Abstract
Malaria constitutes a major public health problem in Zimbabwe, particularly in the north and east bordering Zambia and Mozambique. In Manicaland Province in eastern Zimbabwe, malaria transmission is seasonal and unstable. Over the past decade, Manicaland Province has reported increased malaria transmission due to limited funding, drug resistance and insecticide resistance. The aim of this study was to identify risk factors at the individual and household levels to better understand the epidemiology of malaria and guide malaria control strategies in eastern Zimbabwe. Between October 2012 and September 2014, individual demographic data and household characteristics were collected from cross-sectional surveys of 1,116 individuals residing in 316 households in Mutasa District, one of the worst affected districts. Factors associated with malaria, measured by rapid diagnostic test (RDT), were identified through multilevel logistic regression models. A total of 74 participants were RDT positive. Sleeping under a bed net had a protective effect against malaria despite pyrethroid resistance in the mosquito vector. Multivariate analysis showed that malaria risk was higher among individuals younger than 25 years, residing in households located at a lower household density and in closer proximity to the Mozambique border. The risk factors identified need to be considered in targeting malaria control interventions to reduce host–vector interactions.
Introduction
Zimbabwe lies in the southern fringe of malaria transmission in sub-Saharan Africa.1 Approximately half of the population of 12.9 million lives in malarious areas and malaria transmission occurs in 47 of the 65 districts in the country.2,3 Annually, malaria causes an estimated 380,000 reported cases and 350 deaths.3 The epidemiology of malaria varies in different regions of the country, ranging from year-round transmission in the low-lying areas of the Zambezi and Limpopo valleys, to epidemic-prone areas along the watershed ridge running from the east to southwest of the country.4 Malaria transmission in Manicaland Province on the border with Mozambique is unstable with seasonal epidemics. In 2009, 55,707 confirmed cases of malaria were reported in Manicaland Province, which increased to 192,730 in 2013.3 Manicaland Province reported more than half of the malaria cases and more than one-third of malaria deaths in Zimbabwe.5
Nationally, the annual number of reported cases of malaria declined dramatically from 1.5 million in 2000 to just over 370,000 in 2013.3,5 This has not, however, been the case for Manicaland Province. Within Manicaland Province, the districts of Nyanga and Mutasa are ranked nationally as the districts with the highest incidence of clinical malaria based on passive case-finding data from health centers.5 This increased burden has been attributed to a combination of insecticide resistance, drug resistance, and limited funding, which disrupted malaria control programs that had been effective for more than 50 years.6–8
Better understanding of the epidemiology of malaria in the context of resurgence could support local malaria control strategies and maximize the impact on reducing malaria morbidity and mortality. Serial cross-sectional surveys were conducted from 2012 to 2014 to identify individual- and household-level risk factors for malaria in Mutasa District.
METHODS
Study setting and procedures.
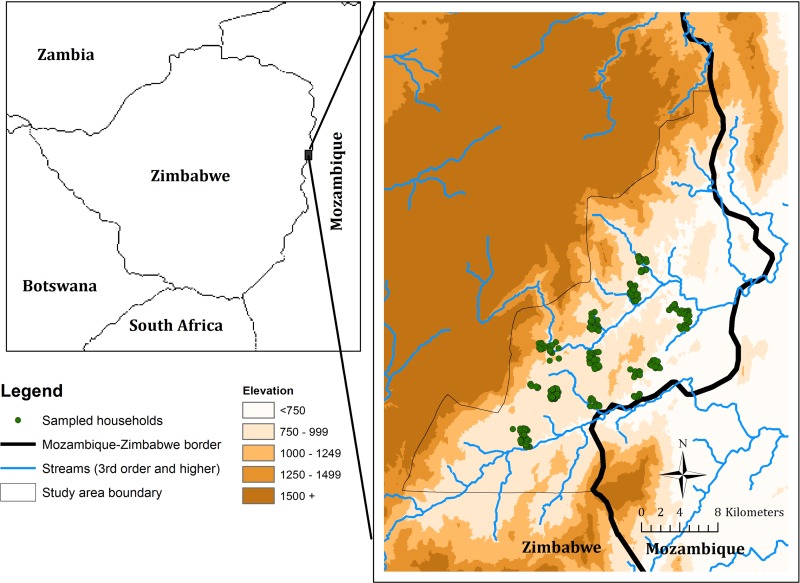
Mutasa District has an elevation ranging from 900 m in the Honde Valley to over 1,500 m in the mountains (Figure 1). Rainfall patterns in Zimbabwe are highly seasonal with a rainy season from November to April and dry season with little to no rainfall from May to October. Mutasa District received 2,352 mm of rainfall during the 2013–2014 rainy season and 96 mm during the dry season as measured in Hauna, the main town in the Honde Valley. The district had an estimated 169,756 residents representing 42,479 households at the time of the 2012 census,2 and the population depends heavily on agriculture for its livelihood. Malaria transmission in the study area is characterized as seasonal and unstable with major outbreaks during the rainy season.9 Plasmodium falciparum is the main malaria species and Anopheles funestus the dominant malaria vector,10 with high levels of resistance to pyrethroid and carbamate insecticides.8 Indoor residual spraying (IRS) and mass distribution of insecticide-treated nets (ITNs) are the main vector control interventions implemented by the National Malaria Control Program and partners. The population covered by pyrethroid-based IRS in Mutasa District was 88% in 2012 and 91% in 2013. In November and December 2014, an organophosphate, pirimiphos-methyl was used for IRS and covered 92% of the population. ITNs were distributed to the general population in 2013 and limited to boarding schools in 2014.
Figure 1.
