Abstract
It has been shown that while commensal bacteria promote Th1, Th17, and Treg cells in lamina propria (LP) in steady-state conditions, they suppress mucosal Th2 cells. However, it is still unclear whether there are specific commensal organisms down-regulating Th2 responses, and the mechanism involved. Here we demonstrate that commensal A4 bacteria, a member of the Lachnospiraceae family, which produce an immunodominant microbiota CBir1 antigen, inhibits LP Th2-cell development. When transferred into the intestines of RAG−/− mice, CBir1-specific T cells developed predominately towards Th1 cells and Th17 cells, but to a lesser extent into Th2 cells. The addition of A4 bacterial lysates to CD4+ T-cell cultures inhibited production of IL-4. A4 bacteria stimulated dendritic cell production of TGF-β, and blockade of TGF-β abrogated A4 bacteria inhibition of Th2-cell development in vitro and in vivo. Collectively, our data show that A4 bacteria inhibit Th2-cell differentiation by inducing dendritic cell production of TGF-β.
Keywords: Commensal A4 bacteria, Th2, dendritic cell, TGF-β
Introduction
The mammalian barrier surfaces, especially the gastrointestinal (GI) tract, harbor trillions of commensal bacteria. It is well established now that dysbiosis results in multiple diseases, such as asthma, diabetes, cancer, and inflammatory bowel disease (IBD) [1–5]. It has been shown that bacterial compositions and metabolites play a vital role in regulating the development of T helper cell lineage in the intestines [6]. For instance, segmented filamentous bacteria (SFB) which reside mostly in the ileum of rodents facilitate the development of Th17 cells in a TLR signaling-independent manner [7]. Bacteroides fragilis induces the development of IL-10-producing Treg cells [8], while Clostridium species promote the differentiation of Helios− Treg cells [9].
The hygiene hypothesis, which was developed decades ago, proposes that a lack of early childhood exposure to infectious agents, symbiotic microorganisms, and parasites increases susceptibility to allergic diseases by suppressing the natural development of the immune system [10, 11]. When it comes to the development of effector T cells specific for commensal bacteria, systemic immune responses are biased to Th2 under Germ-free and neonatal conditions. Furthermore, colonization of commensal bacteria inhibits the intestinal Th2 response [12, 13]. Two elegant studies demonstrated recently that microbiota promoted RORγt+ Treg cells in intestinal lamina propria [14, 15]. While RORγt+ Treg cells were showed to downregulate type 2 immune responses by one report [14], which could count microbiota inhibition of Th2 response in intestines, the other report did not observe such an effect [15]. Thus, the microbiota species which inhibit Th2 response and the mechanisms involved remain unclear. We demonstrated in this report that commensal A4 bacteria, a member of the Lachnospiraceae family isolated from mouse intestinal lumen, inhibited lamina propria Th2 cell development through induction of dendritic cell (DC) production of TGF-β.
