Abstract
Owing to the increase in Salmonella strains with decreased fluoroquinolone susceptibility in the endemic areas, we have been treating enteric fever with intravenous ceftriaxone empirically since 2007. In this study, we reevaluated our treatment protocol. This retrospective cohort study was conducted at a single institute in Tokyo, Japan, between January 2006 and December 2013. Enteric fever was defined as isolation of Salmonella Typhi or Salmonella Paratyphi A, B, and C from the blood and/or stool of patients with fever. Of the 35 patients with imported enteric fever, 28 (80%) had returned from south Asia. Ciprofloxacin-susceptible strains were detected in only 12% of the cases. The isolates showed excellent susceptibility to ampicillin (91%), chloramphenicol (94%), ceftriaxone (97%), and azithromycin (97%). One case of Salmonella Paratyphi B was excluded, and of the remaining 34 patients, 56% were treated with ceftriaxone alone, 26% with ceftriaxone then fluoroquinolone, and 9% with levofloxacin alone. The overall relapse rate was 6.1%; however, among those receiving ceftriaxone monotherapy, the relapse rate was 11% (N = 2). The relapse group was characterized by longer times to treatment initiation (P = 0.035) and defervescence (> 7 days) after treatment initiation (P = 0.022). In such cases, we recommend that ceftriaxone treatment be continued for > 4 days after defervescence or be changed to fluoroquinolone if the strains are found to be susceptible to prevent relapse. Furthermore, ampicillin and chloramphenicol, which are no longer prescribed, may be reconsidered as treatment options in Asia.
Introduction
Every year, an average of 11.9–20.6 million new cases of typhoid fever occur worldwide with the highest incidences in south and southeast Asia,1 resulting in significant morbidity and mortality especially among children.2 In addition, enteric fever (i.e., typhoid fever or paratyphoid fever) is one of the important differential diagnoses of febrile illness in returning travelers. According to the GeoSentinel database of travelers, between 2006 and 2011, 67% of all enteric fever patients were those returning from south Asia.3 This equates to the current epidemiology in Japan: 20–70 cases of enteric fever are being reported in Japan each year, 60–80% of which are imported from south and southeast Asia.
Fluoroquinolones are widely accepted as the optimal treatment of enteric fever in adults.4 They have better clinical outcomes, such as resolution of fever and lower rates of treatment failure, than those of other antibiotics because they have excellent tissue penetration and bactericidal activities against Salmonella enterica subspecies enterica serovar Typhi and Paratyphi A, B, and C in the intracellular stationary stage in monocytes/macrophages. However, isolates susceptible to ciprofloxacin, but resistant to nalidixic acid in the laboratory, showed poor clinical outcomes and treatment failure before 2012 when the treatment was directed by the breakpoint of ciprofloxacin.5,6 Owing to the increasing number of isolates with decreased fluoroquinolone susceptibility found in the endemic areas, we have been treating enteric fever with intravenous ceftriaxone empirically since 2007, depending on the prevalence of strains with decreased fluoroquinolone susceptibility.
To the best of our knowledge, case series that describes the treatment course and outcome of enteric fever in returning travelers is limited. In 2012, the Clinical Laboratory Standard Institute (CLSI) revised the breakpoints of ciprofloxacin: minimum inhibitory concentration (MIC) for susceptibility was changed from ≤ 1 μg/mL to ≤ 0.06 μg/mL. Therefore, in this study, we reevaluated our treatment protocol and determined the clinical characteristics, antibiotic susceptibility, and risk factors for relapse of enteric fever in Japan.
MATERIALS AND METHODS
Subjects.
This retrospective cohort study was conducted by chart review of all cases of enteric fever diagnosed at the National Center for Global Health and Medicine (NCGM), Tokyo, Japan, between January 2006 and December 2013. Enteric fever was defined as isolation of Salmonella Typhi or Salmonella Paratyphi A, B, and C from the blood, urine, stool, or bone marrow samples of patients with fever. Convalescent patients and chronic carriers were excluded in this study, and the Widal test was not used for diagnosis.
Study description and consent.
This research was approved by the institutional review board of NCGM. We obtained participation consent by using an opt-out consent form.
Definitions.
Defervescence was defined as having a body temperature < 37.5°C for > 48 hours without antipyretics. We used the criteria of sepsis adopted by the 2012 Surviving Sepsis Campaign7 and Cunha's criteria for relative bradycardia.8 Relapse was defined as recurrence of symptoms with a positive culture from a sterile site (blood, urine, or bone marrow) or from the stool of a patient with clinical features characteristic of enteric fever. We defined symptomatic treatment failure as > 7 days to defervescence. Early-phase microbiological treatment failure was defined as persistent bacteremia during the initial treatment course. Late-phase microbiological treatment failure was defined as a positive urine or stool culture without any symptoms up to 1 month after the initial treatment course. Convalescent carriers were defined as asymptomatic patients with a positive urine or stool culture for ≥ 1 month up to 1 year after onset. Chronic carriers were defined as asymptomatic patients with a positive culture obtained > 1 year after onset of acute illness.
