Abstract
Primary carcinoma of ureter is an uncommon malignancy. Of which, mostly are transitional cell carcinomas followed by squamous cell carcinomas and adenocarcinomas being the rarest histopathology encountered. We report a case of adenocarcinoma ureter in a middle-aged male along with its clinical scenario. A 62-year-old male, presented with complaints of lower urinary tract symptoms. Computerized tomography urogram showed a soft tissue lesion at the right ureterovesical junction. Cystoscopic biopsy reported villous adenoma. Diethylene triamine pentaacetic acid scan reported nonfunctioning right kidney. He underwent laparoscopic right nephroureterectomy, and histopathology reported adenocarcinoma of the right lower third of ureter, with positive distal and close radial margins. The patient received external beam radiation to the postoperative bed and lymph nodes, and he is disease-free till date.
Keywords: Adenocarcinoma, external beam radiation, primary carcinoma of ureter
INTRODUCTION
Primary carcinoma of ureter is very rare (0.9–1.6% of all urogenital malignancies).[1] Malignant neoplasms of ureter are typically transitional cell carcinomas (approximately 90%), with squamous cell carcinoma being rare (<10%) and adenocarcinomas being rarest (<1%).[2] Approximately, twenty cases of primary adenocarcinoma have been mentioned in the literature.[3] Most common location is lower one-third of ureter and etiological factors being calculus and chronic inflammation. We, hereby, report a case of adenocarcinoma ureter, clinical features and management.
CASE REPORT
A 62-year-old male, presented with complaints of lower urinary tract symptoms, pain in right lower abdomen, off and on fever, and two episodes of painless hematuria. The patient also had a history of stone removal by percutaneous nephrolithotomy technique from the right kidney 6 years prior to presentation. Computerized tomography urogram showed a soft tissue density lesion of size 1.8 cm × 1.6 cm at the right ureterovesical junction, a shrunken right kidney, and few subcentimetric retroperitoneal and mesenteric lymph nodes. Cystoscopic biopsy from the mass reported villous adenoma. Diethylene triamine pentaacetic acid (DTPA) scan revealed faintly visualized right kidney with poor function (glomerular filtration rate [GFR] - 6.19 ml/min) and nonobstructed left kidney with maintained cortical function (GFR - 40.3 ml/min) with adequate bladder activity. In view of nonfunctioning right kidney and lower ureteric tumor, he underwent laparoscopic right nephroureterectomy. Postoperative histopathology reported moderately differentiated adenocarcinoma, mixed papillary and mucinous type of right lower third of ureter of size 1.6 cm × 1.4 cm × 1 cm [Figure 1], the tumor was present at distal resection margin of ureter and distance from radial margin was 2 mm. Invasion of the muscular layer of ureter was also identified. Changes of chronic pyelonephritis were visualized in the right kidney.
Figure 1.
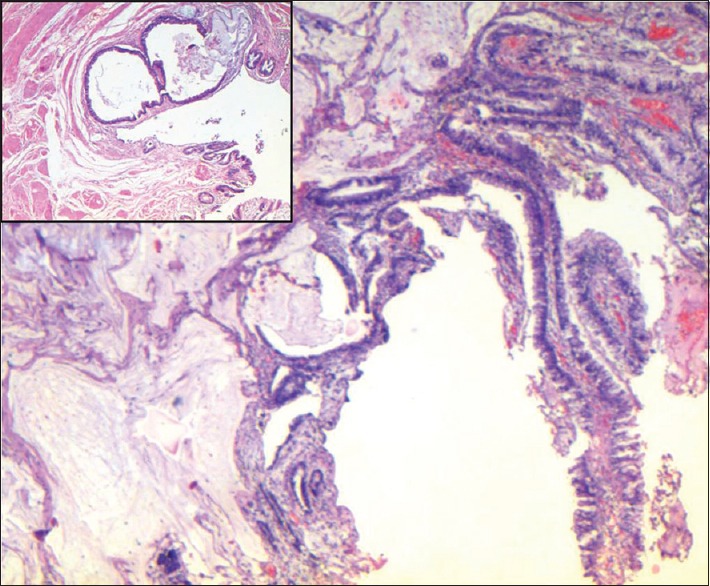
Adenocarcinoma with mixed papillary and mucinous pattern (H and E, ×40). Inset: Tumor in the ureteric wall (H and E, ×200)
Since adenocarcinoma is a rare entity in ureter, the probability of it being coming from any other site was ruled out by whole body positron emission tomography-CT (PET-CT). This test was done to rule out any other origin of the disease as well as to carry out the metastatic work up. Whole body PET-CT scan in the postoperative phase reported the absence of any fluorodeoxyglucose avid disease in the body. In view of positive resection margins and close radial margins, the patient received external beam radiation for a dose of 50.4 Gy/28 fr to the postoperative bed and lymph nodes and a simultaneous boost of 61.6 Gy/28 fr to the region of positive margins, that is, distal resection margin [Figure 2]. There were no postoperative complications and the patient tolerated the radiation well. The patient is still in follow-up and is disease-free for a duration of 2 years and 3 months from the date of diagnosis.