Topographical map of Mutasa District, Zimbabwe, and the distribution of sampled households.
A high-resolution satellite image of the study area obtained in 2011 from DigitalGlobe Services, Inc. (Denver, CO) was imported into ArcGIS 10.2 (Environmental Systems Research Institute, Redlands, CA), and the locations of households were identified manually and digitized. A grid containing 1 × 1 km cells was overlaid onto the image and the first stage of sampling involved selection of grid cells. This selection process ensured adequate geographic distribution of the sample, while considering logistical challenges in reaching remote areas where transport is difficult, especially during the rainy season. In the second stage of sampling, households were randomly selected from within the selected grid cells and assigned to one of two study cohorts: longitudinal and cross-sectional. Households in the prospective longitudinal cohort were surveyed every other month whereas households in the cross-sectional study were surveyed only once during the study period. The process of creating a sampling frame by digitizing households from a high-resolution satellite image was previously validated.11–13
Trained interviewers followed standardized operating procedures that were developed and piloted in the field to ensure reliability. After obtaining written informed consent for study participation from adults and caregivers as well as assent from children older than 7 years, interviewers administered a questionnaire to the head of the household that was used to list all household members and collect basic demographic information. Household members were eligible for inclusion in the study if they were a resident of the selected household regardless of age and pregnancy status. A questionnaire was used to collect data on sociodemographic characteristics, malaria-related knowledge, malaria history, and use of malaria preventative measures. A fingerprick blood sample was obtained for malaria parasite testing using a rapid diagnostic test (RDT) (SD BIOLINE Malaria Ag P.f.; Standard Diagnostic Inc., Kyonggi, Republic of Korea). Those with positive test results were offered treatment with artemisinin-combination therapy according to national guidelines. Responses to the questionnaires and RDT results were recorded and stored electronically on Android tablets using Open Data Kit software (ODK, www.opendatakit.org), then transferred to REDCap 4.1, a secure, web-based application designed to support data capture for research studies.14
Study measures and instruments.
The outcome of interest was whether an individual tested positive or negative for malaria by RDT on the day of enrollment. Supporting data were collected describing features at the individual and household level as well as characteristics of the surrounding environment. Individual-level characteristics included age, sex, history of malaria-related symptoms, malaria knowledge, and use of bed nets and other preventive measures. Age was grouped into five categories: < 5, 5–14, 15–24, 25–49, and 50 or more years. A composite malaria knowledge score was created using responses to survey questions evaluating knowledge of symptoms, causes, and prevention of malaria. Use of a bed net was determined by asking respondents “Do you sleep under a bed net?” and responses were dichotomized into yes/no categories. A history of recent travel within the preceding 4 weeks was collected.
Potential household-level risk factors obtained from questionnaires administered to the heads of the households included: education level of household head, household size, and IRS in the past 6 months. A household wealth index was constructed using a principal components analysis of household characteristics (housing floor material, primary cooking fuel used, and water source) and asset ownership (radio, television, refrigerator, bicycle, motorbike, and car).15,16 The first principal component accounted for 21% of the variability. Greatest weight was given to ownership of a television (0.72), use of electricity as the primary cooking fuel (0.69), and ownership of a refrigerator (0.68). A proxy for household socioeconomic status was created by dividing the wealth index into tertiles defining the poorest, middle, and richest households.
Several variables characterizing the environment surrounding households were obtained. As proximity to environmental and social features can play an important role in understanding spatial disease patterns, Euclidean distances from each household to the nearest health facility, the nearest main road, the Zimbabwe–Mozambique border, and to different categories of streams were calculated in ArcGIS 10.2 using the “Near” tool. The distance from the nearest health facility to each surveyed household was used as a proxy for access to health services. The distance to the nearest nth-order stream, distance to the nearest road, and distance to the Zimbabwe–Mozambique border were proxies for proximity to vector breeding sites, accessibility to transportation, and cross-border migration, respectively.
Construction of a stream network was based on hydrological models from a 90-m resolution digital elevation model (DEM) of the study area from the Shuttle Radar Topographic Mission.17 Although lower order streams typically flow during the rainy season only, higher order streams have greater peak flows, are found at low elevations and flow throughout the year. Within the study area, the highest stream order was four; therefore, distance from a surveyed house to first-, second-, third-, and fourth-order streams were determined. Topographical attributes are potentially relevant for habitat suitability of malaria vectors, thus, aspect, slope, and elevation were extracted from the DEM.13,18 The aspect, which ranges from 0 to 360 degrees, was categorized into three topographically important slope orientations: north-facing, south-facing, and all other slope orientations. In the southern hemisphere, north-facing slopes tend to be warmer and less humid because of more direct sunlight.13,19
Using buffering techniques and the global positioning system (GPS) location of all houses, the number of houses within circular buffers of radii 250, 500, and 1,000 m were calculated to estimate local house density. A LANDSAT™ 8 image from July 2014 with a spatial resolution of 30 m and 11 spectral bands was downloaded from the U.S. Geological Survey Land Processes Distributed Active Archive Center (USGS Earth Resources Observation and Science [EROS] Center, Sioux Falls, SD).20 The normalized difference vegetation index (NDVI) ranges from −1 to 1, with higher values indicating denser vegetation. NDVI was calculated as the ratio of near infrared (NIR) and red spectral bands 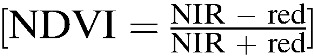 . Land use for the study area was generated by unsupervised land cover classification using LANDSAT 8 image bands 2, 3, 4, 5, 6, and 7; and classified into six groups: water, crop, bare land, impervious, grass, and forest.
