Abstract
We evaluated the clinical efficacy of artesunate–mefloquine (ASMQ) fixed-dose combination to treat uncomplicated malaria in Juruá Valley, the main Plasmodium falciparum transmission hotspot in Brazil. Between November 2010 and February 2013, we enrolled 162 patients aged 4–73 years, with fever or a history of fever, and a single-species P. falciparum infection confirmed by microscopy and polymerase chain reaction (PCR). All 154 patients who completed the 42-day follow-up presented an adequate clinical and parasitologic response. ASMQ was well tolerated and no adverse event caused treatment interruption. Gametocytes were detected in 46.3% patients; 35.2% had gametocytes at enrollment, whereas others developed patent gametocytemia 1–14 days after starting ASMQ. By day 3 of treatment, all subjects had cleared asexual parasitemia, but parasite DNA remained PCR detectable in 37.6% of them. Day-3 PCR positivity was associated with prolonged gametocyte carriage. We found no molecular evidence of resistance to either MQ (pfmdr1 gene amplification) or AS (mutations in selected kelch13 gene domains known to be associated with AS resistance) in the local P. falciparum population. These results strongly support the use of ASMQ as a first-line regimen to treat uncomplicated P. falciparum malaria in northwestern Brazil, but underscore the need for gametocytocidal drugs to reduce the transmission potential of ASMQ-treated patients (ClinicalTrials.gov number NCT01144702).
Introduction
Malaria is endemic to 21 countries in the Americas, with over 400,000 laboratory-confirmed cases each year. Despite a 77% decrease in incidence between 2000 and 2014, Brazil still contributes 42% of all malaria cases in this region; transmission remains entrenched in the Amazon basin, which accounts for 99.5% of the country's malaria burden. Plasmodium vivax is currently the predominant species, followed by Plasmodium falciparum.1
After a series of clinical trials in the early 1980s,2 mefloquine (MQ) was introduced in Brazil in 1987, being widely deployed as a monotherapy for P. falciparum malaria over the next 20 years. Between 2001 and 2007, uncomplicated falciparum malaria in Brazil was treated with quinine plus doxycycline (formerly tetracycline) for 7 days or a single MQ dose (15 mg/kg).3 Clinical surveillance in the late 1990s revealed cure rates of 98.9% for MQ4 and between 77.3% and 100% for quinine plus tetracycline5,6 in northern Mato Grosso, central Brazil, despite emerging evidence for quinine7 and MQ8 resistance in vitro.
The main P. falciparum transmission hotspot in the Amazon basin of Brazil is Juruá Valley, close to the border with Peru. With a combined population of 100,000 inhabitants, three municipalities in this region reported 40% of the 21,000 P. falciparum infections diagnosed countrywide in 2014 (Ministry of Health of Brazil, unpublished data). After a major malaria outbreak in the mid-2000s, artemisinin-based combination therapy (ACT) was introduced for P. falciparum malaria treatment in Juruá Valley in 2006. Between July 2006 and December 2008, the artesunate–mefloquine (ASMQ) fixed-dose combination produced by Farmanguinhos, Rio de Janeiro, Brazil,9 the first locally available ACT, was administered to nearly 24,000 patients with no significant adverse events.10 Until the end of 2008, ASMQ and artemether–lumefantrine (AL) were gradually made available in other endemic areas and became the first-line therapies for falciparum malaria countrywide. However, because of concerns regarding the efficacy of MQ as a partner drug after its long-term use as monotherapy, ASMQ deployment in Brazil is currently limited to areas with very low or no P. falciparum transmission.
Herein, we report on the clinical efficacy of the fixed-dose ASMQ combination in the treatment of uncomplicated P. falciparum malaria in Brazil and show that ASMQ remains highly effective, with adequate clinical and parasitologic response (ACPR) in all subjects followed for 42 days. No molecular evidence of AS or MQ resistance was observed in the local P. falciparum population. However, we found residual parasite DNA detected by polymerase chain reaction (PCR) on day 3 of treatment in over one-third of patients, often associated with persistent gametocyte carriage.
PATIENTS AND METHODS
Study site and design.
Between November 2010 and February 2013, we carried out a single-arm, open-label clinical trial in the urban area of Cruzeiro do Sul (07°37′S; 72°40′W) to assess the efficacy of ASMQ fixed-dose combination in the treatment of uncomplicated P. falciparum malaria (ClinicalTrials.gov number NCT01144702). Cruzeiro do Sul, the most populated municipality in Juruá Valley, northwestern Brazil (81,500 inhabitants), reported 5,321 laboratory-confirmed P. falciparum infections in 2014; 18% of them acquired in the urban area, contributing one-fourth of the malaria burden due to this species countrywide (Ministry of Health of Brazil, unpublished data). The study protocol was written according to the Pan American Health Organization recommendations for in vivo antimalarial drug efficacy trials in areas of low endemicity.11 To calculate the sample size, we assumed an ACPR rate of 98%, similar to that recently reported in Peru,12 with an α error of 5% and a dropout rate of 20%, resulting in a target for recruitment of 101 subjects.
Study subjects.
