Abstract
Wild birds serve as amplifying hosts for many arboviruses, and are thought to be responsible for introducing these viruses into new areas during migration as well as reintroducing them to places where winter temperatures disrupt mosquito-borne transmission. To learn more about four mosquito-borne arboviruses of concern to human or animal health, we tested sera from 997 wild birds of 54 species and 17 families across 44 states of the United States collected from January 1, 2013, through September 30, 2013. Samples were tested for antibody against eastern equine encephalitis, St. Louis encephalitis, West Nile, and Turlock viruses using plaque reduction neutralization tests with an endpoint of 80% or greater. Of the 333 (33.4%) birds that tested positive for antibody to at least one arbovirus, 29.7% were exposed to two or more arboviruses. Exposure to all four arboviruses was detected in Canada geese, double-crested cormorants, mallards, mute swans, laughing gulls, and American coots. Our results suggest that exposure to arboviruses is widespread in the United States across a diversity of wild bird species.
Introduction
Although wild birds are not responsible for directly transmitting arboviruses to humans, they can serve as amplifying hosts, and can transport these viruses during migration to new geographic areas.1 Wild birds may also serve as indicators of the presence and severity of outbreaks of arboviruses in certain areas, which can be helpful for predicting these events in humans and domestic animals. This is important because encephalitic mosquito-borne arboviruses such as eastern equine encephalitis virus (EEEV), West Nile virus (WNV), and St. Louis encephalitis virus (SLEV) can cause severe disease in humans and domestic animals.2,3 EEEV (family Togaviridae, genus Alphavirus) occurs in North and South America and periodically causes severe disease in humans, horses, and exotic gamebirds, which results in high mortality or severe neurologic impairment in most survivors.4,5 WNV (family Flaviviridae, genus Flavivirus) is considered the most widely distributed arbovirus in the world and can be associated with flu-like symptoms or viral encephalitis and neurological disease as well as mortality in humans6 and high mortality in corvids3,7 and other bird species native to North America.8 SLEV (family Flaviviridae, genus Flavivirus) is another important flavivirus that is distributed throughout most of the western hemisphere and although rare, can cause fever, meningitis, encephalitis, or mortality in humans.9 Unlike WNV, SLEV does not cause mortality in birds.10 Turlock virus (TURV) (family Bunyaviridae) can infect birds including domestic poultry, but does not infect humans, which is likely why little is known about potential reservoir hosts and therefore transmission dynamics of this arbovirus.11 The virus has been isolated from passerines11 and various species of forest birds,12 and exposure to the virus has been documented in mute swans (Cygnus olor),13 various gallinaceous species including chickens,11 as well as mallards (Anas platyrhynchos) and greylag geese (Anser anser).14 Our objective was to examine a wide range of wild bird species for antibodies to four mosquito-borne arboviruses to better understand the potential for wild birds to serve as indicators of previous virus activity that may have implications on humans, domestic animals, or bird health.
MATERIALS AND METHODS
Sample collection.
The U.S. Department of Agriculture's (USDA), Animal and Plant Health Inspection Service, Wildlife Services (WS) relocates and removes wild birds from airports and other locations that pose a threat to human health and safety. The National Wildlife Disease Program (a branch of WS), in addition to actively live-capturing and sampling wild birds, opportunistically utilizes this program to obtain samples from various wild bird species for pathogen surveillance and to archive samples for future research. Trapping methods, species selected for sampling, and geographic distribution are left to the discretion of the wildlife biologists within each state. Through this program, blood samples were collected from live birds via the jugular, brachial, or metatarsal vein, or via postmortem intracardiac puncture from euthanized birds; it was then centrifuged at 1,500 rpm for 15 minutes and subsequently ≥ 0.5 mL of serum was transferred to 2-mL cryogenic vials. Samples were refrigerated and then shipped with ice packs within 3 days to the National Wildlife Disease Program where they were stored in the National Wildlife Serum Archive in a −80°C freezer.