. Land use for the study area was generated by unsupervised land cover classification using LANDSAT 8 image bands 2, 3, 4, 5, 6, and 7; and classified into six groups: water, crop, bare land, impervious, grass, and forest.
Statistical analysis.
The analysis was based on the single visit conducted at cross-sectional households and the initial visit to longitudinal households. Subsequent visits to these households were excluded as repeated testing and treating can alter the natural history of malaria transmission within households.21 Descriptive analyses were performed to explore the characteristics of both the outcome and supporting variables using χ2 tests for categorical variables and t tests for continuous variables. The outcome variable, RDT positivity for each individual, was dichotomous for which logistic regression was the appropriate method for analysis. As individuals were nested within households and individuals within a particular household may have been more similar to one another than to individuals in other households, the assumption of independence in standard logistic regression methods is likely to be violated. To account for the hierarchical structure of the data and potential clustering effect of RDT outcomes within households, multilevel logistic regression analysis was used to model RDT positivity as a function of individual and household-level factors. Environmental variables were obtained per household and were considered household-level variables for analysis.
Three consecutive models were fitted to the data. In Model 1 (null model), the probability of an individual testing RDT positive was only a function of the individual's household and modeled by a random intercept allowing estimation of the overall between-household variance for the outcome measure, RDT positivity. Model 2 (individual-level model) included individual-level variables associated with malaria risk. Initially, Model 2 contained all individual-level variables significantly associated with RDT positivity (P < 0.2) in univariate regressions (each variable tested one at a time) and several variables (age, sex, and bed net use) deemed important a priori. Variables not significant at the P < 0.05 level were eliminated one by one in order of least significance. Potential explanatory variables that were not found to be significant in the univariate models were evaluated one by one for inclusion in the model. Model 3 (multilevel model) added household-level variables to Model 2 using the same manual model selection. Within- and cross-level interactions were assessed and variables were tested for multicollinearity. The association of explanatory variables with RDT positivity was quantified by odds ratios (ORs) and 95% confidence intervals (CIs). Environmental data processing and maps were made in ArcGIS version 10.2. Environmental data were linked to parasitological data according to GPS location. Nonspatial data analysis was conducted using STATA 12.0 (StataCorp, College Station, TX) while spatial analyses were performed in R development software (R Core Team, Vienna, Austria) using geoR package.22
Ethical considerations.
The study was approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board (IRB), the Biomedical Research and Training Institute IRB, and the Medical Research Council of Zimbabwe. Discussions were held with chiefs and other community leaders about the study purpose and conduct, and permission was given to visit households in their area. Informed consent was obtained from the head of household before enrollment and written informed consent was obtained from eligible adult participants and caregivers. Assent was obtained from children 7–15 years old.
RESULTS
Descriptive analysis.
A total of 1,187 individuals from 319 households were visited between October 2012 and September 2014. Two households with missing geographical coordinates and 22 individuals with missing RDT results were excluded from the analysis, giving a final sample of 1,161 individuals residing within 316 households. Of the 316 households, only a small proportion used electricity to cook (8.2%), had piped water (34.5%), was situated on north-facing slopes (6.7%), or were located at elevations ≥ 820 m (25.0%). Households were on average 1.7 (interquartile range [IQR] = 0.6–5.0) km from the nearest health facility and 1.3 (IQR = 0.8–3.3) km from the nearest main road. About two-thirds of respondents (61.1%) reported their household ever having been sprayed with insecticide, with just over one half (50.8%) of these households reporting that spraying had occurred within the previous 6 months (Table 1 ).
Table 1.
Characteristics of 316 sampled households in Mutasa District (October 2012–September 2014)
| Characteristic | N | % |
|---|---|---|
| Highest education level of head of household | ||
| None or primary | 129 | 44.0 |
| Secondary or higher | 164 | 56.0 |
| Land use | ||
| Crop | 180 | 57.0 |
| Bare land | 29 | 9.2 |
| Impervious | 4 | 1.3 |
| Grass | 89 | 28.2 |
| Forest | 14 | 4.4 |
| Aspect of slope | ||
| Northern | 21 | 6.7 |
| Southern | 51 | 16.1 |
| All other orientations | 244 | 77.2 |
| Piped water is main source of water | 104 | 34.5 |
| Electricity is main source of cooking energy | 26 | 8.2 |
| Household ever sprayed | 187 | 61.1 |
| Household sprayed in the past 6 months | 95 | 29.8 |
| Median | Lower quartile, upper quartile | |
| Elevation (m) | 786 | 757, 819 |
| Household size | 5 | 3, 8 |
| Distance to first-order stream (km) | 0.6 | 0.4, 0.7 |
| Distance to second-order stream (km) | 1.3 | 1.0, 1.6 |
| Distance to third-order stream (km) | 1.6 | 0.6, 2.3 |
| Distance to forth-order stream (km) | 1.9 | 1.1, 3.8 |
| Distance to nearest health facility (km) | 1.7 | 0.6, 5.0 |
| Distance to main road (km) | 1.3 | 0.8, 3.3 |
| Distance to Zimbabwe–Mozambique border (km) | 6.0 | 4.5, 8.9 |
| Number of households within 250-m buffer | 16 | 8, 42 |
| Number of households within 500-m buffer | 51 | 31, 104 |
| Number of households within 1,000-m buffer | 174 | 137, 242 |
| Normalized difference vegetation index | 0.22 | 0.18, 0.27 |
Of the 1,161 individuals, 189 (16%) were children under 5 years of age and 648 (56%) were females. The rate of bed net ownership was 82%, and the most cited barriers to bed net ownership were cost, heat, and the perception that there were no mosquitoes. Of those with access to a bed net, 52% self-reported actually sleeping under a bed net (Table 2 ). Use of bed nets varied by season with 60% of participants sleeping under a bed net during the rainy season compared with 46% during the dry season (data not shown). A low proportion (9.8%) of the participants reported recent overnight travel (Table 2), with the most common purpose to visit family and friends (data not shown).
