Abstract
Epithelial ovarian cancer represents the most lethal gynecological malignancy in the developed world, and can be divided into five main histological subtypes: high grade serous, endometrioid, clear cell, mucinous and low grade serous. These subtypes represent distinct disease entities, both clinically and at the molecular level. Molecular analysis has revealed significant genetic heterogeneity in ovarian cancer, particularly within the high grade serous subtype. As such, this subtype has been the focus of much research effort to date, revealing molecular subgroups at both the genomic and transcriptomic level that have clinical implications. However, stratification of ovarian cancer patients based on the underlying biology of their disease remains in its infancy. Here, we summarize the molecular changes that characterize the five main ovarian cancer subtypes, highlight potential opportunities for targeted therapeutic intervention and outline priorities for future research.
Keywords: Ovarian cancer, molecular genetics, histological subtypes, molecular subgrouping, ovary
Introduction
Ovarian cancer (OC) represents the most lethal gynecological malignancy in the developed world, with over 21,000 cases diagnosed, accounting for over 14,000 deaths per year in the United States alone 1. The vast majority of ovarian cancers are of epithelial origin, which are typically diagnosed at advanced stage. The current standard of care for epithelial ovarian cancer comprises maximal cytoreductive surgical resection and platinum-taxane combination chemotherapy 2.
A number of clinical parameters influence outcome in OC patients. Age at diagnosis, FIGO stage, disease grade and the presence of ascites are independent factors affecting progression-free-survival (PFS) and overall survival (OS) in OC patients 3- 5. Suboptimal debulking surgery, leaving macroscopic residual disease, also has a significant impact on patient survival 6.
Epithelial OC has historically been grouped according to histology and is currently divided into five main subtypes: high grade serous (HGS), endometrioid, clear cell (CC), low grade serous (LGS) and mucinous OC 7 ( Table 1). It is now recognized that these subtypes have distinct developmental origins: HGS OC predominantly arises from the epithelium of the distal fallopian tubes, while CC and endometrioid OC are associated with endometriosis 8- 16. LGS OC is thought to progress in a step-wise fashion from serous cystadenoma or adenofibroma to serous borderline tumor, and then to LGS OC 17. These histological subtypes display distinct molecular landscapes at both the genomic and transcriptomic level 9, 18- 20. In the face of mounting evidence for the discrete developmental origins and molecular pathogenesis of OC subtypes, there is now a growing appreciation that these five histologically-defined groups represent separate disease entities, and that there is a need for stratification in both the clinical and research setting 7, 21.
Table1.
Characteristic of the five main histological subtypes of OC
| HGS | Endometrioid | Clear cell | Mucinous | LGS | |
| Early stage: FIGO stage I or II; advanced stage: FIGO stage III-IV; amp: amplification | |||||
| Approximate proportion of OC cases | 70% | 10% | 10% | <5% | <5% |
| Overall prognosis | Poor | Favourable | Intermediate | Intermediate | Intermediate |
| Tissue of origin / precursor lesion | Distal fallopian epithelium | Endometriosis | Endometriosis | Poorly defined | Serous borderline tumor |
| Intrinsic chemosensitivity | High | High | Low | Low | Low |
| Associated hereditary syndromes | Germline BRCA1/2 | Lynch syndrome | Lynch syndrome | ||
| Typical stage at diagnosis | 80% advanced stage | 50% early stage | 60% early stage | 80% early stage | Typically advanced stage |
| Frequent molecular abnormalities | Chromosome instability BRCA1, BRCA2 TP53, NF1, RB1 CCNE1 amp. | PTEN, PIK3CA, ARID1A, CTNNB1 | PTEN, PIK3CA, ARID1A, chr20q13.2, amp | KRAS, HER2 amp | KRAS, BRAF |
In keeping with the argument that they are different diseases, these subtypes display different levels of chemosensitivity. CC, mucinous and LGS OC are highly platinum resistant, while HGS OC is often platinum sensitive in the first-line setting 22- 24. Despite the tendency to display therapy resistance, LGS OC is associated with superior clinical outcome compared to HGS, displaying a more indolent disease course, even when diagnosed at advanced stage 25. Endometrioid and CC OC also display generally superior clinical outcome when compared to HGS, which is likely due to their propensity for diagnosis at earlier stage 22, 26- 30.
However, histological subtype alone does not account for the significant clinical heterogeneity seen in OC. Indeed, HGS OC patients matched for disease grade and stage show differential therapy sensitivity, PFS and OS, strongly implicating a molecular basis for the clinical heterogeneity within these histologically-defined groups 31. Extensive molecular characterization of HGS OC has therefore been undertaken to identify subgroups defined by their genomic and transcriptomic characteristics, in the hope of finding a molecular basis for differential clinical outcome and to identify opportunities for targeted therapeutic intervention and treatment stratification.
Approximately one fifth of OC is associated with inherited pathogenic variants in the germline, commonly in BRCA1 or BRCA2 which account for around 75% of hereditary disease 32, 33. The molecular and clinical implications of these defects are discussed below. While BRCA-associated disease is the most common form of hereditary OC, defects in other DNA repair associated genes have also been identified. These include genes that, like BRCA1 and BRCA2, are involved with double stranded DNA repair, such as BARD1, CHEK2, RAD51, PALB2 and BRIP1 33- 36.
Lynch syndrome, caused by inherited defects in genes involved in single stranded mismatch DNA repair (MMR), most commonly predisposes individuals to bowel and endometrial malignancy, but these patients are also at increased risk of OC 37, 38. The most commonly affected genes in this syndrome are MLH1, MSH2, MSH6 and PMS2, and Lynch syndrome patients account for around 10%-15% of hereditary OC 39.
Li-Fraumeni syndrome, caused by an inherited TP53 mutation, accounts for much of the remaining identified hereditary OC cases (around 3%) 33.
Genetic and molecular changes in HGS OC
HGS OC accounts for approximately 70% of OC 7. Of these, only a minority are confined to the ovary at diagnosis. Despite response rates to first-line platinum-based chemotherapy of around 80%, the majority of patients experience disease recurrence which accrues resistance to platinum, and prognosis for advanced stage disease remains poor with a five-year survival of around 30% 40.
DNA sequence
The most frequent molecular defect in HGS OC at the genomic level is almost ubiquitous TP53 mutation 18, 41, 42. The majority of these mutations are missense variants, however around 30% are frameshift, nonsense or splice junction variants which result in complete loss of p53 protein, commonly referred to as 'p53 nulls' 43. Despite this canonical cancer-associated defect, HGS OC does not generally display the classical activating oncogenic mutations typical of other solid tumor types 18, 44. Instead, extensive somatic copy number changes - rooted in chromosome instability and defective DNA repair - scar the genomic landscape 18, 45.
