Abstract
Background and Objectives:
Pregnancy is one of the most important and risky periods in mothers and the fetus life, which plays a key role in health and social activity of the person, family and community. This study is trying to see if there is a relation between increasing weight and urinary tract infection (UTI) in pregnancy by using the open nested case-control study in the city of Shahrekord.
Materials and Methods:
In a nested case-control study, one cohort including 832 patients was examined until week 26 to 30 of pregnancy and their UTIs were studied. The required information was collected by examining the health records of pregnant women and completion of the data registration forms. Data collection was controlled by using SPSS and analyzed by using an independent t-test, Chi-square test, Pearson correlation and logistic regression.
Results:
According to the results of the cohort study with 832 individuals, average weight gain of the group with a UTI was 11.13 ± 3.9 kg and it was 10.63 ± 3.9 kg in the group without UTI, showing no statistically significant difference (P = 0.245). According to the results, genitourinary problems had the highest predictive value for UTIs and the numbers of infertility and the childbirth variables were in the second and third positions, respectively.
Conclusion:
According to the results study we can conclude that screening and treatment of UTIs have been on time and appropriate in health systems of the city of Shahrekord which have lead to the reduction of infant and maternal diseases even with the condition in having no UTI, and continuing this process for screening and treatment is recommended.
Keywords: Obesity, pregnancy, urinary tract infection
INTRODUCTION
Pregnancy is one of the most important and risky periods in mothers and the fetus life, which plays a key role in health and social activity of the person, family and community. Health or disease in this age of mother will affect not only her life quality, but also the life and health of the fetus and the next generation.[1]
50 million women (40%) suffer annually from diseases related to pregnancy, 15% of which experience long-term effects problems. Thus, it seems that 300 million women suffer from pregnancy-related problems worldwide nowadays.[2]
One of the problems during pregnancy is urinary tract infections (UTIs). UTI is a microbial growth in the urinary tract that leads to urinary tract damages.[3] UTI is the most common bacterial infection during pregnancy in 2 to 12% of pregnant women and if there are more than 100,000 organisms per milliliter of non-infected samples, the case will be considered as the UTI.[4,5,6] Also, UTI is the second common disease after anemia in pregnancy, which if it is not handled correctly can have major impacts on the outcome of pregnancy.[7,8] This condition is prevalent in women with weight gain in pregnancy.[9]
In some studies, the relationship between UTI and preterm pregnancy, low birth weight, prenatal mortality, congenital malformations, mental retardation, delay in infants evolution, maternal hypertension, preeclampsia, maternal anemia and infection of the amniotic fluid have been reported.[10,11,12]
Having proper weight before and during pregnancy helps the fetus growth during this period and is essential for continuation of mothers’ health. The relationship between pregnancy outcome and pre-pregnancy weight, weight gain during pregnancy and maternal height has also been shown by one study.[13]
Although factors such as age, number of childbirths, socioeconomic status and personal health are the risk factors of UTI.[14,15,16,17] but the predisposing factors have not been fully identified.[18]
In a cross-sectional descriptive study, the pregnancy weight gain has been cited as a risk factor for UTI, and this has been confirmed.[9]
Because the case-control study which confirms the relationship between the two variables is stronger than cross-sectional descriptive study and since few studies are done on the relationship between emergence of UTIs and the weight gain during pregnancy, both inside and outside the country, this study is trying to see if there is a relation between increasing weight and UTI in pregnancy by using the open nested case-control study in the city of Shahrekord.
MATERIALS AND METHODS
This study is of analytical type, with nested case-control method that is based on a cohort. In this research the confronting data was collected and recorded by the beginning of the study and was available for the researchers. The controls of the disease were selected from the population under study lacking the disease. Specifically, the risk of bias in this study is much less common compared to the case-control study. This method provides faster and more reliable findings than a cohort study.[19] A nested case-control study is an important type of case-control study which chooses samples and controls from a well-defined cohort. In these circumstances, some information about some of the variables under study is available for both of the groups. Therefore, concerns about bias or misconduct in their classification are largely decreased. One of the major advantages of this type of study is its significant recall bias reduction.[20] The studying population included all pregnant women who have been referred to Shahrekord health centers in the first 6 months of 2011and their records were completed (total female = 1027). Entry criteria for this study included: No history of gestational diabetes and chronic diseases like asthma and thyroid, no drug consumptions, being under the supervision of physicians.,[9] being aware of recorded pre-pregnancy BMI, visiting the pregnant woman at 6-10 weeks of gestation, regular BMI measurement and doing their routine urine test at the first visit. The exclusion criteria included incomplete information about weight gain and those with morbid obesity (35 < BMI). This cohort including 832 patients was examined until week 26 to 30 of pregnancy and their UTIs were studied.
