research, especially Klaus Lorenzoni for the biochemical analyses. This work was supported by NIH grants AG18440, AG022074, AG03197 and AG010435.
Daniel Havas, Birgit Hutter-Paier and Manfred Windisch work at JSW Life Sciences, which provides commercial services in the field of neurology including the experimental models of Alzheimer’s disease.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=763).
REFERENCES
- 1.Selkoe D. Amyloid β protein precursor and the pathogenesis of Alzheimer's disease. Cell. 1989;58:611–612. doi: 10.1016/0092-8674(89)90093-7. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe D. Amyloid β-protein deposition as a seminal pathogenic event in AD: an hypothesis. Neurobiol. Aging. 1990;11:299. [Google Scholar]
- 3.Selkoe D. Physiological production of the β-amyloid protein and the mechanisms of Alzheimer's disease. Trends Neurosci. 1993;16:403–409. doi: 10.1016/0166-2236(93)90008-a. [DOI] [PubMed] [Google Scholar]
- 4.Mandelkow E, Mandelkow E. Tau in Alzheimer's disease. Trends Cell. Biol. 1998;8:125–127. doi: 10.1016/s0962-8924(98)01368-3. [DOI] [PubMed] [Google Scholar]
- 5.Trojanowski JQ, Shin RW, Schmidt ML, Lee VM. Relationship between plaques, tangles, and dystrophic processes in Alzheimer's disease. Neurobiol Aging. 1995;16:335–340. doi: 10.1016/0197-4580(94)00176-2. discussion 341–335. [DOI] [PubMed] [Google Scholar]
- 6.DeKosky S, Scheff S. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann. Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 7.DeKosky ST, Scheff SW, Styren SD. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration. 1996;5:417–421. doi: 10.1006/neur.1996.0056. [DOI] [PubMed] [Google Scholar]
- 8.Masliah E. Mechanisms of synaptic dysfunction in Alzheimer's disease. Histol. Histopathol. 1995;10:509–519. [PubMed] [Google Scholar]
- 9.Masliah E, Crews L, Hansen L. Synaptic remodeling during aging and in Alzheimer's disease. J Alzheimers Dis. 2006;9:91–99. doi: 10.3233/jad-2006-9s311. [DOI] [PubMed] [Google Scholar]
- 10.Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- 11.Scheff S, DeKosky S, Price D. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol. Aging. 1990;11:29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- 12.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 13.D'Hooge R, Nagels G, Westland CE, Mucke L, De Deyn PP. Spatial learning deficit in mice expressing human 751-amino acid beta-amyloid precursor protein. Neuroreport. 1996;7:2807–2811. doi: 10.1097/00001756-199611040-00080. [DOI] [PubMed] [Google Scholar]
- 14.Mucke L, Abraham C, Ruppe M, Mallory M, Masliah E. Differential effects of hAPP695 and hAPP751 in a transgenic model of neurodegeneration. Neurobiol. Aging. 1994;15:S33. [Google Scholar]
- 15.Mucke L, Masliah E, Johnson W, Ruppe M, Rockenstein E, Forss-Petter S, Pietropaolo M, Mallory M, Abraham C. Synaptotrophic effects of human amyloid β protein precursors in the cortex of transgenic mice. Brain Res. 1994;666:151–167. doi: 10.1016/0006-8993(94)90767-6. [DOI] [PubMed] [Google Scholar]
- 16.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 17.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemes J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 18.Games D, Masliah E, Lee M, Johnson-Wood K, Schenk D. Neurodegenerative Alzheimer-like pathology in PDAPP 717V-->F transgenic mice. In: Hyman B, Duyckaerts C, Christen Y, editors. Connections, cognition and Alzheimer's disease. Berlin: Springer-Verlag; 1997. pp. 105–119. [Google Scholar]
- 19.Cummings JL. Alzheimer's disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 20.Rowan MJ, Klyubin I, Wang Q, Hu NW, Anwyl R. Synaptic memory mechanisms: Alzheimer's disease amyloid beta-peptide-induced dysfunction. Biochem Soc Trans. 2007;35:1219–1223. doi: 10.1042/BST0351219. [DOI] [PubMed] [Google Scholar]
- 21.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Dam D, De Deyn PP. Drug discovery in dementia: the role of rodent models. Nat Rev Drug Discov. 2006;5:956–970. doi: 10.1038/nrd2075. [DOI] [PubMed] [Google Scholar]
- 24.Arbel M, Solomon B. A novel immunotherapy for Alzheimer's disease: antibodies against the beta-secretase cleavage site of APP. Curr Alzheimer Res. 2007;4:437–445. doi: 10.2174/156720507781788792. [DOI] [PubMed] [Google Scholar]
- 25.Laskowitz DT, Kolls BJ. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2010;74:2026. doi: 10.1212/WNL.0b013e3181e03844. author reply 2026–2027. [DOI] [PubMed] [Google Scholar]
- 26.Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. 2010;6:108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- 28.Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, Mulnard R, Barakos J, Gregg KM, Liu E, Lieberburg I, Schenk D, Black R, Grundman M. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serrano-Pozo A, William CM, Ferrer I, Uro-Coste E, Delisle MB, Maurage CA, Hock C, Nitsch RM, Masliah E, Growdon JH, Frosch MP, Hyman BT. Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology. Brain. 2010;133:1312–1327. doi: 10.1093/brain/awq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vellas B, Black R, Thal LJ, Fox NC, Daniels M, McLennan G, Tompkins C, Leibman C, Pomfret M, Grundman M. Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr Alzheimer Res. 2009;6:144–151. doi: 10.2174/156720509787602852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winblad B, Minthon L, Floesser A, Imbert G, Dumortier T, He Y, Maguire P, Karlsson M, Ostlund H, Lundmark J, Orgogozo J, Graf A, Andreasen N. Results of the first-in-man study with the active Ab immunotherapy CAD-106 in Alzheimer Patients. Alzheimers Dementia. 2009;5:P113–P114. O112-105-105. [Google Scholar]
