Abstract
Objective:
To present an updated description of the relation between Crohn's disease (CD) and Urolithiasis.
Patients and Methods:
A literature search for English-language original and review articles was conducted in Medline, Embase, and Cochrane databases in the month of December 2014 for papers either published or e-published up to that date, addressing the association between CD and urolithiasis as its consequence. All articles published in English language were selected for screening based on the following search terms: “CD,” “renal calculus,” “IBD,” and “urolithiasis.” We restricted the publication dates to the last 15 years (2000–2014).
Results:
In total, 901 patients were included in this review of which 95 were identified as having CD and urolithiasis simultaneously, for a total of 10.5%. Average age was 45.07 years old, irrespective of gender. 28.6% of patients received some kind of medical intervention without any kind of surgical technique involved, 50% of patients were submitted to a surgical treatment, and the remaining 21.4% were submitted to a combination of surgical and medical treatment. Urolithiasis and pyelonephritis incidence ranged from 4% to 23% with a risk 10–100 times greater than the risk for general population or for patients with UC, being frequent in patients with ileostomy and multiple bowel resections. We found that urolithiasis occurred in 95 patients from a total of 901 patients with CD (10.5%); 61.81% in men and 38.19% in women. Stone disease seems to present approximately 4–7 years after the diagnosis of bowel disease and CaOx seems to be the main culprit.
Conclusions:
CD is a chronic, granulomatous bowel disease, with urolithiasis as the most common extraintestinal manifestation (EIM), particularly frequent in patients submitted to bowel surgery. This complication needs to be recognized and addressed appropriately, especially in patients with unexplained renal dysfunction, abdominal pain, or recurrent urinary tract infection. We believe this study to be an updated valuable review as most data related to this kind of EIM refers to articles published before 2000, most of them before 1990. These patients need to be followed up with a specific prevention plan to eliminate or mitigate the risk factors for stone disease, aiming at preventing its formation and its complications, preserving renal function, reducing morbidity, and ultimately improving their quality of life.
Keywords: Calcium oxalate stones, Crohn's disease, extraintestinal manifestations, inflammatory bowel disease, ulcerative colitis
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic inflammatory condition comprising two major disorders: Ulcerative colitis (UC) and Crohn's disease (CD). CD, the latter, is a chronic nonspecific granulomatous disease of unknown cause. It may affect any part of the gastrointestinal (GI) tract from the mouth to the perianal area, but it usually begins in the ileum and proximal colon.[1] Incidence rates range from 3.1 to 20.2 cases per 100,000 person-year in North America, with a prevalence of 201 per 100,000 people. Its prevalence appears to be lower in Asia and the Middle East than in Western Countries. In Europe in particular, prevalence is lower in the south compared to Northern Latitudes.[2] Recent literature on incidence and prevalence rates of CD worldwide have revealed that the Southern Hemisphere countries rates are actually closer to those in the Northern hemisphere.[3] It has a known familial and genetic component, with an earlier onset in the offspring of parents with the disorder.
Environment and lifestyle are especially important and may be determinant to the incidence of the disease. Migration of ethnic groups classically not associated with IBD to high incidence areas leads to increase in the incidence rate and in the risk of developing the disorder.[4]
Etiopathogenesis of CD is still unknown. Current theories refer to an unregulated innate and adaptive immune response, resulting from an imbalance between tolerance to food-derived antigens or commensal microbiota and immune responses to pathogens, with resulting mucosal inflammation. Autoimmunity also seems to play a big part in this disease with genetic research underway to identify relevant susceptibility loci.[5]
Extraintestinal manifestations (EIMs) are common in CD, adding to the burden of disease, with a prevalence varying from 6% to 46%[6,7] and can be divided into reactive manifestations (associated with inflammatory disease activity) and genuine extraintestinal complications (due to metabolic or anatomical abnormalities caused directly by the disease itself).[8] EIM can involve nearly every system: Hepatobiliary, musculoskeletal, dermatologic, ocular, genitourinary, vascular, hematologic, cardiac, pulmonary, endocrine, and metabolic.[9] Close to 36% of patients with IBD have at least one EIM.[10] Some EIM, whether rheumatologic, mucocutaneous or ophthalmologic, have been associated with active colitis. Other manifestations like nephrolithiasis, obstructive uropathy, and cholelithiasis have been observed in patients with small bowel dysfunction. There are also some disorders that are nonspecific (osteoporosis, hepatobiliary disease, and amyloidosis).[11]
Colitis-related EIMs are more frequent in patients with CD involving the colon, which may happen because of the loss of the barrier function of the intestine due to inflammation. Small bowel-related manifestations are commonly associated with ileitis,[12] especially urologic complications. Nephrolithiasis is common in CDs patients and directly related to the activity of bowel disease.