According to ministerial instruction integrated care program, all the pregnant women who go to health centers are routinely sent to the laboratories for urinalysis at 26 to 30 weeks of pregnancy.
The required information was collected by examining the health records of pregnant women and completion of the data registration forms. The data included demographic variables (age, education, women career, blood group and RH), current pregnancy status (multi-fetal condition, unwanted pregnancy, aninterval of less than 3 years between two pregnancies, spotting problems, genitourinary, hyperemesis, preeclampsia, gestational diabetes, pre-pregnancy care), history or current risk of illnesses (renal, diabetes, hypertension), and history of previous pregnancies (stillbirths, miscarriages, premature births, number of pregnancies, number of childbirths).
In this method of case-control study, cases were considered for the individuals with UTIs (urinary tract infection was diagnosed by growth of at least 100,000 colony-forming units of a urinary tract pathogen per milliliter in a culture of a midstream urine sample (4-6). Controls were considered as the cases without UTIs. This 6 months’ cohort was divided into 12 parts containing 15 days (1 or to 15 April, 16 to 31 April, 1 May 15, 16 to 31 and 16 to September 31). Pregnant women with UTIs belonging to each of the 12 segments (depending on the pregnancy beginning) were considered as the case study (n = 109) and three controls were selected from the rest of pregnant women without UTI belonging to the related part (327 control), and finally the weight gain in pregnancy was examined.
The overweight status data was obtained by comparing the case study group with the control group. In the present study, pregnancy BMI was classified according to IOM that women with BMI <19.8 considered thin, 26 >BMI >8.19: Normal, 29 >BMI >26: Overweight and BMI >29 were defined as obese.[21]
By considering the ethical issues in this study, necessary permits were obtained from the Research Council of the University, Health Center of the city and the province and other health centers. The personal information remained confidential and the data were analyzed as a whole.
Data collection was controlled by using SPSS and analyzed by using an independent t-test, Chi-square test, Pearson correlation and logistic regression.
According to the results of the cohort study with 832 individuals, 109 of whom were diagnosed with UTI, and emergence of UTI was recorded to be 13.1% in this study.
RESULTS
Average weight gain of the group with a UTI was 11.13 ± 3.9 kg and it was 10.63 ± 3.9 kg in the group without UTI, showing no statistically significant differences [Table 1].
Table 1.
Average weight gain in the two control and case groups
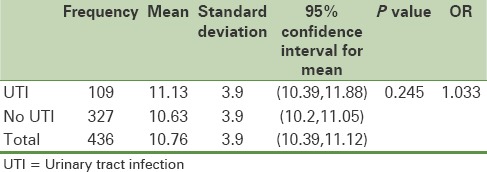
The average age of patients with UTI was 28.37 ± 4.8 and 28.11 ± 5.1 for the group with no UTI, and the age difference was not statistically significant (P = 0.637).
The mean BMI in the beginning of pregnancy for women with infection was 24.63 ± 3.8 and in women without urinary infection was 25.55 ± 3.9. This difference was statistically significant (P = 0.031).
There was no significant relationships between UTIs and the variables of blood group, RH, maternal educational level and job, husband's educational level and job, the year interval of less than 3 years between two pregnancies, multi-fetal, hard childbirth, premature birth, spotting, unwanted pregnancy, abortions, stillbirths, pre-pregnancy care, renal disease, preeclampsia, gestational diabetes and high blood pressure.
There was a significant relationship between urinary infection and variables of parity, number of pregnancies, pregnancy severe vomiting, genitourinary problems, infertility and BMI.
There was no significant relationship between weight gain during pregnancy and variables of blood group and RH, maternal educational level and job, husband's educational level and job, the year interval of less than 3 years between two pregnancies, multi-fetal, hard childbirth, premature birth, spotting, unwanted pregnancy, abortions, stillbirths, pre-pregnancy care, renal disease, preeclampsia, gestational diabetes and high blood pressure.
There was a significant correlation between weight gain during pregnancy and age, number of childbirth, severe vomiting, abortion, infertility, the number of pregnancies, preterm birth, preconception care, husband's occupation and BMI at the beginning of pregnancy.