Eligible subjects were patients with uncomplicated malaria of either sex, with age between 6 months and 75 years, and fever (axillary temperature ≥ 37.5°C) or history of fever in the past 48 hours. Only subjects living in the urban area of Cruzeiro do Sul were eligible, since directly observed therapy and 42-day follow-up would be impractical for study participants living in remote rural sites. All study subjects had to have a P. falciparum single-species infection confirmed by both microscopy and quantitative PCR (qPCR), with parasitemias ranging between 250 and 100,000 asexual parasites/μL of blood, as determined by microscopy. Following a recommendation from the Amazonian Malaria Initiative/Amazon Network for the Surveillance of Antimalarial Drug Resistance technical advisory team in Brazil, we enrolled subjects with relatively low parasite densities (between 250 and 500 asexual parasites/μL) because they represent a large proportion of actual population of patients attending malaria clinics in the country. Given that diagnosis and treatment are provided at no cost in a vast network of health posts, more than two-thirds of malaria episodes in Juruá Valley are treated within 48 hours after the onset of clinical symptoms. As a consequence, local patients rarely present with high parasitemias. Exclusion criteria were severe or complicated malaria, pregnancy or lactation, history of convulsions, any clinically relevant illness other than malaria (including cardiovascular and psychiatric disorders, liver cirrhosis, and chronic renal failure), antimalarial use in the preceding 2 weeks, and known hypersensitivity or allergy to study drugs. Women of childbearing age (11–45 years) were tested for pregnancy before enrollment.
Treatment and follow-up.
The ASMQ fixed-dose combination is supplied by Farmanguinhos as high-dose tablets containing 100 mg AS and 220 mg MQ hydrochloride (200 mg MQ base) and low-dose tablets with 25 mg AS and 55 mg MQ hydrochloride (50 mg MQ base). At enrollment, all study subjects were weighted. They were administered orally, under direct observation by a study physician (first dose) or nurse (second and third doses), with a total average dose of 12 mg/kg of AS and 24 mg/kg of MQ base distributed over three consecutive days (days 0, 1, and 2).13 The weight-adjusted doses for children were 5–8.9 kg of weight, 1 low-dose tablet/day; 9–17.9 kg of weight, 2 low-dose tablets/day; and 18–29.9 kg of weight, 1 high-dose tablet/day. Older children, adolescents, and adults weighing ≥ 30 kg were given 2 high-dose tablets/day. Follow-up visits were made by a study nurse at the patients' homes on days 1, 2, 3, 7, 14, 21, 28, 35, and 42; in addition, patients were advised to return to the health-care facility where they have been enrolled, for clinical and laboratory assessment, whenever they were sick, between the scheduled home visits. Duplicate Giemsa-stained thick smears were obtained at each home visit and whenever patients returned to health-care facilities. Venous blood was collected on days 0, 3, and 42 for determination of hemoglobin levels and platelet counts as well as qPCR-based confirmatory malaria diagnosis.
Laboratory diagnosis.
At least 500 thick smear fields were independently examined on-site by two experienced microscopists, at ×1,000 magnification, before a slide was declared negative by microscopy. All slides (10 duplicate slide sets for subjects who completed the follow-up) were later reviewed by a third reference microscopist, who was blinded to the first readings. Whenever discrepancies between field microscopists were found, we considered the expert microscopist review as the definitive diagnosis. Parasite densities were determined by counting the number of asexual blood-stage parasites against 200 leukocytes, assuming 6,000 leukocytes/μL of blood; estimates used here were obtained by the expert microscopist. We used 200-μL aliquots of venous blood samples to isolate parasite DNA, using QIAamp DNA blood kits (Qiagen, Hilden, Germany) on a QIAcube automated platform (Qiagen), for confirmatory molecular diagnosis of malaria on days 0, 3, and 42. DNA templates were used to amplify a species-specific 100-bp fragment of the P. falciparum and P. vivax 18S ribosomal RNA genes by real-time qPCR. Each 20-μL qPCR reaction mixture contained 2 μL of sample DNA (corresponding to 2 μL of venous blood), 10 μL of 2× Maxima SYBR Green qPCR master mixture (Fermentas, Burlington, Canada), and 0.5 μM of each primer. We used the genus-specific primer P1 (ACG ATC AGA TAC CGT CGT AAT CTT) combined with either of the species-specific primers V1 (CAA TCT AAG AAT AAA CTC CGA AGA GAA A) or F2 (CAA TCT AAA AGT CAC CTC GAA AGA TG), for P. vivax and P. falciparum, respectively.14 Standard curves were prepared with serial 10-fold dilutions of the target sequence, cloned into pGEM-T Easy vectors (Promega, Madison, WI), to allow for species-specific quantitation of parasite loads (number of parasites/μL of blood). We used a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA) for PCR amplification with a template denaturation step at 95°C for 10 minutes, followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C, with fluorescence acquisition at the end of each extension step. Amplification was immediately followed by a melting program consisting of 15 seconds at 95°C, 15 seconds at 60°C, and a stepwise temperature increase of 0.2°C/second until 95°C, with fluorescence acquisition at each temperature transition. Standard curves accompanying each assay indicated that the detection threshold of this diagnostic qPCR is approximately 2 parasites/μL of blood. No-template controls (containing all reagents for amplification except for the DNA template) were run for every qPCR microplate.
Outcome measures and data analysis.
The intention-to-treat (ITT) study population included all enrolled patients who had received at least one treatment dose, whereas the per-protocol (PP) population included only patients who had completed the full 3-day supervised treatment course and had outcome data for the primary efficacy endpoint. Efficacy outcomes were defined in accordance with the World Health Organization guidelines for in vivo antimalarial drug efficacy monitoring.15 The primary outcome was ACPR, defined as absence of asexual blood-stage parasites detected by microscopy in the PP population by day 42, regardless of axillary temperature, with no evidence of earlier treatment failure. Secondary endpoints in the ITT population were parasite clearance time (PCT; time from first dose to the first sample microscopically negative for asexual parasites), fever clearance time (FCT; time from first dose to the first normal temperature reading), and the proportions of patients who had asexual blood stages or gametocytes detected by microscopy during the entire follow-up and the proportion of those with parasite DNA detected by qPCR on days 3 and 42 of treatment. Proportions are given with 95% confidence intervals (95% CI) calculated with Wilson's continuity correction16 and compared with Fisher's exact tests; correlation was evaluated using the nonparametric Spearman correlation test. Mean PCT and FCT were estimated with Kaplan–Meier survival analysis and compared with Mantel–Cox log-rank tests. Continuous variables were compared with Mann–Whitney U tests. Analyses were done using SPSS version 17.0 (SPSS, Chicago, IL), with statistical significance set at the 5% level.