Urolithiasis as an extraintestinal manifestation of Crohn's disease
The relationship between IBD and nephrolithiasis was first described in 1968 by Gelzayd et al.[13] Urolithiasis occurs in both sexes, and it is the most common urinary complication in patients with CD, often clinically unsuspected because of the dominant intestinal or systemic symptoms.[14] Extensive small bowel resection is usually performed for CD, with consequent recurrent calcium oxalate stones (CaOx).[15,16] Nephrolithiasis is more common after small bowel surgery – 4–5.5% (before) versus 15.0–30.5% (after),[17] and it seems to be related to the location of the disease: 7–17% in ileocolic, 6–8% in ileal and 3–5% in colonic disease.[18] Severe hyperoxaluria predominates, as well as low levels of calcium excretion,[19] and treatment strategies in these patients are directed at reducing the magnitude of supersaturation of CaOx by consuming less dietary oxalate (Ox) or giving patients calcium supplementation, as it binds Ox in the intestinal lumen, preventing its absorption.[20]
PATIENTS AND METHODS
Literature search was conducted in Medline, Embase, and Cochrane databases in the month of December 2014. We identified original articles, review articles, and editorials addressing the association between CD and urolithiasis as its consequence.
All articles published in English language were selected for screening based on the following search terms: “CD,” “renal calculus,” “IBD,” and “urolithiasis.” We restricted the publication dates to the last 15 years (2000–2014). Only publications concerning humans were considered. Both genders were included.
Two independent reviewers selected all relevant articles. The authors eliminated independently and simultaneously all duplicates. The relevant articles were selected on the basis of reading the full text manuscripts. The eligibility criteria for inclusion were based on relevance concerning CD associated with urolithiasis. Irrelevant ones were excluded. If there was any doubt concerning the eligibility of a study, abstracts – and if necessary, the full text was examined. If disagreement regarding inclusion occurred, an agreement was reached by discussion. Additional references were identified from the reference lists of these articles. Additional reports, outside the time-frame stipulated that the authors believed were also important for contextualizing CD in this setting, were also included.
We came up with a total of 113 publications identified through the PubMed database using the following search terms: “CD,” “renal calculus,” “IBD,” and “urolithiasis.” Four additional publications were identified through a search in the references of the 113 publications, totaling 117 publications. We excluded duplicates cases, unrelated cases, cases that were outside the time frame we stipulated, case reports and non-English publications, ending up with 10 articles that we included in our systematic review.
Objectives
In this review, the authors investigated the occurrence of urolithiasis in patients with CD, its biochemical and urinary constituents, the location of stones, common management, and other clinical findings including urolithiasis recurrence and follow-up, as well as common risk factors in light of the latest published literature.
RESULTS
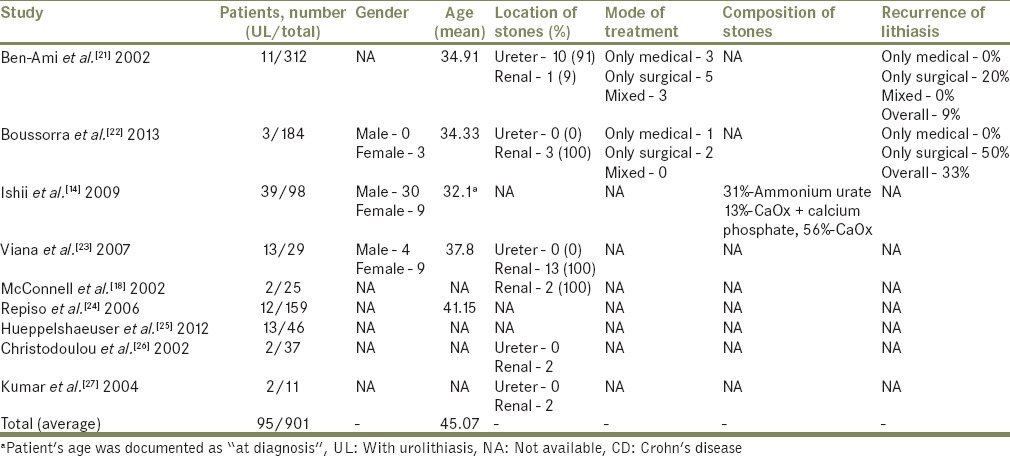
A total of 901 patients were included in this review [Table 1], of which 95 were identified as having CD and urolithiasis, simultaneously, for a total of 10.5%. The average age was 45.07 years old, irrespective of gender. Of the studies that reported some kind of intervention due to urolithiasis, 28.6% described medical intervention only without any kind of surgical technique involved, 50% of patients were submitted to a surgical treatment, and the remaining 21.4% were submitted to an intervention that involved a combination of surgical and medical treatment.
Table 1.
