Abstract
Dengue is responsible for a wide range of clinical manifestations, ranging from asymptomatic infections to severe cases. The alteration of cytokine levels correlated with clinical characteristics can help determine prognostic markers of the disease and the identification of targets for immunotherapy. We measured the viral load, serotype, and cytokine levels of 212 serum samples from patients with acute dengue infection during days 1–4 after the onset of symptoms. The patients were classified as either with hemorrhagic manifestations (HM) or with no hemorrhagic manifestations (NHM). The cytokines interleukin-6 (IL-6), IL-8, and IL-10 were increased (P < 0.05) in the dengue virus+ group, compared with the control group. A higher viral load (P < 0.05) and IL-6 was detected in the HM group compared with the NHM group. Interestingly, the NHM group demonstrated a significant positive correlation between inflammatory (IL-6 and 8) and anti-inflammatory (IL-10) cytokines, whereas the HM group did not. These findings suggest that a disturbance in the balance of inflammatory cytokines IL-6 and IL-8 with the anti-inflammatory cytokine, IL-10, combined with the high levels of IL-6 and viral load, characterize possible mechanisms related to the formation of HM.
Introduction
Dengue virus infection is one of the most widespread mosquito-borne diseases in the world and the lack of a vaccine or licensed antiviral therapy exacerbates this scenario. Dengue viruses (DENVs) are serologically classified into four antigenically distinct serotypes (DENV-1, DENV-2, DENV-3, and DENV-4).1,2 Factors such as virus serotype and virulence, as well as host susceptibility factors, and past DENV infections have been implicated in disease progression and severity.3–6
Symptomatic dengue manifestations typically range from a high fever together with severe headache, eye and joint pain (dengue fever [DF]) to a severe syndrome, which may include hemorrhage and shock (dengue hemorrhagic fever [DHF]). DHF can be divided into four grades of severity, of which the most severe grades III and IV are classified as dengue shock syndrome (DSS). Although this classification system is useful, limitations exist for patient classification due to many clinical and biochemical parameters considered.7–12 The new World Health Organization classification for dengue severity is divided into dengue without warning signs, dengue with warning signs, and severe dengue, but the old system has applicability in many studies and is used here.
The key pathological feature of DHF is increased vascular permeability associated with increased levels of inflammatory cytokines such as Tumor necrosis factor-alpha (TNF-α), Interferon-gamma (IFN-γ), interleukin-1beta (IL-1beta), IL-2, IL-6, IL-8, and anti-inflammatory cytokines such as IL-10.13–16 Other studies have suggested that cytokine levels can be used as predictive factors for severity in dengue.17
High levels of IL-6 have implications for development of pathology in severe dengue, playing an important role in the production of antiplatelet or antiendothelial cell antibodies, as well as elevated levels of tissue plasminogen activator, and a deficiency in coagulation, leading to plasma leakage and bleeding.18
IL-8 is a chemokine that can contribute to platelet activation either by its chemoattractant properties or by its effect on endothelial permeability and has been associated with thrombocytopenia.19,20 IL-8 has also been associated with DHF pathogenesis, due to higher levels in DHF compared with DF, and a correlation with thrombocytopenia and raised alanine transferase.21
IL-10 exhibits anti-inflammatory properties, including the inhibition of inflammatory responses, antigen presentation, and phagocytosis. In some cases, IL-10 may play a role in DENV pathogenesis, reflecting an immunosuppressive function, followed by impaired immune clearance and a persistent infectious effect for acute viral infection.22
Most of these studies have been performed using material from patients with active DHF versus those with classic DF. One important question that remains to be studied is at what stage during disease progression and development these cytokines increase or become out of balance. In many infectious and parasitic diseases, the inflammatory response is augmented and driven by inflammatory cytokines such as those mentioned above. However, the role of anti-inflammatory cytokines, such as IL-10, is key for counteracting and balancing the potentially pathogenic activities of inflammatory cytokines.22,23 Thus, studies designed to determine the profiles and balance between key inflammatory and anti-inflammatory cytokines during DENV infection are critical for helping to understand the cellular and molecular mechanisms that drive disease development.
The search for biological parameters in clinical samples that may support the distinction between severe and nonsevere dengue can be useful for a better understanding of the immunopathogenesis of this infection, as well as identify possible biomarkers of disease progression. Indeed, several studies have presented many cytokines as predictive factors for dengue severity including IFN-γ, TNF-α, IL-6, IL-8, and IL-10.17,24,25 However, the actual use of these potential markers to predict hemorrhagic manifestations (HM) of the disease is not clear due to the wide variation in study designs, timing, methods of sample collection/processing, and the precision in the identification of disease severity. Herein, we investigate cytokine expression profiles together with other parameters including viral load and dengue serotype, in addition to early expression of HM, during the initial 1–4 days of the onset of symptoms, in an attempt to define possible polarized responses in the earliest phases of the onset of disease and identify possible immune profiles related to more severe manifestations.
MATERIALS AND METHODS
Serum samples.
Ethical approval was provided by the Research Ethics Committee of the Instituto de Pesquisa e Ensino da Santa Casa de Belo Horizonte (no. 02126213.8.0000.5138). The samples were selected from the biobank of Ezequiel Dias Foundation (Central Laboratory of Minas Gerais, Brazil), in the year 2013, based on the “Information System for Notifiable Diseases” (SINAN) forms. This form classifies individuals as with HM or with no hemorrhagic manifestations (NHM) during the first 4 days after the onset of symptoms. In addition, the clinician indicates one or more indicators of HM which are epistaxis, hematuria, gingival bleeding, menorrhagia, gastrointestinal bleeding, petechiae, and positive tourniquet test. Patients with one or more of these events were clustered in the group of HM, and those without were grouped as NHM patients.
