Abstract
Following the emergence of Zika in the past decade, there are lessons to be learned from similar emergence events of dengue (DENV) and chikungunya (CHIKV). Specifically, as Zika emerges in the Americas there is a natural tendency to apply the knowledge base of DENV and CHIKV to mitigation and control of a virus with such a similar transmission system. However, there are marked differences that may preclude such broad stroke application of this knowledge base without making potentially faulty assumptions. Herein, Zika virus (ZIKV) transmission is reviewed, and the commonalities among these three arboviruses are discussed. Importantly, the divergence of this particular arbovirus is discussed, as is the need to develop ZIKV-specific knowledge base for mitigation of this disease. Specifically reviewed are 1) emergence and persistence patterns, 2) genetic and phenotypic diversity, 3) vector host range, and finally, 4) alternate transmission routes and added complexity of ZIKV transmission and presentation.
Background
Zika virus (ZIKV) is a newly emergent virus in the western hemisphere, though it was first isolated in Uganda in the 1940s.1,2 ZIKV transmission is similar to two other important arboviruses: dengue (DENV) and chikungunya (CHIKV). Serological evidence throughout 1950–1980s indicated ZIKV also circulated in human populations in Africa, southeast Asia, and the Indian Ocean. These studies also uncovered a high co-occurrence of neutralizing antibody to DENV and CHIKV.3–5 The current circulation of ZIKV in Brazil coincides with endemic DENV and recent establishment of CHIKV. Given the similarities of ZIKV transmission system to the DENV and CHIKV systems, we can use the experiences of the two latter viruses to prepare for incursion of the former. However, differences between ZIKV and these other ecologically similar viruses should be appreciated lest application of DENV or CHIKV specific knowledge result in potentially misleading assumptions regarding ZIKV. Below is a review of ZIKV in the context of lessons learned from DENV and CHIKV regarding several factors that describe or influence transmission factors. Specifically reviewed are 1) emergence and persistence patterns, 2) genetic and phenotypic diversity, 3) vector host range, and finally, 4) alternate transmission routes and added complexity of ZIKV transmission and presentation.
Emergence and persistence patterns.
DENV is a complex of four serotypes that while antigenically related, independently circulate though often together.6 In these hyperendemic situations, the dynamics of DENV are complicated by the four serotypes where there is potential competition for finite vector populations, as well as complex susceptibility profiles of human populations. Alternation of the dominant serotype (“serotype switching”) is associated with increases in severe DENV disease (hemorrhagic fever and shock syndrome), as reported in Ref. 6. Severe DENV disease is attributed to antibody-dependent enhancement whereby antibodies raised to a primary DENV infection cross-react but sub-neutralize subsequent infection with heterologous serotype.6 This means that while rendered unsusceptible to that primary DENV serotype (thus altering dynamics of transmission to that serotype), enhancement may catalyze emergence of heterologous serotypes. Genotypic differences within serotypes may alter the risk of enhancement, indicating that phenotypic heterogeneities as well as genotypic variability are important to DENV transmission7 (see section Genetic and phenotypic diversity).
In contrast, the dynamics of CHIKV transmission indicate that human susceptibility (and loss thereof) is not complicated by incomplete or enhancing immunological statuses humans. In 2005–2006, CHIKV emerged in East Africa and expanded through the Indian Ocean, most notably on La Reunion where hundreds of thousands were infected.8 Most recently, CHIKV emerged in the Americas where the human population was almost completely susceptible,2 infecting over a million people in a relatively short time. Infection with CHIKV is presumed to confer lifelong immunity meaning that all individuals infected with CHIKV become immune and are unavailable for future infections. Thus, recurring CHIKV outbreaks are functionally related to the turnover rate of susceptible humans (in addition to reintroduction rates).