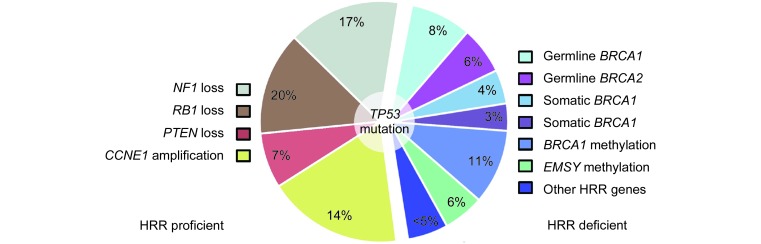
Around half of HGS OC have identifiable germline, somatic or epigenetic defects in the homologous recombination DNA repair (HRR) pathway, the flagship defects being germline or somatic BRCA1 or BRCA2 mutations which together account for approximately 20% of cases 46 ( Figure 1). Around 8% and 6% of HGS OC patients harbor germline BRCA1 and BRCA2 defects, respectively, while somatic changes in each occur in approximately 4% and 3% of cases 18, 47, 48. HRR-deficiency provides a rationale for the use of PARP inhibitors (PARPi), inducing synthetic lethality via inhibition of DNA single stranded break repair mechanisms and induction of error-prone non-homologous end joining (NHEJ) in HRR-deficient tumor cells 49. These agents have proven clinically effective, particularly in the BRCA-mutated HRR-deficient population 50, 51.
Figure1.
Common molecular events identified in HGS OC, including genetic and epigenetic defects in HRR pathway components (right).
Historically, analyses have grouped BRCA1- and BRCA2-defective patients together and have demonstrated improved sensitivity to cytotoxic chemotherapies and superior clinical outcome in this group, despite their propensity to develop visceral metastases and to present with HGS histology 46, 52- 56. However, it has recently emerged that the clinical implications of BRCA1 and BRCA2 defects are distinct, with BRCA1 carriers experiencing only short-term survival advantage while the survival benefit in BRCA2 carriers persists 10 years from diagnosis 57. Notably, BRCA1 can also be epigenetically inactivated, with around 11% of HGS OC showing BRCA1 promoter hypermethylation 18, 58. However, BRCA1-methylated patients are not thought to experience the same survival benefit as those with other BRCA defects, and may even experience reduced disease-free intervals and inferior OS 59.
Structural and copy number changes
HGS OC is characterized by substantial genetic heterogeneity and these tumors display large numbers of structural genomic changes 18, 45, 60. As such, identifying structural variants that represent driver events in tumorigenesis has presented a significant challenge. Such structural changes are now known to be an important mechanism of tumor suppressor gene inactivation in HGS OC, most notably affecting the RB and MAPK/PI3K signalling pathways 18, 45. The TCGA investigators identified defects in RB1 (8% deletion, 2% mutation), NF1 (8% deletion, 4% mutation) and PTEN (7% deletion, <1% mutation) of their HGS OC cohort 18. However, recent whole genome analysis of HGS OC has revealed that RB1 and NF1 are frequent targets of previously unidentified gene breakage events, affecting 20% and 17.5% of cases, respectively, underscoring the role of large structural rearrangements in tumor suppressor inactivation in OC 45.
Approximately 6% of HGS OC displays amplification of the EMSY gene, which encodes a BRCA2-inhibiting protein implicated in DNA repair regulation, as well as in chromatin remodelling and wider transcriptional control 61. These tumors are thought of as likely HRR-defective, and it has therefore been suggested that EMSY-amplified OC may respond well to PARPi therapy 62. However, the question of whether these tumors are truly HRR-deficient remains controversial and the efficacy of PARPi use within this group remains to be established.
Of the non-HRR-deficient HGS OC cases, a significant proportion display amplification of CCNE1, encoding the cell cycle checkpoint regulator cyclin E1. Approximately 14% of HGS OC harbors this abnormality, which has been proposed as a novel therapeutic target 18. It has been suggested that CCNE1 amplification and BRCA1/2 dysfunction occur mutually exclusively, and thus CCNE1-targeted therapies may represent a valuable treatment option in patients who are not candidates for PARPi therapy 45, 63. Furthermore, CCNE1 amplification has been implicated in intrinsic platinum resistance, and may represent a therapeutic target for sensitization of disease that is intrinsically resistant to cytotoxic agents 45, 64.
Gene expression
Because HGS represents the majority of OC, gene expression studies have thus far largely focussed on this subgroup. Indeed, HGS samples have dominated even mixed-histology gene expression studies, owing to its high prevalence relative to other histological subgroups 19. Using supervised and unsupervised analysis, these studies have been successful in generating prognostic gene signatures and discrete molecular subgroups, respectively 18, 19, 65- 71.
Tothill et al. 21 conducted unsupervised gene expression analysis of nearly 300 OC cases, the majority of which were HGS OC. They identified six molecular subgroups, termed C1-C6, four of which (C1, C2, C4 and C5) accounted for nearly all HGS samples. C2 tumors displayed high expression of immune response-related genes, while C5 tumors showed enrichment of genes expressed in mesenchymal development. C1 tumors were characterized by high expression of stromal genes and C4 were characterized by a low stromal response. Multivariate survival analysis comparing C1 tumors versus the other three HGS subgroups revealed that these patients had significantly inferior PFS and OS, implicating an involvement of the stromal response in patient outcome.
Subsequent to the Tothill study, the Cancer Genome Atlas (TCGA) investigators identified four transcriptionally-defined groups within HGS OC 18. These subgroups - termed proliferative, mesenchymal, immunoreactive and differentiated - did not display significant survival differences within the TCGA dataset, but recapitulation of these groups in other datasets has shown clinical relevance, revealing a survival advantage for those in the immunoreactive group 66. The TCGA immunoreactive and mesenchymal groups were consistent with the Tothill C2 and C5 groups, respectively.
While a survival advantage for HGS OC with high levels of immune activation is emerging, these subgrouping methods are yet to be utilized clinically, and we await consensus on molecular subgrouping of HGS OC that can ultimately be taken forward into routine clinical practice.
Numerous gene expression signatures for predicting survival in advanced stage OC have been produced using supervised analyses of mRNA expression data from various platforms. These signatures have been produced using training datasets of varying numbers and many have proven prognostic in independent datasets 65- 73. Among them is the signature produced by the TCGA investigators, which has validated in multiple datasets 18. However, performance of these survival signatures varies between independent cohorts, with poor correlation of risk scores between some studies, and such signatures are yet to be used to inform OC patient management 72.