Microbiological tests.
Bacterial isolates from blood, urine, bone marrow, and stool cultures were identified using the API 20E system (bioMérieux, Marcy l'Etoile, France). Blood cultures were performed using the BACTEC (Becton Dickinson and Company, Franklin Lakes, NJ) automatized blood culture system. The MICs of antibiotics were determined with the microdilution technique, using the dry plate Eiken (Eiken Chemical, Tokyo, Japan). The susceptibility patterns of the isolates to ampicillin, chloramphenicol, trimethoprim–sulfamethoxazole, nalidixic acid, ciprofloxacin, and ceftriaxone were defined according to the guidelines of CLSI M100-S23; for ciprofloxacin, MIC ≥ 1 μg/mL was defined as resistant, MIC 0.12–0.5 μg/mL as intermediate, and MIC ≤ 0.06 μg/mL as susceptible. Susceptibility to azithromycin was defined as MIC ≤ 16 μg/mL according to the guidelines of CLSI M100-S25. This CLSI recommendation for azithromycin only refers to Salmonella Typhi, not Salmonella Paratyphi A, B, or C. The MIC of azithromycin in Salmonella Paratyphi A tends to be higher than that in Salmonella Typhi.9 Consequently, it is currently not clear whether we can use the same breakpoint of azithromycin; however, in this study, we tentatively applied this breakpoint against Salmonella Paratyphi A, B, and C as well.
Treatment and follow-up.
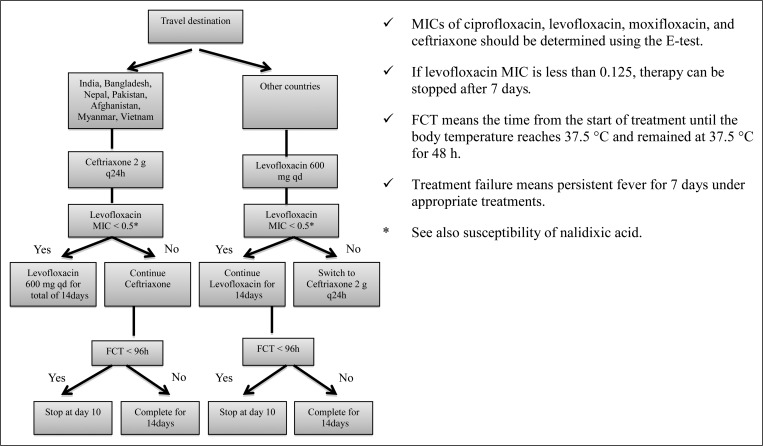
We treated enteric fever depending on the prevalence of strains with decreased fluoroquinolone susceptibility (Figure 1). If a patient returned from a country with high-to-moderate prevalence of decreased fluoroquinolone susceptibility strains, empiric intravenous ceftriaxone was initiated, and then switched to oral levofloxacin for strains found to be susceptible to nalidixic acid. This treatment protocol has been used from 2007 to 2012 at NCGM and is modified according to each case. After the revision of CLSI M100-S23, all patients in 2013 were treated by ceftriaxone empirically regardless of their travel destinations, and ceftriaxone treatment was changed to oral ciprofloxacin depending on the result of the revised susceptibility. We tested three consecutive stool cultures for all patients ≥ 1 month after the initial treatment course to detect convalescent carriers.
Figure 1.
Treatment protocol for uncomplicated enteric fever, Travel Clinic in National Center for Global Health and Medicine, Version 2.0 on June 17, 2008.
Statistical analyses.
All statistical analyses were performed using SPSS Statistics 21 (IBM, Armonk, NY). The Fisher's exact test was used for nominal variables, and the Mann–Whitney test was used for continuous variables to compare the characteristics of patients who relapsed and those who were cured. Statistical significance was set at P < 0.05.
RESULTS
Enteric fever was diagnosed in 35 patients (24 men and 11 women; mean age, 30 years; age range, 4–53 years) during the study period. Most patients were Japanese (94%); there was one Indian and one American. Of the 35 patients, 28 (80%) had returned from south Asia, that is, 19 had returned from India, six from Bangladesh, two from India and Nepal, and one from Nepal. Of the remaining seven, six (17%) had returned from southeast Asia, that is, three from Cambodia, two from Indonesia, and one from Myanmar, and one (3%) had returned from Turkey (Table 1 ). Tourism (46%) and business (37%) were the main reasons for travel; backpackers accounted for half of the patients with tourism as the reason, followed by expatriates (9%), volunteer workers (9%), and those visiting Japan (3%). Only eight patients (23%) had received pretravel consultation, four of whom (11%) had been vaccinated for typhoid fever in the previous 2 years. All patients who had received typhoid vaccination were infected with Salmonella Paratyphi A. None of the patients were taking any immunosuppressive medications or had any immunocompromising underlying diseases, including human immunodeficiency virus infection, liver cirrhosis, chronic kidney disease, diabetes mellitus, or malignancy. None of the patients were on medications that are regarded as risk factors for enteric fever, such as proton pump inhibitors or H2 blockers. Of the 35 patients, 19 (54%) had headache, 17 (49%) had diarrhea, and 10 (27%) had nausea/vomiting (Table 1). None of the patients had symptoms of coma or bloody stool. Only 16 of 34 patients (47%) fulfilled the criteria for sepsis, whereas 22 of 25 patients (88%) experienced relative bradycardia. Rose spots were observed in only two of 35 patients (6%) and eosinopenia (≤ 1% blood eosinophils) was noted in 34 (97%). Of the 32 patients with imaging findings, splenomegaly was noted in 17 (52%); however, none of the patients had gallstones. None had abnormal neurological findings or gastrointestinal perforation. Salmonella Paratyphi A was more common than Salmonella Typhi (54% and 43% patients, respectively). Salmonella Paratyphi B was identified in one patient who returned from Turkey.