The rates of UTIs predictive variables by using logistic regression are shown in Table 2. Since the correlation between variables, number of pregnancies, number of childbirths, BMI and severe vomiting in pregnancy are related to both weight gain and UTI; they can play a confounding role in this study. Thus, the relationship between weight gain and the incidence of UTI and logistic regression analysis was used to control the effects of these variables.
Table 2.
Evaluation of predictive variables on the incidence of urinary tract infection
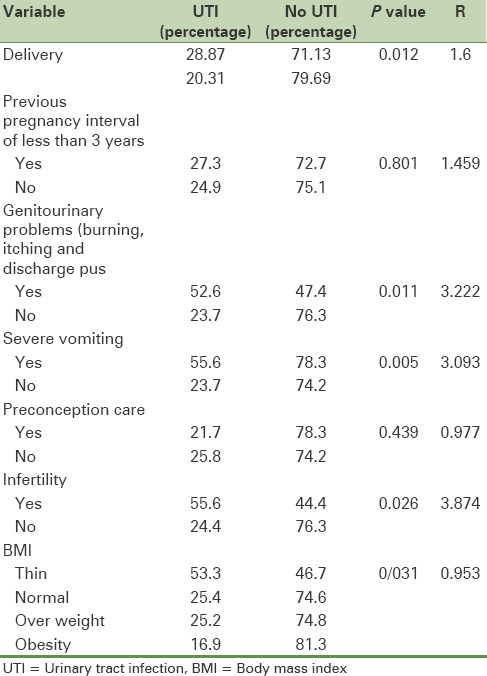
The results of logistic regression analysis showed no correlations between the incidence of UTI and overweight with controlling effects of these variables. According to the results, genitourinary problems had the highest predictive value for UTIs and the numbers of infertility and the childbirth variables were in the second and third positions, respectively. The variables of the interval of less than 3 years between two pregnancies (P = 0.801) and pre-pregnancy care (P = 0.439) had no predictive values for the UTI.
DISCUSSION
This study aimed to determine the correlation between weight gain during pregnancy and UTI of pregnant women in the city of Shahrekord and the results showed that despite significant weight gain during pregnancy, the difference of the average weight gains of the groups with and without UTI was not statistically significant (P = 0.245).
The average weight gain during pregnancy in this study was 10.755. The average weight gain during pregnancy in China was about 11.5 kg and the international standards indicates about 12.5 kg.[22,23] one study have examined the correlation between weight gain during pregnancy and UTI beginning;[9] however, the results of this study were not in conformity with the study done by Ebadi, who examined the relationship between weight gain and UTI in pregnant women in the city of Larestan.[9] The notable factor in this study was that obese women get trauma and less damage of genitalia during sexual relations than in non-obese ones, because of more fat tissues that they have. Also, regarding the average age of women in this study (28.37 ± 4.8), more estrogen preserves genital tissues against damage and infection, but Vessay et al. recorded an increasing prevalence of UTI in obese women for two and three times.[24]
UTI in pregnant women was evaluated in the present study to be 13.1%. Incidence of UTIs during pregnancy has been evaluated to be 28.5% in Pakistan,[25] 48.5% in Nigeria.[26] and 2.5-8.7% in the west population, respectively.[27]
Dysuria, urinary frequency and pain under the belly are the clinical signs of UTI.[10] As in this study, 52.6% of women in the group with UTI and 47.4% of women in the group without UTI showed these symptoms and the differences were statistically significant (P = 0.011). AaliJahan et al. reported a difference in these symptoms between two groups with and without the infections, which was not statistically significant.[28] A number of studies have reported the correlation between UTIs and preeclampsia.[12,29,30,31] The mechanism of catching preeclampsia in women suffering UTI is the direct impact of microorganisms on the walls of blood vessels and the destruction of vascular endothelial which leads to the dysfunction and hardness of blood vessels.[32] However, there was no statistically significant difference between infected and not infected cohorts with the presence of preeclampsia (P = 1), which is consistent and in conformity with the study of Raheleh AaliJahan and Gilstrap.[28,15]