The samples were subjected to viral isolation using 10-mL cell culture tubes at 106 cells/mL (C6/36 clone of Aedes albopictus cells) incubated with 20 μL of serum at 25°C for 10 days. Then, the cell cultures were screened for virus presence by antigen detection immunofluorescence assays, using flavivirus group–reactive antibodies or serotype-specific monoclonal antibodies. Viral replication in C6/36 cells is a key tool useful for viral isolation and typing by immunofluorescence staining, since the increased amount of the virus in these susceptible cells is an important aspect for success of the immunofluorescence reaction.26
We selected a total of 212 serum samples: 91 positive for DENV-1 (39 from HM group and 52 from NHM group), 61 positive for DENV-2 (17 HM and 44 NHM), and 29 positive for DENV-3 (12 HM and 17 NHM). During the period of this study, no DENV-4 positive samples were found. The negative controls for all comparisons consisted of 31 samples (15 samples from negative DENV isolation and polymerase chain reaction (PCR), and 16 samples from healthy individuals).
RNA extraction.
RNA was extracted from the serum samples (140 μL) using QIAamp® Viral RNA Mini Kit (Qiagen, Hilden, Germany), following the manufacturer's protocol. Viral particles were lysed using a lysis solution, and the RNA was precipitated with ethanol and purified using a silica column with affinity for nucleic acids. Finally, the RNA was eluted in 60 μL of diethyl pyrocarbonate (DEPC)–treated water (Sigma, St. Louis, MO) and maintained at −80°C.
One-step quantitative real-time PCR.
DENV serotype consensus RNA standard constructed by cloning the 67–base pair (bp) amplicon of the 3′ untranslated region from DENV.27 In addition, a 64-bp amplicon from human RNase P was cloned and a standard RNAse P RNA was also produced in vitro.28 Both transcribed RNA were generated using MEGAscript® High Yield Transcription Kit (Ambion, Austin, TX) and were purified using the MEGAclear™Kit (Ambion).30 The RNase P internal control was used as an important strategy for accurate quantification of the RNA viral load, allowing us to monitor possible false-negative results (due to RNA degradation) and to correct for variations in the amounts of the initial sample (due to different RNA recovery), thereby allowing for PCR data normalization.29
Standard curves were generated by 10-fold serial dilutions of transcribed RNAs ranging from 107 to 103 copies/μL and used in a one-step quantitative real-time PCR (qRT-PCR).30 Quantitation was performed using the StepOnePlus System™ (Applied Biosystems, Foster City, CA) and SuperScript™ III Platinum® One-Step qRT-PCR System (Invitrogen, Carlsbad, CA). qRT-PCR amplifications were performed using 5 μL of RNA, 0.5 μL of SuperScript III RT/Platinum Taq Mix, 2× reaction mix buffer, 0.4 μM of primers to DENV, 0.2 μM of primers to RNase P, 0.2 μM of TaqMan probe to DENV, 0.1 μM of TaqMan probe to RNase P (Applied Biosystems), 0.25 μL of ROX Reference Dye, 0.5 μL of RNaseOUT™ (Invitrogen), and DEPC-treated water to 25 μL final volume. The cycling program consisted of a reverse transcription step at 50°C for 30 minutes and initial denaturation at 95°C for 2 minutes, followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. The detection limit of the assay was verified from successive dilutions of the standards until detection was impossible or inaccurate. Each reaction set was checked for contamination using negative controls (all reagents included and water instead of RNA). In addition to this negative control, RNA extracted from sera of healthy individuals and DENV-negative samples were included in the assay. All reactions were performed in duplicate. Thus, for each sample, the mean value determined for DENV was normalized by the average quantified value for the internal control (RNase P) as follows: (MeanDENV/MeanRNase P) × 105, where “105” corresponds to the expected amount of RNase P to the quantity of sample used in the qRT-PCR. Therefore, smaller or larger quantities of RNase P detected were normalized to 105, allowing the proportional correction of detected viral load.
Cytokine measurement.
25 μL of undiluted human serum from both DENV-positive and control subjects was used for the determination of the inflammatory cytokines IL-6 and IL-8, together with the anti-inflammatory cytokine, IL-10, using the BD Cytometric Bead Array (CBA) Human Inflammatory Cytokines Kit (BD Biosciences, San Jose, CA) according to the manufacturer's recommendations. In brief, this method uses beads coated with an anticytokine capture antibody, which are then developed with an anticytokine fluorochrome-conjugated antibody. After incubation, the samples are read on a flow cytometer (FACScanto II; BD Biosciences). Standard curves where generated using known quantities of each cytokine for calculation of cytokine concentrations in the sera samples. Importantly, all standard curves returned an R2 of > 0.99. Greater than 90% of 181 DENV-positive samples showed detectable levels of IL-6, IL-8, and IL-10, and all 181 samples where included in the final analysis.
Statistical analysis.
Individuals infected and not infected with DENV were compared using the two-tailed Student's t test. Three or more groups were compared using two-tailed analysis of variance with corrections for multiple comparisons (Tukey). The correlation analyses were performed using Pearson's correlation and were represented with corresponding values of P and R2. Differences were considered significant at P < 0.05 and were indicated by the same letters. The statistical package, Prism v. 6 (GraphPad, San Diego, CA) was used for the analysis.