Figure 2.
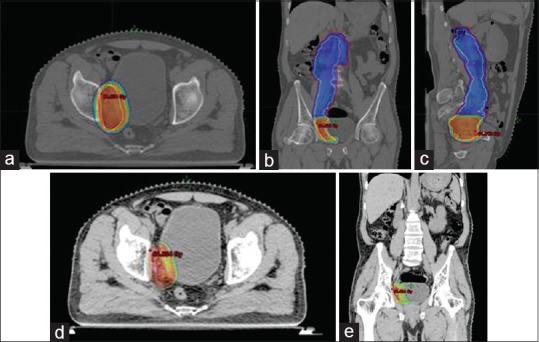
(a) Axial view of the planning computed tomography scan showing dose distribution of 50.4 Gy target volume. (b) Coronal view of the planning computed tomography scan showing dose distribution of 50.4 Gy target volume. (c) Sagittal view of the planning computed tomography scan showing dose distribution of 50.4 Gy target volume. (d) Axial view showing dose distribution of boost area receiving a dose of 61.6 Gy. (e) Coronal view showing dose distribution of boost area receiving a dose of 61.6 Gy
DISCUSSION
Primary adenocarcinoma ureter occurs most often in the age of 60–70 years, with 45% in the lower third of the ureter and 40% accompanied by calculus. Histologically, there are three subcategories; tubulovillous, mucinous, and papillary nonintestinal adenocarcinoma of the renal pelvis.[2] The first two groups representing intestinal adenocarcinoma comprise 93% of cases.[4] A review of adenocarcinomas of the renal pelvis has described villous adenoma-like morphology as a common histological finding in adenocarcinomas of the renal pelvis; this further supports the theory that intestinal type villous adenoma is a precursor to renal pelvis adenocarcinoma. Upper urinary tract stones, with or without recurrent urinary tract infection, are a known risk factor for primary carcinoma of ureter including squamous cell carcinoma, transitional cell carcinoma, and adenocarcinoma. The mechanism is thought to be mediated through chronic inflammation leading to urothelial proliferation and eventual malignant transformation. Chow et al. found significantly increased rates of the renal pelvis and ureter cancer among patients admitted for upper urinary tract stones.[5] The radiological investigations required for making a diagnosis include ultrasonography, intravenous, and retrograde urography, CT, and if needed Doppler study and DTPA scan. Intravenous urography shows the functional status of the affected kidney and combined with retrograde urography, usually shows a filling defect in the renal pelvis and ureter along with hydronephrosis and hydroureter (due to obstruction). Contrast-enhanced CT scan is the investigation of choice and demonstrates the tumor with a total evaluation, for example., appearance, extent, local spread, and its effect on renal function. Preoperative diagnosis of this tumor is difficult, and the final diagnosis is certainly by postoperative histopathology. Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) have been found to be positive in the immunohistochemical staining in some patients, whose serum levels of CEA and CA 19-9 were apparently elevated simultaneously and decreased after the resection of the tumor.[6] The treatment of primary adenocarcinoma includes surgery, chemotherapy, and radiotherapy. Similar to urothelial carcinoma, standard surgery is radical nephroureterectomy with the removal of bladder cuff. It is important to remove the bladder cuff because of high rates of ureteral stump recurrence, reported to be 30–75%.[7] Postoperative adjuvant radiation or chemotherapy may be warranted in selected cases to prevent locoregional recurrence. Various chemotherapeutic regimens include MVAC (Methotrexate, Vinblastin, Adriamycin and Cisplatin) and paclitaxel carboplatin combination. It has been reported that survival in adenocarcinoma ureter depends on histopathological subtypes.[2] Tubulovillous adenocarcinomas are the most aggressive and associated with 5-year survival rate of <30%. Mucinous tumors were associated with 67% survival rate and papillary nonintestinal tumors with nearly a 100% survival rate.