Analysis of P. falciparum mdr1 copy number.
Amplification of the multidrug resistance 1 gene of P. falciparum (pfmdr1), which encodes a P-glycoprotein of the family of adenosine triphosphate–binding cassette transporters, is associated with reduced susceptibility to MQ.17 We thus used a real-time quantitative SYBR green PCR, with the single-copy gene coding for P. falciparum β-tubulin as a reference, to estimate the number of copies of pfmdr1 in pretreatment (day 0) parasites. Plasmids containing amplified fragments of the pfmdr1 and β-tubulin genes, cloned into pGEM-T Easy vectors, were used as calibrators. In brief, PCR was carried out in triplicate, on a StepOnePlus real-time thermal cycler , with the previously described oligonucleotide primer pair17 (pfmdr1–1F and pfmdr1–1R) for pfmdr1 and a newly designed primer pair (tub–F, AAA AAT ATG ATG TGC GCA AGT GA; tub–R, AAC TTC CTT TGT GGA CAT TCT TCC T) for β-tubulin. Each 20-μL reaction mixture contained 5 μL of parasite DNA template (prepared as described above), 10 μL of 2× Maxima SYBR Green qPCR master mixture, and 0.3 μM of each oligonucleotide primer. Amplification included a template denaturation step at 95°C (10 minutes) followed by 40 cycles of 15 seconds at 95°C and 1 minute at 59°C, with fluorescence acquisition at the end of each extension step. Amplification was immediately followed by a melting program consisting of 15 seconds at 95°C, 1 minute at 59°C, and a stepwise temperature increase of 0.3°C/second until 95°C, with fluorescence acquisition at each temperature transition. The PCR efficiency of primer pairs for pfmdr1 and β-tubulin was evaluated using six serial dilutions of the plasmids containing the respective targets and was found to be sufficiently similar to obviate the need for any correction factor. We thus estimated the number of pfmdr1 copies using a comparative threshold method, with the formula ΔΔCt = (Ctpvmdr1 − Ctβ-tubulin)sample − (Ctpvmdr1 − Ctβ-tubulin)calibrator, where Ct is the threshold cycle for each gene. Copy number > 1.6 was defined as a duplication of the pfmdr1 gene. Assays were repeated if the following results were obtained: copy number between 1.3 and 1.6, Ct value > 35, or standard deviation of Ct (for either pfmdr1 or β-tubulin replicates) > 0.2.
Sequence analysis of the kelch13 propeller gene.
We also tested pretreatment parasite DNA samples for point mutations in the kelch13 propeller (k13) gene of P. falciparum that has been associated with emerging artemisinin resistance in southeast Asia.18,19 These mutations are found in the BTB (BR-C, ttk, and bab) and POZ (Pox virus and Zinc finger) (BTB/POZ) domain and in the six repeats of the kelch propeller domain of the k13 gene.19 We used one pair of external primers and five pairs of internal (nested) primers to amplify a fragment comprising the full-length (2,181-bp) k13 gene coding sequence.20 Nested PCR–amplified products were excised from agarose gels, purified using the illustra GFX PCR DNA and Gel Band Purification kits (GE Healthcare Bio-Sciences, Pittsburgh, PA) and were Sanger-sequenced on both strands using an ABI 3130 capillary DNA sequencer (Applied Biosystems). Sequence contigs were assembled using the SeqMan Pro software (DNASTAR, Madison, WI) and aligned against the k13 gene of the 3D7 reference strain (PF3D7_1343700).
GenBank accession numbers.
DNA sequences obtained during this study were deposited in the GenBank database (accession nos. KU200702–KU200841).
Ethical approval.
The institutional review board of Oswaldo Cruz Foundation approved the study protocol (no. 022/2009). Written informed consent was obtained from all study participants or their parents or guardians.
RESULTS
Study population and adverse effects.
The baseline characteristics of the ITT population, which comprised 162 patients with uncomplicated single-species P. falciparum infection recruited at 11 health centers in the urban area of Cruzeiro do Sul, are shown in Table 1. At the time the study was ongoing, the Malaria Expert Committee of Ministry of Health of Brazil started to debate whether ASMQ efficacy in Brazil was declining because of previous widespread use of MQ monotherapy. Given that a drug policy change could result from this debate, we decided to exceed our sample size beyond the original enrollment target of 101 subjects, to provide a more accurate drug efficacy estimate. We failed to obtain complete follow-up data for eight subjects (4.9%): six males and two females with ages ranging from 4 to 68 years. Four of them left the study site after 2–36 days of follow-up, three were censored (on days 16, 28, and 37) because of P. vivax malaria, treated with chloroquine (25 mg/kg of base over three days) plus primaquine (0.5 mg/kg/day of base for 7 days), and one withdrew informed consent on day 3. ASMQ was well tolerated and no study withdrawal was attributable to adverse events related to the study drugs. Most common adverse events, all of them mild, reported by the ITT population between days 1 and 3 were dizziness (88.3%), headache (73.4%), abdominal pain (6.1%), insomnia (3.7%), and blurred vison (3.7%). Dizziness persisted up to 7 (but not beyond) days in 36 of 160 subjects (22.5%), while blurred vison was reported by a single subject (0.6%) who was reassessed on day 7. No patient vomited within 30 minutes of taking the ASMQ doses. Other adverse events were rarely reported.