RESULTS
Of the total samples used in this study, 181 were positive for DENV and 31 were negative controls (Table 1 ). One-step qRT-PCR was used to quantify the viral load in positive samples in both HM and NHM groups. To assess whether viral infection leads to distinct cytokine profiles, the sera levels of IL-6, IL-8, and IL-10 in DENV-positive and negative samples were measured by CBA, stratifying by viral serotype and severity of symptoms as classified by HM versus NHM.
Table 1.
Study group characteristics
| Age (years)* | Male | Female | Total | |
|---|---|---|---|---|
| DENV-1 | 32.5 ± 16.7 | 44 | 47 | 91 |
| DENV-2 | 33.3 ± 16.8 | 25 | 36 | 61 |
| DENV-3 | 37.2 ± 16.2 | 13 | 16 | 29 |
| All DENV-positive samples | 33.5 ± 16.7 | 82 | 99 | 181 |
| Negative controls | 31.3 ± 12.8 | 6 | 25 | 31 |
| All samples | 33.4 ± 15.8 | 88 | 124 | 212 |
DENV = Dengue virus.
Age is shown as mean ± standard deviation.
Comparison of viral load in DENV-1, -2, and -3 positive samples.
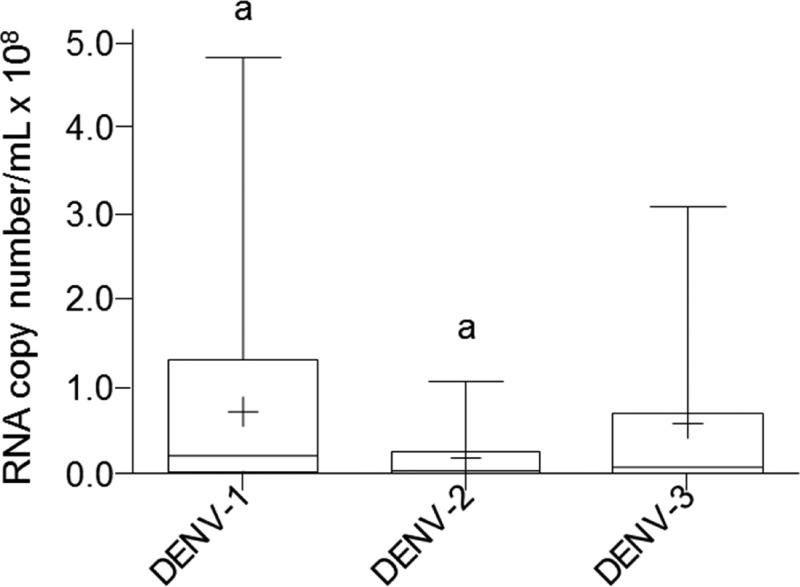
The viral load in positive samples for DENV-1, -2, and -3 was quantified by one-step qRT-PCR and compared. DENV-2 positive samples showed lower levels of viremia (P < 0.05) than DENV-1. However, DENV-3 positive was in an intermediate position, not statistically different from any of the other two groups (Figure 1).
Figure 1.
One-step quantitative real-time polymerase chain reaction (qRT-PCR) analysis of Dengue virus load in sera grouped by viral serotype. The data are shown as box plots with the box extending from the 25th to the 75th percentiles, the line in the middle the median, and the + the mean. The whiskers represent the max/min values. Differences were considered significant at P < 0.05 using the two-tailed analysis of variance with corrections for multiple comparisons (Tukey) and were indicated by the same letters.
Comparison of viral load between HM and NHM patients.
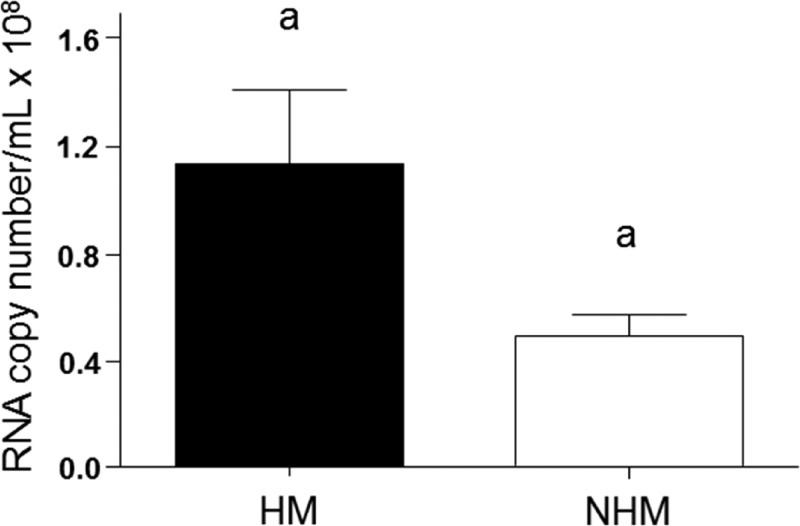
We compared the viral loads between HM and NHM groups to identify correlations between the intensity of viremia, HM, and levels of cytokines. Data from one-step qRT-PCR showed higher viral load in HM than in NHM (Figure 2).
Figure 2.
One-step quantitative real-time polymerase chain reaction(qRT-PCR) analysis of viral RNA to assess viral load in the groups with hemorrhagic manifestations (HM) and with no hemorrhagic manifestations (NHM). The data were analyzed using the two-tailed Student's t test and were considered significant at P < 0.05. The averages of each group are shown in bars and standard error of the mean is shown in the error bars. The same letter indicates significant differences.
Comparison of cytokine profiles between control and DENV-1, -2, and -3 positive samples.