Results and discussion
Commensal A4 bacteria inhibit Th2-cell development
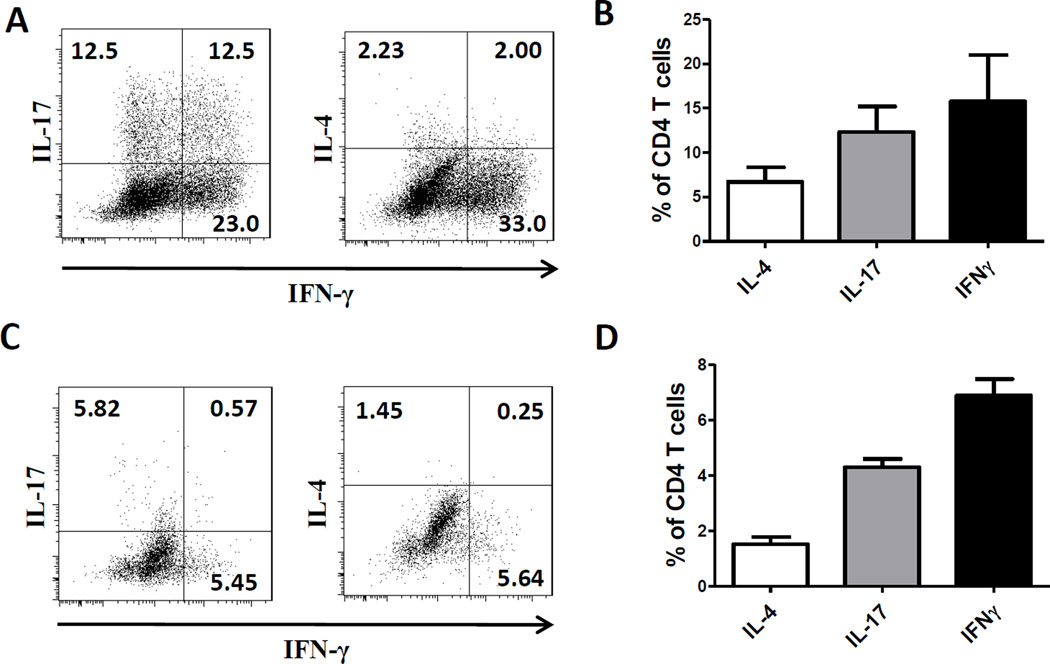
Accumulating evidence indicates that microbiota differentially regulates T-cell responses in intestines. While several species of microbiota have been identified as specifically promoting the development of Th17 or Treg cells, microbiota, in generally, inhibits Th2 responses in intestine. A4 bacteria, a member of the Lachnospiraceae family which produce immunodominant CBir1 antigen in the intestines [16], were isolated from mouse intestinal lumen [17]. When CBir1-specific CD4+ T cells from CBir1 TCR transgenic (Tg) mice were transferred into RAG−/− mice, which contain A4 bacteria in the intestinal lumen, a significant amount of IFN-γ-producing Th1 cells and IL-17-producing Th17 cells, whereas only minimal numbers of IL-4-producing Th2 cells were developed in intestines (Figs. 1A and B). Similar pattern of T cells were detected in spleen, albeit at a lower level (Figs. 1C and D). It is very likely that CBir1 Tg T cells were activated in the intestines and then migrated into spleens, as A4 bacteria only present in the intestinal lumen.
Figure 1.
Development of microbiota-specific T cells in the intestines and spleens. 2×106 CBir1 TCR Tg T cells were transferred into RAG−/− mice. Four weeks later, mice were sacrificed. (A) Intestinal lamina propria CD4+ T-cell expression of IL-4, IFN-γ and IL-17 was determined by flow cytometry in the recipient mice. (B) Frequencies of IL-4+, IFN-γ+, IL-17+ of CD4+ T cells in lamina propria. (C) Representative FACS plots of IL-4, IFN-γ and IL-17 staining from the spleens. (D) Frequencies of IL-4+, IFN-γ+, IL-17+ of CD4+ T cells in spleens. Data are shown as mean + SEM and represent 3 independent experiments pooled from total of 12 mice.
To investigate whether A4 bacteria regulate T cell development, next we cultured B6 CD4+ T cells with splenic APCs and anti-CD3 mAb in the presence or absence of A4 bacterial lysates for 5 days. T cell cytokine production was analyzed by flow cytometry. A4 bacteria promoted T cell production of IFN-γ (Fig. 2A and B). As B6 CD4+ T cells produced only minimum amounts of IL-4 when stimulated with splenic APCs and anti-CD3 mAb under neutral conditions without additional cytokines, to determine the effect of A4 bacteria on IL-4-producing Th2 cell development, we cultured B6 CD4+ T cells with APCs and anti-CD3 mAb in the presence or absence of A4 bacterial lysates under Th2 polarizing conditions with IL-4 and anti-IFN-γ. A4 bacteria inhibited T cell production of IL-4 and IL-5 under Th2 polarization condition (Fig. 2C, 2D and supporting information Figure 1). To further confirm A4 bacterial inhibition of Th2 cell development, we repeated the aforementioned experiments by culturing OT II T cells, which are specific for ovalbumin (OVA), with splenic APCs in the presence or absence of A4 bacterial lysates under Th2 polarizing conditions. A4 bacterial lysates also inhibited OT II T cell development into Th2 cells (data not shown).