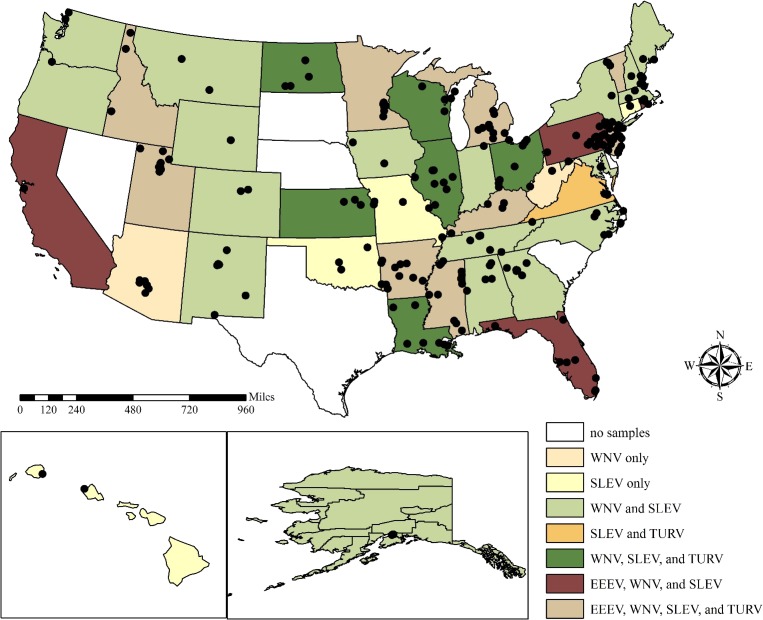
We selected 997 serum samples from the National Wildlife Serum Archive for this project that were originally collected from wild birds from across the United States from January 1, 2013, through September 30, 2013 (Figure 1, Supplemental Table 1). Priority was given to samples collected in counties where EEEV, SLEV, and WNV activity is regularly reported (http://diseasemaps.usgs.gov/mapviewer/), and representative samples were selected from the remaining counties available in the archive. All samples were tested for TURV to expand the current list of avian species known to be infected. Bird-specific (sex, age) and collection site information (county, state, and Global Positioning System coordinates) were available for each sample.
Figure 1.
Sites where wild bird serum samples were collected from January 1, 2013, through September 30, 2013, with exposure by state to eastern equine encephalitis virus (EEEV), West Nile virus (WNV), St. Louis encephalitis virus (SLEV), and Turlock virus (TURV) tested by plaque reduction neutralization test.
Serology testing.
All sera were tested at a 1:20 dilution by plaque reduction neutralization test to determine reciprocal endpoint 80% neutralization (PRNT80) titers for each virus (EEEV, SLEV, WNV, and TURV). Virus strains used to determine antibody titers were WNV (strain 385—isolated from the liver of a snowy owl in New York, 1999; Vero cell passage no. 2), SLEV (strain TBH-28—isolated from a human in Florida, 1962; sucking mouse passage no. 1 and Vero cell passage no. 1), TURV (strain USA 847-32—isolated from a Culex spp. mosquito in California, 1954; Vero cell passage no. 2), and a chimeric SIN/EEEV vaccine strain—its nonstructural protein genes derived from Sindbis virus strain AR339 and structural protein genes from EEEV strain FL93-939 (baby hamster kidney cells passage no. 1 and Vero cell passage no. 1).15 Samples with PRNT80 titers ≥ 20 were considered positive and were titrated to 1:640 to determine the antibody titer against each virus as previously described.16 Results for sera that showed neutralizing activity against two closely related flaviviruses (e.g., WNV and SLEV) were considered inconclusive unless the titer was 4-fold higher for one virus than the other. Testing was performed at the University of Texas Medical Branch in Galveston, TX.
Analysis.
Mean apparent seroprevalence values were calculated with 95% confidence limits (CL) for all samples using a normal approximation for proportional data. A χ2 test was used to assess the association between age class and gender, and prevalence and season. All calculations were conducted in Microsoft Excel™ (Redmond, WA).
RESULTS
We tested samples from 997 wild birds of 54 species and 17 families across 170 counties in 44 states in the United States (Figure 1), of which 33.4% were antibody positive for at least one of the arboviruses examined. The majority of the wild birds sampled (72.8%) were after-hatch-year birds, followed by undetermined (15.8%) and hatch year (11.3%) birds. Sex was undetermined for the majority (77%) of the birds, but the remainder was comprised equally of males and females (11.5% each). Though we examined associations between age class and gender, there were no apparent patterns for any of the pathogens (data not shown).
Of 54 species of the wild birds, there were 39 species for which we detected at least one seropositive sample for one or more of the viruses examined (Tables 1 – 3 ). Of the 333 birds that tested antibody positive for at least one arbovirus, 29.7% had been exposed to two or more arboviruses. Antibody-positive samples were collected during every month of the study (January through September; data not shown), and no seasonal patterns of infection were identified. There were no evident patterns of apparent prevalence for each pathogen by avian family (Table 4 ). Exposure to all four arboviruses was detected in American coots (Fulica americana), Canada geese, double-crested cormorants, laughing gulls (Leucophaeus atricilla), mallards, and mute swans (Tables 1–3).