Table 2.
Characteristics of individuals in Mutasa District at baseline (October 2012–September 2014)
| Characteristics | Total study population | RDT positive | ||
|---|---|---|---|---|
| N = 1,161 | N = 74 | |||
| n | % | n | % | |
| Age (years) | ||||
| < 5 | 189 | 16.3 | 15 | 7.9 |
| 5–14 | 255 | 22 | 21 | 8.2 |
| 15–24 | 220 | 18.9 | 20 | 9.1 |
| 25+ | 497 | 42.8 | 18 | 3.6 |
| Sex | ||||
| Male | 513 | 44.2 | 36 | 7.0 |
| Female | 648 | 55.8 | 38 | 5.9 |
| Fever on the previous day | 65 | 5.6 | 12 | 18.5 |
| Fever in past 2 weeks | 118 | 10.2 | 17 | 14.4 |
| Nausea/vomiting on the previous day | 25 | 2.2 | 4 | 16.0 |
| Nausea/vomiting in past 2 weeks | 59 | 5.1 | 11 | 18.6 |
| Chills on the previous day | 62 | 5.3 | 9 | 14.5 |
| Chills in past 2 weeks | 105 | 9.0 | 18 | 17.1 |
| Visited health facility/post in past month for malaria | 166 | 14.3 | 39 | 23.5 |
| Visited health facility/post in past 6 months for malaria | 413 | 35.6 | 57 | 13.8 |
| Owns a bed net | 948 | 81.7 | 63 | 6.6 |
| Slept under bed net | 493 | 52.1 | 26 | 5.3 |
| Recent overnight travel in past 4 weeks | 114 | 9.8 | 3 | 4.1 |
RDT = rapid diagnostic test.
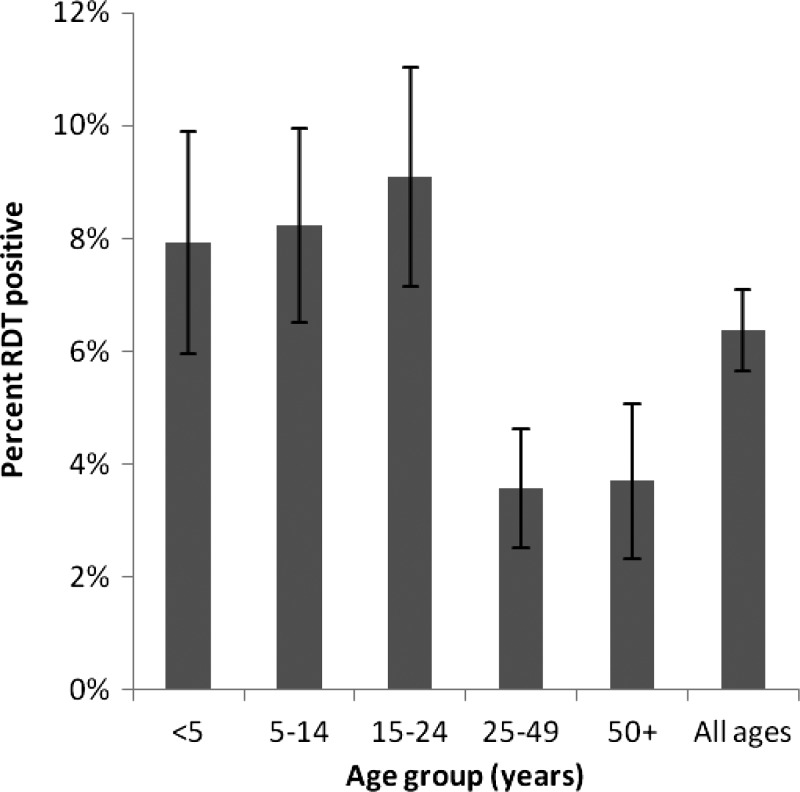
During the study period, 74 individuals from 50 households tested positive for malaria by RDT and 16 of the households included more than one RDT-positive household member at the time of enrollment. The overall crude parasite prevalence by RDT was 6.4% (95% CI = 5.0–7.8%), and was higher during the rainy season compared with the dry season (10% versus 3%, P < 0.05). The highest prevalence in RDT positivity was in young adults 15–24 years of age. There was a sharp decline in RDT positivity between young adults 15–24 years of age and adults 25–49 years of age (Figure 2). Of the RDT-positive individuals, 16% and 23% reported a fever in the prior 48 hours and in the previous 2 weeks, respectively (Table 2).
Figure 2.
Rapid diagnostic test positivity by age group.
Multivariate analysis.
Multilevel, multivariate, logistic regression showed that RDT positivity was associated with age (P value = 0.05) (Table 3 ). Compared with the reference group (age ≥ 50 years), individuals < 5, 5–14, and 15–24 years of age had approximately 2.7 times the odds of RDT positivity (OR = 2.67, 95% CI = 0.94–7.56; OR = 2.61, 95% CI = 0.97–7.06; and OR = 2.70, 95% CI = 1.00–7.29). Individuals 25–49 years old did not have a significantly different risk of RDT positivity than the reference age ≥ 50 years (OR = 0.94, 95% CI = 0.33–2.70). Sleeping under a bed net decreased the odds of a positive malaria RDT (OR = 0.54, 95% CI = 0.29–1.00) despite pyrethroid resistance in An. funestus.