Table 1.
Baseline characteristics of the intention-to-treat study population (n = 162), Cruzeiro do Sul, northwestern Brazil, 2010–2013
| Variable | Value |
|---|---|
| Age (years), mean (range) | 26.6 (4–73) |
| Gender (male:female) | 92:70 |
| Weight (kg), mean (range) | 54.0 (13–94) |
| Temperature (°C), mean (range) | 37.9 (36.5–39.9) |
| Temperature ≥ 37.5°C, n (%) | 141 (87.0) |
| Reported symptoms in the past 48 hours | |
| Fever, n (%) | 159 (98.1) |
| Headache, n (%) | 135 (83.3) |
| Chills, n (%) | 94 (58.0) |
| Abdominal pain, n (%) | 15 (9.9) |
| Dizziness, n (%) | 12 (7.4) |
| Arthralgia, n (%) | 5 (3.1) |
| Vomiting, n (%) | 4 (2.5) |
| Days ill before enrollment, mean (range) | 3.2 (1–31) |
| Hemoglobin (g/100 mL), mean (range) | 13.2 (8.4–28.0) |
| Anemia, n (%) | 35 (21.6) |
| Platelet count × 109/L, mean (range) | 134 (38–273) |
| Thrombocytopenia, n (%) | 100 (61.7) |
| Parasitemia by microscopy (asexual forms/μL) | |
| Mean (range) | 4,581.2 (250–62,069) |
| Median | 1,497.5 |
| Gametocyte carriage, n (%) | 57 (35.2) |
| Parasitemia by qPCR (parasites/μL) | |
| Mean (range) | 4,700.1 (10–53,500) |
| Median | 1,883.4 |
| Total AS dose (mg/kg), mean (range)* | 10.9 (6.4–33.3) |
| Total MQ dose (mg/kg), mean (range)* | 22.0 (14.0–44.0) |
AS = artesunate; MQ = mefloquine; qPCR = quantitative polymerase chain reaction.
One patient who left the study on day 1 (before completing treatment) was excluded from this analysis.
Treatment efficacy.
The PP protocol analysis comprised 154 subjects who were followed up for 42 days (95.1% of those enrolled), with a microscopy-based ACPR rate of 100% (95% CI = 97.0–100%). Of the subjects in the PP population, 60 (39.0%; 95% CI = 31.3–47.2%) and seven (4.5%; 95% CI = 2.0–9.5%) had asexual parasites detected by microscopy on days 1 and 2, respectively; none of them had patent asexual P. falciparum parasitemia observed between days 3 and 42. A single instance of discordant results given by field microscopists was observed: one day-3 slide was considered positive for asexual P. falciparum blood stages by one and negative by the second. After blinded review by the expert microscopist, this slide was declared negative. A good correlation was observed between parasite density estimates on day 0 obtained by the expert microscopist and by qPCR (Spearman correlation coefficient rs = 0.73, P < 0.0001). The overall mean PCT for the ITT population was 1.49 days (95% CI = 1.38–1.60), with no significant difference across gender and age groups. As expected,21 PCT was directly proportional to the initial asexual parasitemia, ranging from 1.33 days (subjects with ≤ 1,000 parasites/μL) to 1.43 days (subjects with parasitemias between 1,001 and 5,000/μL) to 1.81 days (subjects with > 5,000 parasites/μL) (Mantel–Cox log-rank test, P < 0.0001). Interestingly, PCT was shorter among subjects enrolled early in the course of malaria (1–3 days after symptoms started; 1.39 days) compared with those with > 3 days of illness (1.65 days; Mantel–Cox log-rank test, P = 0.037), most likely because of their lower asexual parasitemias at enrollment (median = 1,404.5 versus 4,071.4 parasites/μL; Mann–Whitney U test, P = 0.017). Further comparisons were limited by the fact that PCT estimates were derived from a small number of thick smears analyzed by microscopy. Overall, FCT was 1.49 days (95% CI = 1.51–1.84), with no significant difference according to gender, age, initial parasitemia, and duration of symptoms before enrollment. However, this study was underpowered to detect minor differences in FCT among patient subgroups.
Gametocyte carriage and residual parasite DNA detected by qPCR.