Table 1.
Seroprevalence for EEEV with 95% CL of wild bird species for which at least one bird tested antibody positive by plaque reduction neutralization test
| Species (n) | % Positive (95% CL) |
|---|---|
| Canada goose (Branta candensis) (366) | 1.1 (0.4–2.8) |
| Mallard (Anas platyrhynchos) (58) | 3.5 (1.0–11.7) |
| Mute swan (Cygnus olor) (90) | 5.6 (2.4–12.4) |
| Wood duck (Aix sponsa) (4) | 25.0 (4.6–70.0) |
| Common raven (Corvus corax) (3) | 33.3 (6.2–79.2) |
| Laughing gull (Leucophaeus atricilla) (19) | 5.3 (0.9–24.6) |
| Double-crested cormorant (Phalacrocorax auritus) (79) | 2.5 (0.7–8.8) |
| American coot (Fulica americana) (12) | 16.7 (4.7–44.8) |
| White ibis (Eudocimus albus) (6) | 33.3 (9.7–70.0) |
CL = confidence limits; EEEV = eastern equine encephalitis virus.
Table 3.
Seroprevalence for TURV with 95%CL of wild bird species for which one bird tested antibody positive by plaque reduction neutralization test
| Species (n) | % (95% CL) |
|---|---|
| Canada goose (Branta candensis) (366) | 1.4 (0.6–3.2) |
| Mallard (Anas platyrhynchos) (58) | 3.5 (1.0–11.7) |
| Mute swan (Cygnus olor) (90) | 2.2 (0.6–7.7) |
| Tundra swan (Cygnus columbianus) (1) | 100 (20.7–100) |
| Great blue heron (Ardea herodias) (6) | 16.7 (3.0–56.4) |
| Great egret (Ardea alba) (3) | 66.7 (20.8–93.9) |
| Black-crowned night heron (Nycticorax nycticorax) (3) | 100 (43.9–100) |
| Rock pigeon (Columba livia) (156) | 1.9 (0.7–5.5) |
| Laughing gull (Leucophaeus atricilla) (19) | 5.3 (0.9–24.6) |
| Ring-billed gull (Larus delawarensis) (35) | 5.7 (1.6–18.6) |
| Double-crested cormorant (Phalacrocorax auritus) (79) | 11.4 (6.1–20.3) |
| American coot (Fulica americana) (12) | 25.0 (8.9–53.2) |
CL = confidence limits; TURV = Turlock virus.
Table 4.
Seroprevalence for EEEV, WNV, SLEV, or TURV with 95% CL of wild bird family for which at least one bird tested antibody positive by plaque reduction neutralization test
| Family* (no. of samples tested) | EEEV % (95% CL) | WNV % (95% CL) | SLEV % (95% CL) | TURV % (95% CL) |
|---|---|---|---|---|
| Accipitridae (6) | 0 (0–39.0) | 33.3 (9.7–70.0) | 16.7 (3.0–56.4) | 0 (0–39.0) |
| Anatidae (539) | 2.2 (1.3–3.9) | 14.7 (11.9–17.9) | 15.4 (12.6–18.7) | 1.9 (1.0–3.4) |
| Ardeidae (12) | 0 (0–24.3) | 58.3 (32.0–80.7) | 33.3 (13.8–60.9) | 50 (25.4–74.6) |
| Cathartidae (11) | 0 (0–25.9) | 9.1 (1.6–37.7) | 0 (0–25.9) | 0 (0–25.9) |
| Columbidae (178) | 0 (0–2.1) | 19.7 (14.5–26.1) | 29.8 (23.5–36.9) | 1.7 (0.6–4.8) |
| Corvidae (11) | 9.1 (1.6–37.7) | 54.6 (28.0–78.7) | 36.4 (15.2–64.6) | 0 (0–25.9) |
| Emberizidae (1) | 0 (0–79.4) | 0 (0–79.4) | 100 (20.7–100) | 0 (0–79.4) |
| Falconidae (1) | 0 (0–79.4) | 100 (20.7–100) | 100 (20.7–100) | 0 (0–79.4) |
| Gruidae (4) | 0 (0–49.0) | 0 (0–49.0) | 75 (30.1–95.4) | 0 (0–49.0) |
| Icteridae (6) | 0 (0–39.0) | 66.7 (30.0–90.3) | 50 (18.8–81.2) | 0 (0–39.0) |
| Laniidae (1) | 0 (0–79.4) | 100 (20.7–100) | 100 (20.7–100) | 0 (0–79.4) |