Table 3.
Univariate and multivariate multilevel logistic regression analysis of risk factors for malaria RDT positivity (N = 1,161)
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| Individual-level factors | ||||||
| Age category (years) | 0.03 | 0.05 | ||||
| < 5 | 2.64 | 0.92–7.56 | 2.67 | 0.94–7.56 | ||
| 5–14 | 2.84 | 1.03–7.84 | 2.61 | 0.97–7.06 | ||
| 15–24 | 2.54 | 0.93–6.96 | 2.70 | 1.00–7.29 | ||
| 25–49 | 0.91 | 0.31–2.69 | 0.94 | 0.33–2.70 | ||
| ≥ 50 | Reference | Reference | ||||
| Female | 0.91 | 0.53–1.54 | 0.7 | 0.97 | 0.33–2.70 | 0.91 |
| Sleep under bed net | 0.65 | 0.35–1,19 | 0.1 | 0.54 | 0.29–1.00 | 0.05 |
| Recent overnight travel in past 4 weeks | 0.36 | 0.10–1.32 | 0.1 | – | – | – |
| Household-level factors | ||||||
| Elevation categories (m) | ||||||
| < 800 | Reference | 0.12 | Reference | 0.12 | ||
| 800–849 | 0.78 | 0.35–1.74 | 1.16 | 0.55–2.45 | ||
| ≥ 850 | 0.31 | 0.08–1.12 | 0.32 | 0.09–1.09 | ||
| No. of houses within 250 m (per 10 houses) | 0.89 | 0.80–0.99 | 0.03 | 0.89 | 0.87–0.97 | 0.01 |
| House sprayed with IRS in past 6 months | 3.87 | 2.08–7.18 | < 0.01 | 2.30 | 1.16–4.56 | 0.02 |
| Peak season (December–May) | 5.40 | 2.52–11.40 | < 0.01 | 4.52 | 2.11–9.69 | < 0.001 |
| Distance to Mozambique border (km) | 0.92 | 0.82–1.02 | 0.1 | 0.86 | 0.76–0.97 | 0.01 |
| Distance to the nearest health facility (km) | 0.97 | 0.83–1.14 | 0.7 | – | – | – |
| Distance to second-order stream (km) | 2.37 | 1.25–4.49 | 0.008 | – | – | – |
| Distance to main road (km) | 1.18 | 0.99–1.42 | 0.07 | – | – | – |
| North-facing slope (aspect) | 3.00 | 0.97–9.25 | 0.05 | – | – | – |
| Electricity is main source of cooking energy | 0.11 | 0.01–1.10 | 0.06 | – | – | – |
CI = confidence interval; IRS = indoor residual spraying; RDT = rapid diagnostic test.
At the household level, for every one kilometer increase in distance from the Zimbabwe–Mozambique border, the odds of RDT positivity decreased by 14% (OR = 0.86, 95% CI = 0.76–0.97). The cumulative effect of distance to the Zimbabwe–Mozambique border was substantial as half of study households were located six or more kilometers away from the border; the odds of malaria in individuals from these distant households were 65% lower (OR = 0.35, 95% CI = 0.18–0.65) than among individuals in households close to the border. Furthermore, for every additional 10 houses within 250 m, the odds of RDT positivity decreased by 11% (OR = 0.89, 95% CI = 0.87–0.97). Elevation was associated with lower risk of malaria although it did not reach statistical significance (P = 0.1). Residing in a household ≥ 850 m above sea level reduced the odds of malaria compared with a household < 800 m (OR = 0.32, 95% CI = 0.09–1.09). Individuals in households surveyed during the rainy season were 4.52 times more likely to test positive for malaria (OR = 4.52, 95% CI = 2.11–9.69) than those in households surveyed during the dry season.
The household random effect for the null model (Table 4 , Model 1) showed that RDT positivity was clustered at the household level, confirming the need for a multilevel approach instead of conventional logistic regression. After adding individual-level and household-level variables, the random effect variance decreased from 2.24 (Table 4, Model 1) to 0.69 (Table 4, Model 3). However, the variance of the random intercept remained significant (P = 0.04), suggesting that there are unobserved variables that may further explain heterogeneity across households (Table 4, Model 3).
Table 4.
Household-level random effects summary for the null, individual-level, and multilevel models
| Household random effect | ||||
|---|---|---|---|---|
| Estimate | Standard error | P value | ||
| Model 1: null model | 2.24 | 0.86 | < 0.001 | |
| Model 2: best individual-level model | 2.41 | 0.94 | < 0.001 | |
| Model 3: best multilevel model | 0.69 | 0.52 | 0.04 | |
DISCUSSION
After many years of effective control, Manicaland Province in eastern Zimbabwe experienced a resurgence of malaria in recent years and has now become one of the most affected provinces in the country. This study identified individual- and household-level factors associated with malaria based on cross-sectional surveys involving active case detection in Mutasa District, one of the Districts most affected by malaria in Manicaland. The study highlights the importance of both individual- and household-level factors in determining malaria risk. Malaria risk was significantly higher among individuals who 1) were younger than 25 years, 2) did not sleep under a bed net, 3) were sampled during the rainy season, 4) lived in sparsely populated areas, and 5) lived close to the Zimbabwe–Mozambique border. Several of the findings have clear implications for malaria control in this district that may also be applicable to other settings with similar epidemiology.