Characteristics of patients with CD with documented urolithiasis
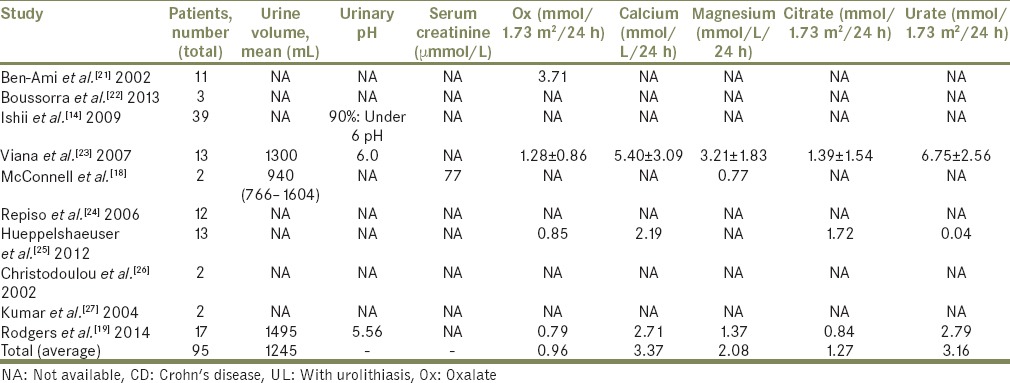
Mean urinary excretion of Ox, other lithogenic and stone inhibitory parameters and urinary saturation indexes in patients with CD with concomitant urolithiasis measured as baseline 24 h urine excretion are shown in Table 2.
Table 2.
Mean urinary excretion of Ox, other lithogenic and stone inhibitory parameters and urinary saturation indexes in patients with CD with concomitant UL measured as baseline 24-h urine excretion
Urolithiasis and pyelonephritis incidence range from 4% to 23%,[21,28] with a risk 10–100 times greater than the risk for general population or even for patients with UC.[22] It is particularly frequent in patients with ileostomy (and with >100 cm of ileum resected) and multiple bowel resections as both cause volume depletion and hyperoxaluria, thus promoting stone formation.[12,19] In our review, we found that urolithiasis occurred in 95 patients from a total of 901 patients with CD (10.5%). In studies where gender distinction was made, urolithiasis was present in 61.81% in men and 38.19% in women. Stone disease seems to present approximately 4–7 years after the diagnosis of bowel disease.[16] Low levels of the anti-lithogenic agents magnesium and citrate have also been implicated as causative factors.[29] Only one study from our review documented the majority of stones as being of CaOx (56%).[14] However, given the strong association of CaOx stones with hyperoxaluric states in general and specifically with enteric forms of hyperoxaluria we can only assume the distribution would be similar.
Nephrolithiasis in CDs patients has been documented with increased frequency in patients over 40 years old at the time of diagnosis.[24] Our calculated average age is similar: 45.07 years old, both in males and females. However, we were not able to relate the duration of the disease neither to the first sign of nephrolithiasis because of limited data nor to alterations in renal function, as of only one of the reviewed papers had data on serum creatinine.[18]
DISCUSSION
Risk factors
Metabolic abnormalities
Metabolic abnormalities may be the initial manifestation of CD, predisposing patients to nephrolithiasis, and this generally occurs in patients with severe, long-standing disease [Figure 1]. Urinary volume, pH, Ox and calcium excretion, citrate, and magnesium are identified as culprits in lithogenesis, but the contribution of each one of these is still debatable.[30] In the study by Viana et al.,[23] most patients had two or more lithogenic risk factors with hypocitraturia and hypomagnesuria being the most common; 38,1% of patients with hypocitraturia and 42.9% with hypomagnesuria had already had ileal surgery. Buño Soto et al.[30] while comparing 24 h urine samples of patients with CD and healthy individuals found significantly lower urinary pH and levels of citric acid and magnesium in the former, but with no significant difference in the levels of Ox, calcium, or uric acid between the two groups. Average Ox excretion, in our review, was almost double the reference value (0.96 mmol/1.73 m2/ 24 h) and urinary calcium was also elevated (3.37 mmol/L/24 h). Urinary citrate, as expected, is lower than normal reference values (1.27 mmol/L/24 h) but urinary magnesium is not (2.08 mmol/L/24 h). All patients with documented pH had values under or = 6, confirming lower urinary pH as a risk factor.
Figure 1.
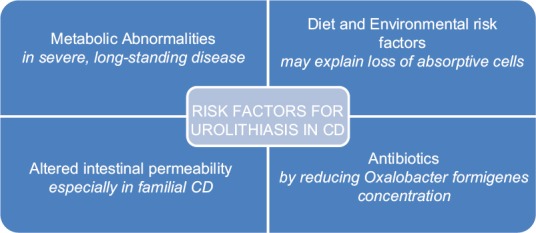
Diagram of urolithiasis risk factors
Altered intestinal permeability
Derangement of the intestinal barrier function needs special consideration. Increased intestinal permeability is one of its manifestations, and it has been associated with several autoimmune disorders, like CD or UC, even prior to these diseases as it appears to be involved in their pathogenesis.[31,32] This has been widely investigated in CD. Using different permeability markers, investigators have substantiated the defective permeability barrier in patients with familial CD and even in relatives who do not harbor the disease.[33] Whether this specific alteration promotes an increase in metabolic risk factors leading to stone lithogenesis is still debatable. CaOx stones may be caused by colonic or ileal Ox hyperabsorption (secondary to intestinal dysfunction) or Ox overproduction. In CD, malabsorption due to inflamed, malfunctioning mucosa, and diarrhea seems to promote enhanced Ox absorption. This would result from the formation of calcium-fatty acid salts, which would decrease the amount of free calcium available to bind Ox in the intestinal lumen.[34] However, this association between intestinal permeability and increased Ox absorption has not been proven, and studies about it are scarce. Increased intestinal permeability can cause hyperabsorption of nutrients, which can lead to the passive diffusion of Ox,[35] but we cannot conclude that there is an increased stone risk solely from this observation, and other mechanisms that may be in play.