Table 1.
Baseline, clinical, and microbiological characteristics of patients with imported enteric fever (N = 35)
| Characteristics | Cases, n (%) |
|---|---|
| Male sex | 24 (69) |
| Age (years), median (range) | 30 (4–53) |
| Japanese | 33 (94) |
| Destination/source of isolate | |
| South Asia | 28 (80) |
| Southeast Asia | 6 (17) |
| Other (Turkey) | 1 (3) |
| Pretravel consultation | 8 (23) |
| Typhoid vaccination within 2 years | 4 (11) |
| Symptoms | |
| Fever | 35 (100) |
| Headache | 19 (54) |
| Diarrhea | 17 (49) |
| Nausea/vomiting | 10 (27) |
| Joint pain | 7 (20) |
| Muscle pain | 4 (11) |
| Constipation | 4 (11) |
| Examination findings | |
| Relative bradycardia (N = 32) | 28 (88) |
| Rose spots | 2 (6) |
| Imaging findings (N = 32) | |
| Splenomegaly | 17 (52) |
| Paraileocecal lymph node swelling | 8 (24) |
| Ileocecal thickening | 4 (11) |
| Laboratory findings | |
| Total leucocytes (× 103/μL), median (range) | 5.1 (1.1–11.7) |
| Eosinopenia (≤ 1%) | 34 (97) |
| Absolute eosinopenia (0/μL) | 19 (54.3) |
| ALT (IU/L), median (range) | 53 (13–560) |
| CRP (mg/L), median (range) | 48 (2.9–212) |
| Fulfilled the sepsis definition (N = 34) | 16 (47) |
| Serovar | |
| Typhi | 15 (43) |
| Paratyphi A | 19 (54) |
| Paratyphi B | 1 (3) |
| Diagnosis | |
| Blood culture | 26 (74) |
| Blood and stool culture | 6 (17) |
| Stool culture | 3 (9) |
| Time to positive blood culture (hours), median (range) (N = 26) | 28 (6–45) |
ALT = alanine transaminase; CRP = C-reactive protein.
Among 33 patients, ciprofloxacin-susceptible strains were detected in only four (12%). Susceptibility to ampicillin (91%), chloramphenicol (94%), ceftriaxone (97%), and azithromycin (97%; Table 2 ) was excellent. In addition, mismatched fluoroquinolone and nalidixic acid susceptibility was found in one patient, that is, nalidixic acid susceptibility was found at an MIC of 8 μg/mL, whereas intermediate ciprofloxacin susceptibility was found at an MIC of 0.12 μg/mL. Multidrug-resistant strains characterized by resistance to ampicillin, chloramphenicol, and trimethoprim–sulfamethoxazole were observed in only two patients (6%). Extended-spectrum beta-lactamase (ESBL)-producing Salmonella Paratyphi A was detected in one patient.10 None of the bacterial strains in patients returning from south Asia were susceptible to ciprofloxacin, but the strains detected in four of six patients returning from southeast Asia (67%) were susceptible to ciprofloxacin. In this study, a strain of Salmonella Paratyphi A with an MIC of 32 μg/mL was tentatively classified azithromycin resistant.
Table 2.
Antibiotic susceptibility of Salmonella Typhi and Salmonella Paratyphi A (N = 33)
| Antibiotics | Susceptibility | ||
|---|---|---|---|
| Susceptible, n (%) | Intermediate, n (%) | Resistant, n (%) | |
| Ampicillin | 30 (91) | 0 | 3 (9) |
| Chloramphenicol | 31 (94) | 0 | 2 (6) |
| TMP–SXM | 26 (79) | NA | 7 (21) |
| Nalidixic acid | 5 (15) | NA | 28 (85) |
| Ciprofloxacin | 4 (12) | 14 (42) | 15 (46) |
| Ceftriaxone | 32 (97) | 0 | 1 (3) |
| Azithromycin | 32 (97) | NA | 1 (3) |
TMP–SXM = trimethoprim–sulfamethoxazole; NA = not available.
