Abstract
Malignant peripheral nerve sheath tumors (MPNSTs) are extremely rare soft tissue sarcomas of ectomesenchymal origin. They are commonly seen in association with neurofibromatosis type 1 (NF-1), but can also occur without a history of NF (isolated MPNST). MPNSTs are most commonly located on the extremities (brachial and sacral plexus), head and neck, and trunk regions and are rarely reported in genitourinary organs. These tumors are aggressive, with a high recurrence rate and distant metastases. MPNST involving the kidney is extremely rare, and review of the literature using PubMed from 2001 to 2014 revealed eight cases of MPNST involving the kidney (seven, primarily involving the kidney and one metastatic MPNST of the kidney). Herein, we describe a case of breast metastases from an MPNST of the kidney without a history of NF-1. The patient was initially diagnosed with a spindle cell neoplasm of the kidney with peripheral nerve sheath differentiation. Eventually, the patient developed a right breast mass that was diagnosed as metastatic MPNST. The patient refused any kind of treatment and died 6 months later in hospice care.
Keywords: Breast metastases, kidney tumor, malignant peripheral nerve sheath tumor, neurofibromatosis, S-100
INTRODUCTION
Malignant peripheral nerve sheath tumors (MPNSTs) account for approximately 10% of all soft tissue sarcomas. They are commonly associated with neurofibromatosis type 1 (NF-1) (50%), but also occur postradiation (10%) or sporadically (40%).[1] Most MPNSTs are aggressive with high rates of recurrence (40%) and distant metastases (60%).[2,3] MPNST arising from the abdominal cavity is extremely rare.[4]
CASE REPORT
An 80-year-old female patient was referred to urology for evaluation of a 3.8 cm × 2.8 cm × 3 cm mass in the left kidney that was discovered incidentally on ultrasound of the abdomen. Computed tomography (CT) of the abdomen and pelvis revealed 3.6 cm uniformly enhancing mass in the left kidney suspicious for a renal neoplasm [Figure 1]. No regional lymphadenopathy was noted. CT-guided needle core biopsy of the left kidney consisted of normal renal parenchyma and one core composed of interlacing bundles of spindle cells with mild nuclear pleomorphism. Necrosis was not identified. Mitotic count was 6 mitoses per 10 high-power fields. Multiple immunohistochemical stains using a polymer detection system with diaminobenzidine chromogen demonstrated diffuse expression of vimentin and S-100 protein [Figure 2], but negative expression of cytokeratin (CK) OSCAR, CK 7, CD10, renal cell carcinoma (RCC), Pax 2, CD34, CD117/c-kit, smooth muscle actin, neurofilament, HMB-45 or Melan-A. The neoplasm was diagnosed as a spindle cell neoplasm with suggestion of peripheral nerve sheath differentiation; recommend resection of the tumor and further pathologic study. Personal and family history was negative for NF-1. The patient was advised treatment but refused surgical intervention. Eight months later, she felt a mass in her right breast, and CT scan of the chest and abdomen revealed a 1.2 cm × 1.2 cm peripherally enhancing lesion in the right breast and increase in the size of the left renal lesion (5.1 cm × 4.8 cm × 5.0 cm). Additional mammogram images showed two masses in the right breast (measuring 2.7 cm [Figure 3] and 1.9 cm). Subsequent ultrasound-guided needle core biopsy of the right breast masses consisted of multiple cores of spindle cell neoplasm, similar to that from the left kidney, but with increased cellularity and increased nuclear pleomorphism [Figure 4]. Mitotic count was 14 mitoses per 10 high-power fields. Areas of geographic tumor necrosis were present. Immunohistochemical stains performed on the breast biopsy demonstrated positive expression of S-100 protein, but a negative expression of CK cocktail, CK MNF116, p63, HMB-45, and Melan-A or MDM2. On further consultation with pathology and review of previous kidney slides, a diagnosis of high-grade MPNST was rendered, most likely primarily in the kidney, and metastatic to the breast. Metastatic workup with nuclear bone scan of the whole body was negative for any other masses. The patient refused any kind of treatment and preferred hospice care. The patient died 6 months later receiving hospice care.
Figure 1.
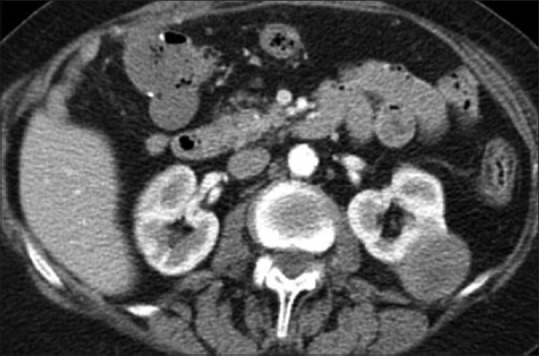
Computed tomography scan abdomen and pelvis demonstrating 3.6 cm uniformly enhancing mass in the left kidney
Figure 2.
