Abstract
Integrin beta3 is critical for tumor invasion, neoangiogenesis, and inflammation making it a promising cancer target. However, preclinical and clinical data of integrin beta3 antagonists have demonstrated no benefit or worse outcomes. We hypothesized that integrin beta3 could affect tumor immunity and evaluated tumors in mice integrin beta3 could affect tumor immunity and evaluated tumors in mice with deletion of integrin beta3 in macrophage lineage cells (β3KOM). β3KOM mice had increased melanoma and breast cancer growth with increased tumor-promoting M2 macrophages and decreased CD8+ T-cells. Integrin beta3 antagonist, cilengitide, also enhanced tumor growth and increased M2 function. We uncovered a negative feedback loop in M2 myeloid cells wherein integrin beta3 signaling favored STAT1 activation, an M1 polarizing signal, and suppressed M2 polarizing STAT6 activation. Finally, disruption of CD8+ T-cells, macrophages, or macrophage integrin beta3 signaling blocked the tumor-promoting effects of integrin beta3 antagonism. These results suggest that effects of integrin beta3 therapies on immune cells should be considered to improve outcomes.
Introduction
Integrins are heterodimeric cell surface receptors that directly bind components of the extracellular matrix (ECM) and participate in cell migration and other important cellular functions (1, 2). Integrin beta3 can heterodimerize with integrin αv or αIIb, and is critical for mature osteoclastic bone resorption, platelet aggregation, and angiogenic endothelial cell function (3, 4). Tumor cells expressing high levels of integrin beta3 exhibit enhanced proliferation and metastasis (5-7). In vitro and in animal models, the integrin αvβ3 antagonist cilengitide was shown to decrease tumor cell proliferation, migration, and neoangiogenesis (8). Mice genetically deleted for integrin beta3 (Itgb3−/−) have decreased metastasis (9). Surprisingly, primary tumor growth is enhanced in Itgb3−/− mice (10). However, cilengitide can enhance tumor growth and angiogenesis if applied suboptimally in preclinical models (11). In clinical trials, integrin beta3 antagonists did not demonstrate improved outcomes in many cancer types (12-14). These findings suggest a need to better understand the role of integrin beta3 in the tumor microenvironment before translating encouraging preclinical discoveries to patients.
We previously reported that integrin beta3 deletion in platelets has no effect on primary tumor growth and angiogenesis, but specific deletion of integrin beta3 in myeloid cells via lysozyme M promoter-driven Cre recombinase (LysM-Cre) results in enhanced tumor growth (15), consistent with both the phenotype observed in Itgb3−/− mice (10) and results from bone marrow transplantation studies (16). Interestingly, in endothelial cells, acute depletion of integrin beta3 transiently inhibits tumor growth and angiogenesis, but long-term deletion has no effect on primary tumor growth (17). These results suggest that integrin beta3 signaling in myeloid cells is most likely to account for the enhanced tumor growth seen in genetic and pharmacologic studies.
Fibroblasts, stromal cells, myeloid cells/macrophages, and lymphocytes are very common tumor-infiltrating cells that play key roles in tumor progression and metastasis (18). Of these, macrophages are among the most abundant recruited host cells in tumors (19). For many solid tumor types, including breast, brain, and skin cancer, high densities of tumor-associated macrophages (TAMs) are generally associated with poor clinical outcome (19, 20). Macrophage recruitment and polarization are dynamic processes in the tumor microenvironment because of the complex milieu of chemoattractant and polarization signals in vivo (20). TAMs that express high levels of major histocompatibility complex (MHC) II usually have a tumoricidal, anti-tumor, M1 phenotype, while TAMs expressing scavenger receptors, such as CD163, CD204, and CD206, are associated with a tumor-promoting and immunosuppressive M2 phenotype (19, 21). Lymphocyte secreted factors, such as interleukin (IL)-4, IL-10, IL-13, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and immunoglobulins, are very strong regulators of macrophage polarization and function (20, 22). M2 TAMs can suppress immune responses by decreasing intra-tumor CD8+ T-cell numbers and have potent pro-angiogenic activity through secretion of factors like VEGF (19, 23, 24). Signal transducers and activators of transcription 1 (STAT1) is a dominant transcriptional factor that regulates M1 macrophage polarization. LPS-activated TLR4 signaling and IFN-γ promote macrophage M1 polarization through activation of STAT1 (25, 26). STAT6 is required to drive M2 macrophage polarization in the presence of IL-4 or IL-13 (25, 26). STAT1 and STAT6 signaling events regulate and inhibit each other and control the polarization and function of macrophages (25, 26).
Beta3 integrins play key roles in myeloid lineage osteoclast differentiation and function and in preclinical models of pathologic inflammation (3, 6). Because loss of integrin beta3 in myeloid cells results in enhanced tumor growth (15), we hypothesized that integrin beta3 may be involved in macrophage polarization and function in the tumor microenvironment. Here, we present data uncovering an anti-tumoral role for integrin beta3 signaling in this context. Integrin beta3 signaling controls the balance between anti-tumor and pro-tumor immune cells through effects on STAT6/STAT1 signaling, which in part explains the mixed results of integrin antagonists in the clinic. Taken together, our findings highlight the important role of TAMs when designing clinical trials with integrin beta3-targeted treatments in cancer.
Materials and Methods
Mice
All animal studies were performed according to the guidelines established by the Animal Studies Committee at Washington University in St. Louis. Wild-type (WT), LysM-Cre knock-in mice and Stat6−/− mice are from The Jackson Laboratory (all C57BL/6J background). Itgb3flox/flox (15) and Itgb3−/− mice (27) on a pure C57BL/6J background were previously reported. All mice are housed under pathogen-free conditions according to the guidelines of the Division of Comparative Medicine, Washington University School of Medicine. Unless noted, all mice were used at 6 to 10 weeks of age.
Cell lines
In 2013, the parental MMTV-PyMT cells (PyMT-B6) (Kindly provided by Dr. DeNardo, Washington University in St. Louis) were isolated from a fully invasive mammary tumor that spontaneous arose at day 120 in a C57bL/6 background MMTV-PyMT mouse, a mouse model that represents an anti-estrogen sensitive, luminal B breast cancer. The tumor was collagenase treated, grown in single-cell suspension on a collagen-coated plate, and cloned to establish the parent PyMT-B6 cell line. Parent PyMT-B6 cells were injected into the mammary fat pad (MFP) tissue of a female C57BL/6J mouse, and after reaching a tumor size approaching 1cm, tumor cells were collagenase treated and cultured in a cell culture dish. The cultured tumor cells were intracardially injected into a 6-week-old female C57BL/6J mouse to establish bone metastases. 12 days post-intracardiac inoculation, the bone tumor was harvested and cultured in a cell culture dish with DMEM media plus 10% FBS to establish the PyMT-BO1 subline, which when compared to the parent PyMT-B6 cells, had a higher incidence of inducing bone metastases after either orthotopic MFP or intracardiac injection. The PyMT-BO1 cells were infected with lentivirus containing the GFP-firefly luciferase genes as described previously (28). GFP-expressing PyMT-BO1 cells were FACS sorted, cultured, and validated for luciferase expression; this cell line was named PyMT-BO1-GFP-Luc. PyMT-B6, PyMT-BO1, and PyMT-BO1-GFP-Luc cells were evaluated by qPCR, and all express the PyMT, Esr1, Esr2, and Itgb3 genes. These cell lines were tested as CD45 negative and pan-Keratin positive by FACS in 2013 and 2015.