One patient had Salmonella Paratyphi B and was excluded from the clinical course evaluation (Table 3 ). Among the remaining 34 patients, 29 (85%) were treated empirically with ceftriaxone and three (9%) with levofloxacin. We divided the 34 patients to four groups: treatment with ceftriaxone alone (19, 56%), ceftriaxone then fluoroquinolone (9, 26%), fluoroquinolone alone (3, 9%), and other (Table 3). In the group treated with ceftriaxone alone, a 4-year-old child treated with cefotaxime was also included. Of the 19 in this group, 18 (95%) returned from south Asia and two (11%) patients relapsed, which only occurred in this group. In those treated with ceftriaxone followed by fluoroquinolone, the median duration of ceftriaxone treatment was 3 days (range, 2–8), and this was changed to levofloxacin (four of nine patients), ciprofloxacin (three of nine), or tosufloxacin (two of nine) treatment. In the group treated with fluoroquinolone alone, all three patients were treated with levofloxacin; however, the levofloxacin susceptibility was intermediate in all patients, that is, MIC was 0.25, 0.5, and 1 μg/mL. The last group (other) included patients treated with azithromycin plus norfloxacin, ceftriaxone changed to azithromycin, and azithromycin alone. A patient treated with azithromycin alone had asymptomatic positive stool culture soon after completion of initial treatment, which was categorized as late-phase microbiological treatment failure in this study. No differences were found between the three groups regarding time to reach defervescence and total treatment period. None of the patients in any group had persistent bacteremia (early-phase microbiological treatment failure). No convalescent carriers or chronic carriers were found, and there were no cases of death, intestinal perforation, or encephalopathy.
Table 3.
Clinical course and outcome of each therapeutic antimicrobial (N = 34)
| Characteristics | Treatment | |||
|---|---|---|---|---|
| Ceftriaxone alone* | Ceftriaxone then fluoroquinolone | Fluoroquinolone alone† | Other‡ | |
| Cases, n (%) | 19 (56) | 9 (26) | 3 (9) | 3 (9) |
| Returning from south Asia, n (%) | 18 (95) | 6 (67) | 2 (67) | 2 (66) |
| Symptomatic treatment failure§, n (%) | 3 (16) | 2 (22) | 1 (33) | 0 (0) |
| Relapse, n (%) | 2 (11) | 0 (0) | 0 (0) | 0 (0) |
| Late-phase microbiological treatment failure | 0 (0) | 0 (0) | 0 (0) | 1 (33) |
| Time from onset to treatment initiation (days), median (range) | 5 (1–27) | 4 (1–9) | 11 (8–25) | NA |
| Time to reach defervescence (days), median (range) | 5 (1–13) | 5 (4–12) | 6 (3–8) | NA |
| Total treatment period (days), median (range) | 13 (9–20) | 14 (8–14) | 12 (10–14) | NA |
| Treatment period after defervescence (days), median (range) | 5 (0–13) | 8 (2–10) | 6 (6–11) | NA |
NA = not applicable.
A 4-year-old child treated with cefotaxime was included.
All three patients were treated with levofloxacin.
Includes patients treated with azithromycin plus norfloxacin, ceftriaxone changed to azithromycin, and azithromycin alone.
Defined as time to reach defervescence > 7 days.
Two patients were excluded from the statistical analysis: one had Salmonella Paratyphi B and the other was categorized as late-phase microbiological treatment failure. Of the remaining 33 patients, two belonged to the relapse group and 31 to the cure group; consequently, the relapse rate was 6% in total. For the two patients who relapsed, the median number of days to relapse after completion of initial treatment was 12 (range, 6–17 days). No significant differences were found between the two groups in terms of travel from south Asia (P = 0.67), typhoid vaccination in the past 2 years (P = 0.78), fulfillment of the sepsis definition (P = 0.76), and time to positive blood culture result (P = 0.96). However, in the relapse group, treatment initiation took longer (P = 0.035) as did time to defervescence after treatment initiation, that is, duration of > 7 days to defervescence (P = 0.022). The cure group took a median of 5 days for defervescence compared with 9 days in the relapse groups (Table 4 ).
Table 4.