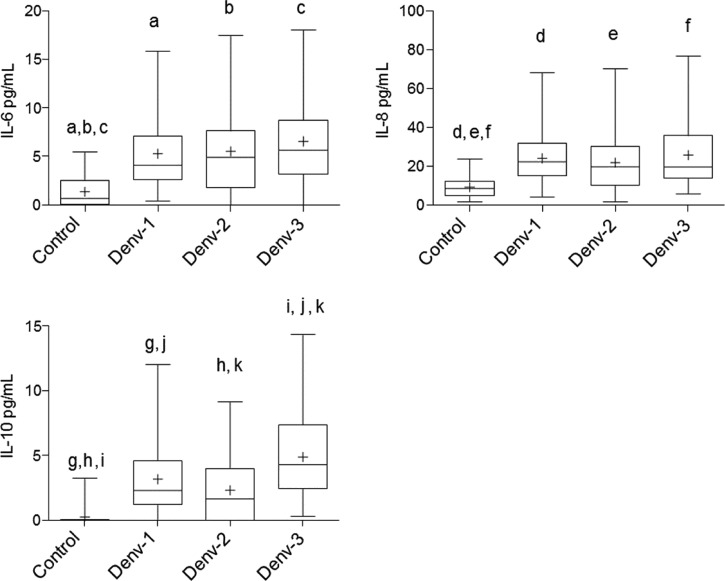
The levels of cytokines IL-6, IL-8, and IL-10 were measured in sera samples and compared between DENV-negative and DENV-positive sera. Given that cytokine levels of healthy sera were not significantly different from DENV- negative sera, Figures 3 and 4 show the average of the 31 negative samples analyzed together (15 samples from negative DENV isolation/PCR results, and 16 samples from healthy individuals).
Figure 3.
Cytokine comparisons between patients positive for Dengue virus (DENV) serotypes 1, 2, and 3 and control individuals. The data are shown as box plots with the box extending from the 25th to the 75th percentiles, the line in the middle the median, and the + the mean. The whiskers represent the max/min values. Differences were considered significant at P < 0.05 using the two-tailed analysis of variance with corrections for multiple comparisons (Tukey) and were indicated by the same letters.
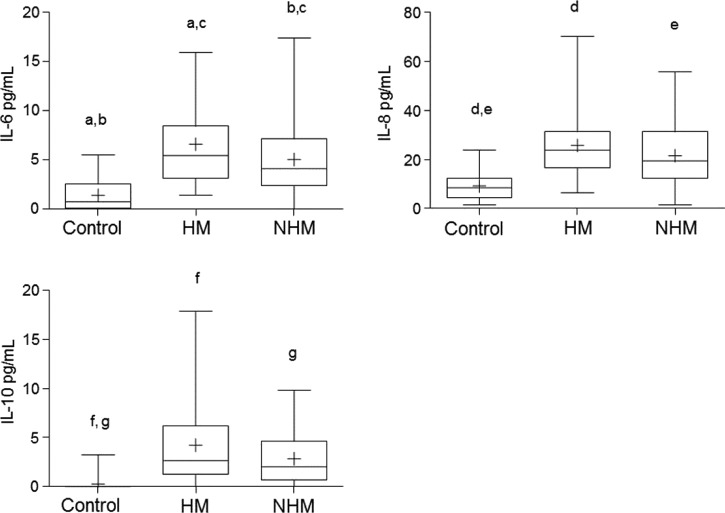
Figure 4.
Cytokine level comparison between patients with hemorrhagic manifestations (HM) and with no hemorrhagic manifestations (NHM). The data are shown as box plots with the box extending from the 25th to the 75th percentiles, the line in the middle the median, and the + the mean. The whiskers represent the max/min values. Differences were considered significant at P < 0.05 using the two-tailed analysis of variance with corrections for multiple comparisons (Tukey) and were indicated by the same letters.
As can be seen in Figure 3, DENV-positive samples for all three serotypes showed serum levels of IL-6, IL-8, and IL-10 higher (P < 0.05) than the controls. However, no significant differences were observed for IL-6 and IL-8 cytokines between the serotypes. The serotype 3 group showed higher levels of IL-10 as compared with serotypes 1 and 2, as well as the control group (P < 0.05).
Comparison of serotype distribution between HM and NHM patients.
There was no significant difference in the distribution of the serotypes amongst the HM and NHM patients. The percentage of HM individuals infected with DENV-1 was 43%, 27% with DENV-2 and 21% with DENV-3. However, this distribution was not significantly different from the distribution of DENV-1, -2, and -3 amongst the NHM patients. (P = 0.08 using the contingency table 2 × 3 and Fisher's exact test).
Comparison of cytokine profiles between HM and NHM patients.
Clinical samples of patients were separated according to the symptoms described in the epidemiological SINAN forms at the time of sample collection during the first 1–4 days after the onset of symptoms, and classified into HM or NHM. All cytokines tested were higher in both HM and NHM groups as compared with the control group. When comparing cytokine levels between HM and NHM groups, only IL-6 showed a higher mean IL-6 concentration in HM than NHM group (Figure 4).