| Laridae (83) | 1.2 (0.2–6.5) | 25.3 (17.2–35.6) | 22.9 (15.2–33.0) | 3.6 (1.2–10.1) |
| Phalacrocoracidae (79) | 2.5 (0.7–8.8) | 31.7 (22.5–42.6) | 20.3 (12.9–30.4) | 11.4 (6.1–20.3) |
| Phasianidae (10) | 0 (0–27.8) | 70 (39.7–89.2) | 10 (1.8–40.4) | 0 (0–27.8) |
| Rallidae (12) | 16.7 (4.7–44.8) | 33.3 (13.8–60.9) | 33.3 (13.8–60.9) | 25 (8.9–53.2) |
| Sturnidae (32) | 0 (0–10.72) | 6.3 (1.7–20.2) | 21.9 (11.0–38.8) | 0 (0–10.7) |
| Threskiornithidae (6) | 33.3 (9.7–70) | 0 (0–39.0) | 0 (0–39.0) | 0 (0–39.0) |
CL = confidence limits; EEEV = eastern equine encephalitis virus; SLEV = St. Louis encephalitis virus; TURV = Turlock virus; WNV = West Nile virus.
Samples from Charadriidae (n = 4) and Recurvirostridae (n = 1) not included (no positives).
Antibody prevalence of EEEV was 2.0% (95% CL: 1.3–3.1), with nine species of wild birds identified as antibody positive from various states (Table 1, Figure 1). Antibody prevalence of WNV was 19.6% (95% CL: 17.22–22.14), with antibody positives identified in 30 species of the wild birds across the United States (Table 2, Figure 1). Antibody prevalence of SLEV was 20.2% (95% CL: 17.79–22.76), and was identified in 31 species of the wild birds across the United States (Table 2, Supplemental Table 1). Antibody prevalence of TURV was 3.4% (95% CL: 2.45–4.73), and was identified in 12 species of the wild birds (Table 3).
Table 2.
Seroprevalence for WNV and SLEV with 95% CL of wild bird species for which at least one bird tested antibody positive by plaque reduction neutralization test
| Species (n) | WNV % (95% CL) | SLEV % (95% CL) |
|---|---|---|
| Red-tailed hawk (Buteo jamaicensis) (6) | 33.3 (9.7–70.0) | 16.7 (3.0–56.4) |
| American green-winged teal (Anas crecca) (3) | 0 (0–56.2) | 66.7 (20.8–93.9) |
| Blue-winged teal (Anas discors) (4) | 0 (0–49.0) | 50.0 (15.0–85.0) |
| Canada goose (Branta candensis) (366) | 9.6 (7.0–13.0) | 11.2 (8.4–14.8) |
| Lesser scaup (Aytha affinis) (2) | 100 (34.2–100) | 0 (0–65.8) |
| Lesser snow goose (Chen caerulenscens) (1) | 100 (20.7–100) | 100 (20.7–100) |
| Mallard (Anas platyrhynchos) (58) | 20.7 (12.3–32.8) | 22.4 (13.6–34.7) |
| Mute swan (Cygnus olor) (90) | 26.7 (18.6–36.6) | 24.4 (16.7–34.3) |
| Northern shoveler (Anas clypeata) (3) | 33.3 (6.2–79.2) | 33.3 (6.2–79.2) |
| Ruddy duck (Oxyura jamaicensis) (1) | 100 (20.7–100) | 0 (0–79.4) |
| Tundra swan (Cygnus columbianus) (1) | 100 (20.7–100) | 100 (20.7–100) |
| Wood duck (Aix sponsa) (4) | 50.0 (15.0–85.0) | 0 (0–49.0) |
| Great blue heron (Ardea herodias) (6) | 16.7 (3.0–56.4) | 33.3 (9.7–70.0) |
| Great egret (Ardea alba) (3) | 100 (43.9–100) | 33.3 (6.2–79.2) |
| Black-crowned night heron (Nycticorax nycticorax) (3) | 100 (43.9–100) | 33.3 (6.2–79.2) |
| Turkey vulture (Cathartes aura) (7) | 14.3 (2.6–51.3) | 0 (0–35.4) |
| Mourning dove (Zenaida macroura) (13) | 23.1 (8.2–50.3) | 7.7 (1.4–33.3) |
| Rock pigeon (Columba livia) (156) | 20.5 (14.9–27.5) | 32.1 (25.2–39.7) |