Adenocarcinoma of ureter is a disease entity that has not been extensively studied and in the absence of clearly established guidelines for the management of postoperative cases, our patient was treated as per the guidelines of transitional cell carcinoma of upper urinary tract. The patient had positive distal margins and close radial margins. Hence, to reduce the risk of recurrence, he was given postoperative adjuvant external beam radiation. He is disease-free after 2 years of follow-up. Primary adenocarcinoma pelvis and ureter are very rare and constitute <1% of all malignancies arising from ureter and renal pelvis. In our case, the patient had a history of stone removal, 4 years prior to presentation and postoperative histopathological report showed changes consistent with chronic pyelonephritis. He underwent surgery followed by adjuvant radiotherapy by image-guided-intensity modulated radiation therapy technique in view of positive margins and is recurrence-free after a follow-up period of 2 years. Due to limited literature and lack of randomized, prospective trials asserting the role of chemotherapy and radiotherapy, management should be individualized, and more cases need to be studied. Randomized, prospective, placebo-controlled trials are required, but are difficult because of the low incidence and prevalence of the disease. However, adjuvant treatment in the form of radiation should be strongly considered in cases with close or positive margins to reduce the chances of locoregional recurrence.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Huben RP, Mounzer AM, Murphy GP. Tumor grade and stage as prognostic variables in upper tract urothelial tumors. Cancer. 1988;62:2016–20. doi: 10.1002/1097-0142(19881101)62:9<2016::aid-cncr2820620924>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Spires SE, Banks ER, Cibull ML, Munch L, Delworth M, Alexander NJ. Adenocarcinoma of renal pelvis. Arch Pathol Lab Med. 1993;117:1156–60. [PubMed] [Google Scholar]
- 3.Wang W, Fu X, Zhou R, He J. Primary Adenocarcinoma of the Ureter. 2010. [Last acccessed on 2013 Nov 30]. Available from: http://www.bjui.org .
- 4.Stein A, Sova Y, Lurie M, Lurie A. Adenocarcinoma of the renal pelvis. Report of two cases, one with simultaneous transitional cell carcinoma of the bladder. Urol Int. 1988;43:299–301. doi: 10.1159/000281360. [DOI] [PubMed] [Google Scholar]
- 5.Chow WH, Lindblad P, Gridley G, Nyrén O, McLaughlin JK, Linet MS, et al. Risk of urinary tract cancers following kidney or ureter stones. J Natl Cancer Inst. 1997;89:1453–7. doi: 10.1093/jnci/89.19.1453. [DOI] [PubMed] [Google Scholar]
- 6.Iwaki H, Wakabayashi Y, Kushima R, Okada Y, Tomoyoshi T. Primary adenocarcinoma of the ureter producing carbohydrate antigen 19-9. J Urol. 1996;156:1437. [PubMed] [Google Scholar]
- 7.Rubenstein MA, Walz BJ, Bucy JG. Transitional cell carcinoma of the kidney 25-year experience. J Urol. 1978;119:594–7. doi: 10.1016/s0022-5347(17)57560-6. [DOI] [PubMed] [Google Scholar]