- 32.Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, Rodriguez Martinez de Liano S, Liu E, Koller M, Gregg KM, Schenk D, Black R, Grundman M. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 33.Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, Diamond DM. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 2001;891:42–53. doi: 10.1016/s0006-8993(00)03186-3. [DOI] [PubMed] [Google Scholar]
- 34.Rockenstein E, Mallory M, Mante M, Sisk A, Masliaha E. Early formation of mature amyloid-beta protein deposits in a mutant APP transgenic model depends on levels of Abeta(1–42) J Neurosci Res. 2001;66:573–582. doi: 10.1002/jnr.1247. [DOI] [PubMed] [Google Scholar]
- 35.Andra K, Abramowski D, Duke M, Probst A, Wiederhold KH, Burki K, Goedert M, Sommer B, Staufenbiel M. Expression of APP in transgenic mice: a comparison of neuron-specific promoters. Neurobiol Aging. 1996;17:183–190. doi: 10.1016/0197-4580(95)02066-7. [DOI] [PubMed] [Google Scholar]
- 36.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 38.King DL, Arendash GW, Crawford F, Sterk T, Menendez J, Mullan MJ. Progressive and gender-dependent cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer's disease. Behav Brain Res. 1999;103:145–162. doi: 10.1016/s0166-4328(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 39.Dumont M, Strazielle C, Staufenbiel M, Lalonde R. Spatial learning and exploration of environmental stimuli in 24-month-old female APP23 transgenic mice with the Swedish mutation. Brain Res. 2004;1024:113–121. doi: 10.1016/j.brainres.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 40.Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RG. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 41.Brown GR, Nemes C. The exploratory behaviour of rats in the hole-board apparatus: is head-dipping a valid measure of neophilia? Behav Processes. 2008;78:442–448. doi: 10.1016/j.beproc.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kliethermes CL, Crabbe JC. Genetic independence of mouse measures of some aspects of novelty seeking. Proc Natl Acad Sci U S A. 2006;103:5018–5023. doi: 10.1073/pnas.0509724103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christen-Zaech S, Kraftsik R, Pillevuit O, Kiraly M, Martins R, Khalili K, Miklossy J. Early olfactory involvement in Alzheimer's disease. Can J Neurol Sci. 2003;30:20–25. doi: 10.1017/s0317167100002389. [DOI] [PubMed] [Google Scholar]
- 44.Kumar-Singh S, Dewachter I, Moechars D, Lubke U, De Jonghe C, Ceuterick C, Checler F, Naidu A, Cordell B, Cras P, Van Broeckhoven C, Van Leuven F. Behavioral disturbances without amyloid deposits in mice overexpressing human amyloid precursor protein with Flemish (A692G) or Dutch (E693Q) mutation. Neurobiol Dis. 2000;7:9–22. doi: 10.1006/nbdi.1999.0272. [DOI] [PubMed] [Google Scholar]
- 45.Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ronnback A, Zhu S, Dillner K, Aoki M, Lilius L, Naslund J, Winblad B, Graff C. Progressive neuropathology and cognitive decline in a single Arctic APP transgenic mouse model. Neurobiol Aging. 2011;32:280–292. doi: 10.1016/j.neurobiolaging.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Tanila H, Puolivali J, Kadish I, van Groen T. Gender differences in the amount and deposition of amyloidbeta in APPswe and PS1 double transgenic mice. Neurobiol Dis. 2003;14:318–327. doi: 10.1016/j.nbd.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Hirata-Fukae C, Li HF, Hoe HS, Gray AJ, Minami SS, Hamada K, Niikura T, Hua F, Tsukagoshi-Nagai H, Horikoshi-Sakuraba Y, Mughal M, Rebeck GW, LaFerla FM, Mattson MP, Iwata N, Saido TC, Klein WL, Duff KE, Aisen PS, Matsuoka Y. Females exhibit more extensive amyloid, but not tau, pathology in an Alzheimer transgenic model. Brain Res. 2008;1216:92–103. doi: 10.1016/j.brainres.2008.03.079. [DOI] [PubMed] [Google Scholar]
- 49.Hu L, Yue Y, Zuo PP, Jin ZY, Feng F, You H, Li ML, Ge QS. Evaluation of neuroprotective effects of long-term low dose hormone replacement therapy on postmenopausal women brain hippocampus using magnetic resonance scanner. Chin Med Sci J. 2006;21:214–218. [PubMed] [Google Scholar]
- 50.Pirskanen M, Hiltunen M, Mannermaa A, Helisalmi S, Lehtovirta M, Hanninen T, Soininen H. Estrogen receptor beta gene variants are associated with increased risk of Alzheimer's disease in women. Eur J Hum Genet. 2005;13:1000–1006. doi: 10.1038/sj.ejhg.5201447. [DOI] [PubMed] [Google Scholar]
- 51.Schuessel K, Leutner S, Cairns NJ, Muller WE, Eckert A. Impact of gender on upregulation of antioxidant defence mechanisms in Alzheimer's disease brain. J Neural Transm. 2004;111:1167–1182. doi: 10.1007/s00702-004-0156-5. [DOI] [PubMed] [Google Scholar]