Heterogeneity
As the emergence of chemoresistant recurrent disease represents the primary cause of mortality in HGS OC patients, intratumor heterogeneity, facilitating selection of pre-existing chemoresistant subclones during treatment, is of great interest. Expansion of these clones represents an avenue for rapid emergency of recurrent disease with therapy resistance 74, 75. Such intratumoral heterogeneity is a prominent feature in HGS OC 76, 77. Indeed, quantitatively assessment of intratumoral heterogeneity may prove clinically informative in HGS OC, with patients suffering from highly heterogeneous disease showing shorter PFS and OS 78.
Existence of genetically and phenotypically diverse subclonal populations within primary HGS disease also present a critical avenue for the failure of novel and existing targeted therapies. Accordingly, the design of novel targeted therapeutic strategies must be centered towards carcinogenic driver mutations at the 'trunk' of the evolving cancer genome.
Molecular changes in acquired therapy resistance in HGS OC
The majority of HGS OC patients have a good clinical response to primary platinum-based chemotherapy. As a result, characterizing mechanisms of acquired resistance in the recurrent disease setting is of great clinical relevance. Reversion of BRCA1 and BRCA2 mutations via secondary genomic events that restore open reading frames, returning HRR proficiency, has been proposed as one mechanism of reducing sensitivity to conventional therapies 45, 79. Accordingly, these BRCA-reverted tumors may well also show decreased sensitivity to targeted PARPi therapy.
Further proposed mechanisms of acquired resistance include upregulation of AKT signalling, promoting cell survival, and increased expression of the ABCB1 drug efflux protein via promoter hijacking 45, 80. Paclitaxel, commonly used in combination with platinum agents in first-line treatment of OC, is a known substrate of ABCB1, and increased expression therefore represents a viable mechanism for reduced accumulation of cytotoxic agents within ABCB1-expressing OC cells 81. These molecular events may well be clinically actionable in the hope of re-sensitizing disease to conventional therapies, although modulation of ABCB1 activity has not yet proven efficacious in advanced stage OC 82.
While some mechanisms of therapy resistance have been elucidated, identification of pathways involved in platinum-resistant recurrent OC remains in its infancy and existing studies have investigated a relatively small number of relapsed disease samples 45, 79, 80. Extensive characterization of the molecular events underpinning subsequent treatment failure have largely been hindered by the failure to acquire multiple, temporally separated biopsies from the same patient throughout the course of their disease. Investigation of large cohorts of such paired samples will undoubtedly uncover further mechanisms of acquired disease resistance which may present further opportunities for therapeutic intervention and re-sensitization of chemoresistant disease.
Epigenetic and microRNA dysregulation in HGS OC
OC research thus far has largely been dominated by analyses at the exomic sequence and transcriptional level. However, progress in defining the epigenomic and microRNA landscapes of OC has been made in recent years.
Promoter hypermethylation and associated gene silencing of BRCA1 is perhaps the most canonical epigenetic defect in HGS OC. While these tumors are considered HRR-deficient and this molecular event appears to occur mutually exclusively with germline or somatic BRCA1/2 mutation, BRCA1-methylated patients may not experience the classical BRCA-associated clinical benefit, as discussed above 18, 58, 59. Accordingly, this epigenetic defect may be of limited clinical interest.
The TCGA investigators reported over 150 genes with increased DNA methylation and associated reduction in gene expression, including BRCA1 18. Clustering of HGS OC samples within their dataset revealed four subtypes based on differential methylation which overlapped significantly with their transcriptionally-defined groups.
Further to DNA methylation analysis, the TCGA investigators reported three HGS OC subtypes defined by their differential microRNA expression profiles, with one of these subtypes displaying superior OS 18. Numerous studies have now found associations between expression of specific microRNAs and clinical parameters, including disease stage, histological subtype and chemoresistance 83- 85.
Improved understanding of how miRNA and epigenomic dysregulation contribute to OC tumorigenesis will undoubtedly further understanding of disease biology, and may well reveal opportunities for therapeutic intervention.
Key future research foci for HGS OC
With characterization of HGS OC at the DNA sequence level having made substantial advances, understanding the clinical implications of each of these molecular events is a clear long term research goal. In particular, the importance of NF1 and RB1 disruption - whether by deletion, mutation or recently identified gene breakage - are of great interest, as together these represent a significant proportion (approximately one third) of HGS OC.
While those patients with germline BRCA1 and BRCA2 mutations have been fairly well characterized in terms of improved sensitivity to platinum and efficacy of PARP inhibitors, the clinical implications of genetic events in other HRR pathway components - including EMSY amplification - remain to be extensively investigated. Further consideration of BRCA1 and BRCA2 defects as distinct entities, and comparison with BRCA1 promoter hypermethylation, will also help address whether all HRR pathway abnormalities convey equal clinical implication - although evidence thus far would suggest otherwise. Because of the relative rarity of non-BRCA HRR pathway aberrations, large retrospective cohorts of molecularly characterized patients with rich clinical annotation will be required.
A key step for future research will be to establish panels of cell lines that represent the spectrum of molecular changes that are now known to occur in HGS OC. This will provide an invaluable pre-clinical resource for investigating novel therapeutic strategies in the context of underlying molecular biology of disease, and will likely uncover potential new biomarkers for sensitivity to both targeted and conventional therapies.
The acquisition and molecular characterization of paired chemosensitive primary and chemoresistant recurrent disease specimens represent an immediate research priority. Sampling from recurrent disease, end stage disease and even post-mortem sampling from rapid autopsy will undoubtedly shed more light upon the molecular mechanisms underpinning therapy-resistant recurrent disease, to which patients ultimate succumb.
In terms of transcriptional profiling of HGS OC, significant advances have been made in finding clinically relevant subgroups. Unsupervised analyses have identified molecular subgroups with distinct clinical outcomes and supervised approaches have produced gene expression signatures predictive of survival. However, a real consensus in subgrouping remains to be established. Reproducibility has in part been hindered by limited sample numbers in training datasets in some studies, differential histological composition of datasets, as well as technical factors such as the diversity of gene expression platforms used and the use of fresh-frozen versus formalin-fixed paraffin-embedded (FFPE) tumor material. The culmination of these pitfalls is that molecular subgrouping is ultimately not currently used to guide management of patients in the clinic. Demonstrating subgroup-specific actionable molecular biology and drug sensitivities in the research setting will be a crucial step in demonstrating the need for such stratification in the clinic.