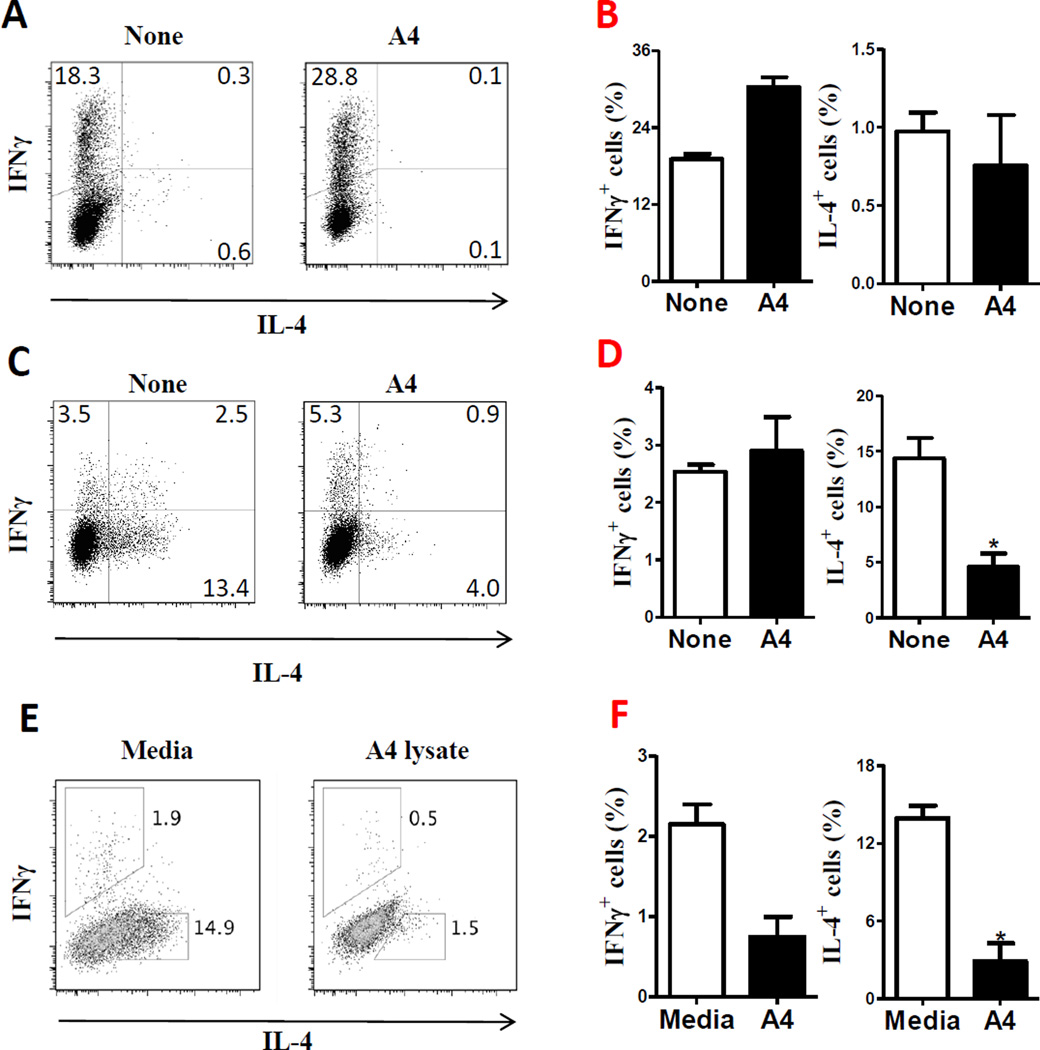
Figure 2.
Effect of A4 bacteria on T-cell differentiation into Th2 cells. (A) B6 CD4+ cells were cultured with α-CD3 (5 µg/ml) and splenic APCs in the presence or absence of A4 lysates (25 µg/ml) for 5 days. CD4+ T-cell expression of IL-4 and IFN-γ was measured by flow cytometry. FACS plots are representative of 3 independent experiments. (B) Relative frequencies of IL-4+ and IFN-γ+ CD4+ T cells of the samples in (A). (C) B6 CD4+ T cells were cultured with α-CD3 (5 µg/ml) and splenic APCs in the presence or absence of A4 lysates (25 µg/ml) under Th2 polarizing conditions (30 ng/ml of IL-4 and 10 µg/ml of anti-IFN-γ mAb) for 5 days. CD4+ T-cell expression of IL-4 and IFN-γ was measured by flow cytometry. FACS plots are representative of 3 independent experiments. (D) Relative frequencies of IL-4+ and IFN-γ+ CD4+ T cells of the samples in (C) (E) Splenic APCs were pretreated with A4 lysate for 20 h, then co-cultured with B6 CD4+ cells with α-CD3 (5 µg/ml) under Th2 polarizing conditions. CD4+ T-cell expression of IL-4 and IFN-γ was determined by flow cytometry. FACS plots are representative of 3 independent experiments. (F) Relative frequencies of IL-4+ and IFN-γ+ CD4+ T cells of the samples in (E). Data are shown as mean ± SEM pooled from 3 independent experiments. *p<0.05, Student’s-t test.