The B16-F10 C57BL/6 murine melanoma cell line (ATCC) was modified to express firefly luciferase (B16F10-Luc) as described (29). In 2015, this cell line was tested as CD45 negative and integrin beta3 positive by FACS.
Mice tumor models
For MFP injection, 1×105 PyMT-BO1-GFP-Luc cells mixed with BD matrigel (BD Biosciences) were injected into MFP tissue of 8-week-old female mice. For subcutaneous injections, 1×106 B16F10-Luc cells in 200uL PBS were injected into the flank of WT and β3KOM mice. Tumor growth was measured at each indicated time point. Tumor size (mm3) was calculated by measuring the longest (L) and shortest (S) distance of tumor tissue, with this formula: (tumor size=0.51*L*S2).
For intracardiac injections, the left ventricular chamber of 6-week-old mice was injected with 1×105 B16F10-Luc cells or 1×105 PyMT-BO1-GFP-Luc cells in 50uL PBS as previously described (30). Bioluminescence imaging (BLI) was used to quantify tumor growth after injection. For the cilengitide treatment, mice were given cilengitide (5mg/kg, Selleckchem) by intraperitoneal injection for the indicated time point. For anti-CSF1 antibody (clone 5A1) treatment, mice were given three doses of antibody (1mg, 0.5mg, and 0.5mg per mouse) by intraperitoneal injection. For CD8+ T-cell depletion, mice were given an intraperitoneal injection of 100 μg anti-CD8α (53-6.7; BioLegend) or rat IgG2b κ-chain isotype-matched control antibody (RTK4530; BioLegend) on the appropriate days.
Flow-cytometric analysis
Tumor tissues were prepared in single-cell suspension for FACS analysis (31), with minor modifications (Supplementary Information).
Primary macrophage culture
Primary BMMs were generated from the femurs and tibias of mice as previously described (30) (Supplementary Information).
Microarray Analysis
Microarray was performed with the Genome Technology Access Center at Washington University School of Medicine (Supplementary Information). The microarray data in this manuscript are available on the GEO database with accession number GSE75882.
RT-qPCR
qPCR was performed using SYBR Advantage mix (Bio-Rad) with mouse-specific primers as specified in Table S4 (Supplementary Information).
Bioluminescence imaging (BLI)
Live BLI analysis was previously described (30), with minor modifications (Supplementary Information).
Western blot
Antibodies and protocol were used from Cell Signaling Technology (Supplementary Information).
Statistical analysis
Data are shown as mean ± SD unless noted otherwise. All experiments were analyzed using two-tailed unpaired Student's t-test for two groups or one-way ANOVA with a Bonferroni post-hoc test for three or more groups by Prism (GraphPad Software). P < 0.05 was considered significant.
Results
Mice with myeloid-specific targeted disruption of integrin beta3 have enhanced primary tumor growth and increased M2/M1 macrophage ratios
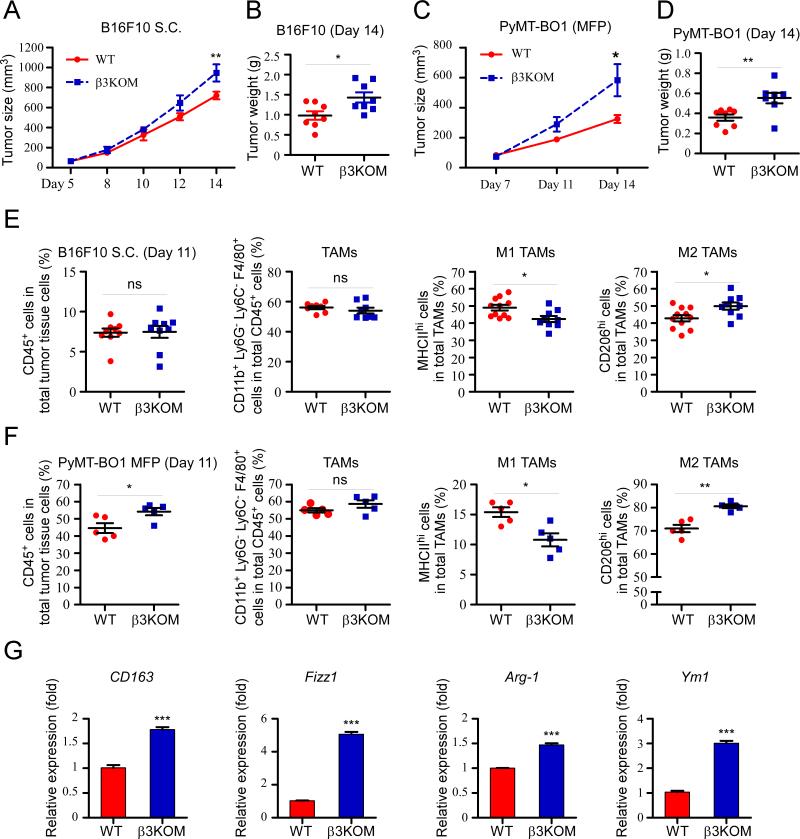
Previous studies have shown that Itgb3−/− mice have enhanced primary tumor growth (10), but the mechanism is largely unknown. Here, we evaluated tumor growth in β3KOM mice lacking integrin beta3 specifically in myeloid cells (LysM-Cre+/−, Itgb3flox/−) and in WT (LysM-Cre+/−, Itgb3flox/+) mice using immunocompetent allograft orthotopic tumor models. We found that in both an orthotopic melanoma subcutaneous tumor model and a breast cancer mammary fat pad (MFP) model, tumor growth was enhanced in β3KOM mice (Figure 1A-D). There was no difference in the number of blood vessels between WT and 3KOM mice as measured by endomucin staining in size matched tumor tissue (Figure S1).
Figure 1. Mice with specific deletion of integrin beta3 in myeloid cells promoted tumor growth with increased M2 TAM infiltration.
(A) Growth curve of subcutaneous injected B16F10-Luc cells (1×106) in 8-week-old male WT (LysM-Cre+/−, Itgb3f/+) and β3KOM (LysM-Cre+/−, Itgb3f/−) mice (n=8). (B) Tumor weight. (C) Growth curve of MFP injected PyMT-BO1-GFP-Luc cells (1×105) in 8-week-old female WT and β3KOM mice (n=6). (D) Tumor weight. (E and F) FACS staining was performed on cells from day 11 tumor tissue. TAMs were gated on CD45+, CD11b+, Ly6G−, Ly6C−, F4/80+ population. In this population, M1 TAMs were gated as MHCIIhi/CD206low cells, and M2 TAMs were gated as MHCIIlow/CD206hi cells. (G) M2 TAM markers CD163, Fizz1, Arg1, and Ym1 mRNA expression in CD11b+ cells isolated from day 11 tumor tissue (n=3). Data represent mean ± SEM. *P < 0.05; **P < 0.01.