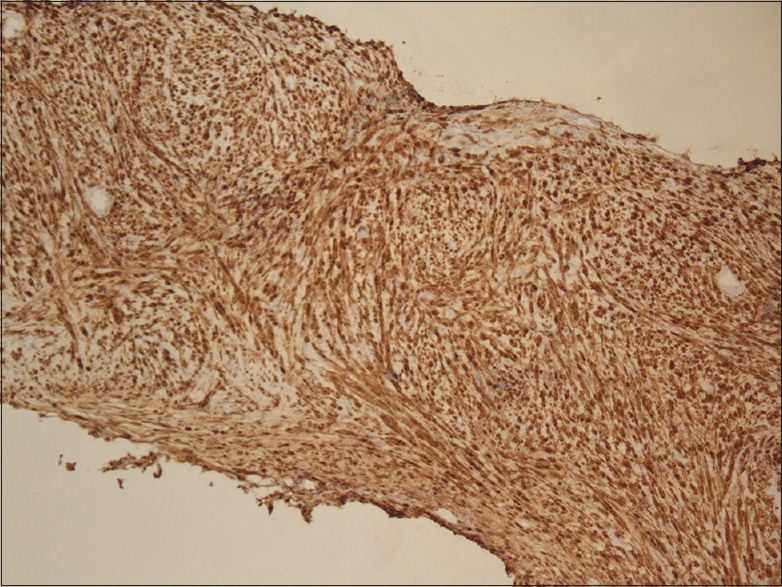
Immunohistochemistry showing kidney tumor cells positive for S-100 protein
Figure 3.
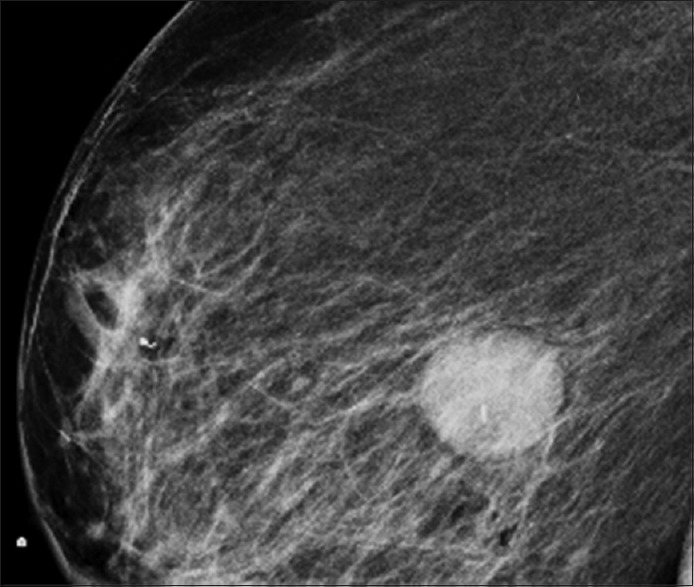
Mammogram showing circumscribed appearing 2.7 cm mass in inner central portion of the right breast
Figure 4.
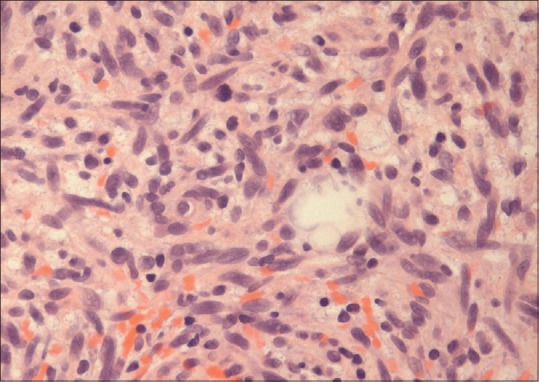
Breast tissue reveals spindle cell morphology with mitosis, high magnification
DISCUSSION
MPNSTs are exceptionally rare soft tissue sarcomas with an overall incidence of 0.001% in the general population and 4.6% in patients with NF-1.[3] MPNST originates from peripheral nerves or sheaths of peripheral nerve fibers and is derived from Schwann cells or perineural cells of neural crest origin.[5,6] It can also arise from preexisting benign nerve sheath tumors such as neurofibromas or perineuromas. The term MPNST replaces a number of previously used names including malignant schwannoma, malignant neurilemmoma, neurogenic sarcoma and neurofibrosarcoma. MPNSTs typically present between 20 and 50 years of age[5] They are most commonly found in the extremities (45%, involving brachial and sacral plexus), followed by the trunk (34%) and the head and neck region (19%). The occurrence of MPNST in the kidney is extremely rare, and review of the literature using PubMed from 2001 to 2014 revealed seven cases of MPNST primarily involving the kidney (renal capsule, renal parenchyma, and renal pelvis),[7,8,9,10,11,12,13] one is metastatic MPNST of the kidney and one is malignant metastatic perirenal schwannoma.[14,15] Interestingly, in all these cases, there was no personal and family history of NF-1 or of radiation exposure described. MPNST can present with new onset of pain, rapidly enlarging mass or may be asymptomatic and found incidentally on imaging studies. Our patient was asymptomatic and incidentally found to have kidney mass on imaging studies. Magnetic resonance imaging (MRI) and CT scan are helpful in visualization of the tumor but cannot differentiate malignant versus benign tumors. MRI is the imaging modality of choice, with findings of tumor size >5 cm, hemorrhage, necrosis, heterogeneity all characteristic of MPNST, and also suggest malignant nature. The definitive diagnosis is made by biopsy with histopathology and immunohistochemical studies. However, attaining the correct diagnosis remains challenging because of the cellular and histopathological similarities with other spindle cell sarcomas such as fibrosarcoma, leiomyosarcoma, and synovial sarcoma. Microscopically, MPNST is comprised of spindle cells reminiscent of Schwann cells with varying degree of mitosis, areas of necrosis, and hemorrhage.