We next tested whether commensal A4 bacteria inhibited Th2-cell development through APCs. First we pretreated splenic APCs with A4 bacteria for 20 h. After washing, pretreated APCs were put into the culture of B6 CD4+ T cells with anti-CD3 under Th2 polarizing conditions. Pretreatment of APCs with A4 bacterial lysates decreased B6 CD4+ T cell production of IL-4 (Fig. 2E and F). Pretreatment of APCs with A4 bacterial lysates was also able to inhibit OT II CD4+ T cell production of IL-4 stimulated with OVA (data not shown). These data indicated that commensal A4 bacteria could inhibit Th2 cell response through interaction with APCs. To determine if other commensal bacteria can also inhibit Th2 response, we cultured B6 CD4+ T cells with APCs and anti-CD3 mAb in the presence or absence of lysates of commensal E. coli isolated from mouse intestinal lumen [17] under Th2 polarizing conditions with IL-4 and anti-IFN-γ. E. coli didn’t inhibit IL-4 production by T cells (supporting information Figures 2A and B).
Commensal A4 bacteria inhibit Th2-cell development by inducing dendritic cell TGF-β
TGF-β is a crucial cytokine that contributes to the maintenance of immune homeostasis through inhibition of immune cell function as well as differentially regulation of development of T cell subsets [18–20]. Although TGF-β has been shown to have the ability to inhibit both Th1 differentiation and Th2 differentiation, a recent report demonstrated that TGF-β ‘preferentially’ inhibited Th2 differentiation expression through inhibition of GATA3 expression [21, 22]. To determine whether A4 bacteria induce dendritic cell production of TGF-β which could mediate A4 inhibition of Th2 cells, we isolated CD11c+ DCs from mesenteric lymph nodes (MLN) of B6 mice, and stimulated them with A4 bacterial lysates for 24 h. TGF-β production was measured in culture supernatant. We found that A4 bacteria stimulated dendritic cell production of TGF-β significantly (Fig. 3A).
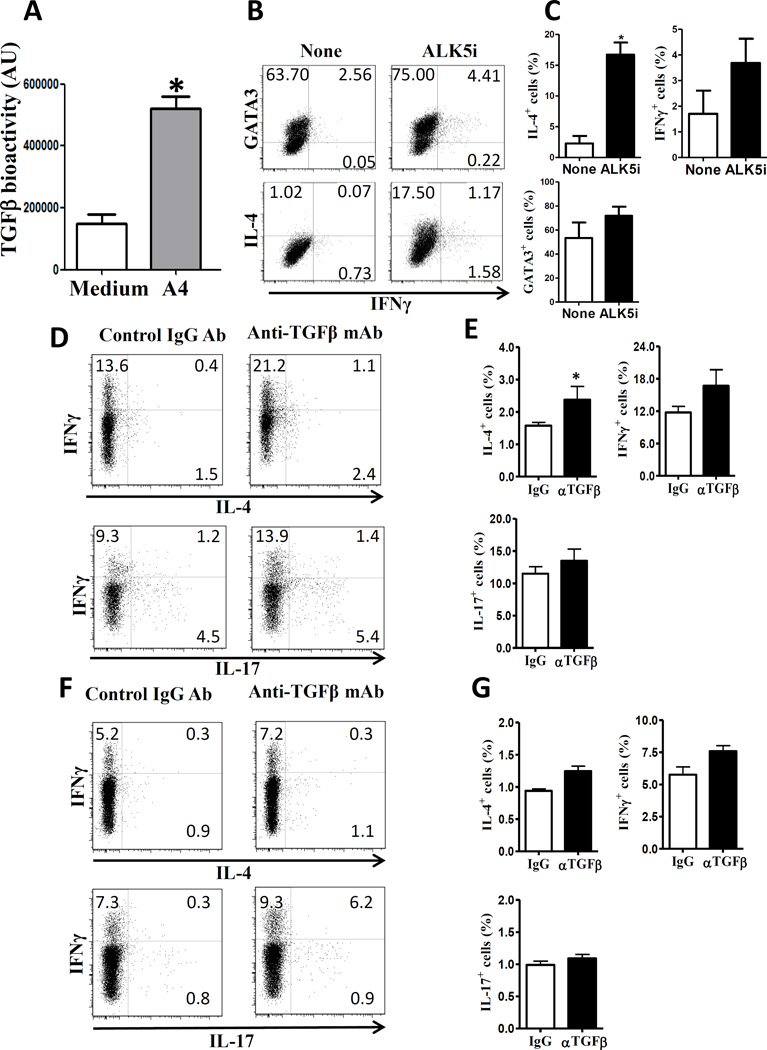
Figure 3.