| Spotted dove (Streptopelia chinensis) (5) | 0 (0–43.5) | 40.0 (11.8–76.9) |
| American crow (Corvus brachyrhynchos) (5) | 80.0 (37.6–96.4) | 60.0 (23.1–88.2) |
| Common raven (Corvus corax) (3) | 0 (0–56.2) | 33.3 (6.2–79.2) |
| Black-billed magpie (Pica hudsonia) (2) | 50.0 (9.5–90.6) | 0 (0–65.8) |
| Fish crow (Corvus ossifragus) (1) | 100 (20.7–100) | 0 (0–79.4) |
| Lark bunting (Calamospiza melanocorys) (1) | 0 (0–79.4) | 100 (20.7–100) |
| American kestrel (Falco sparverius) (1) | 100 (20.7–100) | 100 (20.7–100) |
| Sandhill crane (Grus canadensis) (4) | 0 (0–49.0) | 75.0 (30.1–95.4) |
| Brown-headed cowbird (Molothrus ater) (1) | 100 (20.7–100) | 100 (20.7–100) |
| Great-tailed grackle (Quiscalus mexicanus) (3) | 100 (43.9–100) | 66.7 (20.8–93.9) |
| Northern shrike (Lanius excubitor) (1) | 100 (20.7–100) | 100 (20.7–100) |
| Great black-backed gull (Larus marinus) (9) | 0 (0–29.9) | 44.4 (18.9–73.3) |
| Herring gull (Larus argentatus) (13) | 7.7 (1.4–33.3) | 7.7 (1.4–33.3) |
| Laughing gull (Leucophaeus atricilla) (19) | 36.8 (19.2–59.0) | 21.1 (8.5–43.3) |
| Ring-billed gull (Larus delawarensis) (35) | 37.1 (23.2–53.7) | 28.6 (16.3–45.1) |
| Double-crested cormorant (Phalacrocorax auritus) (79) | 31.7 (22.5–42.6) | 20.3 (12.9–30.4) |
| Gray partridge (Perdix perdix) (3) | 33.3 (6.2–79.2) | 33.3 (6.2–79.2) |
| Wild turkey (Meleagris gallopavo) (7) | 85.7 (48.7–97.4) | 0 (0–35.4) |
| American coot (Fulica americana) (12) | 33.3 (13.8–60.9) | 33.3 (13.8–60.9) |
| European starling (Sturnus vulgaris) (30) | 6.7 (1.9–21.3) | 23.3 (11.8–40.9) |
CL = confidence limits; SLEV = St. Louis encephalitis virus; WNV = West Nile virus.
DISCUSSION
Although Canada geese, double-crested cormorants, mallards, mute swans, laughing gulls, and American coots were the only species in which seropositivity against all four arboviruses was detected (Tables 1–3), this is likely because they were also the species for which the highest numbers of samples were tested. Although we tested a wide variety of wild birds, the distribution was skewed toward the family Anatidae (Table 4). Because these species are often associated with urban and aquatic environments such as marshes, bogs, and sloughs, which are also prime mosquito habitats, a relatively high level of arbovirus exposure is not surprising. For transmission involving avian amplification to be efficient, the susceptible wild bird host must not only develop viremia titers sufficient to infect mosquito vectors, but the host and competent vector species must also overlap in time (during months when mosquitos are most active) and space (in the same geographic area at the same time).17 Canada geese and mallards have been identified as weakly competent reservoir hosts and American coots as not competent for WNV during experimental infections,18 but additional research to evaluate these species and the other three species for their competency as reservoir hosts for EEEV, SLEV, and TURV are recommended.