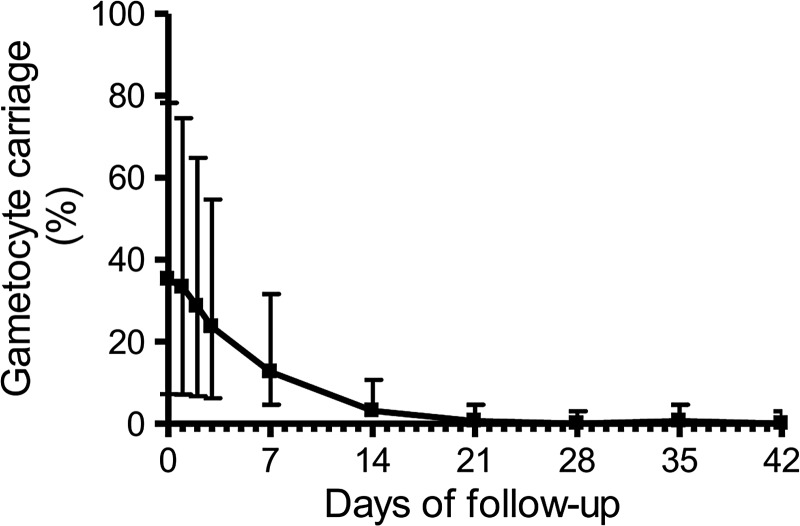
ACTs are thought to prevent the formation of new gametocytes due to their rapid action against asexual blood stages, but are not entirely effective in clearing preformed gametocytes.22 Figure 1 shows the prevalence of gametocyte carriage in the ITT population between days 0 and 42. Seventy-five patients (46.3%) had gametocytes detected by microscopy at least once during the 42-day follow-up; 57 (35.2%) of them had gametocytes at enrollment, whereas all others had patent gametocytemia first detected between 1 and 14 days after starting ASMQ. Note that 23.6% of the ITT population (95% CI = 17.4–31.1%) had gametocytes detected on day 3, although asexual parasites were no longer detected by microscopy. Interestingly, over one-third of day 3 samples (37.6%; 95% CI = 30.3–45.6%) had parasite DNA detected by qPCR, consistent with recent findings in African children treated with AL or dihydroartemisinin–piperaquine (DP).23 Parasite densities determined by qPCR on day 3 were usually low, ranging between 2 and 1,675/μL, with a median of 6/μL. We compared patients with parasite DNA detected on day 3 with qPCR-negative subjects and found no significant difference according to age, gender, prevalence of fever between days 0 and 3, or total AS and MQ dose administered (Table 2 ). On average, subjects with residual parasite DNA had slightly higher initial (day 0) asexual parasitemias detected by microscopy, a difference that did not reach statistical significance, and substantially higher qPCR-determined parasite densities (Table 2). Moreover, subjects with qPCR-detected residual parasite DNA more often carried gametocytes between day 0 and day 7 (Table 2). No patient had parasite DNA detected by qPCR or gametocytes detected by microscopy on day 42.
Figure 1.
Prevalence (%) of gametocyte carriage detected by microscopy before (day 0) and after (days 1–42) treatment of uncomplicated Plasmodium falciparum malaria with artesunate–mefloquine fixed-dose combination. Error bars indicate the 95% confidence intervals.
Table 2.
Characteristics of the study subjects with qPCR-detected parasite DNA on day 3, compared with those who were qPCR-negative, Cruzeiro do Sul, northwestern Brazil, 2010–2013
| Variable | Parasite DNA detected by qPCR on day 3 | ||
|---|---|---|---|
| No (N = 100)* | Yes (N = 61)* | P value† | |
| Age (years), mean (range) | 26.6 (4–70) | 26.5 (6–73) | 0.320 |
| Gender (male:female) | 53:47 | 39:22 | 0.192 |
| Temperature ≥ 37.5°C, n (%) | |||
| Day 0 | 89 (89.0) | 58 (95.1) | 0.253 |
| Day 1 | 24 (24.0) | 10 (16.4) | 0.321 |
| Day 2 | 4 (4.0) | 2 (3.3) | 1.000 |
| Day 3 | 2 (2.0) | 5 (8.2) | 0.105 |
| Microscopy | |||
| Initial asexual parasitemia (/μL) | |||
| Mean (range) | 3,607.4 (250–45,000) | 5,830.0 (250–62,068) | 0.084 |
| Median | 1,369.3 | 1,868.1 | |
| Asexual parasite prevalence, n (%) | |||
| Day 1 | 36 (36.0) | 28 (45.9) | 0.247 |
| Day 2 | 5 (5.0) | 3 (4.9) | 1.000 |
| Gametocyte prevalence, n (%) | |||
| Day 0 | 16 (16.0) | 41 (67.2) | 0.0001 |
| Day 1 | 17 (17.0) | 36 (59.0) | 0.0001 |
| Day 2 | 12 (12.0) | 34 (55.7) | 0.0001 |
| Day 3 | 11 (11.0) | 27 (44.3) | 0.0001 |
| Day 7 (N = 159) | 7/99 (7.1) | 13/60 (21.7) | 0.012 |
| Day 14 (N = 158) | 2/98 (2.0) | 3/60 (5.0) | 0.369 |
| Initial parasitemia by qPCR (parasites/μL) | |||
| Mean (range) | 3,631.6 (10.1–46,858.1) | 5,651.7 (95.7–34,789.4) | 0.017 |
| Median | 1,343.9 | 2,963.6 | |
| Total AS dose (mg/kg), mean (range)‡ | 10.6 (6.7–19.3) | 11.3 (6.4–33.3) | 0.457 |
| Total MQ dose (mg/kg), mean (range)‡ | 23.4 (15.7–42.6) | 24.1 (14.0–44.0) | 0.635 |
AS = artesunate; MQ = mefloquine; qPCR = quantitative polymerase chain reaction.
Number of subjects tested, except if stated otherwise.
Proportions compared with Fisher's exact tests and continuous variables compared with Mann–Whitney U test.
One patient who left the study on day 1 (before completing treatment) was excluded from this analysis.
Molecular markers of MQ and AS resistance.