Tumor-associated macrophages (TAMs), osteoclasts, and myeloid-derived suppressor cells are LysM+ myeloid cells that have been demonstrated to support tumor growth through immune suppression, promotion of bone resorption, and stimulation of neoangiogenesis (19, 32, 33). We asked whether loss of integrin beta3 in myeloid cells altered immune cell infiltration of primary tumor tissue. Tumor-infiltrating immune cells from WT and β3KOM mice were analyzed by flow cytometry. To avoid effects from varying tumor size, we analyzed similar size tumors before statistically significant changes in tumor growth were observed (day 11 for B16F10 and PyMT-BO1). Myeloid cell populations were defined by fluorescence-activated cell sorting (FACS) as previously described (31) and by the FACS gating strategy detailed in Figure S2. In both tumor types, while the overall number of tumor-infiltrating CD45+ cells and TAMs (CD45+ CD11b+ Ly6C− Ly6G− F4/80+) was not significantly different between WT and β3KOM mice (Figure 1E, 1F), β3KOM tumors displayed a significant increase in the number of tumor-promoting M2 TAMs (CD206hi) and a significant decrease in M1 TAMs (MHCIIhi) (Figure 1E, 1F). Gene expression of FACS-sorted CD11b+ cells from tumors established in β3KOM mice and WT mice revealed upregulation of the M2 TAM markers CD163, Fizz1, Arg1, and Ym1 in β3KOM CD11b+ cells as compared to WT CD11b+ cells (Figure 1G). Furthermore, there was a significant decrease in CD8+ T-cell number in β3KOM tumors as compared to WT tumors (Table S1). These results suggest that genetic deletion of integrin beta3 in myeloid cells increases the M2/M1 TAM ratio in both orthotopic melanoma and breast cancer models.
Integrin beta3 knockout M2 macrophages have enhanced tumor-promoting function
To determine whether the increased number of M2 macrophages alone can explain the increased tumor growth seen in β3KOM mice, or whether these β3KOM M2 TAMs also display enhanced function, we adoptively transferred ex vivo polarized WT and Itgb3−/− M2 macrophages directly into B16F10 tumors. We found that the Itgb3−/− M2 macrophages were more potent in promoting tumor growth than WT M2 macrophages (Figure 2A). To account for the effects of integrin loss on M2 cell migration and homing, we adoptively transferred bone marrow macrophages (BMMs) through intracardiac injection into mice bearing PyMT-BO1 MFP tumors. Again, we observed that breast cancer bearing mice that received Itgb3−/− BMMs had larger tumors (Figure 2B). Together, these data suggest that integrin beta3 is a negative regulator of tumor-promoting function in M2 polarized macrophages.
Figure 2. Integrin beta3 knockout M2 macrophages have enhanced tumor-promoting function, and β3KOM macrophages promote tumor growth in part through CD8+ T-cells.
(A) In WT mice, at day 6 after subcutaneous B16F10 tumor establishment, 3×106 IL-4-induced WT or Itgb3−/− M2 macrophages were injected into the tumor tissue. Tumor weight was measured at day 14. (B) At day 6 and day 9 after PyMT-BO1-GFP-Luc tumor cell injection, 5×106 WT or Itgb3−/− ex vivo M2 macrophages were intracardially injected into tumor bearing mice. MFP tumor size and weight were measured. (n=6) (C) 8-week-old male WT and β3KOM mice were subcutaneously injected with 1×106 B16F10 tumor cells. Anti-CD8 antibody was given by i.p. injection one day before tumor cell injection and 7 days after first antibody injection. Tumor growth was monitored and measured for 2 weeks. (D) Tumor weight measured on day 14. (Control, n=8; Anti-CD8, n=5). Data represent mean ± SEM. *P < 0.05; **P < 0.01; ***P<0.001.
Despite comparable numbers of Foxp3+ Tregs, we also found that CD8+ T-cell numbers were significantly decreased in β3KOM tumors compared to WT tumors (Table S1). To determine whether CD8+ T-cells contributed to the enhanced tumor growth in β3KOM mice, we depleted CD8+ T-cells in WT and β3KOM mice by anti-CD8 antibody injection and monitored tumor growth. As expected, depletion of CD8+ T-cells enhanced tumor growth in WT mice; however, there was no additional enhancement of tumor growth in β3KOM mice (Figure 2C, 2D). These data show that CD8+ T-cells in the tumor microenvironment contributed to the enhanced tumor growth phenotype observed in β3KOM mice.
Pharmacologic blockade of integrin αvβ3 enhanced tumor growth and increased M2 TAM infiltration
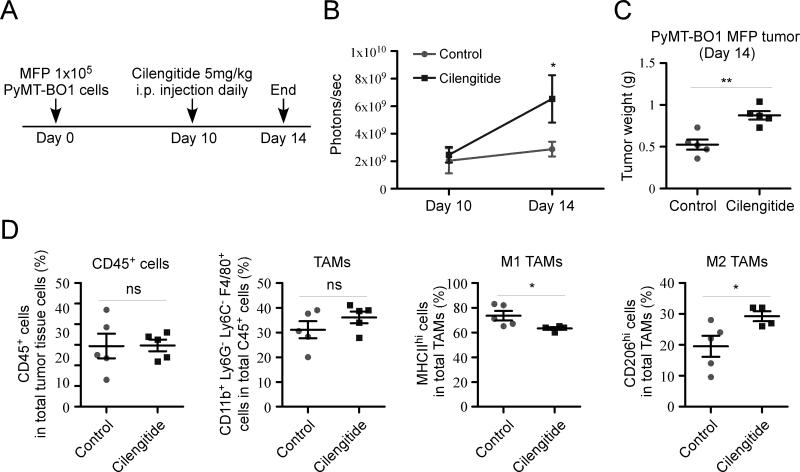
Recent clinical trials with cilengitide have failed to improve survival in glioblastoma, non-small-cell lung cancer, metastatic melanoma, prostate cancer, and advanced non-resectable pancreatic cancer (12-14, 34-36). Since the β3KOM mice exhibit enhanced tumor growth and increased M2 macrophage numbers (Figure 1), we evaluated the effect of pharmacologic blockade of integrin beta3 on TAMs by establishing PyMT-BO1 MFP tumors in WT immunocompetent mice. Treatment with cilengitide, initiated when the tumors were fully established (Day 10, ~200mm3), significantly enhanced tumor growth (Figure 3 A-C). Analysis of the tumor-infiltrating myeloid cell population by FACS revealed that cilengitide treatment increased M2 TAMs in the tumor tissue (Figure 3D). Interestingly, cilengitide administration to early stage tumors (less than 100 mm3) did not increase tumor burden; however, it did not decrease tumor burden either (Figure S3). Day 10 tumors had almost double the number of myeloid cells and exhibited 50% decreases in T-cell numbers compared to day 6 tumors (Figure S3), making them potentially more susceptible to the myeloid cell effects of cilengitide. These results show that cilengitide treatment can adversely affect breast cancer tumor growth in immunocompetent mice and results in increased tumor-infiltrating M2 TAMs.