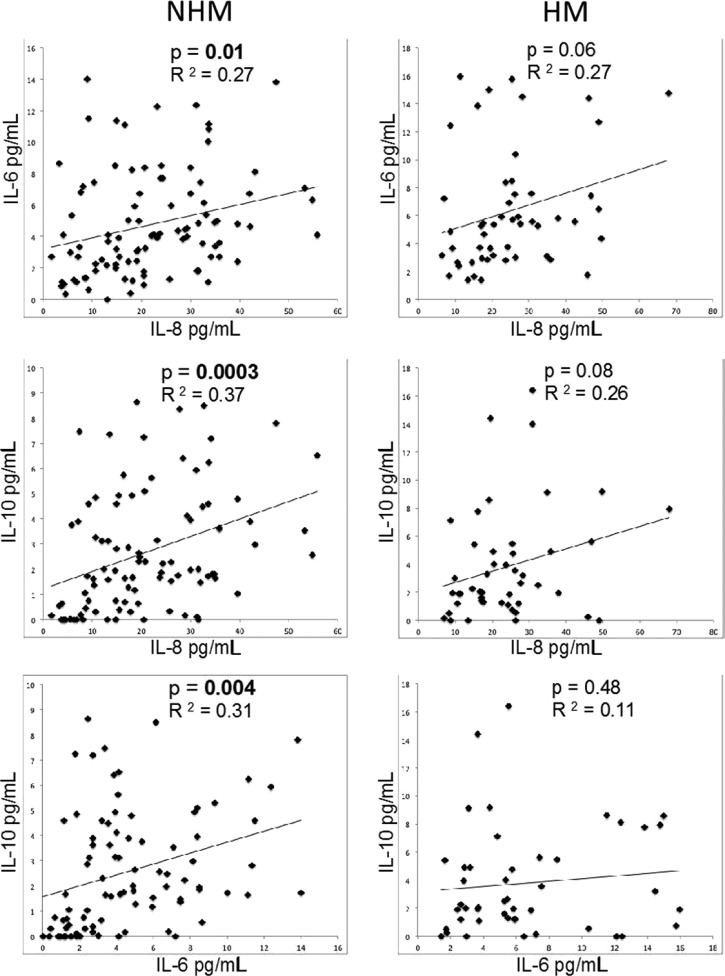
To further study the immunoregulatory mechanisms that operate in HM and NHM patients, correlations were performed between inflammatory and anti-inflammatory cytokines within each clinical group. The two inflammatory cytokines, IL-6 and IL-8 demonstrated a positive correlation in the NHM group (R2 = 0.27, P = 0.01) and a similar correlation in the HM group, but with a P value that did not reach our cutoff for significance of 0.05 (R2 = 0.27, P = 0.06) (Figure 5, top row). Interestingly, the NHM group showed a significant correlation for the production of the inflammatory cytokines IL-6 (R2 = 0.31, P = 0.004) and IL-8 (R2 = 0.37, P = 0.0003) when compared with the anti-inflammatory cytokine IL-10 (Figure 5, left column), whereas the HM group did not show this positive correlation between the inflammatory cytokines (IL-6 and IL-8) and anti-inflammatory cytokine, IL-10 (Figure 5, right column).
Figure 5.
Patients with hemorrhagic manifestations (HM) lost the coregulated expression of anti-inflammatory (interleukin-10 [IL-10]) and inflammatory (IL-6 or IL-8) cytokines. Correlations between IL-6, IL-8, and IL-10 cytokines in the groups of patients with hemorrhagic manifestations (HM—right column) and with no hemorrhagic manifestations (NHM—Left column) were performed. Each point represents the measurements for the two indicated cytokines for a given individual. The data was analyzed using Spearman's correlation coefficient with statistical significance at P < 0.05. Significant results are in bold.
DISCUSSION
This study integrated the profile of serum levels of cytokines from individuals with acute DENV infection with viral load, serotype, and dengue hemorrhagic manifestation parameters in an attempt to better understand the immunoregulatory events associated with less and more severe forms of early DENV infection. Moreover, it is hoped that this study can contribute to the discovery of possible biomarkers of disease severity.
Several studies have been conducted to identify clinical patterns and laboratory predictors of dengue severity correlating them with the viral load.31,32 We observed a significant increase in viral RNA quantified in HM group as compared with the NHM group. These data are consistent with literature data,4,31,32 that showed a higher viral load in individuals with DHF when compared with patients with DF, suggesting a correlation between high viral load and the occurrence of HM. The viral load range quantified in our study were also compatible with the data observed by other authors.27,33 Interestingly, our studies were performed in the very early stages of disease (days 1–4 after the onset of symptoms) and the groups were defined by the presence of HM rather than DHF versus DF. This indicates that even amongst individuals with early HM, there is a bias toward higher viral loads.
To investigate whether a particular serotype was correlated with distinct viral load and disease progression, we compared the viral load and HM of different serotypes. The DENV-1 had higher viral load than DENV-2, and there was no difference in relation to DENV-3. These data are insufficient to confirm a direct influence of the serotypes on viral load. However, 43% of individuals infected with DENV-1 had HM, whereas only 27% of individuals infected with serotype DENV-2 or DENV-3 had HM (P = 0.08, comparing DENV-1 to DENV-2, or DENV-1 to DENV-3). The data imply that the differences between serotypes and viral load can contribute to determine disease progression.4,9,34
In our study, we examined the levels of immunoregulatory cytokines IL-6, IL-8, and IL-10 to gain greater insights into possible roles for these cytokines in the earliest phases of disease development, between days 1 and 4 of the onset of symptoms.