Given numerous reports of the impact of tumoral-immune response on PFS and OS, understanding if and how these above mentioned molecular features of OC influence the interaction with the host immune system will also be of interest 86, 87. Indeed, recent data have shown that BRCA1/2-mutated OCs display higher levels of tumor-infiltrating lymphocytes, indicating these tumors may be more immunogenic, consistent with the survival advantage experienced by this patient group 88. Such analyses may well prove informative in relation to the efficacy of immune checkpoint inhibitor therapy, and establishing biomarkers of tumor immunogenicity may help stratify patients who are likely to benefit from these, and other, immunotherapeutic strategies.
Genetic and molecular changes in non-HGS OC
Endometrioid, CC, LGS and mucinous subtypes account for nearly all remaining OC cases, representing around 10%, 10%, <5% and <5% of OC cases, respectively. These rarer subtypes represent distinct disease entities from both HGS OC and from one another. Collectively, and conversely to HGS OC, they harbor activating oncogenic mutations more typical of solid tumors but do not display high rates of TP53 mutation 89, 90.
Endometrioid OC
Of the OC histological subtypes, endometrioid OC represents the group with most favorable clinical outcome: they have a tendency to be diagnosed at earlier stage versus HGS OC and are generally sensitive to platinum in the first line setting 27, 28. Endometrioid OC is associated with endometriosis, and their gene expression profiles bear resemblance to that of endometrial tissue 9, 16, 91. Together, endometrioid and CC OC represent the majority of Lynch syndrome-associated OC cases 92- 94.
Historically, endometrioid OC has been subclassified into either low or high grade disease. However, it is now recognized that high grade endometrioid OC more closely resembles HGS OC both molecularly and clinically, while low grade endometrioid OC represents a more distinct 'true endometrioid' OC subtype 95.
Mutations deregulating the PI3K pathway are common in this latter subtype: around 20% of cases harbor PTEN tumor suppressor gene mutations and around 30% display activating PIK3CA mutations 96- 98. Around 30% of endometrioid OC displays mutations in the chromatin-remodelling associated gene ARID1A, a suggested tumor suppressor gene 99, 100. A minority also harbor somatic mutations in the PPP2R1A gene, encoding a subunit of protein phosphatase 2A 101.
Endometrioid OC also commonly displays activated Wnt signalling, with around half showing CTNNB1 mutation 96, 97, 102.
Clear cell OC
As with endometrioid ovarian cancer, CC OC is associated with endometriosis and has a tendency to be diagnosed at earlier stage versus HGS OC 22, 29, 30. However, CC OC frequently displays intrinsic platinum resistance and advanced stage CC OC remains a great clinical challenge, with inferior PFS and OS compared to advanced stage HGS OC 22, 30. CC OC accounts for the majority of non-endometrioid cases associated with inherited MMR deficiency 93, 94.
Like endometrioid OC, CC carcinomas harbor defects in PTEN (in around 10% of cases), PIK3CA (in around 50% of cases), and ARID1A (in around 50% of cases), consistent with the shared molecular pathogenesis and developmental origins of these carcinomas 97, 99, 103- 106. Similarly, a minority display somatic PPP2R1A mutation 101. However, unlike endometrioid OC, around a third of CC tumors show amplification of chr20q13.2 and do not generally harbor Wnt-activating CTNNB1 mutations 97, 107.
Mucinous OC
Mucinous OC was once thought to account for a significant number of OC cases, but it is now recognized that few are true primary mucinous OC, while the rest represent metastases from other malignancies, most commonly from the gastrointestinal tract 9, 108. In comparison to HGS OC, mucinous OC tends to present at earlier stage 109, 110. However, this subtype frequently displays platinum resistance in the first line setting, and advanced stage mucinous OC is associated with particularly poor OS 24, 111.
The molecular pathogenesis of mucinous OC remains relatively poorly understood, and extensive characterization of this subtype has largely been hindered by its low prevalence compared to other histological OC subtypes. However, KRAS mutation and HER2 gene amplification are known common events in mucinous OC, with around 50% and 20% of cases displaying these defects, respectively 109, 112.
LGS OC
LGS OC is characterized by young age at diagnosis, indolent disease course, and prolonged OS versus HGS OC, even when diagnosed at advanced stage 25. Over 60% of LGS OC harbor MAPK pathway-activating KRAS or BRAF mutations, accounting for around 30% of cases each, but almost invariably do not harbor TP53 mutations 113- 115. Furthermore, KRAS mutation may be associated with more aggressive, recurrent disease versus BRAF-mutated LGS OC 116, 117.
Key future research foci for non-HGS OC
Recognition of non-HGS OC subtypes as distinct disease entities has been a critical step in OC research. Although mucinous, CC and endometrioid OC is more commonly diagnosed at early stage versus HGS OC, advanced stage cases present a significant clinical challenge 22, 27- 30, 111. Clinical studies now need to be performed in a subtype-specific fashion in order to properly characterize the underlying biology within each of these histotypes at both the genomic and transcriptional level.
In particular, the characterization of non-HGS OC subtypes that display resistance to the platinum-based chemotherapies are needed, as these agents remain a cornerstone of OC treatment irrespective of histological subtype. These studies hope to uncover underlying disease biology that may be actionable through the use of novel or existing targeted therapies, with the aim of improving treatment either directly through selective cytotoxicity or indirectly through sensitizing disease to conventional therapies.
Our current understanding of the biology behind these rarer subtypes has already revealed avenues for potential implementation of targeted therapies: LGS and mucinous OC may well benefit from MAPK pathway inhibitors, while CC and endometrioid OC may benefit from agents targeting the PI3K pathway. Indeed, the MEK1/2 inhibitor selumetinib has shown promising results in patients with recurrent LGS OC 118. Targeting HER2 has already proven a successful treatment strategy in HER2-amplified breast cancer, and the use of therapies such as the monoclonal antibody trastuzumab may also prove useful in treating HER2-amplified mucinous OC 119. Investigating the potential therapeutic efficacy of these and other agents in genomically characterized disease models will be an important step toward therapy stratification within these rarer OC subtypes.
Perhaps the greatest challenge for single-histotype studies of non-HGS OC will be acquiring large cohorts of these rarer subtypes. Historic samples will need to undergo rigorous pathology review to ensure the accuracy of histotype assignment. Sample numbers will be a particular challenge for studies looking to identify transcriptionally-defined molecular subgroups by unsupervised analysis, as these investigations will require large training and validation cohorts. Indeed, acquiring uniformly staged, graded and treated cohorts of OC remains a challenge even in HGS disease.
Conclusions
OC is a substantial cause of morbidity and mortality in the developed world. A number of clinical features are known to affect PFS and OS rates in OC, including disease stage, grade and surgical outcome. The five main histologically-defined subtypes of OC are now recognized as separate diseases and display differences in stage at diagnosis, responses to platinum-based chemotherapies as well as OS.