Figure 3. Cilengitide treatment enhanced tumor growth and increased M2 TAM population.
(A) Experimental schema. 1×105 PyMT-BO1-GFP-Luc cells were injected into MFP of 8-week-old female WT mice (n=5). Starting at day 10 after tumor cell injection, mice were treated daily with cilengitide for 5 days. At day 14, all mice were sacrificed. (B) Tumor growth was measured at the indicated time points by BLI. (C) Tumor weight at day 14. (D) TAM populations in tumor tissue were analyzed by FACS. Data represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Tumor cells and M2 macrophages from β3KOM mice have diminished immune response signatures
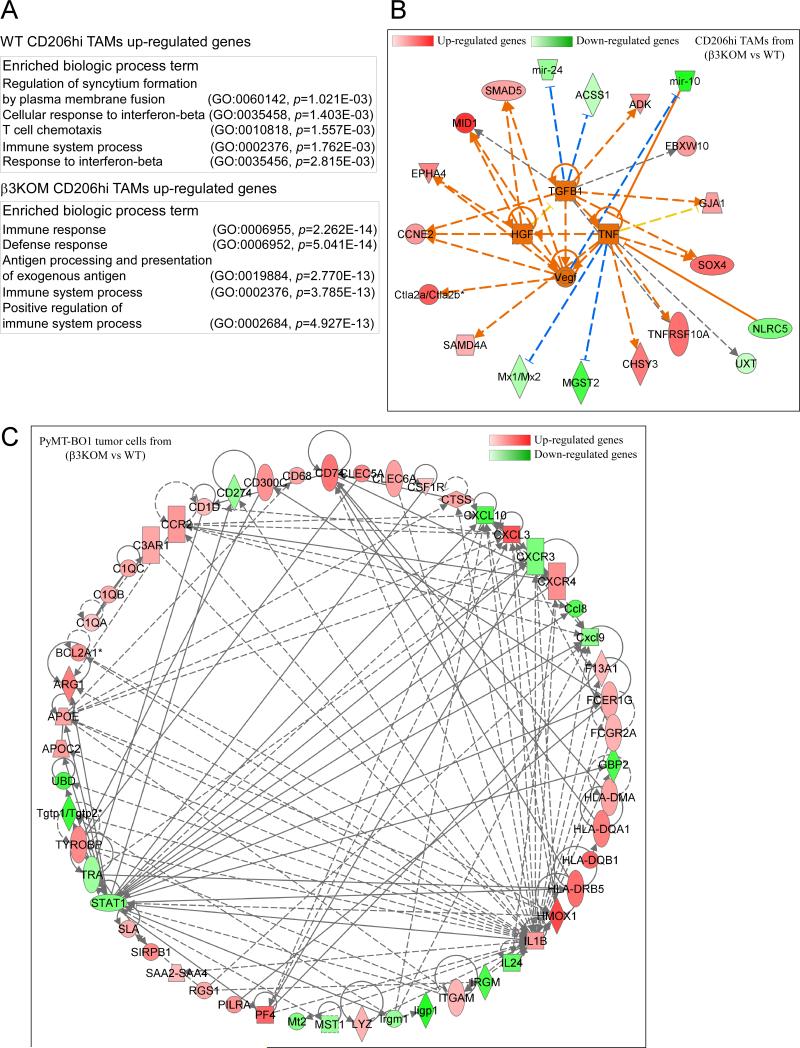
To better understand the impact of integrin beta3 signaling in myeloid cells on the tumor microenvironment, we compared the gene expression profiles of FACS-isolated GFP+ PyMT-BO1 MFP tumor cells and M2 TAMs (CD11b+ Gr1− F4/80+ CD206+) from WT and β3KOM tumor tissue. For the microarray experiment data analysis, an unadjusted p-value cutoff of less than 0.01 was selected to generate lists of differentially expressed genes (DEGs) (accession number GSE75882). For the CD206hi TAMs WT vs. β3KOM comparison, 122 unique annotated genes were identified (Table S2). Term enrichment (produced by the PANTHER classification system) for genes upregulated in M2 TAMs from the β3KOM tumor identified many immune-related biological processes to be significantly overrepresented (Figure 4A). A more diverse list of biological processes was identified from the down-regulated genes. Among them, interferon-beta response genes were found to be significantly down regulated in β3KOM M2 TAMs (Figure 4A).
Figure 4. Gene expression profiling of tumor cells and M2 macrophages from β3KOM mice reveals diminished immune response signatures.
Pathway-based analysis of microarray data. (A) Biological process, term enrichment results produced by PANTHER classification system (GO) for both up and downregulated genes in β3KOM tumors. (B) Upstream analysis of WT CD206hi macrophages vs. β3KOM CD206hi macrophages, in which genes upregulated in β3KOM are shown in red and genes downregulated in β3KOM are shown in green (the darker the more extreme the increase/decrease). For the edges, orange indicates predicated activation, blue indicates predicted inhibition, yellow indicates findings inconsistent with the state of the downstream molecule, and grey indicates that the effect is unknown. Solid edges indicate direct interactions, dashed edges indirect interactions. (C) Interaction network from the DEGs of WT tumor cells vs. β3KOM tumor cells. Solid grey edges indicate direct interactions, dashed grey edges indirect interactions.
The list of DEGs generated from the comparison of CD206hi TAMs in WT vs. β3KOM mice was used for pathway analyses with IPA® Ingenuity. An upstream analysis was performed and four genes (Tgfb1, Hgf, Tnfa, and Vegf) were predicted to be upregulated in β3KOM with an activation Z-score of greater than 2.0 (Figure 4B). TGFβ1, HGF, TNFα, and VEGF may generally promote tumor growth and are related to TAM function. All genes connected directly to these four upstream genes were placed in a network, which also included mir-10 (also called mir99a). Mir-10 was the most down-regulated gene/microRNA in β3KOM and has been shown to inhibit TNF (37). Cytotoxic T lymphocyte antigen-2α and -2β (Ctla2a, Ctla2b) were also up-regulated in β3KOM CD206hi TAMs; of the two, Ctla2a was shown to inhibit effector T-cell function (38). Together, these data suggest that loss of integrin beta3 results in a tumor-promoting, immunosuppressive gene expression signature in TAMs.
Gene expression analysis of isolated tumor cells established in WT vs. β3KOM mice identified 130 unique annotated genes (Table S2). Changes in tumor cell gene expression reflected myeloid integrin beta3-dependent changes to the tumor microenvironment. An interaction network consisting of 53 nodes was subsequently generated from the DEGs in tumor cells from WT vs. β3KOM mice (Figure 4C). In this network, Stat1 (downregulated in β3KOM) and Il1b (upregulated in β3KOM) are two highly connected nodes. Among the downregulated genes in tumor cells isolated from β3KOM mice, Stat1 and Cxcl10 belong to the interferon-γ pathway. Taken together, these data indicate that interferon-related pathways are downregulated in the γ3KOM tumor microenvironment. STAT1 is an important regulator in interferon-related pathways (39), suggesting that integrin beta3 may be involved in the regulation of STAT1 pathway genes in the tumor microenvironment.