IL-6 and IL-8 are significantly involved in the inflammatory response to infection. IL-6 is an intercellular signaling molecule, traditionally associated with the control and coordination of the immune response, and secreted primarily by macrophages and lymphocytes in response to injury or infection.35,36 IL-8 has important effector functions such as the ability of activation and recruitment of neutrophils,37 attraction of natural killer cells, basophilic T cells, and some types of eosinophils to sites of infection.38 High levels of IL-8 have been linked to pleural spill during different infectious processes.13 IL-10 is an anti-inflammatory cytokine involved in the regulation of various inflammatory processes.35
The immunological profile showed a significant increase in the levels of IL-6, IL-8, and IL-10 in the DENV-positive group (P < 0.05), in agreement with previous studies.3,13,39 All serotypes showed increased plasma levels of IL-6, IL-8, and IL-10. Patients with severe forms of dengue present high levels of circulating pro-inflammatory cytokines leading to endothelial activation and vascular leak with hemorrhage and shock. The levels of IL-6 are significantly higher in severe forms of dengue.6,21,39
Higher levels of IL-10 were found in samples positive for DENV-3 than for DENV-1 and DENV-2. These differences suggest that viral serotypes may have distinct host–pathogen interactions, which could imply changes in disease progression. This adds to the concept that different serotypes can induce distinct inflammatory responses due to their unique antigenic variation.4,40
The imbalanced and deregulated cell-mediated immunity is a pivotal component in DENV infection.41 Activation of T lymphocytes leads to the production of pro-inflammatory cytokines (i.e., TNF-α, IFN-γ) that may be pathogenic in the context of excessive T-cell activation, which is commonly observed in severe dengue.6 However, in our studies, we examined a window in time only 1–4 days after the onset of symptoms, when the innate response might play a more crucial role in generating the immune cytokine profile.
Cytokine analysis showed higher serum levels of IL-6 (P < 0.05) in the HM group. IL-8 and IL-10 showed different levels only when comparing infected and control groups, but not among those infected (HM and NHM). Therefore, it is suggested that, unlike IL-8 and IL-10, IL-6 cytokine may be related to the most serious clinical stage of this disease, which was also evidenced by Levy and others39 and may be a good biomarker candidate of dengue severity. Additional studies are needed to confirm whether high serum levels of IL-6 can be used as a biomarker to predict the evolution to severe dengue.
The balance between inflammation and anti-inflammation is critical for infection control.22 It has been increasingly recognized that the inflammatory response and the production of regulatory cytokines play key roles in the development of severe clinical manifestations, for which the rapid increase in cytokine levels seems to be a predominant factor in disease severity.3,42 Inflammatory cytokines help fight the virus and anti-inflammatory cytokines such as IL-10 can avoid excessive inflammation (usually damaging the host organism).35 Increased levels of serum IL-10 may be a useful prognostic hallmark in DHF/DSS patients. Aberrant IL-10 expression may also be involved in DENV pathogenesis, particularly for DENV infection/replication under antibody-dependent enhancement as demonstrated in vitro.23 Correlation analyses were performed between inflammatory (IL-6 and IL-8) and anti-inflammatory (IL-10) cytokines in both groups (HM and NHM) to identify the response profile of HM and NHM groups. Interestingly, the NHM group demonstrated a significant correlation with the production of both inflammatory cytokines, and also with the production of the regulatory cytokine IL-10, which was not observed with the HM group. These data suggest that the intensification of the disease (with the appearance of HM) may be due to the loss of the fine balance required between pro- and anti-inflammatory immune responses.
In conclusion, the imbalance in the serum levels of IL-6, IL-8, and IL-10 observed in the HM group, may have added to the high levels of IL-6 and viral load, which in turn could explain, in part, the cellular and molecular mechanisms of disease progression toward HM. Furthermore, the imbalance can be seen as an alert to the risk of disease progression toward HM. The identification of altered levels of cytokines in DENV infection correlated with clinical characteristics of patients presented herein reveal a potential prognostic marker of clinical disease, and can help to identify key targets for immunotherapy.
Footnotes
Financial support: This work was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), the Fundação Ezequiel Dias (FUNED), Instituto Mario Penna, and CNPq, INCT-DT/CNPq.
Authors' addresses: Felipe Campos de Melo Iani, Myrian Morato Duarte, and Ana Luisa Furtado Cury, Divisão de Epidemiologia e Controle de Doenças/Instituto Octávio Magalhães (IOM), Fundação Ezequiel Dias (FUNED), Belo Horizonte, Brazil, E-mails: felipe.iani@funed.mg.gov.br, myrian.duarte@funed.mg.gov.br, and ana.cury@funed.mg.gov.br. Sérgio Caldas and Alzira Batista Cecílio, Divisão de Plataformas Tecnológicas/Diretoria de Pesquisa e Desenvolvimento, Fundação Ezequiel Dias (FUNED), Belo Horizonte, Brazil, E-mails: sergio.caldas@funed.mg.gov.br and alzira.cecilio@funed.mg.gov.br. Pedro Augusto Carvalho Costa and Lis R. Antonelli, Centro de Pesquisas René Rachou (CPqRR), Fundação Oswaldo Cruz (FIOCRUZ), Belo Horizonte, Brazil, E-mails: costa@cpqrr.fiocruz.br and lisantonelli@cpqrr.fiocruz.br. Kenneth J. Gollob, Instituto de Ensino e Pesquisa, Hospital Santa Casa; Institutos Nacionais de Ciências e Tecnologia-Doenças Tropicais; Núcleo de Ensino e Pesquisa, Instituto Mario Penna, Belo Horizonte, Minas Gerais, Brazil, Belo Horizonte, Minas Gerais, Brazil, E-mail: kjgollob@gmail.com.