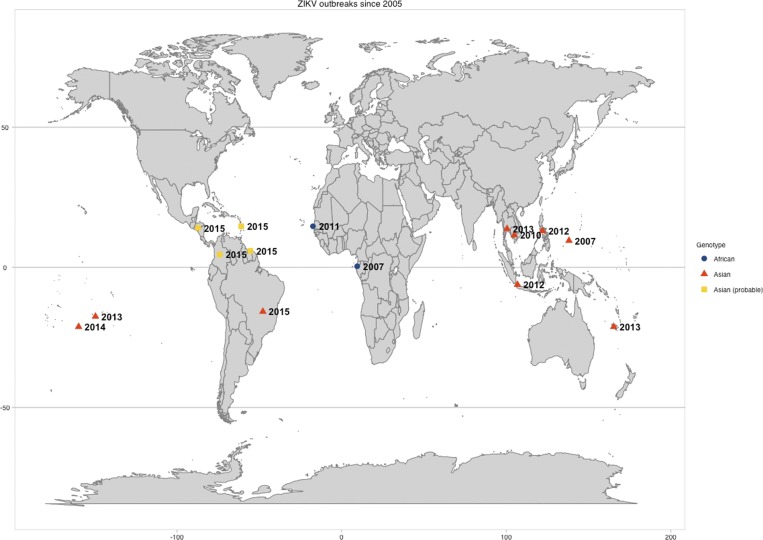
Similar to both DENV and CHIKV, reports of ZIKV outbreaks were sporadic until the latter part of the twentieth century, with an increase in the last decade especially. The largest outbreak recorded occurred in 2007 on the Island of Yap where over 5,000 human infections were estimated.9 Since, there have been additional outbreaks (Figure 1) and it appears that ZIKV infections convey lasting immunity, like CHIKV. However, as ZIKV is a member of the Japanese encephalitis virus (JEV) serocomplex, where it closely groups with Spondweni virus.10 Within the JEV group, there may be some cross-reactivity and potential cross-neutralization to other members of the JEV group.11 There is conflicting information regarding the potential for ZIKV cross-reactivity specifically with DENV. Some studies have demonstrated cross-reaction with DENV,12,13 while others demonstrated that enzyme-linked immunosorbent assay for IgM and IgG in ZIKV acute and convalescent serum samples do not cross-react with DENV.14–16 Thus, there is still need for clarification of clinical protection and immunogenic cross-reactivity of ZIKV, DENV, and potentially other JEV group members.
Figure 1.
This map shows the location and year of Zika outbreaks since 2005 that have been published or reported to ProMed Mail (archive nos. 20151223.3886435 and 20151213.3858300). The shape and color of the markers indicate which genotype was linked to that outbreak. Asian (probable) refers to those reported ProMed cases that are likely linked to the Asian outbreak in Brazil but have not been confirmed.
Genetic and phenotypic diversity.
Relative efficiency of arboviruses in vector populations is an important driver of transmission dynamics. DENV serotypes and genotypes often display variability in transmission phenotype, and genotypes within serotypes also show heterogeneity in vector competence.17 In the last 20 years, the American genotype of DENV2 has been displaced by the Asian and cosmopolitan genotypes.17 Similarly, the Asian genotype of CHIKV and prototypic ECSA lineage was thought to be largely displaced by an emergent sub-lineage East Central South African (ECSA) due in part to its increased efficiency in the vector, Aedes albopictus.8 However, the unexpected emergence of the Asian genotype throughout the Americas in 2013–2014 indicates that the Asian genotype is not inefficient and quite capable of seeding and supporting an epidemic.2
Similar to DENV and CHIKV, ZIKV is subdivided into two phylogenetically distinct genotypes—Asian and African—as well as further subdivision of the African genotype into two sub-lineages:West African and East African.14,18 In the last decade, eleven reported outbreaks of ZIKV (Figure 1) have been attributed primarily to strains of the Asian genotype (with the exception of Gabon 2007 and Senegal 2011).9,14–16,18,19 From these data it would be easy to suggest that the Asian genotype is more transmissible and thus has the capability (and is likely) to displace the African genotype, as the American genotype of DENV2 was displaced by other more fit DENV2 variants. However, the emergence of the Asian CHIKV genotype over the ECSA-V sub-lineage would warn us against this assumption. To date, there are no studies directly comparing infection kinetics of ZIKV genotypes in human samples, animal models, or vector populations. Such data would support a hypothesis of in vivo differences and potentially explain the dominance of the Asian genotype. Without studies to characterize the genetic and phenotypic diversity of ZIKV, such an assumption is premature.
Vector host range.