Diet and environmental risk factors
Inherent absorption characteristics from each and diet are also relevant. A high dietary protein intake can increase urinary Ox,[36] along with lower dietary calcium and magnesium that cause reduced binding with Ox in the GI tract, making it more available. Altered composition of the intestinal microbiota along with altered expression of tight junctions proteins, seen in IBD patients,[37] can also promote an increase in permeability, especially the paracellular route, as Ox is absorbed in the intestine by both a paracellular and a transepithelial transport.[31] It is the severe loss of absorptive cells that may be the primary reason for alterations in transport processes seen in IBDs, especially in their acute stage, as macromolecules can permeate the barrier at an increased rate via, for example, breaks in the integrity of the epithelium.[38] We still need to identify the controlling mechanisms for Ox metabolism in IBD patients.
Stone formation in ulcerative colitis
The other main IBD is UC, an idiopathic, chronic IBD, along with CD that starts in the rectum and extends proximally, continuously, throughout the colon. Inflammation in UC is characteristically restricted to the mucosal surface. Mechanisms of stone formation are different in CD and UC. Hyperoxaluria, typically associated with distal ileal CD or ileal resection, is not common in UC. Stone formation in UC is related to disease activity: Diarrhea, dehydration, and lower urinary volumes.[39] Colectomy with a construction of the ileal-pouch anal anastomosis in UC seems to further increase the risk of lithiasis with approximately <30% of patients with UC eventually require this procedure.[40] The reported frequency of nephrolithiasis ranges from 0.2% to 11.0% in noncolectomy UC patients and from 8.4% to 40.0% in UC patients with total colectomy and ileostomy.[17] In fact, there is a tendency to uric acid stones among patients with colon resection compared with small bowel resection or bypass, or in the absence of surgery. Two factors may be responsible: The lower urine pH values found in colon resection patients and the lower urinary volume. The lowest urine volumes are found among patients who have had colon surgery, adding to the risk of uric acid stones via increased uric acid supersaturation.[16] The loss of magnesium due to chronic diarrhea and a low urine citrate level from metabolic acidosis may contribute further to the risk of nephrolithiasis.[17] Metabolic alterations from mucosal inflammation of the pouch alone do not seem to constitute a risk to nephrolithiasis.[16,17]
Antibiotics
The use of antibiotics is another interesting risk factor to consider.[17] They may reduce Oxalobacter formigenes concentration, as stated before, resulting in higher Ox absorption from the gut with an increased risk of Ox stones. On the other hand, lack of antibiotic use relates to poorer control of bowel movements, which results in loss of alkaline fluids (and consequent metabolic acidosis), a lower serum bicarbonate level, acidic urine, and an increased susceptibility to calcium and uric acid nephrolithiasis. These patients may also develop more urinary tract infections with a higher risk of struvite stones.
In addition, Cury et al.[39] and Gelzayd et al. also noted that besides surgery, the extent of disease relates to a more severe diarrhea. Therefore, these patients are at risk regardless of their surgical status.[13,40]
Bariatric surgery
There is a group of patients who suffer from the same metabolic modifications that CDs patients do, after surgery. Evidence shows that bariatric surgery has been associated with metabolic changes that alter kidney stone risk in a similar fashion to CD following surgery, as the pathophysiology of stone disease and hyperoxaluria in this high-risk population are the same.
Obesity (and its complications) affects close to one-third of American adults[41] with bariatric surgery being nowadays the most successful surgical treatment option. Its goal is to restrict and/or to reduce the absorption of food. Procedures that promote malabsorption include biliopancreatic diversion with duodenal switch and the Roux-en-Y gastric bypass (RYGB), the most commonly used procedure in the United States.[42] Renal complications of bariatric surgery were only reported recently, by Nelson et al.[43] Studies concerning metabolic imbalance of hyperoxaluria, CaOx stones, and Ox nephropathy in this context soon followed, and the subject is still a hot topic in the medical community. One of the first manifestations of metabolic derangement, and the most significant is hyperoxaluria.[16] In a paper by Wu et al.,[44] the first urinary changes noted following bariatric surgery were an increase in Ox and calcium excretion, with a supersaturation of calcium Ox and a decrease in total urinary volume.[45] In a similar fashion to what happens in CD, the levels of urinary citrate in patients who have undergone malabsorption procedures may be 50% or less of those found the urine of normal subjects urine, leading to chronic acidosis, further increasing stone risk.[44] Saponification of fat-soluble vitamins and calcium ions leads to steatorrhea and nutrient loss, diminishing calcium availability for binding to Ox.[46] Not only does RYGB reduce the gastric reservoir (restricting food intake) but it is also responsible for a bypass to a sizable length of GI tract that includes the distal stomach, the duodenum, and the proximal jejunum, thus diminishing nutrient absorption. Another reason may exist: Exposure to unconjugated bile salts and long-chain fatty acids may increase the permeability of the GI tract to Ox.[47]
Gastric banding, on the other hand, is achieved by placing an inflatable tube around the stomach thus reducing stomach reservoir size. It is deemed to be less invasive with lesser morbidity than a malabsorptive procedure and minimizes the urinary metabolic changes when compared to the latter.[48,49]
Bowel disease patients cannot be distinguished from common stone formers by comprehensive stone risk measurements if they have not yet undergone bowel resection, the event being the precipitating factor for the alterations in metabolic profile: The development of hyperoxaluria, along with hypocalciuria, hypocitraturia, and hypomagnesuria the same changes observed during bariatric surgery that involve small bowel resection. In fact, a study by Parks et al. from 2003[16] reveals that urolithiasis risk profile in bowel disease patients who have never been submitted to surgery, is similar in metabolic profile to patients without underlying bowel disease. In contrast to what happens after bariatric surgery or small bowel resection, the main metabolic change present in patients who undergo colon resection is a decrease in both urinary volume and pH. These patients form uric acid stones, with Ox and calcium levels in the normal range. Calcium stones also occur from low urine volume but also from decreased urinary ionic strength, hypocitraturia due to malabsorption, hypomagnesiuria, hyperoxaluria and hypercalciuria from steroid use, immobilization, or acidosis.