Impact of TGF-β in the Th2-cell inhibition in vitro and in vivo. (A) CD11c+ cells from MLN were stimulated with A4 lysates (10 µg/ml). TGF-β was measured from the supernatant of a 24 h culture by SEAP. Data are shown as mean ± SEM pooled from 2 independent experiments. *p<0.05, Student’s-t test. (B) OTII CD4+ T cells were cultured with CD11c+ cells, that were pre-treated with A4 bacterial lysates, and OVA peptide (1µg/ml) with or without ALK5i (1 µM) under Th2-polarizing conditions. On day 5, IL-4, GATA3 and IFN-γ expressions were measured by flow cytometry. (C) Relative frequencies of IL-4+, IFN-γ+ and GATA3+ CD4+ T cells of the samples in (B). Data are shown as mean ± SEM pooled from 2 independent experiments. *p<0.05, Student’s-t test. (D–G) Two-week-old B6 mice were injected with anti-TGF-β Ab or control antibody for 6 weeks. CD4+ T-cell expression of IL-4 and IFN-γ was measured by flow cytometry. (D) FACS profile and (E) summary of IL-4 and IFN-γ expression in lamina propria. (F) FACS profile and (G) summary of IL-4 and IFN-γ expression in spleens. Data are shown as mean ± SEM of 10 mice pooled from 2 independent experiments. *p<0.05, Mann-Whitney U test.
To determine if TGF-β is involved in the Th2 inhibition of A4 bacteria, ALK5i, a specific inhibitor of TGF-β receptor I kinase, was used to block TGF-β signaling. OTII CD4+ T cells were cultured with CD11c+ DCs, which were pretreated with A4 lysates, and OVA peptide in the presence or absence of ALK5i under Th2 polarizing conditions. As shown in Figures 3B and C, blockade of TGF-β abrogated A4 bacteria inhibition of OTII T cell GATA3 expression and IL-4 production. Collectively, these data demonstrated that A4 bacteria induction of DC TGF-β possibly mediates A4 bacterial inhibition of Th2 cell development.
Blockade of TGF-β promotes Th2-cell development in the intestines
To determine the role of TGF-β in Th2 response in the intestines, we treated 2-week-old B6 mice for 6 weeks with anti-TGF-β Ab or control antibody to initiate microbiota colonization in the intestines. The mice did not develop inflammation in the intestines and other organs when sacrificed (data not shown). As expected, lamina propria T cell IFN-γ production was increased after treatment with anti-TGF-β Ab in the intestines. Interestingly, blockade of TGF-β also increased lamina propria T cell IL-4 production (Figs. 3D and E). In contrast, T cell production of IL-4 in the spleen (Figs. 3F and G) was not affected by anti-TGF-β Ab treatment. These data demonstrated that TGF-β inhibits a Th2 response in the intestines.