However, substantial clinical heterogeneity remains even within these histological groups, particularly within HGS which represents the majority of OC. As such, the majority of the research effort thus far has focused on this subtype, elucidating clinically meaningful subgroups at both the genomic and transcriptomic level, despite extreme genomic heterogeneity. The challenge remains for these subgroups to be taken forward into the clinic, and we await a consensus on clinically meaningful transcriptomic subgroups that validate in the wealth of publicly available HGS OC gene expression data. At present, only BRCA status is routinely used clinically, with germline BRCA1 and BRCA2 genetic testing now in place at a number of centers as a biomarker for the use of PARPi therapy. Rarer genomic defects in HRR pathway components remains an area of great interest, and the field awaits data on whether these patients are truly HRR deficient and likely to benefit from PARP inhibition.
The clinical implications of more recently identified genomic defects, including NF1 and RB1 loss, remain to be established. Together with CCNE1 amplification, these defects account for much of HRR-proficient HGS OC, and finding novel therapeutic strategies to improve clinical outcome in these patients is an area of substantial unmet need.
Recurrent HGS OS with acquired chemoresistance is the ultimate cause of the majority of patient mortality. Therefore, investigating its molecular drivers is an urgent research priority. The acquisition and molecular characterization of matched primary and recurrent samples promise to reveal opportunities for using novel therapeutic strategies and re-sensitizing to cytotoxic agents.
Non-HGS OC is characterized by more classical oncogenic mutations, and subtype-specific studies of endometrioid, CC, LGS and mucinous OC are now needed to further stratify these subtypes at both the transcriptomic and genomic level. The real challenge for these investigations will be acquiring sufficiently large cohorts to make meaningful conclusions that can pave the way for stratification of therapy, which will undoubtedly require international collaborative efforts.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- Farley J, Brady WE, Vathipadiekal V, Lankes HA, Coleman R, Morgan MA, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. 2013;14:134–40. doi: 10.1016/S1470-2045(12)70572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013 Oct;24 Suppl 6:vi24-32.
- Chan JK, Urban R, Cheung MK, Osann K, Husain A, Teng NN, et al. Ovarian cancer in younger vs older women: a population-based analysis. Br J Cancer. 2006;95:1314–20. doi: 10.1038/sj.bjc.6603457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer RO. Survival and treatment differences by age. Cancer. 1993;71:524–9. doi: 10.1002/cncr.2820710206. [DOI] [PubMed] [Google Scholar]
- Chan JK, Tian C, Monk BJ, Herzog T, Kapp DS, Bell J, et al. Prognostic factors for high-risk early-stage epithelial ovarian cancer: a Gynecologic Oncology Group study. Cancer. 2008;112:2202–10. doi: 10.1002/cncr.23390. [DOI] [PubMed] [Google Scholar]
- du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115:1234–44. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460:237–49. doi: 10.1007/s00428-012-1203-5. [DOI] [PubMed] [Google Scholar]
- Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- Marquez RT, Baggerly KA, Patterson AP, Liu J, Broaddus R, Frumovitz M, et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005;11:6116–26. doi: 10.1158/1078-0432.CCR-04-2509. [DOI] [PubMed] [Google Scholar]
- Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek JM, Van Diest PJ, Zweemer RP, Jansen JA, Menko FH, Gille JJ, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–6. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- Falconer H, Yin L, Grönberg H, Altman D. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015; 107. pii: dju410.
- Kuhn E, Kurman RJ, Vang R, Sehdev AS, Han GM, Soslow R, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma-evidence supporting the clonal relationship of the two lesions. J Pathol. 2012;226:421–6. doi: 10.1002/path.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, et al. Transformation of the fallopian tube secretory epithelium leads to High-Grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24:751–65. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somigliana E, Vigano' P, Parazzini F, Stoppelli S, Giambattista E, Vercellini P. Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol Oncol. 2006;101:331–41. doi: 10.1016/j.ygyno.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Vang R, Shih IeM, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009;16:267–82. doi: 10.1097/PAP.0b013e3181b4fffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- Zorn KK, Bonome T, Gangi L, Chandramouli GV, Awtrey CS, Gardner GJ, et al. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clin Cancer Res. 2005;11:6422–30. doi: 10.1158/1078-0432.CCR-05-0508. [DOI] [PubMed] [Google Scholar]
- Vaughan S, Coward JI, Bast RC, Berchuck A, Berek JS, Brenton JD, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–25. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–9. [PubMed] [Google Scholar]
- Schmeler KM, Sun CC, Bodurka DC, Deavers MT, Malpica A, Coleman RL, et al. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2008;108:510–4. doi: 10.1016/j.ygyno.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Hess V, A'hern R, Nasiri N, King DM, Blake PR, Barton DP, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol. 2004;22:1040–4. doi: 10.1200/JCO.2004.08.078. [DOI] [PubMed] [Google Scholar]
- Gershenson DM, Sun CC, Lu KH, Coleman RL, Sood AK, Malpica A, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006;108:361–8. doi: 10.1097/01.AOG.0000227787.24587.d1. [DOI] [PubMed] [Google Scholar]
- Gilks CB, Ionescu DN, Kalloger SE, Koebel M, Irving J, Clarke B, et al. Tumor cell type can be reproducibly diagnosed and is of Independent prognostic significance in patients with maximally debulked ovarian carcinoma. Hum Pathol. 2008;39:1239–51. doi: 10.1016/j.humpath.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Aysal A, Karnezis A, Medhi I, Grenert JP, Zaloudek CJ, Rabban JT. Ovarian endometrioid adenocarcinoma: incidence and clinical significance of the morphologic and immunohistochemical markers of mismatch repair protein defects and tumor microsatellite instability. Am J Surg Pathol. 2012;36:163–72. doi: 10.1097/PAS.0b013e31823bc434. [DOI] [PubMed] [Google Scholar]