Management
To avoid serious sequelae from a renal stone disease associated CD, which may include loss of renal function, early recognition, and adequate therapy are very important. A multimodal intervention, with a dietary, medical, and surgical approach is important [Figure 2]. Prevention of future kidney stones, in this population, through health education, diet, and medication is imperative to preserve kidney function and prevent morbidity. Initial conservative medical therapy of urolithiasis in CD is similar to that used in ordinary patients. It is effective in most cases, but specific surgical, urological treatment (such as extracorporeal shock wave lithotripsy, ureteroscopic lithotripsy, and percutaneous nephrolithotomy) should be considered.[21,28]
Figure 2.
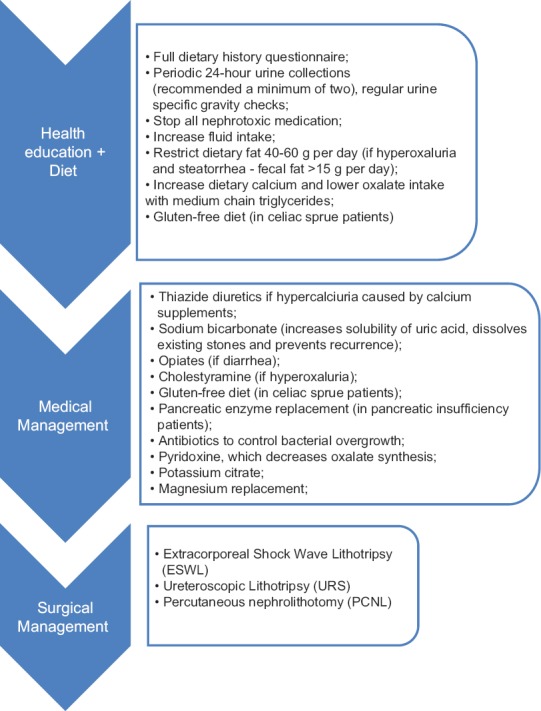
Management of renal stone disease in patients with CD
It is of the utmost importance to begin an evaluation by identifying metabolic risk factors contributing to increased urinary supersaturation. A careful anamnesis with diet history should be performed, together with the collection of two or more 24 h urines and evaluation of the nature and extent of the bowel disease. Renal function, given by serum creatinine and urea, along with serial urinalysis should be performed in all patients. 24 h fecal fat measurement may prove helpful as well. Whenever renal dysfunction is identified, all potentially nephrotoxic medications should be stopped, or the need for their use reevaluated.[15]
Patients should be encouraged to increase their fluid intake to ensure a high urine output (the goal is a daily urine volume of approximately 2 L). They can do their self-monitoring if instructed to collect 24 h urine. They can also be counseled to check their urine specific gravity at specific intervals using urine test strips, aiming to avoid excessively concentrated urines.[15]
Acid urine may be a contributor to an increased uric acid stone formation. Therapy with an alkalinizing agent such as sodium bicarbonate has some advantages. It increases the solubility of uric acid, dissolves existing stones, and prevents recurrences.[26]
Physicians should employ nonspecific methods for control of diarrhea (for example opiates), in order to decrease GI fluid loss. The specific treatment of hyperoxaluria is cholestyramine, a low fat and low Ox diet with medium-chain triglycerides, calcium supplements, a gluten-free diet (in celiac sprue patients), pancreatic enzyme replacement (in pancreatic insufficiency patients), and antibiotics to control bacterial overgrowth.[50] In addition, pyridoxine, which decreases Ox synthesis, may be helpful.[50] Patients with hyperoxaluria and steatorrhea (fecal fat >15 g/day) in particular should restrict the fat in their diet to 40–60 g/day. This will lower Ox absorption and can also have a beneficial effect on lowering secretory diarrhea. Urine volume may rise, and urinary Ox may even go down even further. Cholestyramine, which will bind bile salts in the gut, should be used only in patients without significant steatorrhea after conservative measures have failed.[26]
Calcium supplements may be useful for lowering urinary Ox, especially in patients with lactose intolerance who have a low calcium intake due to the restriction of dairy products. Increasing dietary calcium could progressively lower urinary Ox excretion by binding dietary Ox in the gut. Thiazide diuretics may be necessary to control the hypercalciuria caused by calcium supplements.[26]
Hypocitraturia can be managed with potassium citrate and magnesium replacement, and the latter can also be used for magnesium deficiency or hypomagnesuria. Citrate (and other alkali) can be given to avoid uric acid crystallization. Ileostomy patients or with a history of multiple bowel resections should be monitored closely for the development of calculi by means of urinalysis, ultrasound, or/and kidneys-ureters-bladder.[25]
After successful management, prevention of stone recurrence is imperative. Stone/crystalline analysis along with blood and urine laboratory analysis must be performed, with an appropriate treatment and prevention plan designed based on these findings.[26]
Some critic is due to many of the EIMs may influence the intensity with which they are sought by clinicians, with a direct effect on their prevalence. Some can even have a subclinical course without any symptoms. Furthermore, the frequency of these manifestations increases with follow-up, being higher in prospective series than in descriptive, transverse studies.[15,21]