Despite the importance of competent vector identification, in only two of the 11 outbreaks of the past decade have the vector(s) been identified: Ae. albopictus was incriminated as the primary vector in Gabon and Aedes hensilii in the Island of Yap outbreak9,19; Aedes aegypti has been implicated in the ongoing transmission in the Americas.20 The relative efficiency of ZIKV genotypes in vector populations has not been fully explored, as vector competence studies have thus far used the African genotype. Still, mosquito population diversity does play an important role in transmission potential. For example, both Ae. aegypti and Ae. albopictus from Singapore were found to be highly efficient at transmitting an African strain of ZIKV, with 100% saliva infectious after 14 days postexposure.21,22 However, Senegalese strains of Ae. aegypti showed no ability to transmit ZIKV of the same genotype.23
Relatedly, the documented vector host range associated with ZIKV outbreaks is more diverse than that of DENV or CHIKV. First isolated from a forest mosquito (Aedes africanus), ZIKV is transmitted efficiently by nine Aedes mosquito species as well as Anopheles species.19 The relative role of these mosquitoes in future emergence and persistence needs further study. The continued expansion of Ae. albopictus does open the possibility of transmission in more temperate regions. In addition, several of these ZIKV competent mosquitoes are more associated with sylvatic cycles rather than the urban and peri-urban ecologies of Ae. aegypti and Ae. albopictus. Could this indicate that there are similarly competent forest-associated vectors in South and Central America? If that is the case, there is potential for establishment of a reservoir system, as ZIKV-specific antibody has been detected in nonhuman primates as well as rodents.24,25 Though virus has not been isolated from rodents or non-human primates (NHP) in recent surveys, the virus was originally identified from a sentinel monkey, indicating NHP could potentially support sylvatic transmission.
Alternate routes and added complexity.
While many lessons learned can inform hypotheses regarding ZIKV transmission, there are the lessons that remain unanswered for all three arboviruses. Most notable is the distribution of asymptomatic cases and thus the total force of infection within human populations. High rates of subclinical disease lead to underestimates in total DENV incidence, though the actual proportion varies considerably.26 CHIKV was thought to have a higher presentation rate compared with DENV, but recent reports have estimated as much as 47% of infections are asymptomatic.27 ZIKV asymptomatic rates were estimated to be relatively high during the Yap Island outbreak,9 though in subsequent outbreaks these were not reported. Until there is comprehensive knowledge of asymptomatic rates—not only the prevalence of, but also mechanisms behind them—assessments of total transmission will be underestimates.
As DENV and CHIKV are already established in the areas where ZIKV is reported and/or has the potential to establish, there is a high likelihood for co-circulation. There remain questions regarding the interplay among arboviruses with such similar ecologies. Arbovirus coinfection in mosquitoes do not often result in dually infectious mosquitoes, though there are few exceptions.28 Since DENV, CHIKV, and ZIKV share the same urban vectors in areas such as the Americas (Ae. aegypti and Ae. albopictus), competitive dynamics are likely as they co-circulate in a finite vector population. This may alter epidemic patterns of already-established viruses such as DENV or of the future (re)emergence of other viruses like CHIKV, yellow fever, or Spondweni viruses.
While DENV and CHIKV are primarily transmitted through the bite of infectious mosquitoes, there are alternative means of human-to-human ZIKV transmission. ZIKV has been detected in urine and saliva, and there are case reports of sexual and perinatal transmission.29–31 Though isolated incidents, alternate means of transmission (and thus potential virus perpetuation) do suggest that the factors involved in emergence potential of ZIKV may be more complex than the DENV and CHIKV transmission systems. Further, recent surges in microcephaly incidence in Brazil has coincided with the emergence of ZIKV, over 1,700 cases as of the writing of this article.20 While the epidemiological observations certainly suggest an association, causation has not been definitely established: ZIKV RNA has been confirmed in only one case, though testing continues.20 It is notable that in no other previous ZIKV outbreak has there been an association made with increases in microcephaly. However, the reports are compelling and the Brazilian government has issued a warning suggesting that women wait to get pregnant. In light of the potential sexual transmission, campaigns to help pregnant women or women trying to get pregnant avoid mosquitoes should also include their partners, as well as for men seeking to donate or bank semen. Regardless, the education infrastructure is likely to have a challenge in the years to come when this comparatively larger population of children with special needs looks to enter the education system. Thus, the social implications of ZIKV look to be more long term and multifaceted than either DENV or CHIKV.