After the introduction of WNV to the United States in 1999, high mortality rates were observed in American crows (Corvus brachyrhynchos) both in the field7,19 and under experimental conditions.18 Mortality was estimated at 65% for one population of American crows in Stillwater, OK in 2003,20 and at a national level crow mortality was presumed underestimated since not all dead birds were reported. This extensive mortality was predicted to result in a subsequent decline in American crow populations nationally.20 The seroprevalence we observed was higher than a study conducted in the winter months of 2004–2006 that identified WNV antibodies in 16.5% of fish crows (Corvus ossifragus) and 5.7% of American crows.21
Previous studies that examined WNV in Canada geese in Georgia22 and Illinois23 reported much lower seroprevalence (1.2% in each state) than we detected (9.6%; Table 2). Although samples were collected from 2000 to 2004 in the Georgia study, no positive Canada geese were identified until 2002, with a few more detected in the remaining years of the study.22 In Illinois, despite reporting more human cases of WNV than anywhere else in the country, the prevalence of WNV in Canada geese was still very low. The apparent delay in detection was likely due to the time lapse between the initial introduction to the United States in the summer of 1999,24 and the time it took for the arbovirus to spread and become endemic. Since our study was conducted so many years after the initial detection of WNV, it is not surprising that antibody prevalence was much higher. Many of the Canada geese that we tested were sampled in urban locations, which may have been another reason for the apparent increase in prevalence in this species.
Our sample set was comprised of only eight samples from Alaska, but we identified SLEV in an American green-winged teal (Anas crecca), mallard, and sandhill crane (Grus canadensis) collected in the state, as well as both SLEV and WNV in a Canada goose and a lesser snow goose (Chen caerulescens). Although these are all migratory species and exposure likely occurred during migration since they were all sampled in the spring, this is the first report to include evidence of either of these arboviruses in wild birds in Alaska.
WNV was not detected in any of the serum samples collected in Hawaii, but this is not surprising because this virus has not been reported in Hawaii and its introduction would likely have visible impacts on native birds and human health.25 However, two spotted doves (Streptopelia chinensis) sampled at an airport in Hawaii were SLEV antibody positive, a surprise considering that spotted doves are resident birds. One possible explanation is cross-reactivity of another flavivirus, a common phenomenon among flaviviruses (e.g., SLEV or Japanese encephalitis virus).6 Interestingly, a similar detection in this same species at a Hawaiian airport was identified previously.26 Spotted doves have been introduced to other areas,27 suggesting the possibility that the two antibody-positive birds may have been introduced from another part of the world where SLEV is prevalent. Introduction of other non-native bird species to Hawaii has been well documented.28
The higher national seroprevalence of SLEV compared with WNV was also surprising, considering the latter virus has become more commonly detected in human and mosquito surveillance in the United States since its arrival (http://www.cdc.gov/sle/technical/epi.html). It also demonstrates that SLEV transmission is continuing to occur despite the apparently low number of cases reported in humans.29 Serosurveillance of wild birds is useful for better understanding the virus distribution and geographic extent by the avian hosts because humans are often asymptomatic. On a national level, SLEV occurrence was also higher than the WNV antibody prevalence, which may be due to the sporadic surveillance that occurs for arboviruses focused on certain mosquitoes collected in urban regions, with little systematic avian sampling. In this study, WNV seropositivity was high among birds throughout the country except in Hawaii, consistent with its active enzootic circulation throughout most of the North and South America.6
Although infection with EEEV has been well documented in some passerine species,5,17 the only passerine species we identified as antibody positive was a common raven (Corvus corax) in Utah. The majority (60%) of the samples that were antibody positive for EEEV were from species within the family Anatidae, which is likely because the majority of the samples tested were also from this family (Table 4). However, this may have also been due to the small sample size of passerines (N = 50), and the fact that the species composition of the passerines that were tested consisted primarily of crows and blackbirds. Another surprise finding was that EEEV seroprevalence was identified in three western states (California, Idaho, and Utah; Figure 1, Supplemental Table 1); neither enzootic circulation, nor human and veterinary cases, have been reported in the western United States aside from an equine case that is believed to have resulted from the administration of an incompletely inactivated vaccine.30 However, antibody detections primarily occurred in migratory birds (mallards, Canada goose) that likely migrated after exposure. The remaining exposure occurred in a common raven (C. corax), in which the route of exposure is unclear because ravens are resident birds. However, the true western boundary of this arbovirus may have expanded since EEEV has been reported in Texas, Oklahoma, Missouri, Arkansas, Minnesota, and Nova Scotia over the last few years (http://www.cdc.gov/EasternEquineEncephalitis/tech/epi.html#casesbystate). Also, there has been evidence that EEEV has been transported beyond its enzootic range previously, although it was transported southward.31 Further studies to redefine the western boundary of this arbovirus are recommended.