Comparison of imported enteric fever patients in the relapse and cure groups (N = 33)
| Characteristics | Relapse group (N = 2) | Cure group (N = 31) | P value |
|---|---|---|---|
| Age (years), median (range) | 42 (31–53) | 30 (4–56) | 0.19 |
| Male, n (%) | 2 (100) | 20 (67) | 0.48 |
| Returning from south Asia, n (%) | 2 (100) | 25 (78) | 0.67 |
| Typhoid vaccination within 2 years, n (%) | 0 (0) | 3 (1) | 0.78 |
| Time from onset to treatment initiation (days), median (range) | 7 (7–7) | 5 (0–27) | 0.035 |
| Fulfilled the sepsis definition, n (%) | 1 (33) | 14 (43) | 0.76 |
| Total leucocytes (× 103/μL), median (range) | 4.4 (3.2–5.6) | 5.3 (1.1–11.7) | 0.39 |
| CRP (mg/L), median (range) | 65 (32–99) | 49 (11–218) | 0.88 |
| Time to positive blood culture (hours), median (range) (N = 25) | 17 (16–18) | 16 (6–45) | 0.96 |
| Time to reach defervescence (days), median (range) | 9 (5–13) | 5 (2–12) | 0.24 |
| Total treatment period (days), median (range) | 13 (11–15) | 14 (8–21) | 0.66 |
| Treatment period after defervescence (days), median (range) | 4 (2–4) | 8 (1–16) | 0.14 |
| Symptomatic treatment failure*, n (%) | 1 (50) | 5 (16) | 0.022 |
CRP = C-reactive protein.
Defined as time to reach defervescence > 7 days.
DISCUSSION
In this study, we evaluated the epidemiological and clinical characteristics of imported cases of enteric fever in Japan. To the best of our knowledge, this is one of the largest case series that describes the treatment course and outcome of imported enteric fever in returning travelers.
A previous study reported a higher incidence of imported enteric fever in young men,5 which may be related to men's preferred travel and meal habits. Six of our patients had returned from southeast Asia, including three from Cambodia who had Salmonella Paratyphi A, which likely reflects the outbreak of paratyphoid fever that occurred in Cambodia in 2013.11,12 The strongest risk factor for enteric fever in travelers is considered to be traveling to visit friends and relatives.13,14 However, none of our patients traveled for such purpose in this study, which may indicate that there are not many immigrants from the endemic areas in Japan. Additionally, the importance of pretravel consultation is still not yet widely recognized. Therefore, further initiatives to provide pretravel health information are required. Moreover, typhoid fever is a vaccine-preventable disease, whereas the typhoid vaccinations such as the Vi capsular polysaccharide vaccine and the TY21a vaccine have, unfortunately, not been licensed in Japan.
The rate of occurrence of enteric fever symptoms in this study is similar to that reported in the literature (Table 1).15–17 The rates of occurrence of relative bradycardia and splenomegaly were also similar to those in a previous study.15 However, the rate for rose spots is reported to vary widely, from 2.6–4%5,17 up to 49%,15 and it was only 6% (2/35 patients) in the present study. We speculate that this wide variation could be the result of differences in time from disease onset to treatment initiation, because rose spots generally develop 1–2 weeks after onset. In addition, one article reported that all patients had elevated liver function test results;18 however, in our study, 16% of patients had results within the normal range. Classical signs of enteric fever such as relative bradycardia and eosinopenia were noted in 88% and 97% patients, respectively. However, these are not specific diagnostic predictors of enteric fever and can also be seen in other infections.
Isolation of Salmonella Typhi or Salmonella Paratyphi A in the stool alone may not be sufficient to confirm the diagnosis of enteric fever because it may represent a carrier state. However, in the presence of pyrexia or a reliable history of febrile episodes, stool cultures may be diagnostic when blood cultures fail, as seen in three of our patients. In endemic countries, approximately 80% of enteric fever cases are caused by Salmonella Typhi, with Salmonella Paratyphi accounting for the remaining 20%. However, the incidence of Salmonella Paratyphi infection among travelers from Nepal tends to be higher and the ratio is reversed,19 as noted in the present study. This disproportion may be due to vaccines, which protect against Salmonella Typhi only, as well as the likelihood of Salmonella Typhi infections originating from household environments and Salmonella Paratyphi infections originating from street food.20
In this study, ciprofloxacin-susceptible strains were detected in only 12% of patients (Table 2). This included all fluoroquinolone-resistant strains isolated from those returning from south Asia. A study in the Netherlands showed decreased ciprofloxacin susceptibility and resistance in 32.8% and 1.7% cases, respectively.9 The increase in strains with decreased susceptibility to fluoroquinolones, especially in the endemic areas, is a serious problem that leads to treatment failure and severe or fatal outcomes such as intestinal perforation, encephalopathy, and hemodynamic shock.17 Accordingly, recent articles have recommended intravenous ceftriaxone or oral azithromycin for empiric treatment of enteric fever in returning travellers.21 In fact, in our study, the isolates showed excellent susceptibility to ceftriaxone (97%) and azithromycin (97%). By contrast, one study reported azithromycin-resistant strains (MIC > 16 μg/mL) in 16.1% of patients.9 Moreover, we have experienced a case of ESBL-producing Salmonella Paratyphi A.10 If the azithromycin-resistant strains and ESBL-producing organisms become widespread, treatment options could become severely limited.22 However, it should be noted that ampicillin and chloramphenicol were effective in 91% and 94% cases, respectively, suggesting that antibiotics that are no longer being used as treatment of enteric fever may be effective treatment options again, as mentioned in a previous report.22 This result might also suggest the effective strategy of revising the primary antimicrobial regimen in a timely manner to fight against the antibiotic-resistant strains of enteric fever.