- Storey DJ, Rush R, Stewart M, Rye TA, Williams AR, Smyth JF. Endometrioid epithelial ovarian cancer - 20 years of prospectively collected data from a single center. Cancer. 2008;112:2211–20. doi: 10.1002/cncr.23438. [DOI] [PubMed] [Google Scholar]
- Takano M, Kikuchi Y, Yaegashi N, Kuzuya K, Ueki M, Tsuda H, et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006;94:1369–74. doi: 10.1038/sj.bjc.6603116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M, Takano M, Goto T, Kato M, Sasaki N, Tsuda H, et al. Clear cell histology as a poor prognostic factor for advanced epithelial ovarian cancer: a single institutional case series through central pathologic review. J Gynecol Oncol. 2013;24:37–43. doi: 10.3802/jgo.2013.24.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- King MC, Marks JH, Mandell JB, New York Breast Canc Study Grp. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–6. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A. 2011;108:18032–7. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveday C, Turnbull C, Ramsay E, Hughes D, Ruark E, Frankum JR, et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet. 2011;43:879–82. doi: 10.1038/ng.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadei S, Norquist BM, Walsh T, Stray S, Mandell JB, Lee MK, et al. Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res. 2011;71:2222–9. doi: 10.1158/0008-5472.CAN-10-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl A, Hellebrand H, Wiek C, Erven V, Wappenschmidt B, Niederacher D, et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet. 2010;42:410–4. doi: 10.1038/ng.569. [DOI] [PubMed] [Google Scholar]
- Watson P, Riley B. The tumor spectrum in the lynch syndrome. Fam Cancer. 2005;4:245–8. doi: 10.1007/s10689-004-7994-z. [DOI] [PubMed] [Google Scholar]
- Robinson KL, Liu T, Vandrovcova JA, Clendenning M, Frebourg TA, Kinzler KW, et al. Lynch syndrome (hereditary nonpolyposis colorectal cancer) diagnostics. J Natl Cancer Inst. 2007;99:291–9. doi: 10.1093/jnci/djk051. [DOI] [PubMed] [Google Scholar]
- Lynch HT, Casey MJ, Snyder CL, Bewtra C, Lynch JF, Butts M, et al. Hereditary ovarian carcinoma: heterogeneity, molecular genetics, pathology, and management. Mol Oncol. 2009;3:97–137. doi: 10.1016/j.molonc.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman JD, Yemelyanova A, Cosin JA, Smith A, Kurman RJ. Survival rates for international federation of gynecology and obstetrics stage iii ovarian carcinoma by cell type: A study of 262 unselected patients with uniform pathologic review. Int J Gynecol Cancer. 2012;22:367–71. doi: 10.1097/IGC.0b013e31823c6f80. [DOI] [PubMed] [Google Scholar]
- Salani R, Kurman RJ, Giuntoli R, Gardner G, Bristow R, Wang TL, et al. Assessment of TP53 mutation using purified tissue samples of ovarian serous carcinomas reveals a higher mutation rate than previously reported and does not correlate with drug resistance. Int J Gynecol Cancer. 2008;18:487–91. doi: 10.1111/j.1525-1438.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- Ahmed AA, Etemadmoghadam D, Temple J, Lynch AG, Riad M, Sharma R, et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. 2010;221:49–56. doi: 10.1002/path.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, Shih IM, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24:1248–53. doi: 10.1038/modpathol.2011.85. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–58. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–94. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- Bolton KL, Chenevix-Trench G, Goh CA, Ramus SJ, Karlan BY, Lambrechts D, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382–90. doi: 10.1001/jama.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–16. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- Kanchi KL, Johnson KJ, Lu C, McLellan MD, Leiserson MD, Wendl MC, et al. Integrated analysis of germline and somatic variants in ovarian cancer. Nat Commun. 2014;5:3156. doi: 10.1038/ncomms4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(adp-ribose) polymerase (parp) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci U S A. 2011;108:3406–11. doi: 10.1073/pnas.1013715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–61. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- Scott CL, Swisher EM, Kaufmann SH. Poly (adp-ribose) polymerase inhibitors: Recent advances and future development. J Clin Oncol. 2015;33:1397–406. doi: 10.1200/JCO.2014.58.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, et al. Germline and somatic mutations in homologous recombination genes predict Platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–75. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodnova TV, Sokolenko AP, Ivantsov AO, Iyevleva AG, Suspitsin EN, Aleksakhina SN, et al. High response rates to neoadjuvant platinum-based therapy in ovarian cancer patients carrying germ-line BRCA mutation. Cancer Lett. 2015;369:363–7. doi: 10.1016/j.canlet.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Gourley C, Michie CO, Roxburgh P, Yap TA, Harden S, Paul J, et al. Increased incidence of visceral metastases in Scottish patients with BRCA1/2-defective ovarian cancer: an extension of the ovarian BRCAness phenotype. J Clin Oncol. 2010;28:2505–11. doi: 10.1200/JCO.2009.25.1082. [DOI] [PubMed] [Google Scholar]
- Piek JM, Torrenga B, Hermsen B, Verheijen RH, Zweemer RP, Gille JJ, et al. Histopathological characteristics of BRCA1- and BRCA2-associated intraperitoneal cancer: a clinic-based study. Fam Cancer. 2003;2:73–8. doi: 10.1023/a:1025700807451. [DOI] [PubMed] [Google Scholar]
- Gilks CB, Prat J. Ovarian carcinoma pathology and genetics: recent advances. Hum Pathol. 2009;40:1213–23. doi: 10.1016/j.humpath.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Candido-Dos-Reis FJ, Song H, Goode EL, Cunningham JM, Fridley BL, Larson MC, et al. Germline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer. Clin Cancer Res. 2015;21:652–7. doi: 10.1158/1078-0432.CCR-14-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin RL, Nemeth E, Tran H, Shvartsman H, Cass IA, Karlan BY. BRCA1 promoter region hypermethylation in ovarian carcinoma: A population-based study. Cancer Res. 2000;60:5329–33. [PubMed] [Google Scholar]
- Chiang JW, Karlan BY, Cass L, Baldwin RL. BRCA1 promoter methylation predicts adverse ovarian cancer prognosis. Gynecol Oncol. 2006;101:403–10. doi: 10.1016/j.ygyno.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Wang ZC, Birkbak NJ, Culhane AC, Drapkin R, Fatima A, Tian R, et al. Profiles of genomic instability in high-grade serous ovarian cancer predict treatment outcome. Clin Cancer Res. 2012;18:5806–15. doi: 10.1158/1078-0432.CCR-12-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes-Davies L, Huntsman D, Ruas M, Fuks F, Bye J, Chin SF, et al. Emsy links the BRCA2 pathway to sporadic breast and ovarian cancer. Cell. 2003;115:523–35. doi: 10.1016/s0092-8674(03)00930-9. [DOI] [PubMed] [Google Scholar]