Integrin beta3 favors STAT1 activation and suppresses STAT6 signaling in macrophages
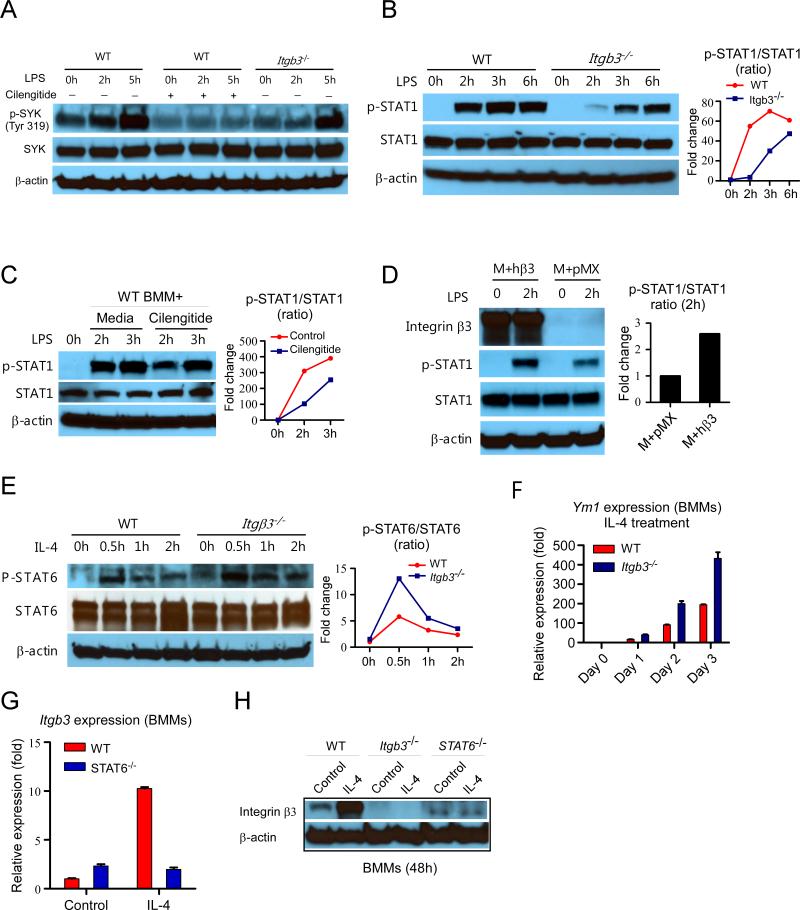
We found that macrophages lacking integrin beta3 had downregulation of interferon (STAT1) pathway genes in vivo (Figure 4). We evaluated the role of integrin beta3 signaling when macrophages were polarized in M1 (LPS-STAT1) or M2 (IL-4-STAT6) conditions. After LPS treatment, Itgb3−/− BMMs had decreased phosphorylation of STAT1 compared to WT (Figure 5A, 5B). The mRNA expression of the STAT1 downstream gene Ccl5 was also decreased in Itgb3−/− BMMs after LPS treatment (Figure S4A). Spleen tyrosine kinase (SYK) phosphorylation is usually associated with integrin activation (40). Genetic or pharmacological (cilengitide) disruption of integrin beta3 significantly decreased SYK phosphorylation in macrophages (Figure 5A, 5C). We evaluated the effect of LPS on integrin beta3 expression and activation in BMMs and found that in a short time course, LPS had little effect on integrin beta3 expression (Figure S5A). However, LPS had a potent effect on integrin beta3 activation as measured by binding of IntegriSense-680, a ligand for activated integrin αvβ3 (Figure S5C). To determine whether decreased STAT1 signaling was caused by the loss of integrin beta3, we performed a rescue experiment. Integrin beta3 was transduced into Itgb3−/− BMMs and rescued the defect in STAT1 phosphorylation after LPS treatment (Figure 5D). To determine if the expression of other integrins was altered by genetic disruption of Itgb3, we compared the mRNA expression of other integrin genes between polarized and non-polarized WT and Itgb3−/− BMMs (Figure S6). Interestingly, the mRNA expression of some integrins was indeed affected, suggesting that integrin beta3 disruption can modulate expression of other integrins. That said, ITGAV and ITGB5, which together bind the same RGD motif as integrin αvβ3, were not significantly changed after disruption of ITGB3, and were thus unlikely to have compensatory effects in Itgb3−/− BMMs. Together, these results demonstrate that disruption of integrin beta3 caused decreased phosphorylation of both the integrin beta3 signaling molecule SYK and the M1 polarizing molecule STAT1 in macrophages.
Figure 5. Integrin beta3 signaling favors STAT1 activation and suppression of STAT6 signaling in macrophages.
(A) Western blots of p-SYK and SYK in LPS-stimulated WT and Itgb3−/− BMMs. (B) Western blots of p-STAT1 and STAT1 in LPS-stimulated WT and Itgb3−/− BMMs. (C) Western blots of p-STAT1 and STAT1 in LPS-stimulated WT BMMs with or without cilengitide pretreatment. (D) Western blots of p-STAT1 and total STAT1 in WT BMMs overexpressing integrin beta3. M+pMX: macrophage treated with empty pMX vector. M+hβ3: macrophage treated with human integrin beta3 constructed pMX vector. (E) Western blots of p-STAT6 and total STAT6 in IL-4 stimulated WT and Itgb3−/− BMMs. (F) Ym1 mRNA expression after IL-4 treatment of WT and Itgb3−/− BMMs. (G) Itgb3 mRNA expression level after IL-4 treatment in Stat6−/− macrophages. (H) Western blot analysis of integrin beta3 expression in WT, Itgb3−/−, and Stat6−/− BMMs after 5ng/ml IL-4 treatment for 48 hours.
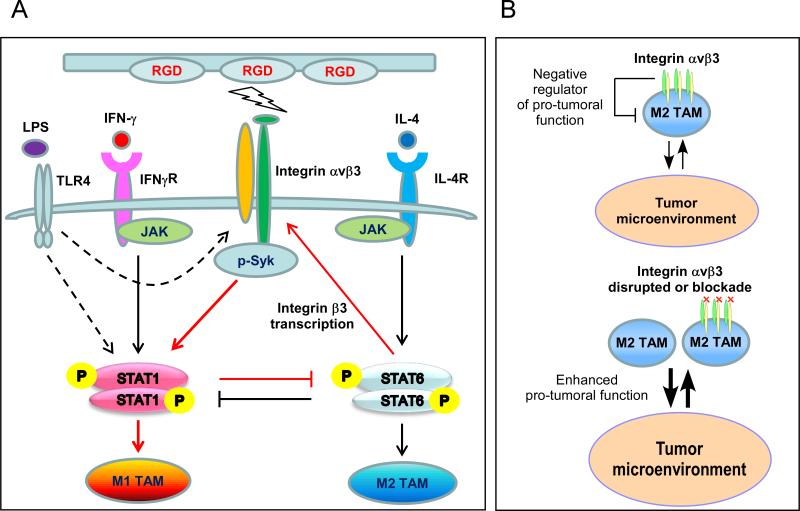
β3KOM mice had increased M2 macrophages with enhanced tumor-promoting function (Figure 1), so we evaluated the M2 polarizing STAT6 signaling pathway in Itgb3−/− BMMs. In contrast to WT, Itgb3−/− BMMs treated with the M2 polarization factor IL-4 showed enhanced STAT6 signaling, and increased expression of STAT6 downstream gene Ym1 (Figure 5E, 5F, S4B). Enhanced STAT6 signaling was also observed in WT BMMs pretreated with cilengitide (Figure S7). IL-4 has been shown to increase integrin beta3 mRNA expression in BMMs (41). We found that while IL-4 induced integrin beta3 gene and protein expression in WT BMMs, this was abrogated in Stat6−/− BMMs (Figure 5G, 5H). Taken together, we find that integrin beta3 signaling regulates the balance of p-STAT1 and p-STAT6. These results show an integrin beta3 negative feedback loop within M2 macrophages, in which integrin beta3 expression is induced by STAT6 signaling, but that integrin beta3 signaling favors STAT1 activation and suppression of STAT6 signaling (Figure 6).