Concluding remarks
Data are emerging relative to the species of microbiota which specifically regulate development of different types of immune responses. SFB has been shown to preferably induce Th17 cells in the intestines, whereas Bacteroides fragilis and Clostridium species specifically promote IL-10 producing Tregs and Foxp3+ Tregs respectively [8, 9]. In our study, by using a microbiota-specific CBir1 TCR Tg mice model, we demonstrated that A4 bacteria, which stimulate CBir1 Tg T cells, were able to inhibit IL-4 production of T cells in vitro. However, commensal E. coli, isolated from mouse intestines, did not inhibit T cell IL-4 production. Thus, A4 bacteria could be one of multiple species of commensal bacteria which downregulate Th2 responses in the intestines. Under different cytokine milieu, TGF-β can induce T cell differentiation into Treg and as well as into Th17 cells. In contrast, TGF-β also inhibits the differentiation of naïve T cells into Th1 and Th2 cells. Our data demonstrated that A4 bacteria stimulated DC production of TGF-β, and blockade of TGF-β abrogated A4 inhibition of T cell production of IL-4 in vitro, but only minimally affected IFN-γ production, indicating that TGF-β could mediate A4 bacteria inhibition of Th2 responses. In addition to A4 bacteria, multiple species of commensal bacteria have also been shown to induce TGF-β production in the intestine, we are currently investigating if any commensal bacteria with capability to induce DC TGF-β production could inhibit Th2 responses, or additional features of A4 bacteria are also contributing to their ability to inhibit Th2 responses. it is very likely that a group of commensal bacteria are capable to inhibit intestinal Th2 response via induction of TGF-β in the local environment.
Materials and Methods
Mice
C57BL/6 and OT-II B6 mice were purchased from Jackson Laboratory and housed in the animal resource center at UTMB. CBir1 flagellin-specific TCR transgenic (CBir1-Tg) mice [23] were maintained in the animal resource center at UTMB. All experiments were reviewed and approved by the Institutional Animal Care and Use Committees of UTMB.
Reagents
RPMI 1640, HEPES, penicillin/streptomycin, β-Mercaptoethanol (β-ME), sodium pyruvate and L-glutamine were all purchased from Life Technologies. Anti-TGF-β and control IgG were from Bioxcell. Th2-polarizing cytokines were from R&D Systems. Anti-mCD3 and fluorochrome-conjugated anti-mouse antibodies were all from Biolegend.
Culture of A4 bacteria
A4 bacteria were isolated and cultured in an anaerobe chamber (Coy Laboratories, Ann Arbor, MI) using a gas mix of 90% N2, 5% CO2, and 5% H2 as previous described [17]. Briefly, A4 isolates were placed into 10 mL of E medium for anaerobes (ATCC medium 602), and mixed in an anaerobe chamber.
Isolation of CD4+ T cells and naïve CD4+ CD62L+ T cells
CD4+ T cells were isolated by using anti-mouse CD4-magnetic beads (BD Biosciences) [24]. CD4+CD62L+ naïve T cells were isolated by using the CD4+CD62L+ T cell isolation kit from Miltenyi Biotec according to the instructions provided by the manufacturer [25].
Primary T cell culture
For Th2-polarizing conditions, B6 T cells were cultured with anti-CD3, irradiated splenic CD4− APCs, IL-4 (30 ng/ml), and anti-IFN-γ (10 µg/ml) for 5 days. OT-II T cells were cultured with OVA peptide (1 µg/ml), irradiated splenic CD4− APCs, IL-4 (10 ng/ml), and anti-IFN-γ (10 µg/ml) for 5 days.
Flow cytometry
As described previously [8], cells were stimulated for 5 h with PMA (50 ng/mL) and ionomycin (750 ng/mL). Staining was performed for CD4, IL-4, IFN-γ, IL-17, and GATA-3 by using fluorescence-conjugated anti-mouse antibodies [23]. The data was analyzed based on the gating strategy as shown in supporting information Figure 1.
Isolation of LP lymphocytes
LP lymphocytes were isolated in the colon by using a Percoll gradient as previously described [24]. The cell yield was typically ~2×106 lymphocytes per mouse with > 90% cell viability.
TGF-β bioassay
As described previously [26], MFB-F11 cells are embryonic fibroblasts from TGF-b1−/− mice which are stably transfected with a reporter plasmid consisting of TGF-β- responsive, Smad-binding elements coupled to a secreted alkaline phosphatase reporter gene. Using the manufacturer’s instructions, secreted alkaline phosphatase activity was measured by using a Great EscApe SEAP Chemiluminescence kit 2.0 (Clontech), which represents biologically active TGF--β activity.