References
- 1.World Health Organization and Special Programme for Research and Training in Tropical Diseases . Geneva, Switzerland: 2009. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control–New edition.http://www.ncbi.nlm.nih.gov/books/NBK143154/ PREFACE. Available at. [PubMed] [Google Scholar]
- 2.Chen R, Vasilakis N. Dengue: Quo tu et quo vadis? Viruses. 2011;3:1562–1608. doi: 10.3390/v3091562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clyde K, Kyle JL, Harris E. Recent advances in deciphering viral and host determinants of dengue virus replication and pathogenesis. J Virol. 2006;80:11418–11431. doi: 10.1128/JVI.01257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, Nisalak A. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 5.OhAinle M, Balmaseda A, Macalalad AR, Tellez Y, Zody MC, Saborío S, Nuñes A, Lennon NJ, Birren BW, Gordon A, Henn MR, Harris E. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci Transl Med. 2011;3:114ra128. doi: 10.1126/scitranslmed.3003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guabiraba R, Ryffel B. Dengue virus infection: current concepts in immune mechanisms and lessons from murine models. Immunology. 2014;141:143–156. doi: 10.1111/imm.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deen JL, Harris E, Wills B, Balmaseda A, Hammond SN, Rocha C, Dung NM, Hung NT, Hien TT, Farrar JJ. The WHO dengue classification and case definitions: time for a reassessment. Lancet. 2006;368:170–173. doi: 10.1016/S0140-6736(06)69006-5. [DOI] [PubMed] [Google Scholar]
- 8.Phuong CX, Nhan NT, Kneen R, Thuy PT, Van Thien C, Nga NT, Thuy TT, Solomon T, Stepniewska K, Wills B. Clinical diagnosis and assessment of severity of confirmed dengue infections in Vietnamese children: is the World Health Organization classification system helpful? Am J Trop Med Hyg. 2004;70:172–179. [PubMed] [Google Scholar]
- 9.Balmaseda A, Hammond SN, Perez L, Tellez Y, Saborio SI, Mercado C, Cuadra R, Rocha J, Pérez MA, Silva S, Rocha C, Harris E. Serotype-specific differences in clinical manifestations of dengue. Am J Trop Med Hyg. 2006;74:449–456. [PubMed] [Google Scholar]
- 10.Bandyopadhyay S, Lum LC, Kroeguer A. Classifying dengue: a review of the difficulties in using the WHO case classification for dengue haemorrhagic fever. Trop Med Int Health. 2006;11:1238–1255. doi: 10.1111/j.1365-3156.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- 11.Rigau-Pérez JG. Severe dengue: the need for new case definitions. Lancet Infect Dis. 2006;6:297–302. doi: 10.1016/S1473-3099(06)70465-0. [DOI] [PubMed] [Google Scholar]
- 12.Narvaez F, Gutierrez G, Perez MA, Elizondo D, Nuñez A, Balmaseda A, Harris E. Evaluation of the traditional and revised WHO classifications of dengue disease severity. PLoS Negl Trop Dis. 2011;5 doi: 10.1371/journal.pntd.0001397.e1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghupathy R, Chaturvedi UC, Al-Sayer H, Elbishbishi EA, Agarwal R, Nagar R, Kapoor S, Misra A, Mathur A, Nusrat H, Azizieh F, Khan MA, Mustafa AS. Elevated levels of IL-8 in dengue hemorrhagic fever. J Med Virol. 1998;56:280–285. doi: 10.1002/(sici)1096-9071(199811)56:3<280::aid-jmv18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Lew R, Innis BL, Kurane I, Rothman AL, Ennis FA. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J Infect Dis. 1999;179:755–762. doi: 10.1086/314680. [DOI] [PubMed] [Google Scholar]
- 15.Kittigul L, Temprom W, Sujirarat D, Kittigul C. Determination of tumor necrosis factor-alpha levels in dengue virus infected patients by sensitive biotin-streptavidin enzyme-linked immunosorbent assay. J Virol Methods. 2000;90:51–57. doi: 10.1016/s0166-0934(00)00215-9. [DOI] [PubMed] [Google Scholar]
- 16.Fink JG, Vasudevan SG. Role of T cells, cytokines and antibody in dengue fever and dengue hemorrhagic fever. Rev Med Virol. 2006;16:263–275. doi: 10.1002/rmv.507. [DOI] [PubMed] [Google Scholar]
- 17.Bozza FA, Cruz OG, Zagne SM, Azeredo EL, Nogueira RM, Assis EF, Bozza PT, Kubelka CF. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect Dis. 2008;8:86. doi: 10.1186/1471-2334-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rachman A, Rinaldi I. Coagulopathy in dengue infection and the role of interleukin-6. Acta Med Indones. 2006;38:105–108. [PubMed] [Google Scholar]
- 19.Lee YR, Liu MT, Lei HY, Liu CC, Wu JM, Tung YC, Lin YS, Yeh TM, Chen SH, Liu HS. MCP-1, a highly expressed chemokine in dengue haemorrhagic fever/dengue shock syndrome patients, may cause permeability change, possibly through reduced tight junctions of vascular endothelium cells. J Gen Virol. 2006;87:3623–3630. doi: 10.1099/vir.0.82093-0. [DOI] [PubMed] [Google Scholar]
- 20.Talavera D, Castillo AM, Dominguez MC, Gutierrez AE, Meza I. IL8 release, tight junction and cytoskeleton dynamic reorganization conducive to permeability increase are induced by dengue virus infection of microvascular endothelial monolayers. J Gen Virol. 2004;85:1801–1813. doi: 10.1099/vir.0.19652-0. [DOI] [PubMed] [Google Scholar]