Of particular significance is the finding that the closer the individuals live to the Zimbabwe–Mozambique border, the higher their risk of malaria. Several regional studies found a higher risk of malaria closer to international borders. In Mpumalanga Province, South Africa, individuals living within 5 km of the Mozambique border had four times the risk of malaria compared with individuals residing further from the border.23 Similarly in Namibia, the highest risk of malaria was found along the border with Angola, which is considered malaria endemic.24,25 The increased risk of malaria transmission along international borders has been attributed to poorer access to health care, differences in malaria endemicity, the porosity of borders, frequent human population movement, and suitable climatic conditions for malaria transmission.26 Interestingly, proximity to a health facility was not associated with malaria. This lack of association may be because of the relatively good health infrastructure in Mutasa District. With 45 private and public health facilities, including several along the border with Mozambique, most participants resided within 2 km of a health facility providing malaria diagnosis and treatment free of charge. On the other hand, across the border in Mozambique, such services currently are not available.
The conducive ecological environment for malaria transmission along the border, and cross-border migration of infected individuals may also explain the higher risk for malaria in this area. The elevation in Mutasa District generally slopes from west to east, with low-lying areas near the border with Mozambique (Figure 1), and the risk of malaria is higher at lower elevations. Mutasa District has experienced considerable human population movement to and from Mozambique, for example, during the Mozambican civil war and more recently, during the economic crisis in Zimbabwe. The border with Mozambique is porous and individuals move between countries for employment, to access health care and to visit relatives. These migrants may serve as a human reservoir of malaria parasites.27,28 Malaria control in western Mozambique is largely dependent on case management, with limited IRS and distribution of ITNs only in peri-urban areas.29 The movement of parasite-carrying individuals from the higher transmission setting of Mozambique may be undermining malaria control in eastern Zimbabwe, as suggested by studies in other countries where malaria resurgence has been attributed in part to frequent human population movement across international borders.24,30 Regionally, several intercountry collaborations have been developed to address cross-border malaria, including the Lubombo Spatial Development Initiative between Mozambique, South Africa, and Swaziland,31 the Trans-Kunene Malaria Initiative between Namibia and Angola,25 and the Trans-Zambezi Malaria Initiative between Angola, Botswana, Namibia, Zambia, and Zimbabwe.24 The success of these regional initiatives has been hampered, however, by lack of political will, limited funding, slow mobilization of resources, and poor coordination.25,32 The strong relationship between malaria and proximity to the Zimbabwe–Mozambique border provides yet another argument for the importance of regional collaborations in controlling malaria. Stronger intercountry collaborations will be especially important as malaria goals shift from control to elimination.
Malaria RDT positivity was associated with younger age. Compared with the reference group (age 50 and older), individuals < 25 years were significantly more likely to be RDT positive and the risk of malaria infection was highest for adolescents and young adults. This might be a result of different exposure rates and other behavioral risk factors in the younger population. In fact, individuals 15–24 years old reported the lowest usage of bed nets, followed by 5–14 year olds. Sleeping under a bed net was associated with a lower risk of malaria, which is consistent with evidence from randomized controlled trials.33 This reduced risk is in spite of the high levels of pyrethroid resistance in Mutasa District,8 and so is presumably because of the physical barrier effect of ITNs rather than their insecticidal properties. Approximately 58% of individuals did not have access to or did not use a bed net, increasing their odds of acquiring malaria by 46%. Malaria interventions including ITN distribution need to target younger populations, perhaps through school-based interventions to achieve higher ITN coverage.
IRS reduces malaria transmission by reducing the life span of mosquitoes and by reducing the density of mosquitoes in sprayed households.34,35 Recent IRS of a house was expected to be associated with a significant protective effect against malaria infection. However, IRS within the previous 6 months was in fact associated with increased odds of malaria. One plausible explanation for this seemingly paradoxical result is that IRS was applied where the risk of malaria was greater. In a resource-limited setting, targeting higher prevalence areas can maximize impact.36 According to Zimbabwe's Malaria Strategic Plan, only some districts are targeted for IRS based on previous transmission patterns and incidence data.3 Another explanation is that insecticide resistance that has been reported nationally may be undermining IRS efficacy. Results of insecticide susceptibility tests in 2013 and 2014 showed that the main malaria vector in Mutasa District, An. funestus was resistant in bioassays to both pyretheroids and carbamates.8 Finally, recent IRS was self-reported, and may have been misclassified. It is likely that misclassification would be nondifferential with respect to the outcome, RDT positivity, thus biasing the results toward the null.
The results presented here should be interpreted in light of several limitations. Given that the use of bed nets and recent residual spraying of household were self-reported, these exposure variables may be subject to social desirability bias and recall bias. In the case of use of bed nets, any bias may be minimal as interviewers requested to see the bed nets in households reporting ownership of at least one bed net, although the presence of a net was not necessarily related to effective use.
Although data on a variety of potential confounding factors were collected, the final model only explained 69% of the between-household variance. This may be because of the relatively small number of RDT-positive individuals, limiting the power to detect association, and other factors not measured in this study that contribute toward the understanding of malaria transmission in an area such as Mutasa District. For example, agriculture is the main activity in this district and engaging in agriculture-related activities has been associated with increased malaria risk, particularly among migrant laborers.37,38 Understanding the movement of individuals, not just at large spatial scales such as across international borders, but locally, may also be important to understanding the epidemiology of malaria. No association between recent travel and malaria risk was identified, although the power to detect such an association was low given the infrequency of reported overnight travel. Given the potential importance of cross-border malaria transmission, more research attempting to quantify the magnitude of the impact of cross-border movement on malaria transmission in eastern Zimbabwe is necessary.