CONCLUSIONS
Following the emergence of CHIKV in the Americas, a spate of cases in Brazil did not clinically fit the definition of either DENV or CHIKV, prompting officials to molecularly investigate ZIKV.28 ZIKV was confirmed in seven of eight patients and determined to be of the Asian lineage, which has also been circulating in the Philippines since 2012.14,28 This was the first cases of autochthonous transmission of ZIKV in the region, and cases have been reported now in several Central and South American countries.19 There have been traveler-associated ZIKV cases, most recently originating from this Brazilian outbreak.32,33 According to the U.S. Centers for Disease Control, there were nearly 2,300 cases of returned traveler–associated CHIKV in 2014 and over 550 in 2015 as of the writing of this review. As ZIKV continues to expand throughout the Americas, it presents another likely source of travel medicine woes.
The knowledge base of ZIKV is considerably smaller than that of DENV and even CHIKV, which itself lags behind DENV. Given the similarities among the three arboviruses and the likely vectors to be associated with autochthonous transmission, it is natural and necessary that we look back at the DENV and CHIKV experiences. However, there are a number of differences that indicate that these experiences may not provide a complete list of answers, and we should take care to investigate the important questions regarding transmission and not fall into patterns of assumption. Going forward, there needs to be thoughtful investigation of 1) the interplay of ZIKV and other circulating arboviruses on human infectivity and immunological cross-reactivity; 2) the capacity for alternate transmission routes to alter expectation of ZIKV emergence, expansion, and/or persistence; 3) ZIKV-specific dynamics in known and potentially competent vector species; and 4) the capacity for a reservoir system and/or sylvatic transmission cycle. We should recognize that these and other co-circulating arboviruses cannot be studied in isolation if we are to fully realize comprehensive approaches to transmission control and disease mitigation.
Footnotes
Author's address: Rebecca C. Christofferson, Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, E-mail: rcarri1@lsu.edu.
References
- 1.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 2.Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, De Lamballerie X. Chikungunya in the Americas. Lancet. 2014;383:514. doi: 10.1016/S0140-6736(14)60185-9. [DOI] [PubMed] [Google Scholar]
- 3.Olson JG, Ksiazek TG, Gubler DJ, Lubis SI, Simanjuntak G, Lee VH, Nalim S, Juslis K, See R. A survey for arboviral antibodies in sera of humans and animals in Lombok, Republic of Indonesia. Ann Trop Med Parasitol. 1983;77:131–137. doi: 10.1080/00034983.1983.11811687. [DOI] [PubMed] [Google Scholar]
- 4.Rodhain F, Gonzalez JP, Mercier E, Helynck B, Larouze B, Hannoun C. Arbovirus infections and viral haemorrhagic fevers in Uganda: a serological survey in Karamoja district, 1984. Trans R Soc Trop Med Hyg. 1989;83:851–854. doi: 10.1016/0035-9203(89)90352-0. [DOI] [PubMed] [Google Scholar]
- 5.Adekolu-John EO, Fagbami AH. Arthropod-borne virus antibodies in sera of residents of Kainji Lake Basin, Nigeria 1980. Trans R Soc Trop Med Hyg. 1983;77:149–151. doi: 10.1016/0035-9203(83)90053-6. [DOI] [PubMed] [Google Scholar]
- 6.Lee KS, Lai YL, Lo S, Barkham T, Aw P, Ooi PL, Tai JC, Hibberd M, Johansson P, Khoo SP, Ng LC. Dengue virus surveillance for early warning, Singapore. Emerg Infect Dis. 2010;16:847–849. doi: 10.3201/eid1605.091006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watts DM, Porter KR, Putvatana P, Vasquez B, Calampa C, Hayes CG, Halstead SB. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet. 1999;354:1431–1434. doi: 10.1016/S0140-6736(99)04015-5. [DOI] [PubMed] [Google Scholar]
- 8.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3:e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 10.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato R, Hamada N, Kashiwagi T, Imamura Y, Hara K, Nishimura M, Kamimura T, Takasaki T, Watanabe H, Koga T. Dengue hemorrhagic fever in a Japanese traveler with pre-existing Japanese encephalitis virus antibody. Trop Med Health. 2015;43:85–88. doi: 10.2149/tmh.2014-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979;83:213–219. doi: 10.1017/s0022172400025997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monath TP, Craven RB, Muth DJ, Trautt CJ, Calisher CH, Fitzgerald SA. Limitations of the complement-fixation test for distinguishing naturally acquired from vaccine-induced yellow fever infection in flavivirus-hyperendemic areas. Am J Trop Med Hyg. 1980;29:624–634. doi: 10.4269/ajtmh.1980.29.624. [DOI] [PubMed] [Google Scholar]