- Bast RC, Mills GB. Personalizing therapy for ovarian cancer: BRCAness and beyond. J Clin Oncol. 2010;28:3545–8. doi: 10.1200/JCO.2010.28.5791. [DOI] [PubMed] [Google Scholar]
- Etemadmoghadam D, Weir BA, Au-Yeung G, Alsop K, Mitchell G, George J, et al. Synthetic lethality between CCNE1 amplification and loss of BRCA1. Proc Natl Acad Sci U S A. 2013;110:19489–94. doi: 10.1073/pnas.1314302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadmoghadam D, Defazio A, Beroukhim R, Mermel C, George J, Getz G, et al. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary chemoresistance in ovarian carcinomas. Clin Cancer Res. 2009;15:1417–27. doi: 10.1158/1078-0432.CCR-08-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentink S, Haibe-Kains B, Risch T, Fan JB, Hirsch MS, Holton K, et al. Angiogenic mRNA and microRNA gene expression signature predicts a novel subtype of serous ovarian cancer. PLoS One. 2012;7:e30269. doi: 10.1371/journal.pone.0030269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecny GE, Wang C, Hamidi H, Winterhoff B, Kalli KR, Dering J, et al. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J Natl Cancer Inst. 2014;106:106. doi: 10.1093/jnci/dju249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfakianos GP, Iversen ES, Whitaker RA, Schildkraut JL, Murphy SK, Marks JR, et al. Validation of ovarian cancer gene expression signatures for survival and subtype in formalin fixed paraffin embedded tissues. Gynecol Oncol. 2013;129:159–64. doi: 10.1016/j.ygyno.2012.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonome T, Levine DA, Shih J, Randonovich M, Pise-Masison CA, Bogomolniy F, et al. A gene signature predicting for survival in suboptimally debulked patients with ovarian cancer. Cancer Res. 2008;68:5478–86. doi: 10.1158/0008-5472.CAN-07-6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok SC, Bonome T, Vathipadiekal V, Bell A, Johnson ME, Wong KK, et al. A gene signature predictive for outcome in advanced ovarian cancer identifies a survival factor: microfibril-associated glycoprotein 2. Cancer Cell. 2009;16:521–32. doi: 10.1016/j.ccr.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara K, Tsunoda T, Shigemizu DA, Hatae M, Fujiwara HA, Katabuchi H, et al. High-Risk ovarian cancer based on 126-Gene expression signature is uniquely characterized by downregulation of antigen presentation pathway. Clin Cancer Res. 2012;18:1374–85. doi: 10.1158/1078-0432.CCR-11-2725. [DOI] [PubMed] [Google Scholar]
- Yoshihara K, Tajima A, Yahata T, Kodama S, Fujiwara H, Suzuki M, et al. Gene expression profile for predicting survival in Advanced-Stage serous ovarian cancer across two Independent datasets. PLoS One. 2010;5:e9615. doi: 10.1371/journal.pone.0009615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron L, Haibe-Kains B, Culhane AC, Riester M, Ding J, Wang XV, et al. Comparative meta-analysis of prognostic gene signatures for late-stage ovarian cancer. J Natl Cancer Inst. 2014;106:106. doi: 10.1093/jnci/dju049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riester M, Wei W, Waldron L, Culhane AC, Trippa L, Oliva E, et al. Risk prediction for late-stage ovarian cancer by meta-analysis of 1525 patient samples. J Natl Cancer Inst. 2014;106:106. doi: 10.1093/jnci/dju048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford A, Jamal-Hanjani M, Hicks JA. Implications of intratumor heterogeneity for treatment stratification. J Pathol. 2014;232:264–73. doi: 10.1002/path.4270. [DOI] [PubMed] [Google Scholar]
- Burrell RA, Mcgranahan N, Bartek J, Swanton C. The causes and Consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338–45. doi: 10.1038/nature12625. [DOI] [PubMed] [Google Scholar]
- Bashashati A, Ha G, Tone A, Ding JA, Roth A, Rosner J, et al. Distinct evolutionary trajectories of primary high-grade serous ovarian cancers revealed through spatial mutational profiling. J Pathol. 2013;231:21–34. doi: 10.1002/path.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SL, Ng C, Melnyk N, Garcia M, Temple J, Langdon S, et al. Genomic analysis of genetic heterogeneity and evolution in high-grade serous ovarian carcinoma. Oncogene. 2010;29:4905–13. doi: 10.1038/onc.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz RF, Ng CK, Cooke SL, Newman S, Temple J, Piskorz AM, et al. Spatial and temporal heterogeneity in high-grade serous ovarian cancer: a phylogenetic analysis. PLoS Med. 2015;12:e1001789. doi: 10.1371/journal.pmed.1001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norquist B, Wurz KA, Pennil CC, Gross J, Sakai W, Karlan BY, et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol. 2011;29:3008–15. doi: 10.1200/JCO.2010.34.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronach EA, Chen M, Maginn EN, Agarwal R, Mills GB, Wasan H, et al. DNA-PK mediates AKT activation and apoptosis inhibition in clinically acquired Platinum resistance. Neoplasia. 2011;13:1069–80. doi: 10.1593/neo.111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther. 2006;112:457–73. doi: 10.1016/j.pharmthera.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Lhomme C, Joly F, Walker JL, Lissoni AA, Nicoletto MO, Manikhas GM, et al. Phase III study of valspodar (psc 833) combined with paclitaxel and carboplatin compared with paclitaxel and carboplatin alone in patients with stage IV or suboptimally debulked stage III epithelial ovarian cancer or primary peritoneal cancer. J Clin Oncol. 2008;26:2674–82. doi: 10.1200/JCO.2007.14.9807. [DOI] [PubMed] [Google Scholar]
- Vecchione A, Belletti B, Lovat F, Volinia S, Chiappetta G, Giglio S, et al. A microrna signature defines chemoresistance in ovarian cancer through modulation of angiogenesis. Proc Natl Acad Sci U S A. 2013;110:9845–50. doi: 10.1073/pnas.1305472110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- Zhang L, Volinia S, Bonome T, Calin G, Greshock J, Yang N, et al. Genomic and epigenetic alterations deregulate microrna expression in human epithelial ovarian cancer. Proc Natl Acad Sci U S A. 2008;105:7004–9. doi: 10.1073/pnas.0801615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Regnani G, Makrigiannakis A, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated withfavorable prognosis in ovarian cancer Proc Natl Acad Sci U S A. 2005; 102: 18538-43.