Simple Euclidean distance to the Zimbabwe–Mozambique border, which was used as a proxy for cross-border migration, does not account for the actual physical travel path used, access to transportation, and the time of travel, and so these data may underestimate actual distance and time applicable to migration. However, in the absence of digital data on road network for the study area and actual travel paths, the distance to the border was taken to be a reasonable proxy for cross-border migration, and has previously been used as a surrogate measure for migration and importation of malaria cases.39
Using multilevel modeling, individual and household characteristics were identified that were predictive of malaria risk, implying that combined interventions targeting “at risk” individuals and “high risk” areas may effectively reduce malaria. This study underscores the need for strong regional initiatives to control malaria. Results from this study can be used by malaria control managers to define priority populations and areas for intensified efforts. Increasing coverage of malaria interventions and undertaking intercountry programs will be key to regaining malaria control in Mutasa and other districts in Zimbabwe.
ACKNOWLEDGMENTS
We would like to thank the field team for their assistance in data collection and the community in Mutasa District for participating in this study.
Footnotes
Financial support: This work was supported by the Division of Microbiology and Infectious Diseases, National Institutes of Allergy and Infectious Diseases, National Institutes of Health as part of the International Centers of Excellence for Malaria Research (U19 AI089680).
Authors' addresses: Mufaro Kanyangarara and Luke C. Mullany, Department of International Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, E-mails: mkanyan1@jhu.edu and lmullany@jhu.edu. Edmore Mamini, Sungano Mharakurwa, Shungu Munyati, and Peter R. Mason, Biomedical Research and Training Institute, Harare, Zimbabwe, E-mails: edmoremamini@gmail.com, smharak1@jhu.edu, smunyati@brti.co.zw, and pmason@brti.co.zw. Lovemore Gwanzura, Department of Medical Laboratory Sciences, College of Health Sciences, University of Zimbabwe, Harare, Zimbabwe, E-mail: gwanzura@mweb.co.zw. Tamaki Kobayashi, Timothy Shields, Frank C. Curriero, and William J. Moss, Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, E-mails: tkobaya2@jhu.edu, tshield2@jhu.edu, fcurriero@jhu.edu, and wmoss1@jhu.edu. Susan Mutambu, National Institute of Health Research, Harare, Zimbabwe, E-mail: mutambusl@gmail.com.
References
- 1.Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA, Snow RW. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 2010;7:e1000290. doi: 10.1371/journal.pmed.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimbabwe National Statistics Agency (ZNSA) Census 2012: Preliminary Report. Harare, Zimbabwe: National Statistics Agency; 2012. [Google Scholar]
- 3.President's Malaria Initiative (PMI) Zimbabwe Malaria Operational Plan FY 2015. Washington, DC: President's Malaria Initiative; 2015. [Google Scholar]
- 4.Craig MH, Snow RW, le Sueur D. A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol Today. 1999;15:105–111. doi: 10.1016/s0169-4758(99)01396-4. [DOI] [PubMed] [Google Scholar]
- 5.Ministry of Health and Child Welfare (MOHCW) Zimbabwe National Health Profile 2012. Harare, Zimbabwe: Ministry of Health and Child Welfare; 2012. [Google Scholar]
- 6.Mharakurwa S, Thuma PE, Norris DE, Mulenga M, Chalwe V, Chipeta J, Munyati S, Mutambu S, Mason PR. Malaria epidemiology and control in southern Africa. Acta Trop. 2012;121:202–206. doi: 10.1016/j.actatropica.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mharakurwa S, Mutambu SL, Mudyiradima R, Chimbadzwa T, Chandiwana SK, Day KP. Association of house spraying with suppressed levels of drug resistance in Zimbabwe. Malar J. 2004;3:35. doi: 10.1186/1475-2875-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi KS, Christian R, Nardini L, Wood OR, Agubuzo E, Muleba M, Munyati S, Makuwaza A, Koekemoer LL, Brooke BD, Hunt RH, Coetzee M. Insecticide resistance and role in malaria transmission of Anopheles funestus populations from Zambia and Zimbabwe. Parasit Vectors. 2014;7:464. doi: 10.1186/s13071-014-0464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moss WJ, Norris DE, Mharakurwa S, Scott A, Mulenga M, Mason PR, Chipeta J, Thuma PE. Challenges and prospects for malaria elimination in the southern Africa region. Acta Trop. 2012;121:207–211. doi: 10.1016/j.actatropica.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das S, Henning TC, Simubali L, Hamapumbu H, Nzira L, Mamini E, Makuwaza A, Muleba M, Norris DE, Stevenson JC. Underestimation of foraging behaviour by standard field methods in malaria vector mosquitoes in southern Africa. Malar J. 2015;14:12. doi: 10.1186/s12936-014-0527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowther SA, Curriero FC, Shields T, Ahmed S, Monze M, Moss WJ. Feasibility of satellite image-based sampling for a health survey among urban townships of Lusaka, Zambia. Trop Med Int Health. 2009;14:70–78. doi: 10.1111/j.1365-3156.2008.02185.x. [DOI] [PubMed] [Google Scholar]
- 12.Sutcliffe CG, Kobayashi T, Hamapumbu H, Shields T, Kamanga A, Mharakurwa S, Thuma PE, Glass G, Moss WJ. Changing individual-level risk factors for malaria with declining transmission in southern Zambia: a cross-sectional study. Malar J. 2011;10:324. doi: 10.1186/1475-2875-10-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss WJ, Hamapumbu H, Kobayashi T, Shields T, Kamanga A, Clennon J, Mharakurwa S, Thuma PE, Glass G. Use of remote sensing to identify spatial risk factors for malaria in a region of declining transmission: a cross-sectional and longitudinal community survey. Malar J. 2011;10:163. doi: 10.1186/1475-2875-10-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 16.Rutstein SO, Johnson K. The DHS Wealth Index. Calverton, MD: ORC Macro, MEASURE DHS; 2004. [Google Scholar]
- 17.Shuttle Radar Topography Mission NASA, (SRTM) SRTM Digital Elevation Model. http://www2.jpl.nasa.gov/srtm/ Available at. Accessed October 1, 2015.