- 14.Alera MT, Hermann L, Tac-An IA, Klungthong C, Rutvisuttinunt W, Manasatienkij W, Villa D, Thaisomboonsuk B, Velasco JM, Chinnawirotpisan P, Lago CB, Roque VG, Jr, Macareo LR, Srikiatkhachorn A, Fernandez S, Yoon IK. Zika virus infection, Philippines, 2012. Emerg Infect Dis. 2015;21:722–724. doi: 10.3201/eid2104.141707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21:1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heang V, Yasuda CY, Sovann L, Haddow AD, Travassos da Rosa AP, Tesh RB, Kasper MR. Zika virus infection, Cambodia, 2010. Emerg Infect Dis. 2012;18:349–351. doi: 10.3201/eid1802.111224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong PM, Rico-Hesse R. Differential susceptibility of Aedes aegypti to infection by the American and Southeast Asian genotypes of dengue type 2 virus. Vector Borne Zoonotic Dis. 2001;1:159–168. doi: 10.1089/153036601316977769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pyke AT, Daly MT, Cameron JN, Moore PR, Taylor CT, Hewitson GR, Humphreys JL, Gair R. Imported Zika virus infection from the Cook Islands into Australia, 2014. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.4635a54dbffba2156fb2fd76dc49f65e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, Fontenille D, Paupy C, Leroy EM. Zika virus in Gabon (Central Africa)–2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8:e2681. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ProMED-mail Zika virus—Americas (04). 20151211.3855107 2015.
- 21.Li MI, Wong PSJ, Ng LC, Tan CH. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl Trop Dis. 2012;6:e1792. doi: 10.1371/journal.pntd.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong PS, Li MZ, Chong CS, Ng LC, Tan CH. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis. 2013;7:e2348. doi: 10.1371/journal.pntd.0002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diagne CT, Diallo D, Faye O, Ba Y, Faye O, Gaye A, Dia I, Faye O, Weaver SC, Sall AA, Diallo M. Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infect Dis. 2015;15:492. doi: 10.1186/s12879-015-1231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darwish MA, Hoogstraal H, Roberts TJ, Ahmed IP, Omar F. A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Trans R Soc Trop Med Hyg. 1983;77:442–445. doi: 10.1016/0035-9203(83)90106-2. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe ND, Kilbourn AM, Karesh WB, Rahman HA, Bosi EJ, Cropp BC, Andau M, Spielman A, Gubler DJ. Sylvatic transmission of arboviruses among Bornean orangutans. Am J Trop Med Hyg. 2001;64:310–316. doi: 10.4269/ajtmh.2001.64.310. [DOI] [PubMed] [Google Scholar]
- 26.Chastel C. Eventual role of asymptomatic cases of dengue for the introduction and spread of dengue viruses in non-endemic regions. Front Physiol. 2012;3:70. doi: 10.3389/fphys.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakkhara P, Chongsuvivatwong V, Thammapalo S. Risk factors for symptomatic and asymptomatic chikungunya infection. Trans R Soc Trop Med Hyg. 2013;107:789–796. doi: 10.1093/trstmh/trt083. [DOI] [PubMed] [Google Scholar]
- 28.Rohani A, Potiwat R, Zamree I, Lee HL. Refractoriness of Aedes aegypti (Linnaeus) to dual infection with dengue and chikungunya virus. Southeast Asian J Trop Med Public Health. 2009;40:443–448. [PubMed] [Google Scholar]
- 29.Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19 PMID:24721538. [PubMed] [Google Scholar]
- 30.Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17:880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gourinat AC, O'Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21:84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Summers DJ, Acosta RW, Acosta AM. Zika virus in an American recreational traveler. J Travel Med. 2015;22:338–340. doi: 10.1111/jtm.12208. [DOI] [PubMed] [Google Scholar]
- 33.Tappe D, Rissland J, Gabriel M, Emmerich P, Gunther S, Held G, Smola S, Schmidt-Chanasit J. First case of laboratory-confirmed Zika virus infection imported into Europe, November 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.4.20685. PMID:24507467. [DOI] [PubMed] [Google Scholar]