Although TURV is widely distributed from Canada south to Ecuador,32 none of the birds from Hawaii (N = 10) tested seropositive, which may be due to small sample size or because Hawaii is west of the longitudinal gradient of TURV distribution. Our national-level apparent prevalence of TURV (3.4%) was higher than another study conducted in Texas that identified an apparent prevalence of 0.4% (15 of 3,964) in house sparrows (Passer domesticus).33 Our dataset did not include any house sparrows or any samples from Texas, but it appears that, in addition to house sparrows, various species have the potential to be exposed to TURV (Table 4). The highest number of positives detected was in double-crested cormorants; this represents, to our knowledge, the first evidence of TURV infection in this species. We recommend further sampling of this species to determine whether double-crested cormorants could be important for the maintenance of this virus.
Serology studies are somewhat limited in that they often do not contribute to the understanding of the timing or location of infections, and do not allow assessment of reservoir host competence status of individuals or species. Even though other studies have reported resident birds as more likely to serve as amplifying hosts of arboviruses than migrants,32 we identified serological evidence of past infection in several migrant species, and evidence of EEEV in western states in migrating species, suggesting that further studies are warranted to assess their competence as amplifying hosts. We also identified a relatively high percentage of antibody positive birds (29.7%) with evidence of infection with multiple arboviruses, which has been reported previously.12,13 This may be because mosquitos are the vector for all of the arboviruses we examined and the risk of exposure is likely to be high in the wild birds that use habitats capable of supporting high densities of mosquitoes (e.g., wetlands). To more thoroughly understand the potential risk posed by these avian species, additional studies are recommended to examine viral shedding, especially because some individuals do not develop detectable viremia titers despite the development of antibodies.34 In addition, multi-year studies that focus on hatch year birds are recommended to ensure a more clear interpretation of serological results and their implications since exposure from May to June could be used as a predictor of human risk in subsequent months. A better understanding of the dynamics and interrelationships between wild birds and mosquitos are necessary to help predict arboviral epidemics in humans.
Supplementary Material
ACKNOWLEDGMENTS
We thank all of the wildlife biologists and technicians who participated by collecting the samples included in this article. We also thank the Centers for Disease Control and Prevention and the UTMB World Reference Center for Emerging Viruses and Arboviruses for provision of virus strains used in the serology testing.
Disclaimer: Mention of trade names or commercial products in this work is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture
Footnotes
Authors' addresses: Kerri Pedersen, U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, Fort Collins, CO, E-mail: kerri.pedersen@aphis.usda.gov. David R. Marks, U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, Okemos, MI, E-mail: david.r.marks@aphis.usda.gov. Eryu Wang, Gillian Eastwood, and Scott C. Weaver, Department of Microbiology and Immunology, Institute for Human Infections and Immunity, University of Texas Medical Branch, Galveston, TX, E-mails: erwang@utmb.edu, gill2g@hotmail.com, and sweaver@utmb.edu. Samuel M. Goldstein, U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, Honolulu, HI, E-mail: samuel.m.goldstein@aphis.usda.gov. David R. Sinnett, U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, Palmer, AK, E-mail: david.r.sinnett@aphis.usda.gov. Thomas J. DeLiberto, U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, National Wildlife Research Center, Fort Collins, CO, E-mail: thomas.j.deliberto@aphis.usda.gov.