- 18.Atieli HE, Zhou G, Lee M-C, Kweka EJ, Afrane Y, Mwanzo I, Githeko AK, Yan G. Topography as a modifier of breeding habitats and concurrent vulnerability to malaria risk in the western Kenya highlands. Parasit Vectors. 2011;4:241. doi: 10.1186/1756-3305-4-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black PE. Watershed Hydrology. Vol. 3. Water Encyclopedia; 2005. pp. 472–479. [Google Scholar]
- 20.U.S. Geological Survey (USGS) Land Processes Distributed Active Archive Center, (LP DAAC) MODIS Land Products. https://lpdaac.usgs.gov/dataset_discovery/modis/modis_products_table Available at.
- 21.Sutcliffe CG, Kobayashi T, Hamapumbu H, Shields T, Mharakurwa S, Thuma PE, Louis TA, Glass G, Moss WJ. Reduced risk of malaria parasitemia following household screening and treatment: a cross-sectional and longitudinal cohort study. PLoS One. 2012;7:e31396. doi: 10.1371/journal.pone.0031396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro PJ, Jr, Diggle PJ. geoR: a package for geostatistical analysis. R News. 2001;1:14–18. [Google Scholar]
- 23.Booman M, Durrheim DN, La Grange K, Martin C, Mabuza AM, Zitha A, Mbokazi FM, Fraser C, Sharp BL. Using a geographical information system to plan a malaria control programme in South Africa. Bull World Health Organ. 2000;78:1438–1444. [PMC free article] [PubMed] [Google Scholar]
- 24.Noor AM, Uusiku P, Kamwi RN, Katokele S, Ntomwa B, Alegana VA, Snow RW. The receptive versus current risks of Plasmodium falciparum transmission in northern Namibia: implications for elimination. BMC Infect Dis. 2013;13:184. doi: 10.1186/1471-2334-13-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith Gueye C, Gerigk M, Newby G, Lourenco C, Uusiku P, Liu J. Namibia's path toward malaria elimination: a case study of malaria strategies and costs along the northern border. BMC Public Health. 2014;14:1190. doi: 10.1186/1471-2458-14-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wangdi K, Gatton ML, Kelly GC, Clements AC. Cross-border malaria: a major obstacle for malaria elimination. Adv Parasitol. 2015;89:79–107. doi: 10.1016/bs.apar.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Pindolia DK, Garcia AJ, Wesolowski A, Smith DL, Buckee CO, Noor AM, Snow RW, Tatem AJ. Human movement data for malaria control and elimination strategic planning. Malar J. 2012;11:205. doi: 10.1186/1475-2875-11-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cosner C, Beier JC, Cantrell RS, Impoinvil D, Kapitanski L, Potts MD, Troyo A, Ruan S. The effects of human movement on the persistence of vector-borne diseases. J Theor Biol. 2009;258:550–560. doi: 10.1016/j.jtbi.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conteh L, Sharp BL, Streat E, Barreto A, Konar S. The cost and cost-effectiveness of malaria vector control by residual insecticide house-spraying in southern Mozambique: a rural and urban analysis. Trop Med Int Health. 2004;9:125–132. doi: 10.1046/j.1365-3156.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 30.Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, Moonen B. Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11:122. doi: 10.1186/1475-2875-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharp BL, Kleinschmidt I, Streat E, Maharaj R, Barnes KI, Durrheim DN, Ridl FC, Morris N, Seocharan I, Kunene S, La Grange JJ, Mthembu JD, Maartens F, Martin CL, Barreto A. Seven years of regional malaria control collaboration–Mozambique, South Africa, and Swaziland. Am J Trop Med Hyg. 2007;76:42–47. [PMC free article] [PubMed] [Google Scholar]
- 32.The Roll Back Malaria Partnership. Adams H, Kunene S, Mouzin E, Novotny J, Phillips AA. Focus on Swaziland. Geneva, Switzerland: Roll Back Malaria Partnership Secretariat; 2012. [Google Scholar]
- 33.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;2:CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010;4:CD006657. doi: 10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mabaso ML, Sharp B, Lengeler C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Trop Med Int Health. 2004;9:846–856. doi: 10.1111/j.1365-3156.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 36.Carter R, Mendis KN, Roberts D. Spatial targeting of interventions against malaria. Bull World Health Organ. 2000;78:1401–1411. [PMC free article] [PubMed] [Google Scholar]
- 37.Ghebreyesus TA, Witten KH, Getachew A, Yohannes AM, Tesfay W, Minass M, Bosman A, Teklehaimanot A. The community-based malaria control programme in Tigray, northern Ethiopia. A review of programme set-up, activities, outcomes and impact. Parassitologia. 2000;42:255–290. [PubMed] [Google Scholar]
- 38.Martens P, Hall L. Malaria on the move: human population movement and malaria transmission. Emerg Infect Dis. 2000;103:1–7. doi: 10.3201/eid0602.000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleinschmidt I, Sharp BL, Clarke GP, Curtis B, Fraser C. Use of generalized linear mixed models in the spatial analysis of small-area malaria incidence rates in Kwazulu Natal, South Africa. Am J Epidemiol. 2001;153:1213–1221. doi: 10.1093/aje/153.12.1213. [DOI] [PubMed] [Google Scholar]