[4] Approximately, 80–85% of MPNSTs are spindle cell tumors characterized by diffuse spindle-shaped cells with a fascicular pattern that resemble fibrosarcoma.[16] The remaining 15% exhibit epithelioid, or heterogeneous differentiation; the latter includes rhabdomyoblastic, cartilaginous, osteogenic and rarely, glandular, smooth muscle cell, and liposarcomatous components.[16] Recently, Gallego et al., reported a case of malignant triton tumor of the kidney.[17] Malignant triton tumor refers to MPNSTs with rhabdomyosarcomatous differentiation characterized by both neural and skeletal muscle differentiation.[18] It is extremely rare to have two or more heterogeneous components in a single MPNST. Suresh et al. described a case of MPNST with divergent differentiation with foci of epithelioid, rhabdomyoblastic, osteogenic, and lipogenic differentiation.[18] MPNST stains positive for S-100 protein (nerve sheath origin), Leu 7, vimentin, myelin basic protein, and neuron-specific enolase.[4] In general, a combination of antigens is used to help exclude other spindle cell lesions and to confirm the diagnosis of MPNST. MPNSTs most commonly metastasize to the lungs, followed by the bone and the pleura.[19] Metastatic workup is done using imaging studies, mainly with CT scan of chest and bone scan, as it commonly metastasizes to the lungs and the bones. Metastases to the breast from extramammary tumors are much less come with respect to primary breast tumor (0.5–2% of all breast cancers). Metastatic RCC to the breast is extremely rare, accounting for 3% of cases.[20] Breast metastases from an MPNST kidney have not been previously described in literature, and to the author's knowledge this is the first case of metastatic MPNST of the breast. Complete surgical excision with wide negative margins is the treatment of choice in MPNST. Routine nodal dissection is not indicated. MPNSTs have limited response to chemotherapy and radiotherapy.[21] However, Oncologists recommend postoperative systemic therapy to give the best chance of survival. Despite all treatments, prognosis remains poor with 5 years survival rates of 40–66%. Ducatman et al. studied MPNST in patients with and without NF and reported 5-year survival rates of 16% and 53%, respectively.[3] Our patient died 18 months after the initial diagnosis of the kidney mass. Primary MPNST of the breast is also a rare entity with only nine cases reported in the literature, and most of them are treated with surgical excision of the tumor.