In this study, severe vomiting of pregnancy showed a statistically significant relationship with urine infection (P = 0.005). However, this factor was not significant in AaliJahan's study.[28] Severe vomiting can lead to decreasing fluid intake by the mother, during pregnancy and hence the urinary tract function would be affected. Thus, the decrease in urinary output, leading to long-term accumulation of urine in the urinary tract and the ground for bacteria and infection will be provided.[33] Pregnancy number factor is significantly associated with UTI in pregnancy, as it has been frequently an agent for increasing and in fact doubling a marked UTI.[34,35] The relationship between UTI and pregnancy numbers is due to the physiological changes that occur in the urinary tract during pregnancy period.[36] In this study, the number of pregnancies had a significant relationship with UTI. Therefore, the results of this study disagrees with the results of other studies as of Hamdan's study on UTI in Sudan,[37] or Masinde study in Tanzania,[38] Turpin's study in Ghana,[39] and Hazhir's investigations on asymptomatic UTIs of pregnant women in Iran.[40] However, it agreed with UTI studies of Okonko in from Nigeria,[26] Enayat's study on asymptomatic UTI in pregnant women,[41] and Haider's study in Pakistan.[42]
In this study, the interval of less than 3 years had no significant correlation with UTI (P = 0.801). Undermining food supplies of pregnant women due to pregnancies and having not enough time for reconstructing body supplies lead to lack of essential nutrition and as a result, lack of resistance of mothers’ bodies toward infections.[43] In many cases with interval of less than three years between pregnancies, pregnancy care begins with delays, due to the unwanted pregnancy, high maternal engagement in taking care of previous children and lack of awareness to menstrual arrears due to amenorrhea or irregularities in the first few months after childbirth.[43] In AaliJahan et al., study, a relationship between UTI and 3 years interval between pregnancies was reported.[28]
Because of women screening for in 6-10 weeks before pregnancy UTI, they do not receive any proper care or receive very early care before pregnancy which may increase the risk of UTI in these women. However, we did not find a significant relationship between pre-pregnancy care and catching UTI in this study (P = 0.439).
Also, no significant correlation was seen between the age of pregnant women and affecting UTI. Similar findings to Haider,[42] Okonko,[26] Vszkan,[34] and AaliJahan[28] reports are obtained. However, there was a significant correlation between the age factor and UTI in Vessey investigation.[24]
The different results about the presence or absence of a correlation between risk factors of UTI among pregnant women in different studies may be due to the differences in methodologies, the study population and the study sample volume.
Most notably, maternal factors especially weight gain during pregnancy did not have statistically significant association with UTI. Therefore, we can conclude that screening and treatment of UTIs have been on time and appropriate in health systems of the city of Shahrekord which have lead to the reduction of infant and maternal diseases even with the condition in having no UTI, and continuing this process for screening and treatment is recommended. The results of this project can be useful for health centers in terms of its merits, and for pregnant women should be screened for UTIs.
It is recommended that further studies to be done on the rate of weight gain during pregnancy once every 3 months, and its relationship with UTIs to be measured.
Financial support and sponsorship
This article supported by Isfahan University of Medical Sciences- Deputy of Research.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Hereby we would like to express our especial thanks to the Research Council of Isfahan University of Medical Sciences, co-vice president of health care in Chahar-Mahal va Bakhtiari and specially Shahrekord health center staffs for their unwavering support in this study.
REFERENCES
- 1.Safari M, Saadatmand N, Azarman M. Food Intake Pattern and Related Factors in Women Referred to Medical and Health Centers of Yasouj. Dena Q J Yasuj Fac Nurs Midwifery. 2007;2:27–37. [Google Scholar]
- 2.Albers L. “Evidence” and midwifery practice. J Midwifery Womens Health. 2001;46:130–6. doi: 10.1016/s1526-9523(01)00114-3. [DOI] [PubMed] [Google Scholar]