We determined pfmdr1 gene copy number in day 0 P. falciparum samples from 161 subjects (99.4% of the ITT population) and found no single instance of pfmdr1 gene amplification. Moreover, we obtained 140 high-quality, full-length sequences of the k13 gene (corresponding to 86.4% of the ITT population) from day 0 parasites. Sixty-one (43.6%) samples had a single nonsynonymous (A→C) nucleotide substitution, causing the K189T amino acid change; no other mutations were found in the k13 gene. The K189T mutation, which is common in African isolates, maps to the P. falciparum-specific N-terminal domain of the k13 gene product, upstream of the BTB/POZ and propeller domains.19 Parasites carrying the K189T mutation do not display longer parasite clearance half-life after artemisinin treatment.24 Therefore, we found no molecular evidence of either AS or MQ resistance in our day 0 P. falciparum samples.
DISCUSSION
This is the first evaluation of the clinical efficacy of ASMQ since its introduction in Juruá Valley, Brazil, in 2006. Herein, we show that three daily doses of the ASMQ fixed-dose combination remain highly effective for uncomplicated malaria in the main P. falciparum transmission hotspot in Brazil. Moreover, ASMQ was relatively well tolerated, with mild adverse effects (mainly dizziness) that did not cause treatment interruption. The high prevalence of dizziness (88%) after treatment is a matter of concern, which prevents ASMQ use in patients with a history of neuropsychiatric disease. Uncomplicated P. falciparum malaria patients commonly reported dizziness before any treatment; for example, a recent case series found a prevalence of 75% at presentation in Brazil.25 However, dizziness and other neurologic and psychiatric symptoms are often reported to be associated with MQ administration either for malaria chemoprophylaxis or treatment, mostly in subjects reporting previous neuropsychiatric disease. For this reason, since 2013, the Food and Drug Administration of the United States requests MQ to carry a black box warning on its potential neurological and psychiatric side effects in the drug label (http://www.fda.gov/downloads/Drugs/DrugSafety/UCM362232.pdf).
Overall, our results are consistent with a series of clinical trials showing > 98% efficacy of AS and MQ in neighboring Peru.12,26,27 Moreover, we found no molecular evidence of resistance to either MQ (pfmdr1 gene amplification)17 or AS (mutations in the BTB/POZ and propeller domains of the k13 gene)18,19 in the local P. falciparum population. Taken together, the clinical efficacy results combined with molecular data strongly support the use of ASMQ as a first-line regimen to treat uncomplicated P. falciparum malaria in this region. Moreover, these results have potential implications for neighboring countries where ASMQ remains as the first-line treatment of P. falciparum infections.
The Malaria Expert Committee of Ministry of Health of Brazil has repeatedly raised concerns about using MQ as an ACT partner drug after two decades of its widespread deployment as a monotherapy countrywide. Despite the absence of definitive clinical evidence of declining ASMQ efficacy in Brazil, the Malaria Expert Committee recommended in July 2012 that AL should replace ASMQ as the first-line regimen for P. falciparum malaria treatment in most of the Amazon, including Juruá Valley, limiting ASMQ use to areas of the country with very low (annual parasite index < 0.1 case/1,000 inhabitants) or no autochthonous malaria transmission (Ministry of Health of Brazil, unpublished information). This decision has several implications. First, local production of ASMQ fixed-dose combination was achieved in 2006 after years of successful collaboration with the Drugs for Neglected Diseases initiative and several other international partners,9 with a major financial investment by the state-owned pharmaceutical company Farmanguinhos. Now this highly effective ACT is little used in Brazil, while the National Malaria Control Program has to import large quantities of AL. Second, AL is administered twice a day (total of 8 tablets/day for adults), compared with a single daily dose of ASMQ, likely reducing patients' compliance with the regimen and making directly observed treatment less feasible. Finally, there are no publicly available data from randomized and controlled clinical trials showing that AL is either equivalent or superior to ASMQ to treat uncomplicated P. falciparum malaria in Brazil.
Although ASMQ was highly effective in clearing asexual blood-stage parasitemia detected by microscopy by day 3 after treatment, we hypothesized that residual subpatent parasitemia might persist for a few more days. In fact, we found residual parasite DNA detectable by qPCR on day 3 in more than one-third of ASMQ-treated patients, as recently reported in Kenyan children treated with AL or DP.23 The presence of residual parasite DNA, also termed “residual parasitemia,”23 was associated with prolonged gametocyte carriage in both studies. Indeed, the Kenyan study further demonstrated that children with residual parasitemias were more likely to infect mosquitoes than those who were PCR-negative by day 3.23 These findings suggest that preformed gametocytes persisting after ASMQ treatment are the main source of parasite DNA amplified by qPCR on day 3. Nevertheless, we cannot rule out that low-level, residual submicroscopic asexual parasitemias might also be present, leading to the continuous release of newly formed gametocytes in the bloodstream, despite ASMQ administration. Our results further support the use of a single dose of primaquine to clear gametocytes after ACT, reducing the transmission potential of treated patients.22 In fact, a gametocytocidal dose of primaquine (0.75 mg/kg) was reintroduced in the treatment of P. falciparum malaria in Brazil in early 2012, before the completion of this study.
We conclude that ASMQ remains highly effective in Juruá Valley, the area of Brazil where it is most needed. Comparative clinical trials are now required to determine the relative role of ASMQ, AL, and other ACTs in the current efforts to eliminate P. falciparum malaria in this country.
ACKNOWLEDGMENTS
We are grateful to Ana Carolina Santelli, José Lázaro de Brito Ladislau, and Paola B. Marchesini (National Malaria Control Program, Ministry of Health of Brazil); Oscar Mesones Lapouble (Pan American Health Organization) for overall support; Cor Jesus F. Fontes (Federal University of Mato Grosso, Brazil) for helpful suggestions regarding the study design; Haine Felipe da Silva and André Daher (Farmanguinhos) for study drug supply; and the Health Secretary of Acre State and the Health Secretary of Cruzeiro do Sul for their logistic support during fieldwork.