The overall relapse rate was 6.1% (2 of 33) in this study, which is similar to that reported by a previous study (3–6%).21 Some articles mention a higher relapse rate with ceftriaxone treatment (5–17%) than with fluoroquinolone treatment, although the relapse rate in this study was lower than that in previous studies,23–25 despite using mainly ceftriaxone. By changing the perspective, the relapse rate of treatment with ceftriaxone alone was found to be 11% (2 of 19) in this study. However, no relapse occurred in groups treated with ceftriaxone then fluoroquinolone and fluoroquinolone alone. Therefore, these results may support our recommendation on initiating empiric ceftriaxone treatment and changing to fluoroquinolone later if the strains are susceptible. As mentioned in introduction, fluoroquinolone treatment generally has better clinical outcomes than other antibiotics; however, no differences were found in this study (Table 3). This may be because intermediate fluoroquinolone susceptibility was found in all three patients receiving fluoroquinolone monotherapy or because the number of cases receiving this treatment were too small. Furthermore, interestingly, all three patients treated with levofloxacin alone were cured, despite intermediate susceptibility. It cannot be generalized to the larger population owing to the small number of cases in this study; however, this poses a good clinical question with respect to the revised breakpoint of fluoroquinolones and the differences between in vitro and in vivo antibacterial activity.
Although decreased fluoroquinolone susceptibility has been considered as a risk factor for relapse,17,26 no large-scale study has analyzed the risk factors for relapse with ceftriaxone treatment. In the present study, the relapse group showed longer times from disease onset to treatment initiation (P = 0.035) and time to defervescence (> 7 days) after treatment initiation (P = 0.022) compared with the cure group. Consequently, we suspect that the amount of bacteria on initial visit is a risk factor for relapse; however, no statistical differences were found between the two groups in terms of time to confirmation of a positive blood culture (P = 0.96). Generally, the relapse group had a higher occurrence of severe complications such as persistent bacteremia, enteric perforation, and sepsis, although disease severity did not differ significantly between the two groups in our study. Additionally, no clear evidence exists on the optimal treatment period of ceftriaxone. One study on children with enteric fever recommended ceftriaxone treatment of at least 5 days after defervescence.5,27 In our study, we could not find that treatment of > 4 days after defervescence may reduce the risk for relapse.
This study has some limitations. It was retrospective and limited by the relatively low number of enteric fever cases. In addition, the treatment period (median, 14 days; range, 8–21 days) and choice of antibiotics differed among cases. We also did not evaluate factors that increase the risk for acquiring enteric fever, virulence of the bacterial strain, and host factors such as human leucocyte antigen type and evidence of Helicobacter pylori infection. Further studies are needed to reduce biases and to clarify the risk factors for relapse.
CONCLUSIONS
Empiric ceftriaxone and subsequent switching to fluoroquinolones, if susceptible, may be an effective treatment protocol for enteric fever. Ampicillin and chloramphenicol, which are no longer prescribed, are likely to be effective and should be reconsidered as alternative treatment options among selected patients for enteric fever in Asia. The greater the delay in initiating treatment and the longer the time to defervescence (> 7 days) may influence relapse. In such cases, we recommend that ceftriaxone treatment be continued for > 4 days after defervescence or be changed to fluoroquinolone if the strains are found to be susceptible to prevent relapse.
ACKNOWLEDGMENTS
We thank the physicians, nurses, and clinical staff at the Disease Control and Prevention Center for their excellent work.
Footnotes
Financial support: This work is supported by a grant from the Ministry of Health, Labor, and Welfare (H24-shinkou-ippan-013).
Authors' addresses: Takashi Matono and Yasuyuki Kato, Disease Control and Prevention Center, National Center for Global Health and Medicine, Toyama, Shinjuku-ku, Tokyo, Japan, E-mails: sincedec2012@gmail.com and ykato@hosp.ncgm.go.jp. Masatomo Morita and Hidemasa Izumiya, Department of Bacteriology I, National Institute of Infectious Diseases, Toyama, Shinjuku-ku, Tokyo, Japan, E-mails: mmorita@niid.go.jp and izumiya@nih.go.jp. Kei Yamamoto, Satoshi Kutsuna, Nozomi Takeshita, and Kayoko Hayakawa, Disease Control and Prevention Center, National Center for Global Health and Medicine, Toyama, Shinjuku-ku, Tokyo, Japan, E-mails: nicepoko@gmail.com, sonare.since1192@gmail.com, nozomitake@gmail.com, and kayokohayakawa@gmail.com. Kazuhisa Mezaki, Microbiology Laboratory, National Center for Global Health and Medicine, Toyama, Shinjuku-ku, Tokyo, Japan, E-mail: kmezaki@hosp.ncgm.go.jp. Maho Kawamura and Noriko Konishi, Department of Microbiology, Tokyo Metropolitan Institute of Public Health, Hyakunincho, Shinjuku-ku, Tokyo, Japan, E-mails: Maho_Kawamura@member.metro.tokyo.jp and Noriko_Konishi@member.metro.tokyo.jp. Yasutaka Mizuno, Department of Infectious Diseases, Tokyo Medical University, Shinjuku-ku, Tokyo, Japan, E-mail: mizuno@tokyo-med.ac.jp. Shuzo Kanagawa and Norio Ohmagari, Disease Control and Prevention Center, National Center for Global Health and Medicine, Toyama, Shinjuku-ku, Tokyo, E-mails: skanagaw@hosp.ncgm.go.jp and nohmagari@hosp.ncgm.go.jp.