- Strickland KC, Howitt BE, Shukla SA, Rodig S, Ritterhouse LL, Liu JF, et al. Association and prognostic significance of brca1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of pd-1/pd-l1 in high grade serous ovarian cancer. Oncotarget. 2016 Feb 9. doi: 10.18632/oncotarget.7277. [Epub ahead of print]
- Shih IeM, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–8. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurman RJ, Shih IM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer-Shifting the paradigm. Hum Pathol. 2011;42:918–31. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz de la Cuesta R, Eichhorn JH, Rice LW, Fuller AF, Nikrui N, Goff BA , Nikrui N, Goff BA. Histologic transformation of benign endometriosis to early epithelial ovarian cancer. Gynecol Oncol. 1996;60:238–44. doi: 10.1006/gyno.1996.0032. [DOI] [PubMed] [Google Scholar]
- Liu J, Albarracin CT, Chang KH, Thompson-Lanza JA, Zheng W, Gershenson DM, et al. Microsatellite instability and expression of hMLH1 and hMSH2 proteins in ovarian endometrioid cancer. Mod Pathol. 2004;17:75–80. doi: 10.1038/modpathol.3800017. [DOI] [PubMed] [Google Scholar]
- Pal T, Permuth-Wey J, Kumar A, Sellers TA. Systematic review and meta-analysis of ovarian cancers: estimation of Microsatellite-High frequency and characterization of mismatch repair deficient tumor histology. Clin Cancer Res. 2008;14:6847–54. doi: 10.1158/1078-0432.CCR-08-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui MH, Ryan P, Radigan J, Ferguson SE, Pollett A, Aronson M, et al. The histomorphology of lynch syndrome-associated ovarian carcinomas toward a subtype-specific screening strategy. Am J Surg Pathol. 2014;38:1173–81. doi: 10.1097/PAS.0000000000000298. [DOI] [PubMed] [Google Scholar]
- Schwartz DR, Kardia SL, Shedden KA, Kuick R, Michailidis G, Taylor JM, et al. Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Cancer Res. 2002;62:4722–9. [PubMed] [Google Scholar]
- Mcconechy MK, Ding JR, Senz J, Yang W, Melnyk N, Tone AA, et al. Ovarian and endometrial endometrioid carcinomas have distinct CTNNB1 and PTEN mutation profiles. Mod Pathol. 2014;27:128–34. doi: 10.1038/modpathol.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Yamazaki M, Takahashi HA, Suzuki E, Tsuruta T, Saegusa M. Distinct beta-Catenin and PIK3CA mutation profiles in endometriosis-associated ovarian endometrioid and clear cell carcinomas. Am J Clin Pathol. 2015;144:452–63. doi: 10.1309/AJCPZ5T2POOFMQVN. [DOI] [PubMed] [Google Scholar]
- Obata K, Morland SJ, Watson RH, Hitchcock A, Chenevix-Trench G, Thomas EJ, et al. Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res. 1998;58:2095–7. [PubMed] [Google Scholar]
- Wiegand KC, Shah SP, Al-Agha OM, Zhao YJ, Tse K, Zeng T, et al. ARID1A mutations in Endometriosis-Associated ovarian carcinomas. N Engl J Med. 2010;363:1532–43. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan B, Wang TL, Shih IM. ARID1A, a factor that promotes formation of SWI/SNF-Mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 2011;71:6718–27. doi: 10.1158/0008-5472.CAN-11-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih IM, Panuganti PK, Kuo KT, Mao TL, Kuhn E, Jones S, et al. Somatic mutations of PPP2R1A in ovarian and uterine carcinomas. Am J Pathol. 2011;178:1442–7. doi: 10.1016/j.ajpath.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KR, Shih IM. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Wang TL, Shih IM, Mao T, Roden R, Glas R, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–31. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, Kubo T, et al. Loss of heterozygosity on 10q23. Loss of heterozygosity on 10q23. 3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: Possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052–6. [PubMed] [Google Scholar]
- Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–81. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- Kuo KT, Mao TL, Jones S, Veras E, Ayhan A, Wang TL, et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol. 2009;174:1597–601. doi: 10.2353/ajpath.2009.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo KT, Mao TL, Chen X, Feng YA, Wang YE, Glas R, et al. DNA copy numbers profiles in Affinity-Purified ovarian clear cell carcinoma. Clin Cancer Res. 2010;16:1997–2008. doi: 10.1158/1078-0432.CCR-09-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaino RJ, Brady MF, Lele SM, Michael H, Greer B, Bookman MA. Advanced stage mucinous adenocarcinoma of the ovary is both rare and highly lethal a gynecologic oncology group study. Cancer. 2011;117:554–62. doi: 10.1002/cncr.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglesio MS, Kommoss S, Tolcher MC, Clarke B, Galletta L, Porter H, et al. Molecular characterization of mucinous ovarian tumors supports a stratified treatment approach with HER2 targeting in 19% of carcinomas. J Pathol. 2013;229:111–20. doi: 10.1002/path.4088. [DOI] [PubMed] [Google Scholar]
- Brown J, Frumovitz M. Mucinous tumors of the ovary: current thoughts on diagnosis and management. Curr Oncol Rep. 2014;16:389. doi: 10.1007/s11912-014-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamias A, Psaltopoulou T, Sotiropoulou M, Haidopoulos D, Lianos E, Bournakis E, et al. Mucinous but not clear cell histology is associated with inferior survival in patients with advanced stage ovarian carcinoma treated with platinum-paclitaxel chemotherapy. Cancer. 2010;116:1462–8. doi: 10.1002/cncr.24915. [DOI] [PubMed] [Google Scholar]
- Gemignani ML, Schlaerth AC, Bogomolniy F, Barakat RR, Lin O, Soslow R, et al. Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol Oncol. 2003;90:378–81. doi: 10.1016/s0090-8258(03)00264-6. [DOI] [PubMed] [Google Scholar]
- Singer G, Stohr R, Cope L, Dehari R, Hartmann AA, Wang TL, et al. Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis - A mutational analysis with immunohistochemical correlation. Am J Surg Pathol. 2005;29:218–24. doi: 10.1097/01.pas.0000146025.91953.8d. [DOI] [PubMed] [Google Scholar]
- Sieben NL, Macropoulos P, Roemen GM, Kolkman-Uljee SM, Jan FG, Houmadi R, et al. In ovarian neoplasms,braf,but not kras, mutations are restricted to low-grade serous tumors. J Pathol. 2004;202:336–40. doi: 10.1002/path.1521. [DOI] [PubMed] [Google Scholar]
- Singer G, Oldt R, Cohen Y, Wang BG, Sidransky D, Kurman RJ, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–6. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- Wong KK, Tsang YT, Deavers MT, Mok SC, Zu Z, Sun C, et al. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol. 2010;177:1611–7. doi: 10.2353/ajpath.2010.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang YT, Deavers MT, Sun CC, Kwan SY, Kuo E, Malpica A, et al. KRAS (but not BRAF) mutations in ovarian serous borderline tumor are associated with recurrent low-grade serous carcinoma. J Pathol. 2013;231:449–56. doi: 10.1002/path.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]