Figure 6. Model of integrin beta3 signaling pathway in tumor-associated macrophages.
(A) A negative feedback loop, in which integrin beta3 expression is induced in M2 macrophages by STAT6 activation, but integrin beta3 signaling suppresses STAT6 and promotes STAT1 activation. (B) In the tumor microenvironment, integrin beta3 signaling is a negative regulator of M2 TAM function. Blocking integrin beta3 signaling enhances tumor-promoting function in M2 TAMs.
Myeloid cells are required for increased tumor growth after cilengitide treatment
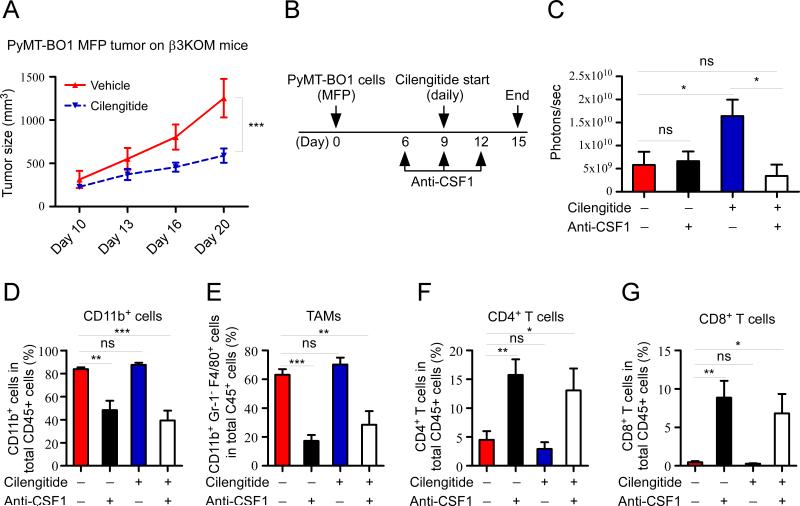
Because there were increased numbers of M2 tumor-promoting macrophages in cilengitide-treated tumors, we asked if the direct effects of cilengitide on myeloid cells were required for enhanced tumor growth. We administered cilengitide to tumor bearing β3KOM mice, where only LysM+ myeloid cells would be unresponsive to cilengitide. In contrast to the larger MFP tumors observed when cilengitide was administered to WT mice, we found that cilengitide treatment significantly reduced tumor burden in β2KOM mice (Figure 7A). These results show that integrin beta3 expression on TAMs is required for some of the cilengitide-induced tumor-promoting effects.
Figure 7. Myeloid cells are required for increased tumor growth after cilengitide treatment.
(A) PyMT-BO1-GFP-Luc MFP tumor established on β3KOM mice (n=5). From day 10 after tumor cell injection, mice were treated daily with cilengitide for 5 days. Tumor growth was measured at the indicated time points. (B) Experimental schema. 1×105 PyMT-BO1-GFP-Luc cells were injected into MFP of 8-week-old female WT mice at day 0. Anti-CSF1 antibody treatment started on day 6 by i.p. injection of 1mg of antibody per mouse, followed by 0.5mg of antibody on day 9 and day 12. Cilengitide treatment started on day 9 for 5 days with a dosage of 5mg/kg per mouse by i.p. injection (n=7 or 8 per group). (C) Tumor burden was measured by BLI at day 15. (D-G) Tumor-infiltrating myeloid cells and T-cells were measured by FACS at day 15. Data represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Next, we used anti-CSF1 antibody to decrease the number of macrophages and myeloid cells in tumor bearing mice and evaluated the effect of cilengitide treatment. Co-administration of cilengitide with anti-CSF1 resulted in no enhancement of tumor growth compared to cilengitide alone (Figure 7B, 7C). Analysis of tumor-infiltrating cells demonstrated that anti-CSF1 antibody treatment significantly decreased myeloid cell numbers in the tumor, with an expected concomitant increase in CD4+ and CD8+ T-cell numbers in the tumors in WT mice (Figure 7D-G). Anti-CSF1 antibody also decreased tumor-infiltrating myeloid cells and increased CD4+ and CD8+ T-cells in tumor-bearing β3KOM mice (Figure S8). Taken together, these data show that macrophage/myeloid lineage cells are required for the tumor-enhancing effects of cilengitide and that co-administration of cilengitide and anti-macrophage therapy reverses the tumor-enhancing effects of integrin beta3 blockade.
Discussion
In this study, we provide new evidence for the role of integrin beta3 in tumor-associated macrophage (TAM) polarization and function. We found that modulating integrin beta3 levels either genetically or pharmacologically resulted in enhanced tumor growth and increased M2 macrophage numbers and tumor-promoting function in the tumor microenvironment. We found that integrin beta3 signaling favored M1 polarizing STAT1 signaling and suppressed M2 polarizing STAT6 signaling. However, integrin beta3 gene expression was suppressed by STAT1 and induced by STAT6 signaling, creating a negative feedback loop for M2 polarization. Thus, loss of integrin beta3 signaling promoted an immune suppressive tumor environment through increased M2 TAM polarization and function and decreased CD8+ T-cell numbers. Finally, disruption of macrophages blocked the tumor-promoting effects of cilengitide, demonstrating an immediate way to improve the efficacy of integrin beta3 therapies in cancer.