References
- 1.Mogasale V, Maskery B, Ochiai RL, Lee JS, Mogasale VV, Ramani E, Kim YE, Park JK, Wierzba TF. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health. 2014;2:e570–e580. doi: 10.1016/S2214-109X(14)70301-8. [DOI] [PubMed] [Google Scholar]
- 2.Ochiai RL, Acosta CJ, Danovaro-Holliday MC, Baiqing D, Bhattacharya SK, Agtini MD, Bhutta ZA, Canh do G, Ali M, Shin S, Wain J, Page AL, Albert MJ, Farrar J, Abu-Elyazeed R, Pang T, Galindo CM, von Seidlein L, Clemens JD, Domi Typhoid Study Group A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull World Health Organ. 2008;86:260–268. doi: 10.2471/BLT.06.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensenius M, Han PV, Schlagenhauf P, Schwartz E, Parola P, Castelli F, von Sonnenburg F, Loutan L, Leder K, Freedman DO, GeoSentinel Surveillance Network Acute and potentially life-threatening tropical diseases in western travelers—a GeoSentinel multicenter study, 1996–2011. Am J Trop Med Hyg. 2013;88:397–404. doi: 10.4269/ajtmh.12-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Effa EE, Lassi ZS, Critchley JA, Garner P, Sinclair D, Olliaro PL, Bhutta ZA. Fluoroquinolones for treating typhoid and paratyphoid fever (enteric fever) Cochrane Database Syst Rev. 2011;5:CD004530. doi: 10.1002/14651858.CD004530.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel TA, Armstrong M, Morris-Jones SD, Wright SG, Doherty T. Imported enteric fever: case series from the hospital for tropical diseases, London, United Kingdom. Am J Trop Med Hyg. 2010;82:1121–1126. doi: 10.4269/ajtmh.2010.10-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassing RJ, Goessens WH, Mevius DJ, van Pelt W, Mouton JW, Verbon A, van Genderen PJ. Decreased ciprofloxacin susceptibility in Salmonella Typhi and Paratyphi infections in ill-returned travellers: the impact on clinical outcome and future treatment options. Eur J Clin Microbiol Infect Dis. 2013;32:1295–1301. doi: 10.1007/s10096-013-1878-9. [DOI] [PubMed] [Google Scholar]
- 7.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 8.Cunha BA. The diagnostic significance of relative bradycardia in infectious disease. Clin Microbiol Infect. 2000;6:633–634. doi: 10.1046/j.1469-0691.2000.0194f.x. [DOI] [PubMed] [Google Scholar]
- 9.Hassing RJ, Goessens WH, van Pelt W, Mevius DJ, Stricker BH, Molhoek N, Verbon A, van Genderen PJ. Salmonella subtypes with increased MICs for azithromycin in travelers returned to The Netherlands. Emerg Infect Dis. 2014;20:705–708. doi: 10.3201/eid2004.131536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mawatari M, Kato Y, Hayakawa K, Morita M, Yamada K, Mezaki K, Kobayashi T, Fujiya Y, Kutsuna S, Takeshita N, Kanagawa S, Ohnishi M, Izumiya H, Ohmagari N. Salmonella enterica serotype Paratyphi A carrying CTX-M-15 type extended-spectrum beta-lactamase isolated from a Japanese traveller returning from India, Japan, July 2013. Euro Surveill. 2013;18:20632. doi: 10.2807/1560-7917.es2013.18.46.20632. [DOI] [PubMed] [Google Scholar]
- 11.Tourdjman M, Le Hello S, Gossner C, Delmas G, Tubiana S, Fabre L, Kerléguer A, Tarantola A, Fruth A, Friesema I, Thorstensen Brandal L, Lawrence J, Fisher I, Dufour M, Weill FX, de Valk H. Unusual increase in reported cases of paratyphoid A fever among travellers returning from Cambodia, January to September 2013. Euro Surveill. 2013;18:20594. doi: 10.2807/1560-7917.es2013.18.39.20594. [DOI] [PubMed] [Google Scholar]
- 12.Saitoh T, Morita M, Shimada T, Izumiya H, Kanayama A, Oishi K, Ohnishi M, Sunagawa T. Increase in paratyphoid fever cases in Japanese travellers returning from Cambodia in 2013. Epidemiol Infect. 2015;144:602–606. doi: 10.1017/S0950268815001648. [DOI] [PubMed] [Google Scholar]