A limitation of this study was that it was a retrospective evaluation from different institutions where variables were not always the same. Lack of data collected in Tables 1 and 2 shows that there is still information missing when dealing with these particular patients and warrants further studies with larger samples. We suggest a multi-institutional study, with a recruitment of an adequate number of patients in order to have a clear understanding of the best methods for the prevention, diagnosis, and management of urolithiasis in CD. In addition, it is our understanding that two different subsets of CD patients should be considered, as their management differs. Patients with urolithiasis who need specific medical or surgical management versus patients who are stone free and for whom preventive measures may suffice.
CONCLUSIONS
Often clinically underestimated, urologic complications in patients with CD are not rare and need to be correctly recognized and reported in order to receive appropriate treatment, as well as a thorough periodic urological evaluation. Clinicians should have this diagnosis in mind when faced with patients with unexplained renal dysfunction, abdominal pain, or recurrent urinary tract infection. We believe this study to be an updated valuable review as most data related to this kind of EIM refers to articles published before 2000, most of them before 1990. It is hard to compare studies or draw conclusions in this setting as patients with CD without urolithiasis are usually grouped together along patients with urolithiasis, with this finding being almost incidental during the patient's follow-up. It should be interesting to analyze urinary metabolic risk factors since the first diagnosis of CD until, eventually, small bowel surgery of any kind, and again afterward, or when urolithiasis is first documented, in order to understand the specific mechanisms involved in renal and ureteral stones genesis. These patients need to be followed up with a specific prevention plan to eliminate or mitigate the risk factors for stone disease, aiming at preventing its formation and its complications, preserving renal function, reducing morbidity, and ultimately improving the quality of life.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 2.Kappelman MD, Rifas-Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–9. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Aamodt G, Jahnsen J, Bengtson MB, Moum B, Vatn MH IBSEN Study Group. Geographic distribution and ecological studies of inflammatory bowel disease in southeastern Norway in 1990-1993. Inflamm Bowel Dis. 2008;14:984–91. doi: 10.1002/ibd.20417. [DOI] [PubMed] [Google Scholar]
- 4.Hou JK, El-Serag H, Thirumurthi S. Distribution and manifestations of inflammatory bowel disease in Asians, Hispanics, and African Americans: A systematic review. Am J Gastroenterol. 2009;104:2100–9. doi: 10.1038/ajg.2009.190. [DOI] [PubMed] [Google Scholar]
- 5.Laass MW, Roggenbuck D, Conrad K. Diagnosis and classification of Crohn's disease. Autoimmun Rev. 2014;13:467–71. doi: 10.1016/j.autrev.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Mendoza JL, Lana R, Taxonera C, Alba C, Izquierdo S, Díaz-Rubio M. Extraintestinal manifestations in inflammatory bowel disease: Differences between Crohn's disease and ulcerative colitis. Med Clin (Barc) 2005;125:297–300. doi: 10.1157/13078423. [DOI] [PubMed] [Google Scholar]
- 7.Primas C, Novacek G, Schweiger K, Mayer A, Eser A, Papay P, et al. Renal insufficiency in IBD – prevalence and possible pathogenetic aspects. J Crohns Colitis. 2013;7:e630–4. doi: 10.1016/j.crohns.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Danese S, Semeraro S, Papa A, Roberto I, Scaldaferri F, Fedeli G, et al. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005;11:7227–36. doi: 10.3748/wjg.v11.i46.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ardizzone S, Puttini PS, Cassinotti A, Porro GB. Extraintestinal manifestations of inflammatory bowel disease. Dig Liver Dis. 2008;40(Suppl 2):S253–9. doi: 10.1016/S1590-8658(08)60534-4. [DOI] [PubMed] [Google Scholar]