- 21.Priyadarshini D, Gadia RR, Tripathy A, Gurukumar KR, Bhagat A, Patwardhan S, Mokashi N, Vaidya D, Shah PS, Cecilia D. Clinical findings and pro-inflammatory cytokines in dengue patients in western India: a facility-based study. PLoS One. 2010;5:e8709. doi: 10.1371/journal.pone.0008709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai T, Chuang Y, Lin Y, Wan S, Chen C, Lin C. An emerging role for the anti-inflammatory cytokine interleukin-10 in dengue virus infection. J Biomed Sci. 2013;20:40. doi: 10.1186/1423-0127-20-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ubol S, Halstead SB. How innate immune mechanisms contribute to antibody-enhanced viral infections. Clin Vaccine Immunol. 2010;17:1829–1835. doi: 10.1128/CVI.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srikiatkhachorn A, Green S. Markers of dengue disease severity. Curr Top Microbiol Immunol. 2010;338:67–82. doi: 10.1007/978-3-642-02215-9_6. [DOI] [PubMed] [Google Scholar]
- 25.Kumar Y, Liang C, Bo Z, Rajapakse JC, Ooi EE, Tannenbaum SR. Serum proteome and cytokine analysis in a longitudinal cohort of adults with primary dengue infection reveals predictive markers of DHF. PLoS Negl Trop Dis. 2012;6:e1887. doi: 10.1371/journal.pntd.0001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarman RG, Nisalak A, Anderson KB, Klungthong C, Thaisomboonsuk B, Kaneechit W, Kalayanarooj S, Gibbons RV. Factors influencing dengue virus isolation by C6/36 cell culture and mosquito inoculation of nested PCR-positive clinical samples. Am J Trop Med Hyg. 2011;84:218–223. doi: 10.4269/ajtmh.2011.09-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurukumar KR, Priyadarshini D, Patil JA, Bhagat A, Singh A, Shah PS, Cecilia D. Development of real time PCR for detection and quantitation of dengue viruses. Virol J. 2009;6:10. doi: 10.1186/1743-422X-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization (WHO) CDC protocol of realtime RTPCR for Swine Influenza A(H1N1). Version 2009. 2009. http://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf World Health Organization, Atlanta, GA. Available at. Accessed April 15, 2016.
- 29.Caldas S, Caldas IS, Diniz LF, Lima WG, Oliveira RP, Cecílio AB, Ribeiro I, Talvania A, Bahia MT. Real-time PCR strategy for parasite quantification in blood and tissue samples of experimental Trypanosoma cruzi infection. Acta Trop. 2012;123:170–177. doi: 10.1016/j.actatropica.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Cecílio AB, Caldas S, Oliveira RA, Santos ASB, Richardson M, Naumann GB, Schneider FS, Alvarenga VG, Estevão-Costa MI, Fuly AL, Eble JA, Sanchez EF. Molecular characterization of Lys49 and Asp49 phospholipases A2 from snake venom and their antiviral activities against dengue virus. Toxins. 2013;5:1780–1798. doi: 10.3390/toxins5101780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, Rothman AL, Libraty DH. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189:990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
- 32.Wang WK, Chen HL, Yang CF, Hsieh SC, Juan CC, Chang SM, Yu CC, Lin LH, Huang JH, King CC. Slower rates of clearance of viral load and virus-containing immune complexes in patients with dengue hemorrhagic fever. Clin Infect Dis. 2006;43:1023–1030. doi: 10.1086/507635. [DOI] [PubMed] [Google Scholar]
- 33.Libraty DH, Acosta LP, Tallo V, Segubre-Mercado E, Bautista A, Potts JA, Jarman RG, Yoon IK, Gibbons RV, Brion JD, Capeding RZ. A prospective nested case-control study of dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med. 2009;6:e1000171. doi: 10.1371/journal.pmed.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guilarde AO, Turchi MD, Siqueira JB, Feres VC, Rocha B, Levi JE, Souza VA, Boas LS, Pannuti CS, Martelli CM. Dengue and dengue hemorrhagic fever among adults: clinical outcomes related to viremia, serotypes, and antibody response. J Infect Dis. 2008;197:817–824. doi: 10.1086/528805. [DOI] [PubMed] [Google Scholar]
- 35.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–746. doi: 10.1016/j.jaci.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 36.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, Appella E, Kung HF, Leonard EJ, Oppenheim JJ. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988;167:1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burke SM, Issekutz TB, Mohan K, Lee PW, Shmulevitz M, Marshall JS. Human mast cell activation with virus-associated stimuli leads to the selective chemotaxis of natural killer cells by a CXCL8-dependent mechanism. Blood. 2008;111:5467–5476. doi: 10.1182/blood-2007-10-118547. [DOI] [PubMed] [Google Scholar]
- 39.Levy A, Valero N, Espina LM, Añez G, Arias J, Mosquera J. Increment of interleukin 6, tumour necrosis factor alpha, nitric oxide, C-reactive protein and apoptosis in dengue. Trans R Soc Trop Med Hyg. 2010;104:16–23. doi: 10.1016/j.trstmh.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 40.Lanciotti RS, Gubler DJ, Trent DW. Molecular evolution and phylogeny of dengue-4 viruses. J Gen Virol. 1997;78:2279–2284. doi: 10.1099/0022-1317-78-9-2279. [DOI] [PubMed] [Google Scholar]
- 41.Costa VV, Fagundes CT, Souza DG, Teixeira MM. Inflammatory and innate immune responses in dengue infection: protection versus disease induction. Am J Pathol. 2013;182:1950–1961. doi: 10.1016/j.ajpath.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 42.Chaturvedi UC. The curse of dengue. Indian J Med Res. 2006;124:467–470. [PubMed] [Google Scholar]