- 3.Maclean AB. Urinary Tract Infection in Pregnancy. Int J Antimicrob Agents. 2001;17:273–6. doi: 10.1016/s0924-8579(00)00354-x. [DOI] [PubMed] [Google Scholar]
- 4.Pereira EV, Figueiro EA, Oliveira MV, Fernandes AC, Moura Fe CS, Coelho LR, et al. Urinary tract infection in high risk pregnant women. Revista DePartologia Tropic. 2013;42:21–9. [Google Scholar]
- 5.Lee M, Bozzo P, Einarson A, Koren G. Urinarytract infections in pregnancy. Can Fam Physician. 2008;54:853–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Blomberg B, Olsen BE, Hinderaker SG, Langeland N, Gasheka P, Jureen R, et al. Antimicrobial resistance in urinary bacterial isolates from pregnant women in rural Tanzania: Implications for public health. Scand J Infect Dis. 2005;37:262–8. doi: 10.1080/00365540410021045. [DOI] [PubMed] [Google Scholar]
- 7.Mittal P, Wing DA. Urinary tract infections in pregnancy. Clin Perinatol. 2005;32:749–64. doi: 10.1016/j.clp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Mashfeghi Z. Tehran: Tehran University of Medical Sciences; 2002. Analysis of relationship of urinarybacteria without early sign in pregnant mothers referred to the well-known hospitals in Shiraz.[dissertation] [Google Scholar]
- 9.Ebadi M, Rahmanian P. The relationship between weight gain during pregnancy urinary tract infections in pregnant women larestan city. World Journal of Microbiology. 2009;2:177–82. [Google Scholar]
- 10.Cunningham FG, Leveno KJ, Hauth JC. Vol. 3. Tehran: Arjmand Medical Publisher; 2010. Bloom, Rouse, Spong. Williams Obstetrics. 23th Edition Translation: Valadan M, Razaghi S, FaghaniJadidi N, Ghorbani M; pp. 489–91. [Google Scholar]
- 11.Cleves M, Malik S, Yang S, Carter TC, Hobbs CA. Maternal urinary tract infections and selected cardiovascular malformations. Birth defect research: Clin Mol Teratol. 2008;82:464–73. doi: 10.1002/bdra.20460. [DOI] [PubMed] [Google Scholar]
- 12.Mazor-Dray E, Levy A, Schlaeffer F, Sheiner E. Maternal urinary tract infection: Is it independently associated with adverse pregnancy outcome. J Matern Fetal Neonatal Med. 2009;22:124–8. doi: 10.1080/14767050802488246. [DOI] [PubMed] [Google Scholar]
- 13.Solimanizadeh L, Solimanizadeh A, Naseri N. Mother BMI and Pregnancy Outcomes. Payesh, J Iran Inst Health Sci Res. 2006;4:243–8. [Google Scholar]
- 14.Cunningham FG, Leveno KJ, Hauth JC. 23th ed. New York: McGraw-Hill Professional; 2009. Williams Obstetrics; pp. 489–91. [Google Scholar]
- 15.Gilstrap LC, 3rd, Ramin SM. Urinary tract infections during pregnancy. Obstet Gynecol Clin North Am. 2001;28:581–91. doi: 10.1016/s0889-8545(05)70219-9. [DOI] [PubMed] [Google Scholar]
- 16.Al-Haddad AM. Urinary tract infection among pregnant women in Al-Mukalla district, Yemen. East Mediterr Health J. 2005;11:505–10. [PubMed] [Google Scholar]
- 17.Hooton TM, Scholes D, Hughes JP, Winter C, Roberts PL, Stapleton AE, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468–74. doi: 10.1056/NEJM199608153350703. [DOI] [PubMed] [Google Scholar]
- 18.Cronise K, Kelly SJ. Maternal urinary tract infection alters water maze performance in the offspring. Neurotoxicol Teratol. 2001;23:374–9. doi: 10.1016/s0892-0362(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 19.Langholz B. Case-Control Study, Nested. In: Arbitrage P, Colton T, editors. Encyclopedia of Biostatistics. Chichester, UK: John Wiley and Sons; 2005. pp. 646–65. [Google Scholar]
- 20.Nasseri K. 1st ed. Tehran: Gap; 2010. Thesaurus epidemiology (Epidemiology) publications, chat; p. 209. [Google Scholar]
- 21.Panahandeh Z, Pour Ghasemi M, AsgharNia M. Body Mass Index and Prenatal Weight Gain. J Gilan Univ Med Sci. 2006;57:15–20. [Google Scholar]
- 22.Wong W, Tang NL, Lau TK, Wong TW. A new recommendation for maternal weight gain in Chinese women. J Am Diet Assoc. 2000;100:791–6. doi: 10.1016/S0002-8223(00)00230-3. [DOI] [PubMed] [Google Scholar]
- 23.Dawes MG, Gross T. Perinatal problem of the obese mother and her infant. Obs Gyn. 1995;66:299–305. [PubMed] [Google Scholar]