Disclaimer: The funders had no role in study design, data collection and interpretation, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This clinical study was supported by the Amazonian Malaria Initiative (AMI)/Amazon Network for the Surveillance of Antimalarial Drug Resistance (RAVREDA) through the Pan American Health Organization (PAHO; BR/LOA/0900212.001), Ministry of Health of Brazil, and Oswaldo Cruz Foundation (cooperation agreements 25380.4120/09-26 and 82/2012). AMI is a partnership of the U.S. Agency for International Development with PAHO, Centers for Disease Control and Prevention (CDC), the Management Sciences for Health's Rational Pharmaceutical Management Plus (MSH/RPM Plus) program, the U.S. Pharmacopoeia, and Links Media, Inc. RAVREDA was organized in 2001 by several countries with PAHO's support to respond to the challenge of antimalarial drug resistance in the Amazon. Laboratory work was partially supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (grant 404067/2012-3). Laís C. Salla, Priscila T. Rodrigues, and Marcelo U. Ferreira are supported by CNPq scholarships.
Authors' addresses: Simone Ladeia-Andrade, Laboratory of Parasitic Diseases, Oswaldo Cruz Institute/Oswaldo Cruz Foundation (Fiocruz), , Rio de Janeiro, Brazil and Amazonian Malaria Initiative/Amazon Network for the Surveillance of Antimalarial Drug Resistance, Regional Hospital of Juruá Valley, Cruzeiro do Sul, Brazil, E-mail: shawam@uol.com.br. Gladson Naber P. de Melo and Rita de Cássia de Souza-Lima, Amazonian Malaria Initiative/Amazon Network for the Surveillance of Antimalarial Drug Resistance, Regional Hospital of Juruá Valley, Cruzeiro do Sul, Brazil, E-mails: gladson_melo@hotmail.com and draritalima@yahoo.com.br. Laís C. Salla, Melissa S. Bastos, Priscila T. Rodrigues, and Marcelo U. Ferreira, Department of Parasitology, Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil, E-mails: lais.salla@usp.br, melissabastos@yahoo.com.br, priscilathihara@usp.br, and muferrei@usp.br. Francisco das Chagas O. Luz, Center for Tropical Medicine, Campus Universitário Darcy Ribeiro, University of Brasília, Brasília, Brazil, E-mail: franciluz@yahoo.com.br.
References
- 1.World Health Organization . World Malaria Report 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 2.Souza JM. Mefloquine clinical trials—therapeutical experience with mefloquine alone and combination (MSP) in Brazilian male subjects with falciparum malaria. Mem Inst Oswaldo Cruz. 1986;81((Suppl II)):259–268. [Google Scholar]
- 3.Ministry of Health of Brazil . Manual de Terapêutica da Malária. Brasília, Brazil: Ministry of Health of Brazil; 2001. http://portal.saude.gov.br/portal/arquivos/pdf/manu_terapeutica_malaria.pdf Available at. [Google Scholar]
- 4.Cerutti C, Jr, Durlacher RR, de Alencar FE, Segurado AA, Pang LW. In vivo efficacy of mefloquine for the treatment of falciparum malaria in Brazil. J Infect Dis. 1999;180:2077–2080. doi: 10.1086/315141. [DOI] [PubMed] [Google Scholar]
- 5.Duarte EC, Fontes CJ, Gyorkos TW, Abrahamowicz M. Randomized controlled trial of artesunate plus tetracycline versus standard treatment (quinine plus tetracycline) for uncomplicated Plasmodium falciparum malaria in Brazil. Am J Trop Med Hyg. 1996;54:197–202. doi: 10.4269/ajtmh.1996.54.197. [DOI] [PubMed] [Google Scholar]
- 6.de Alencar FE, Cerutti C, Jr, Durlacher RR, Boulos M, Alves FP, Milhous W, Pang LW. Atovaquone and proguanil for the treatment of malaria in Brazil. J Infect Dis. 1997;175:1544–1547. doi: 10.1086/516497. [DOI] [PubMed] [Google Scholar]
- 7.Zalis MG, Pang L, Silveira MS, Milhous WK, Wirth DF. Characterization of Plasmodium falciparum isolated from the Amazon region of Brazil: evidence for quinine resistance. Am J Trop Med Hyg. 1998;58:630–637. doi: 10.4269/ajtmh.1998.58.630. [DOI] [PubMed] [Google Scholar]
- 8.Pratt-Riccio LR, Chehuan YF, Siqueira MJ, das Graças Alecrim M, Bianco-Junior C, Druilhe P, Brasseur P, de Fátima Ferreira-da-Cruz M, Carvalho LJ, Daniel-Ribeiro CT. Use of a colorimetric (DELI) test for the evaluation of chemoresistance of Plasmodium falciparum and Plasmodium vivax to commonly used anti-plasmodial drugs in the Brazilian Amazon. Malar J. 2013;12:281. doi: 10.1186/1475-2875-12-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells S, Diap G, Kiechel JR. The story of artesunate-mefloquine (ASMQ), innovative partnerships in drug development: case study. Malar J. 2013;12:68. doi: 10.1186/1475-2875-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santelli AC, Ribeiro I, Daher A, Boulos M, Marchesini PB, dos Santos RL, Lucena MB, Magalhães I, Leon AP, Junger W, Ladislau JL. Effect of artesunate-mefloquine fixed-dose combination in malaria transmission in Amazon basin communities. Malar J. 2012;11:286. doi: 10.1186/1475-2875-11-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan American Health Organization . Washington, DC: Pan American Health Organization; 1998. Evaluación de la Eficacia Terapéutica de los Medicamentos para el Tratamiento del Paludismo por Plasmodium falciparum sin Complicaciones en las Américas. [Google Scholar]