- 10.Rankin GB, Watts HD, Melnyk CS, Kelley ML., Jr National Cooperative Crohn's Disease Study: Extraintestinal manifestations and perianal complications. Gastroenterology. 1979;77(4 Pt 2):914–20. [PubMed] [Google Scholar]
- 11.Su CG, Judge TA, Lichtenstein GR. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:307–27. doi: 10.1016/s0889-8553(01)00019-x. [DOI] [PubMed] [Google Scholar]
- 12.Levine JB, Lukawski-Trubish D. Extraintestinal considerations in inflammatory bowel disease. Gastroenterol Clin North Am. 1995;24:633–46. [PubMed] [Google Scholar]
- 13.Gelzayd EA, Breuer RI, Kirsner JB. Nephrolithiasis in inflammatory bowel disease. Am J Dig Dis. 1968;13:1027–34. doi: 10.1007/BF02233547. [DOI] [PubMed] [Google Scholar]
- 14.Ishii G, Nakajima K, Tanaka N, Hara H, Kato M, Ishii N. Clinical evaluation of urolithiasis in Crohn's disease. Int J Urol. 2009;16:477–80. doi: 10.1111/j.1442-2042.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- 15.Worcester EM. Stones from bowel disease. Endocrinol Metab Clin North Am. 2002;31:979–99. doi: 10.1016/s0889-8529(02)00035-x. [DOI] [PubMed] [Google Scholar]
- 16.Parks JH, Worcester EM, O’Connor RC, Coe FL. Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int. 2003;63:255–65. doi: 10.1046/j.1523-1755.2003.00725.x. [DOI] [PubMed] [Google Scholar]
- 17.Mukewar S, Hall P, Lashner BA, Lopez R, Kiran RP, Shen B. Risk factors for nephrolithiasis in patients with ileal pouches. J Crohns Colitis. 2013;7:70–8. doi: 10.1016/j.crohns.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 18.McConnell N, Campbell S, Gillanders I, Rolton H, Danesh B. Risk factors for developing renal stones in inflammatory bowel disease. BJU Int. 2002;89:835–41. doi: 10.1046/j.1464-410x.2002.02739.x. [DOI] [PubMed] [Google Scholar]
- 19.Rodgers AL, Allie-Hamdulay S, Jackson GE, Sutton RA. Enteric hyperoxaluria secondary to small bowel resection: Use of computer simulation to characterize urinary risk factors for stone formation and assess potential treatment protocols. J Endourol. 2014;28:985–94. doi: 10.1089/end.2014.0077. [DOI] [PubMed] [Google Scholar]
- 20.Hylander E, Jarnum S, Nielsen K. Calcium treatment of enteric hyperoxaluria after jejunoileal bypass for morbid obesity. Scand J Gastroenterol. 1980;15:349–52. doi: 10.3109/00365528009181482. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Ami H, Ginesin Y, Behar DM, Fischer D, Edoute Y, Lavy A. Diagnosis and treatment of urinary tract complications in Crohn's disease: An experience over 15 years. Can J Gastroenterol. 2002;16:225–9. doi: 10.1155/2002/204614. [DOI] [PubMed] [Google Scholar]
- 22.Boussorra H, Sallami S, Said Y, Chebil M, Najjar T. Evaluation of urolithiasis in Crohn's disease in Tunisian patients. Tunis Med. 2013;91:440–3. [PubMed] [Google Scholar]
- 23.Viana ML, Pontes RM, Garcia WE, Fávero ME, Prete DC, Matsuo T. Crohn's disease and kidney stones: Much more than coincidence? Arq Gastroenterol. 2007;44:210–4. doi: 10.1590/s0004-28032007000300006. [DOI] [PubMed] [Google Scholar]
- 24.Repiso A, Alcántara M, Muñoz-Rosas C, Rodríguez-Merlo R, Pérez-Grueso MJ, Carrobles JM, et al. Extraintestinal manifestations of Crohn's disease: Prevalence and related factors. Rev Esp Enferm Dig. 2006;98:510–7. doi: 10.4321/s1130-01082006000700004. [DOI] [PubMed] [Google Scholar]
- 25.Hueppelshaeuser R, von Unruh GE, Habbig S, Beck BB, Buderus S, Hesse A, et al. Enteric hyperoxaluria, recurrent urolithiasis, and systemic oxalosis in patients with Crohn's disease. Pediatr Nephrol. 2012;27:1103–9. doi: 10.1007/s00467-012-2126-8. [DOI] [PubMed] [Google Scholar]
- 26.Christodoulou DK, Katsanos KH, Kitsanou M, Stergiopoulou C, Hatzis J, Tsianos EV. Frequency of extraintestinal manifestations in patients with inflammatory bowel disease in Northwest Greece and review of the literature. Dig Liver Dis. 2002;34:781–6. doi: 10.1016/s1590-8658(02)80071-8. [DOI] [PubMed] [Google Scholar]
- 27.Kumar R, Ghoshal UC, Singh G, Mittal RD. Infrequency of colonization with Oxalobacter formigenes in inflammatory bowel disease: Possible role in renal stone formation. J Gastroenterol Hepatol. 2004;19:1403–9. doi: 10.1111/j.1440-1746.2004.03510.x. [DOI] [PubMed] [Google Scholar]
- 28.Sato S, Sasaki I, Naito H, Funayama Y, Fukushima K, Shibata C, et al. Management of urinary complications in Crohn's disease. Surg Today. 1999;29:713–7. doi: 10.1007/BF02482314. [DOI] [PubMed] [Google Scholar]
- 29.Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med. 1992;327:1141–52. doi: 10.1056/NEJM199210153271607. [DOI] [PubMed] [Google Scholar]