- 24.Vessey MP, Metcalfe MA, McPherson K, Yeates D. Urinary tract infection in relation to diaphragm use and obesity. Int J Epidemiol. 1987;16:441–84. doi: 10.1093/ije/16.3.441. [DOI] [PubMed] [Google Scholar]
- 25.Sheikh M, Khan M, Khatoon A, Arain G. Incidence of urinary tract infection during pregnancy. East Mediterr Health J. 2000;6:265–71. [PubMed] [Google Scholar]
- 26.Okonko IO, Ijandipe LA, Ilusanya OA, Donbraye-Emmanuel OB, Ejembi J, Udeze AO, et al. Incidence of urinary tract infection (UTI) among Pregnant women in Ibadan, South-Western Nigeria. Afr J Biotechnol. 2009;6:6649–57. [Google Scholar]
- 27.Sweet RL. Bacteriuria and pyelonephritis during pregnancy. Semin Perinatol. 1977;1:25–40. [PubMed] [Google Scholar]
- 28.Allijahan R, Purfarzy F, Salimi S, T Tahmasby P, Moradi S, Hazrati S. Iran: Shiraz University of Medical Sciences; 1391. Incidence and risk factors associated with urinary tract infection in pregnant women in Ardabil city. South Medical Journal, Environmental Research Institute-Medical Persian Gulf. [Google Scholar]
- 29.Sheiner E, Mazor-Drey E, Levy A. Asymptomaticbateriuria during pregnancy. J Matern Fetal Neonatal Med. 2009;22:423–7. doi: 10.1080/14767050802360783. [DOI] [PubMed] [Google Scholar]
- 30.Benhidy F, Acs N, Puho EH, Czeizel AE. Pregnancy complications and birth outcomes of pregnant women with urinary tract infections and related drug treatments. Scand J Infect Dis. 2007;39:390–7. doi: 10.1080/00365540601087566. [DOI] [PubMed] [Google Scholar]
- 31.Mazor-Dray E, Levy A, Schlaeffer F, Sheiner E. Maternal urinary tract infection: Is independently associated with advers pregnancy outcome? J Matern Fetal Neonatal Med. 2009;22:124–8. doi: 10.1080/14767050802488246. [DOI] [PubMed] [Google Scholar]
- 32.Berad A, Santos F, Ferreia E, Perreault S. Urinary tract infection during pregnancy. Int J Antimicrob Agents. 2002;19:522–38. [Google Scholar]
- 33.Beetz R. Mild dehydration: A risk factor of urinary tract infection. Eur J Clin Nutr. 2003;57:52–8. doi: 10.1038/sj.ejcn.1601902. [DOI] [PubMed] [Google Scholar]
- 34.Sescon NIC, Garingalao-Molina FD, Ycasiano CEJ, Saniel MC, Manalastas RM. Prevalence of Asymptomatic Bacteriuria and Associated Risk Factors in Pregnant Women. Phil J Microbial Infect Dis. 2003;32:63–9. [Google Scholar]
- 35.Cram LF, Zapata MI, Toy EC, Baker B., 3rd Genitourinary infections and their association with preterm labor. Am Fam Physician. 2002;6:241–8. [PubMed] [Google Scholar]
- 36.Nandy P, Thakur AR, Ray CS. Characterization of bacterial strains isolated through microbial profiling of urine samples. On Line J Biol Sci. 2007;6:44–51. [Google Scholar]
- 37.Hamdan HZ, Zaid AH, Ali SK. Epidemiology of urinary tract infections and antibiotic sensitivity among pregnant women at Khartoum North Hospital. Ann Clin Microbiol Antimicrob. 2011;6:1–5. doi: 10.1186/1476-0711-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masinde A, Gumodoha B, Kilonzo A, Mshana SE. Prevalence of urinary tract infection among women at Bugando Medical Center, Mwanza, Tanzania. Tanzania J Health Res. 2009;6:154–9. doi: 10.4314/thrb.v11i3.47704. [DOI] [PubMed] [Google Scholar]
- 39.Turpin C, Minkah B, Danso K, Frimpong E. Asymptomatic Bacteriuria in pregnant women attending antenatal clinic at KomfoAnokye teaching hospital, Kumasi, Ghana. Ghana Med J. 2007;6:26–9. [PMC free article] [PubMed] [Google Scholar]
- 40.Hazhir S. Asymptomatic bacteriuria in pregnant women. Urol J (Tehran) 2007;6:24–7. [PubMed] [Google Scholar]
- 41.Enayat K, Fariba F, Bahram N. Asymptomatic bacteriuria among pregnant women referred to outpatient clinics in Sanandaj, Iran. Int Braz J Urol. 2008;6:699–707. doi: 10.1590/s1677-55382008000600004. [DOI] [PubMed] [Google Scholar]
- 42.Haider G, Zehra N, Munir AA, Haider A. Risk factors of urinary tract infection in pregnancy. J Pak Med Assoc. 2010;6:213–6. [PubMed] [Google Scholar]
- 43.Klerman LV, Cliver SP, Goldenberg RL. The impact of short interpregnancy intervals on pregnancy outcomes in a low income population. Am J Public Health. 1998;88:1182–5. doi: 10.2105/ajph.88.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]