- 12.de Oliveira AM, Chavez J, de Leon GP, Durand S, Arrospide N, Roberts J, Cabezas C, Marquiño W. Efficacy and effectiveness of mefloquine and artesunate combination therapy for uncomplicated Plasmodium falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2011;85:573–578. doi: 10.4269/ajtmh.2011.11-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Health of Brazil . Guia Prático de Tratamento da Malária no Brasil. Brasília, Brazil: Ministry of Health of Brazil; 2010. http://bvsms.saude.gov.br/bvs/publicacoes/guia_pratico_malaria.pdf Available at. [Google Scholar]
- 14.Kimura M, Kaneko O, Liu Q, Zhou M, Kawamoto F, Wataya Y, Otanie S, Yamaguchi Y, Tanabe K. Identification of the four species of human malaria parasites by nested PCR that targets variant sequences in the small subunit rRNA gene. Parasitol Int. 1997;46:91–95. [Google Scholar]
- 15.World Health Organization . Geneva, Switzerland: World Health Organization; 2003. Assessment and Monitoring of Antimalarial Drug Efficacy for the Treatment of Uncomplicated Falciparum Malaria. [Google Scholar]
- 16.Newcombe R. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Price RN, Uhlemann AC, Brockman A, McGready R, Ashley E, Phaipun L, Patel R, Laing K, Looareesuwan S, White NJ, Nosten F, Krishna S. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Ménard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Ménard D. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mita T, Tachibana SI, Hashimoto M, Hirai M. Plasmodium falciparum kelch 13: a potential molecular marker for tackling artemisinin-resistant malaria parasites. Expert Rev Anti Infect Ther. 2016;14:125–135. doi: 10.1586/14787210.2016.1106938. [DOI] [PubMed] [Google Scholar]
- 20.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ, Tracking Resistance to Artemisinin Collaboration (TRAC) Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White NJ. The parasite clearance curve. Malar J. 2011;10:278. doi: 10.1186/1475-2875-10-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White NJ, Ashley EA, Recht J, Delves MJ, Ruecker A, Smithuis FM, Eziefula AC, Bousema T, Drakeley C, Chotivanich K, Imwong M, Pukrittayakamee S, Prachumsri J, Chu C, Andolina C, Bancone G, Hien TT, Mayxay M, Taylor WR, von Seidlein L, Price RN, Barnes KI, Djimdé A, ter Kuile F, Gosling R, Chen I, Dhorda MJ, Stepniewska K, Guérin P, Woodrow CJ, Dondorp AM, Day NP, Nosten FH. Assessment of therapeutic responses to gametocytocidal drugs in Plasmodium falciparum malaria. Malar J. 2014;13:483. doi: 10.1186/1475-2875-13-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beshir KB, Sutherland CJ, Sawa P, Drakeley CJ, Okell L, Mweresa CK, Omar SA, Shekalaghe SA, Kaur H, Ndaro A, Chilongola J, Schallig HD, Sauerwein RW, Hallett RL, Bousema T. Residual Plasmodium falciparum parasitemia in Kenyan children after artemisinin-combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J Infect Dis. 2013;208:2017–2024. doi: 10.1093/infdis/jit431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miotto O, Amato R, Ashley EA, MacInnis B, Almagro-Garcia J, Amaratunga C, Lim P, Mead D, Oyola SO, Dhorda M, Imwong M, Woodrow C, Manske M, Stalker J, Drury E, Campino S, Amenga-Etego L, Thanh TN, Tran HT, Ringwald P, Bethell D, Nosten F, Phyo AP, Pukrittayakamee S, Chotivanich K, Chuor CM, Nguon C, Suon S, Sreng S, Newton PN, Mayxay M, Khanthavong M, Hongvanthong B, Htut Y, Han KT, Kyaw MP, Faiz MA, Fanello CI, Onyamboko M, Mokuolu OA, Jacob CG, Takala-Harrison S, Plowe CV, Day NP, Dondorp AM, Spencer CC, McVean G, Fairhurst RM, White NJ, Kwiatkowski DP. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet. 2015;47:226–234. doi: 10.1038/ng.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.da Silva-Nunes M, Ferreira MU. Clinical spectrum of uncomplicated malaria in semi-immune Amazonians: beyond the “symptomatic” vs “asymptomatic” dichotomy. Mem Inst Oswaldo Cruz. 2007;102:341–347. doi: 10.1590/s0074-02762007005000051. [DOI] [PubMed] [Google Scholar]
- 26.Marquino W, Huilca M, Calampa C, Falconi E, Cabezas C, Naupay R, Ruebush TK., 2nd Efficacy of mefloquine and a mefloquine-artesunate combination therapy for the treatment of uncomplicated Plasmodium falciparum malaria in the Amazon basin of Peru. Am J Trop Med Hyg. 2003;68:608–612. doi: 10.4269/ajtmh.2003.68.608. [DOI] [PubMed] [Google Scholar]
- 27.Gutman J, Green M, Durand S, Rojas OV, Ganguly B, Quezada WM, Utz GC, Slutsker L, Ruebush TK, 2nd, Bacon DJ. Mefloquine pharmacokinetics and mefloquine-artesunate effectiveness in Peruvian patients with uncomplicated Plasmodium falciparum malaria. Malar J. 2009;8:58. doi: 10.1186/1475-2875-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]