- 30.Buño Soto A, Torres Jiménez R, Olveira A, Fernández-Blanco Herraiz I, Montero García A, Mateos Antón F. Lithogenic risk factors for renal stones in patients with Crohn's disease. Arch Esp Urol. 2001;54:282–92. [PubMed] [Google Scholar]
- 31.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–20. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibson PR. Increased gut permeability in Crohn's disease: Is TNF the link? Gut. 2004;53:1724–5. doi: 10.1136/gut.2004.047092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, et al. Genetic basis for increased intestinal permeability in families with Crohn's disease: Role of CARD15 3020insC mutation? Gut. 2006;55:342–7. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cirillo M, Iudici M, Marcarelli F, Laudato M, Zincone F. Nephrolithiasis in patients with intestinal diseases. G Ital Nefrol. 2008;25:42–8. [PubMed] [Google Scholar]
- 35.Dobbins JW. Nephrolithiasis and intestinal disease. J Clin Gastroenterol. 1985;7:21–4. doi: 10.1097/00004836-198502000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Zarembski PM, Hodgkinson A. Some factors influencing the urinary excretion of oxalic acid in man. Clin Chim Acta. 1969;25:1–10. doi: 10.1016/0009-8981(69)90218-6. [DOI] [PubMed] [Google Scholar]
- 37.Takaishi H, Matsuki T, Nakazawa A, Takada T, Kado S, Asahara T, et al. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol. 2008;298:463–72. doi: 10.1016/j.ijmm.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Manokas T, Fromkes JJ, Sundaram U. Effect of chronic inflammation on ileal short-chain fatty acid/bicarbonate exchange. Am J Physiol Gastrointest Liver Physiol. 2000;278:G585–90. doi: 10.1152/ajpgi.2000.278.4.G585. [DOI] [PubMed] [Google Scholar]
- 39.Cury DB, Moss AC, Schor N. Nephrolithiasis in patients with inflammatory bowel disease in the community. Int J Nephrol Renovasc Dis. 2013;6:139–42. doi: 10.2147/IJNRD.S45466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gustavsson A, Halfvarson J, Magnuson A, Sandberg-Gertzén H, Tysk C, Järnerot G. Long-term colectomy rate after intensive intravenous corticosteroid therapy for ulcerative colitis prior to the immunosuppressive treatment era. Am J Gastroenterol. 2007;102:2513–9. doi: 10.1111/j.1572-0241.2007.01435.x. [DOI] [PubMed] [Google Scholar]
- 41.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 42.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–17. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 43.Nelson WK, Houghton SG, Milliner DS, Lieske JC, Sarr MG. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: Potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:481–5. doi: 10.1016/j.soard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Wu JN, Craig J, Chamie K, Asplin J, Ali MR, Low RK. Urolithiasis risk factors in the bariatric population undergoing gastric bypass surgery. Surg Obes Relat Dis. 2013;9:83–7. doi: 10.1016/j.soard.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 45.Duffey BG, Alanee S, Pedro RN, Hinck B, Kriedberg C, Ikramuddin S, et al. Hyperoxaluria is a long-term consequence of Roux-en-Y Gastric bypass: A 2-year prospective longitudinal study. J Am Coll Surg. 2010;211:8–15. doi: 10.1016/j.jamcollsurg.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez RD, Canales BK. Kidney stone risk following modern bariatric surgery. Curr Urol Rep. 2014;15:401. doi: 10.1007/s11934-014-0401-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Froeder L, Arasaki CH, Malheiros CA, Baxmann AC, Heilberg IP. Response to dietary oxalate after bariatric surgery. Clin J Am Soc Nephrol. 2012;7:2033–40. doi: 10.2215/CJN.02560312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar R, Lieske JC, Collazo-Clavell ML, Sarr MG, Olson ER, Vrtiska TJ, et al. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery. 2011;149:654–61. doi: 10.1016/j.surg.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiner R, Blanco-Engert R, Weiner S, Matkowitz R, Schaefer L, Pomhoff I. Outcome after laparoscopic adjustable gastric banding-8 years experience. Obes Surg. 2003;13:427–34. doi: 10.1381/096089203765887787. [DOI] [PubMed] [Google Scholar]
- 50.Dilauro S, Crum-Cianflone NF. Ileitis: When it is not Crohn's disease. Curr Gastroenterol Rep. 2010;12:249–58. doi: 10.1007/s11894-010-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