Statistical analysis
For comparisons between samples, levels of significance were determined by Student’s t test or Mann-Whitney U test in Prism 5.0 (Graphpad). Where appropriate, mean ± SEM is represented on graphs. *p < 0.05; **p < 0.01.
Supplementary Material
Acknowledgments
This work was supported by NIH grants DK098370 and DK105585, and John Sealy Memorial Endowment Fund.
Abbreviations
- IL
interleukin
- Th
T helper cell
- LP
lamina propria
Footnotes
Conflict of Interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010;8:171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noverr MC, Huffnagle GB. The 'microflora hypothesis' of allergic diseases, Clinical and experimental allergy. Adv. Exp. Med. Biol. 2005;35:1511–1520. doi: 10.1111/j.1365-2222.2005.02379.x. [DOI] [PubMed] [Google Scholar]
- 4.Gomes AC, Bueno AA, de Souza RG, Mota JF. Gut microbiota, probiotics and diabetes. Nutr. J. 2014;13:60. doi: 10.1186/1475-2891-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, Cao AT, Cong Y. Microbiota regulation of inflammatory bowel disease and colorectal cancer. Semin. Cancer Bio. 2013;23:543–552. doi: 10.1016/j.semcancer.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furusawa Y, Obata Y, Hase K. Commensal microbiota regulates T cell fate decision in the gut. Semin. Immunopathol. 2015;37:17–25. doi: 10.1007/s00281-014-0455-3. [DOI] [PubMed] [Google Scholar]
- 7.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 8.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. P. Natl. Acad. Sci. USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 11.Rook GA. Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology. 2009;126:3–11. doi: 10.1111/j.1365-2567.2008.03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanov II, Littman DR. Modulation of immune homeostasis by commensal bacteria. Curr. Opin. Microbiol. 2011;14:106–114. doi: 10.1016/j.mib.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bamias G, Okazawa A, Rivera-Nieves J, Arseneau KO, De La Rue SA, Pizarro TT, Cominelli F. Commensal bacteria exacerbate intestinal inflammation but are not essential for the development of murine ileitis. J. Immunol. 2007;178:1809–1818. doi: 10.4049/jimmunol.178.3.1809. [DOI] [PubMed] [Google Scholar]
- 14.Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, et al. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science. 2015;349:989–993. doi: 10.1126/science.aac4263. [DOI] [PubMed] [Google Scholar]
- 15.Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, et al. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science. 2015;349:993–997. doi: 10.1126/science.aaa9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J. Clin. Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duck LW, Walter MR, Novak J, Kelly D, Tomasi M, Cong Y, Elson CO. Isolation of flagellated bacteria implicated in Crohn's disease. Inflamm. Bowel Dis. 2007;13:1191–1201. doi: 10.1002/ibd.20237. [DOI] [PubMed] [Google Scholar]
- 18.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 19.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 21.Heath VL, Murphy EE, Crain C, Tomlinson MG, O'Garra A. TGF--beta1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur. J. Immunol. 2000;30:2639–2649. doi: 10.1002/1521-4141(200009)30:9<2639::AID-IMMU2639>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Kuwahara M, Yamashita M, Shinoda K, Tofukuji S, Onodera A, Shinnakasu R, Motohashi S, et al. The transcription factor Sox4 is a downstream target of signaling by the cytokine TGF--beta and suppresses T(H)2 differentiation. Nat. Immunol. 2012;13:778–786. doi: 10.1038/ni.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. P. Natl. Acad. Sci. USA. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng T, Qin H, Wang L, Benveniste EN, Elson CO, Cong Y. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J. Immunol. 2011;186:6313–6318. doi: 10.4049/jimmunol.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Yao S, Dann SM, Qin H, Elson CO, Cong Y. ERK differentially regulates Th17- and Treg-cell development and contributes to the pathogenesis of colitis. Eur. J. Immunol. 2013;43:1716–1726. doi: 10.1002/eji.201242889. [DOI] [PubMed] [Google Scholar]
- 26.Cao AT, Yao S, Gong B, Elson CO, Cong Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J. Immunol. 2012;189:4666–4673. doi: 10.4049/jimmunol.1200955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.