M1 TAMs are essential participants in Th1 responses and have potent immunestimulatory capacity (25). On the other hand, M2 TAMs help maintain an immunosuppressive environment and promote tumor growth by facilitating angiogenesis, tumor cell invasion, metastasis, and chemotherapeutic resistance (19, 25, 33, 42). We found that integrin beta3 knockout TAMs had elevated expression of M2 macrophage markers and enhanced tumor-promoting function in vivo when compared to WT TAMs (Figure 1, 2). We also noticed a significant decrease in CD8+ T-cell number in the tumor tissue when integrin beta3 was disrupted in myeloid cells (Table S1). In the tumor microenvironment, CD8+ T-cells play a central role in anti-tumor cellular immune responses. High CD8+ T-cells, low CD4+ T-cells, and low CD68+ TAMs in the tumor microenvironment correlated with better survival for breast cancer patients (42). In vivo, β3KOM TAMs have elevated TGF-β signaling downstream gene expression, suggesting increased TGF-β in the β3KOM tumor microenvironment (Figure 4B). In vitro, Itgb3−/− BMMs have higher levels of IL-10 mRNA expression after LPS treatment when compared with WT BMMs (data not shown). Finally, while CD8+ T-cell depletion in WT mice enhanced tumor growth, the same treatment in β3KOM mice resulted in no significant difference in tumor size (Figure 2C, 2D), indicating that the enhanced tumor-promoting function of integrin beta3-null TAMs is mediated by suppressive effects on the CD8+ T-cell response. Therefore, our data describe a functional role for integrin beta3 in the macrophagedependent regulation of immune suppression within tumors.
Several integrin beta3 antagonists have been developed for clinical use in cancer, including peptide antagonists, such as cilengitide, and neutralizing antibodies, such as Vitaxin and c7E3 (43). Treating integrin αvβ3-expressing tumor cells with cilengitide in vitro reduces tumor cell proliferation and invasion (8, 43). Based on these data, integrin beta3 inhibition initially appeared to be an exciting cancer therapeutic target, particularly after cilengitide treatment displayed positive results in some animal tumor models (8, 43). However, clinical trials with cilengitide on glioblastoma, non-small-cell lung cancer, metastatic melanoma, prostate cancer, and advanced pancreatic cancer showed no improvement in outcomes, even while cilengitide was well tolerated in patients (12-14, 34-36). We noticed that in WT mice with early stage mammary tumors (day 6, less than 100mm3), cilengitide treatment did not increase or decrease tumor growth (Figure S3). However, in established mammary tumors (day 10, ~200mm3), cilengitide treatment significantly enhanced tumor growth (Figure 3). We compared the number of tumor-infiltrating T-cells and myeloid cells in both early and established mammary tumors; established tumors had almost double the number of infiltrating myeloid cells and a 50% decrease in infiltrating T-cell numbers compared to early stage tumors (Figure S3). This result suggests that late stage tumors with an increased number of TAMs may be more susceptible to the pro-tumor effect of integrin beta3 blockade. Importantly, cilengitide treatment decreased tumor burden in mice genetically lacking integrin beta3 on myeloid cells (Figure 7A). Cilengitide has been shown to inhibit tumor cell proliferation (8). We subsequently confirmed that cilengitide treatment inhibits PyMT-BO1 cell proliferation in vitro (Figure S9A), a direct inhibitory role that may explain the decreased tumor burden we observed in cilengitide-treated β3KOM mice. Taken together, tumor-promoting M2 macrophages played an important role in integrin beta3 disruption-induced enhancement of tumor growth (cilengitide treatment and β3KOM). Moreover, when mice were pretreated with anti-CSF1 antibody to decrease myeloid cell numbers, this cilengitide-induced tumor progression was significantly abrogated (Figure 7C). Thus, anti-integrin beta3 (cilengitide) treatment has several roles in the tumor microenvironment, directly inhibiting tumor proliferation and neoangiogenesis while indirectly promoting tumor progression through stimulation of macrophage M2 polarization and tumor-promoting function.
We found that LPS LPS-induced phosphorylation of SYK and STAT1 was strongly reduced in Itgb3−/− BMMs. SYK is an important signaling molecule to transduce integrin beta3 outside-in signaling (40). In addition, it has been shown that p-SYK can activate STAT1 (44). A similar decrease in SYK and STAT1 activation was observed if WT BMMs were pre-treated with integrin αvβ3 inhibitor cilengitide (Figure 5A-C). Furthermore, Itgb3−/− BMMs and WT BMMs pretreated with cilengitide displayed an increase in STAT6 activation upon IL-4 treatment (Figure 5E, S7). These data demonstrate that integrin beta3 downstream signaling enhances STAT1 activation, the key M1 polarizing signal, and negatively regulates STAT6 activation, the key M2 polarizing signal. Thus, integrin beta3 activation and ligand binding induce SYK phosphorylation and then STAT1 activation in macrophages. LPS (TLR4 signaling) administration to macrophages induced integrin beta3 activation and enhanced STAT1 signaling; disruption of integrin beta3 (such as β3KOM) diminished this enhancement of STAT1 signaling. Defining the precise signaling pathway through which integrin beta3, LPS, and STAT1 interact in macrophages is underway.
It is important to understand effects on M2 TAMs and other myeloid cells when administrating integrin beta3-targeted therapy or anti-tumor immune therapy, particularly in patients whose tumors express high numbers of infiltrating macrophages. Infiltrating TAMs in cancer are more likely to polarize towards an M2 phenotype with tumor progression (21, 24). TAM burden correlates with poor survival in many cancer types (19, 20). TAM-targeted therapy is now being considered as an important facet of successful cancer treatment. We found that anti-CSF1 therapy prevented the increase in tumor growth associated with cilengitide treatment in breast cancer. Single agent anti-CSF1 antibody was effective to decrease TAMs by 30-50% and increase T-cells in established tumors. However, it did not significantly decrease tumor burden. Most breast cancer cells, including PyMT-BO1 cells, secrete MCSF. It is possible that anti-CSF1 antibody as dosed in our experiments did not completely block the MCSFR signaling on tumor-infiltrating macrophages. Also, anti-CSF1 treatment targets both tumor-promoting M2 TAMs and tumor-suppressive M1 TAMs, thus diminishing the anti-tumor properties of the latter. Accordingly, longer treatment times or blockade of MCSFR signaling pathways may have stronger effects on the tumor microenvironment in terms of rebalancing the T-cell population and reducing tumor burden.
Taken together, we propose that M2 TAMs are a key player in the decreased efficacy of integrin αvβ3-targeted therapies and that concurrent treatment with macrophage antagonists represents a possible strategy to improve their clinical effectiveness. Our results suggest that when giving integrin beta3-targeted therapy, myeloid cell function should be considered.
Supplementary Material
Acknowledgements
We would like to thank Dr. William Frazier, Dr. Patrick Ross, Dr. Sheila Stewart, Dr. Michael Tomasson, and Dr. Duanwen Shen for their valuable expert suggestions and criticism. We thank Crystal Idleburg, Lynne Collins, and Julie Prior for their expert technical assistance. We thank the Musculoskeletal Research center for histology and microCT (NIH P30-AR057235), the Molecular Imaging Center at Washington University (NIH/NCI P50-CA09056), DDRCC Morphology core (Grant # P30 DK52574), The Barnes-Jewish Foundation, The St. Louis Men's Group Against Cancer, and Hope Center Alafi Neuroimaging Lab (NIH Shared Instrumentation Grant # S10 RR027552).