CONCLUSION
To the author's knowledge, this is the first case of MPNST of the kidney with metastases to the breast. MPNST tends to be rapidly growing with the potential to recur and metastasize. The definitive diagnosis is made by histopathology and immunostaining. However, preoperative diagnosis of renal MPNST remains challenging, which was indeed what happened in this case. Radiographic evaluation is extremely important for metastatic workup of MPNST, being an aggressive tumor. Prognosis remains poor despite all treatments.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Beer TC. Department of Neurosurgery Faculty Papers; 2012. Malignant Peripheral Nerve Sheath Tumor (MPNST): An Overview with Emphasis on Pathology, Imaging and Management Strategies. Paper 18. 2012 [Google Scholar]
- 2.Murphey MD, Smith WS, Smith SE, Kransdorf MJ, Temple HT. From the archives of the AFIP. Imaging of musculoskeletal neurogenic tumors: Radiologic-pathologic correlation. Radiographics. 1999;19:1253–80. doi: 10.1148/radiographics.19.5.g99se101253. [DOI] [PubMed] [Google Scholar]
- 3.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–21. doi: 10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Miguchi M, Takakura Y, Egi H, Hinoi T, Adachi T, Kawaguchi Y, et al. Malignant peripheral nerve sheath tumor arising from the greater omentum: Case report. World J Surg Oncol. 2011;9:33. doi: 10.1186/1477-7819-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rober PE, Smith JB, Sakr W, Pierce JM., Jr Malignant peripheral nerve sheath tumor (malignant schwannoma) of urinary bladder in von Recklinghausen neurofibromatosis. Urology. 1991;38:473–6. doi: 10.1016/0090-4295(91)80242-y. [DOI] [PubMed] [Google Scholar]
- 6.Eltoum IA, Moore RJ, 3rd, Cook W, Crowe DR, Rodgers WH, Siegal GP. Epithelioid variant of malignant peripheral nerve sheath tumor (malignant schwannoma) of the urinary bladder. Ann Diagn Pathol. 1999;3:304–8. doi: 10.1016/s1092-9134(99)80027-6. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto H, Naito K, Hiragino T, Bara Y. Retroperitoneal malignant schwannoma: A case report. Hinyokika Kiyo. 2002;48:307–9. [PubMed] [Google Scholar]
- 8.Okumi M, Tanaka M, Noma M, Ichimaru N, Kobayashi Y, Itoh K. A case of malignant peripheral nerve sheath tumor of the kidney. Hinyokika Kiyo. 2004;50:421–4. [PubMed] [Google Scholar]
- 9.Jankulovski N, Stankov O, Banev S, Petrovski D, Mickovski A, Mitevski A, et al. Isolated malignant peripheral nerve sheath tumor of kidney capsule. Prilozi. 2008;29:361–69. [PubMed] [Google Scholar]
- 10.Voznesensky MA, Yamase H, Taylor JA., 3rd Malignant peripheral nerve sheath tumor of the renal pelvis. Urol Int. 2009;83:370–2. doi: 10.1159/000241687. [DOI] [PubMed] [Google Scholar]
- 11.Khandelwal A, Gupta A, Khandelwal K. Malignant peripheral nerve sheath tumor of kidney. Iran J Kidney Dis. 2011;5:373. [PubMed] [Google Scholar]
- 12.Alharbi B. Malignant peripheral nerve sheath tumor of kidney – A case report. Int J Surg Case Rep. 2013;4:914–6. doi: 10.1016/j.ijscr.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouellet S, Doueik A, Sabbagh R. Malignant peripheral nerve sheath tumour of the renal parenchyma presenting as a fast growing atypical renal cyst. Can Urol Assoc J. 2013;7:E601–4. doi: 10.5489/cuaj.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cachay M, Sousa-Escandón A, Gibernau R, Benet JM, Valcacel JP. Malignant metastatic perirenal schwannoma. Scand J Urol Nephrol. 2003;37:443–5. doi: 10.1080/00365590310019026. [DOI] [PubMed] [Google Scholar]
- 15.Williams SB, Szlyk GR, Manyak MJ. Malignant peripheral nerve sheath tumor of the kidney. Int J Urol. 2006;13:74–5. doi: 10.1111/j.1442-2042.2006.01238.x. [DOI] [PubMed] [Google Scholar]
- 16.Guo A, Liu A, Wei L, Song X. Malignant peripheral nerve sheath tumors: Differentiation patterns and immunohistochemical features – A mini-review and our new findings. J Cancer. 2012;3:303–9. doi: 10.7150/jca.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallego A, Pontones JL, Ramos D, Boronat F. Malignant triton tumor of the kidney. New location not previously reported. Urol Int. 2015 doi: 10.1159/000434639. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Suresh TN, Harendra Kumar ML, Prasad CS, Kalyani R, Borappa K. Malignant peripheral nerve sheath tumor with divergent differentiation. Indian J Pathol Microbiol. 2009;52:74–6. doi: 10.4103/0377-4929.44971. [DOI] [PubMed] [Google Scholar]
- 19.Geller DS, Gebhardt M. Malignant peripheral nerve sheath tumors (MPNSTs) Electron Sarcoma Update Newsl. 2006. [Last accessed on 2015 Nov 10]. p. 3. Available from: http://sarcomahelp.org/learning_center/mpnst.html .
- 20.Alzaraa A, Vodovnik A, Montgomery H, Saeed M, Sharma N. Breast metastasis from a renal cell cancer. World J Surg Oncol. 2007;5:25. doi: 10.1186/1477-7819-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grobmyer SR, Reith JD, Shahlaee A, Bush CH, Hochwald SN. Malignant peripheral nerve sheath tumor: Molecular pathogenesis and current management considerations. J Surg Oncol. 2008;97:340–9. doi: 10.1002/jso.20971. [DOI] [PubMed] [Google Scholar]