- 13.Ackers ML, Puhr ND, Tauxe RV, Mintz ED. Laboratory-based surveillance of Salmonella serotype Typhi infections in the United States: antimicrobial resistance on the rise. JAMA. 2000;283:2668–2673. doi: 10.1001/jama.283.20.2668. [DOI] [PubMed] [Google Scholar]
- 14.Angell SY, Cetron MS. Health disparities among travelers visiting friends and relatives abroad. Ann Intern Med. 2005;142:67–72. doi: 10.7326/0003-4819-142-1-200501040-00013. [DOI] [PubMed] [Google Scholar]
- 15.Kuvandik C, Karaoglan I, Namiduru M, Baydar I. Predictive value of clinical and laboratory findings in the diagnosis of the enteric fever. New Microbiol. 2009;32:25–30. [PubMed] [Google Scholar]
- 16.Lutterloh E, Likaka A, Sejvar J, Manda R, Naiene J, Monroe SS, Khaila T, Chilima B, Mallewa M, Kampondeni SD, Lowther SA, Capewell L, Date K, Townes D, Redwood Y, Schier JG, Nygren B, Tippett Barr B, Demby A, Phiri A, Lungu R, Kaphiyo J, Humphrys M, Talkington D, Joyce K, Stockman LJ, Armstrong GL, Mintz E. Multidrug-resistant typhoid fever with neurologic findings on the Malawi-Mozambique border. Clin Infect Dis. 2012;54:1100–1106. doi: 10.1093/cid/cis012. [DOI] [PubMed] [Google Scholar]
- 17.Parry CM, Thompson C, Vinh H, Chinh NT, Phuong le T, Ho VA, Hien TT, Wain J, Farrar JJ, Baker S. Risk factors for the development of severe typhoid fever in Vietnam. BMC Infect Dis. 2014;14:73. doi: 10.1186/1471-2334-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klotz SA, Jorgensen JH, Buckwold FJ, Craven PC. Typhoid fever. An epidemic with remarkably few clinical signs and symptoms. Arch Intern Med. 1984;144:533–537. doi: 10.1001/archinte.144.3.533. [DOI] [PubMed] [Google Scholar]
- 19.Connor BA, Schwartz E. Typhoid and paratyphoid fever in travellers. Lancet Infect Dis. 2005;5:623–628. doi: 10.1016/S1473-3099(05)70239-5. [DOI] [PubMed] [Google Scholar]
- 20.Vollaard AM, Ali S, van Asten HA, Widjaja S, Visser LG, Surjadi C, van Dissel JT. Risk factors for typhoid and paratyphoid fever in Jakarta, Indonesia. JAMA. 2004;291:2607–2615. doi: 10.1001/jama.291.21.2607. [DOI] [PubMed] [Google Scholar]
- 21.Basnyat B, Maskey AP, Zimmerman MD, Murdoch DR. Enteric (typhoid) fever in travelers. Clin Infect Dis. 2005;41:1467–1472. doi: 10.1086/497136. [DOI] [PubMed] [Google Scholar]
- 22.Wain J, Hendriksen RS, Mikoleit ML, Keddy KH, Ochiai RL. Typhoid fever. Lancet. 2015;385:1136–1145. doi: 10.1016/S0140-6736(13)62708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frenck RW, Nakhla I, Sultan Y, Bassily SB, Girgis YF, David J, Butler TC, Girgis NI, Morsy M. Azithromycin versus ceftriaxone for the treatment of uncomplicated typhoid fever in children. Clin Infect Dis. 2000;31:1134–1138. doi: 10.1086/317450. [DOI] [PubMed] [Google Scholar]
- 24.Thaver D, Zaidi AK, Critchley J, Azmatullah A, Madni SA, Bhutta ZA. A comparison of fluoroquinolones versus other antibiotics for treating enteric fever: meta-analysis. BMJ. 2009;338:b1865. doi: 10.1136/bmj.b1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler T. Treatment of typhoid fever in the 21st century: promises and shortcomings. Clin Microbiol Infect. 2011;17:959–963. doi: 10.1111/j.1469-0691.2011.03552.x. [DOI] [PubMed] [Google Scholar]
- 26.Chinh NT, Parry CM, Ly NT, Ha HD, Thong MX, Diep TS, Wain J, White NJ, Farrar JJ. A randomized controlled comparison of azithromycin and ofloxacin for treatment of multidrug-resistant or nalidixic acid-resistant enteric fever. Antimicrob Agents Chemother. 2000;44:1855–1859. doi: 10.1128/aac.44.7.1855-1859.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatli MM, Aktas G, Kosecik M, Yilmaz A. Treatment of typhoid fever in children with a flexible-duration of ceftriaxone, compared with 14-day treatment with chloramphenicol. Int J Antimicrob Agents. 2003;21:350–353. doi: 10.1016/s0924-8579(02)00388-6. [DOI] [PubMed] [Google Scholar]