Financial Support:
X. Su, A.K. Esser, S.R. Amend, J. Xiang, Y. Xu, J. Tomasson and K.N. Weilabecher were supported by NIH RO1-CA097250. J. Xiang, Y. Xu, M. Hurchla and K.N. Weilbaecher were supported by NIH PO1-CA100730. A.K. Esser, M.H. Ross, F. Fontana and K.N. Weilabecher were supported by NIH RO1-CA154737. M.H. Ross was supported by the training grant (NIH 5T32GM007067-39) and (NIH T32AR060719). A.K. Esser was supported by the training grant (NIH 5T32CA113275-07). W. Zou and S.L. Teitelbaum received grants support from NIH R01-AR057037, R37-AR046523 and Shriners Hospitals for Children grant 85400-STL. J.G. Schneider received grant support from the German Research Society (DFG SCH682/3-1), the Fonds Nationale de La Recherche de Luxembourg (CORE Itgb3VascIn), and the EU (CIG303682).
Footnotes
The authors disclose no potential conflicts of interest.
Reference List
- 1.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim C, Ye F, Ginsberg MH. Regulation of integrin activation. Annu Rev Cell Dev Biol. 2011;27:321–45. doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- 3.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–25. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 5.Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, et al. Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider JG, Amend SR, Weilbaecher KN. Integrins and bone metastasis: integrating tumor cell and stromal cell interactions. Bone. 2011;48:54–65. doi: 10.1016/j.bone.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pecheur I, Peyruchaud O, Serre CM, Guglielmi J, Voland C, Bourre F, et al. Integrin alpha(v)beta3 expression confers on tumor cells a greater propensity to metastasize to bone. FASEB J. 2002;16:1266–8. doi: 10.1096/fj.01-0911fje. [DOI] [PubMed] [Google Scholar]
- 8.Mas-Moruno C, Rechenmacher F, Kessler H. Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med Chem. 2010;10:753–68. doi: 10.2174/187152010794728639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakewell SJ, Nestor P, Prasad S, Tomasson MH, Dowland N, Mehrotra M, et al. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci U S A. 2003;100:14205–10. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- 12.Kim KB, Prieto V, Joseph RW, Diwan AH, Gallick GE, Papadopoulos NE, et al. A randomized phase II study of cilengitide (EMD 121974) in patients with metastatic melanoma. Melanoma Res. 2012;22:294–301. doi: 10.1097/CMR.0b013e32835312e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manegold C, Vansteenkiste J, Cardenal F, Schuette W, Woll PJ, Ulsperger E, et al. Randomized phase II study of three doses of the integrin inhibitor cilengitide versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer. Invest New Drugs. 2013;31:175–82. doi: 10.1007/s10637-012-9842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100–8. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 15.Morgan EA, Schneider JG, Baroni TE, Uluckan O, Heller E, Hurchla MA, et al. Dissection of platelet and myeloid cell defects by conditional targeting of the beta3-integrin subunit. FASEB J. 2010;24:1117–27. doi: 10.1096/fj.09-138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taverna D, Moher H, Crowley D, Borsig L, Varki A, Hynes RO. Increased primary tumor growth in mice null for beta3- or beta3/beta5-integrins or selectins. Proc Natl Acad Sci U S A. 2004;101:763–8. doi: 10.1073/pnas.0307289101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steri V, Ellison TS, Gontarczyk AM, Weilbaecher K, Schneider JG, Edwards D, et al. Acute depletion of endothelial beta3-integrin transiently inhibits tumor growth and angiogenesis in mice. Circ Res. 2014;114:79–91. doi: 10.1161/CIRCRESAHA.114.301591. [DOI] [PubMed] [Google Scholar]
- 18.Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med (Berl) 2013;91:411–29. doi: 10.1007/s00109-013-1021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–72. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 25.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–61. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 27.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–38. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–12. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 29.Baker M, Reynolds LE, Robinson SD, Lees DM, Parsons M, Elia G, et al. Stromal Claudin14-heterozygosity, but not deletion, increases tumour blood leakage without affecting tumour growth. PLoS One. 2013;8:e62516. doi: 10.1371/journal.pone.0062516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su X, Floyd DH, Hughes A, Xiang J, Schneider JG, Uluckan O, et al. The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. J Clin Invest. 2012;122:3579–92. doi: 10.1172/JCI38576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–69. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–55. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De PM, Lewis CE. Cancer: Macrophages limit chemotherapy. Nature. 2011;472:303–4. doi: 10.1038/472303a. [DOI] [PubMed] [Google Scholar]
- 34.Alva A, Slovin S, Daignault S, Carducci M, Dipaola R, Pienta K, et al. Phase II study of cilengitide (EMD 121974, NSC 707544) in patients with non-metastatic castration resistant prostate cancer, NCI-6735. A study by the DOD/PCF prostate cancer clinical trials consortium. Invest New Drugs. 2012;30:749–57. doi: 10.1007/s10637-010-9573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisele G, Wick A, Eisele AC, Clement PM, Tonn J, Tabatabai G, et al. Cilengitide treatment of newly diagnosed glioblastoma patients does not alter patterns of progression. J Neurooncol. 2014;117:141–5. doi: 10.1007/s11060-014-1365-x. [DOI] [PubMed] [Google Scholar]
- 36.Friess H, Langrehr JM, Oettle H, Raedle J, Niedergethmann M, Dittrich C, et al. A randomized multi-center phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancer. BMC Cancer. 2006;6:285. doi: 10.1186/1471-2407-6-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tufekci KU, Oner MG, Genc S, Genc K. MicroRNAs and Multiple Sclerosis. Autoimmune Dis. 2011;2011:807426. doi: 10.4061/2011/807426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugita S, Horie S, Nakamura O, Maruyama K, Takase H, Usui Y, et al. Acquisition of T regulatory function in cathepsin L-inhibited T cells by eye-derived CTLA-2alpha during inflammatory conditions. J Immunol. 2009;183:5013–22. doi: 10.4049/jimmunol.0901623. [DOI] [PubMed] [Google Scholar]
- 39.Khodarev NN, Roizman B, Weichselbaum RR. Molecular pathways: interferon/stat1 pathway: role in the tumor resistance to genotoxic stress and aggressive growth. Clin Cancer Res. 2012;18:3015–21. doi: 10.1158/1078-0432.CCR-11-3225. [DOI] [PubMed] [Google Scholar]
- 40.Zou W, Kitaura H, Reeve J, Long F, Tybulewicz VL, Shattil SJ, et al. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176:877–88. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitazawa S, Ross FP, McHugh K, Teitelbaum SL. Interleukin-4 induces expression of the integrin alpha v beta 3 via transactivation of the beta 3 gene. J Biol Chem. 1995;270:4115–20. doi: 10.1074/jbc.270.8.4115. [DOI] [PubMed] [Google Scholar]
- 42.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Millard M, Odde S, Neamati N. Integrin targeted therapeutics. Theranostics. 2011;1:154–88. doi: 10.7150/thno/v01p0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tassiulas I, Hu X, Ho H, Kashyap Y, Paik P, Hu Y, et al. Amplification of IFN-alpha-induced STAT1 activation and inflammatory function by Syk and ITAM-containing adaptors. Nat Immunol. 2004;5:1181–9. doi: 10.1038/ni1126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.