References
- 1.Brown CR, O'Brien VA. Are wild birds important in the transport of arthropod-borne viruses? Ornithol Monogr. 2011;71:1–64. [Google Scholar]
- 2.Calisher CH. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev. 1994;7:89. doi: 10.1128/cmr.7.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- 4.Molaei G, Andreadis TG, Armstrong PM, Thomas MC, Deschamps T, Cuebas-Incle E, Montgomery W, Osborne M, Smole S, Matton P, Andrews W, Best C, Cornine F, 3rd, Bidlack E, Texeira T. Vector-host interactions and epizootiology of eastern equine encephalitis virus in Massachusetts. Vector-Borne Zoonot. 2013;13:312–323. doi: 10.1089/vbz.2012.1099. [DOI] [PubMed] [Google Scholar]
- 5.Weaver SC, Scott TW, Rico-Hesse R. Molecular evolution of eastern equine encephalomyelitis virus in North America. Virology. 1991;182:774–784. doi: 10.1016/0042-6822(91)90618-l. [DOI] [PubMed] [Google Scholar]
- 6.Roehrig JT. West Nile virus in the United States—a historical perspective. Viruses. 2013;5:3088–3108. doi: 10.3390/v5123088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caffrey C, Weston TJ, Smith SC. High mortality among marked crows subsequent to the arrival of West Nile virus. Wildl Soc Bull. 2003;31:870–872. [Google Scholar]
- 8.McLean RG. West Nile virus in North American birds. Ornithol Monogr. 2006;60:44–64. [Google Scholar]
- 9.Tsai TF. Arboviral infections in the United States. Infect Dis Clin North Am. 1991;5:73–102. [PubMed] [Google Scholar]
- 10.McLean RG, Ubico SR, Docherty DE, Hansen WR, Sileo L, McNamara TS. West Nile virus transmission and ecology in irds. Ann N Y Acad Sci. 2001;951:54–57. doi: 10.1111/j.1749-6632.2001.tb02684.x. [DOI] [PubMed] [Google Scholar]
- 11.Scott TW, McLean RG, Francy DB, Mitchell CJ, Card CS. Experimental infections of birds with Turlock Virus. J Wildl Dis. 1983;19:82–85. doi: 10.7589/0090-3558-19.2.82. [DOI] [PubMed] [Google Scholar]
- 12.Shope RE, De Andrade AH, Bensabath G, Causey OR, Humphrey PS. The epidemiology of EEE, WEE, SLE and Turlock viruses, with special reference to birds, in a tropical rain forest near Belem, Brazil. Am J Epidemiol. 1966;84:467–477. doi: 10.1093/oxfordjournals.aje.a120659. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen K, Marks DR, Arsnoe DM, Bevins SN, Wang E, Weaver SC, Mickley RM, DeLiberto TJ. Antibody prevalence of select arboviruses in mute swans (Cygnus olor) in the Great Lakes region and Atlantic coast of the United States. Am J Trop Med Hyg. 2014;91:1247–1249. doi: 10.4269/ajtmh.14-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolman JM, Folk C, Hudec K, Reddy GN. Serologic examination of birds from the area of southern Moravia for the presence of antibodies against arboviruses of the groups alfa, flavo, Uukuniemi, Turlock and Bunyamwera supergroup II wild living birds. Folia Parasitol (Praha) 1976;23:251–255. [PubMed] [Google Scholar]
- 15.Aguilar PV, Paessler S, Carrara A-S, Baron S, Poast J, Wang E, Moncayo AC, Anishchenko M, Watts D, Tesh RB, Weaver SC. Variation in interferon sensitivity and induction among strains of eastern equine encephalitis virus. J Virol. 2005;79:11300–11310. doi: 10.1128/JVI.79.17.11300-11310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaty BJ, Calisher CH, Shope RE. Arboviruses. In: Lennette EH, Lennette DA, Lennette ET, editors. Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections. Washington, DC: American Public Health Association; 1995. pp. 189–212. [Google Scholar]
- 17.Stamm DD. Relationships of birds and arboviruses. Auk. 1966;83:84–97. [Google Scholar]
- 18.Komar N, Langevin S, Hinten S, Nemeth NM, Edwards E, Hettler DL, Davis B, Bowen RA, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaremych SA, Warner RE, Mankin PC, Brawn JD, Raim A, Novak R. West Nile virus and high death rate in American crows. Emerg Infect Dis. 2004;10:709–711. doi: 10.3201/eid1004.030499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caffrey C, Smith SC, Weston TJ. West Nile virus devastates an American crow population. Condor. 2005;107:128–132. [Google Scholar]
- 21.Wilcox BR, Yabsley MJ, Ellis AE, Stallknecht DE, Gibbs SE. West Nile virus antibody prevalence in American crows (Corvus brachyrhynchos) and fish crows (Corvus ossifragus) in Georgia, USA. Avian Dis. 2007;51:125–128. doi: 10.1637/0005-2086(2007)051[0125:WNVAPI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Gibbs SE, Allison AB, Yabsley MJ, Mead DG, Wilcox BR, Stallknecht DE. West Nile virus antibodies in avian species of Georgia, USA: 2000–2004. Vector Borne Zoonotic Dis. 2006;6:57–72. doi: 10.1089/vbz.2006.6.57. [DOI] [PubMed] [Google Scholar]
- 23.Ringia AM, Blitvich BJ, Koo H-Y, Van de Wyngaerde M, Brawn JD, Novak RJ. Antibody prevalence of West Nile virus in birds, Illinois, 2002. Emerg Infect Dis. 2004;10:1120–1124. doi: 10.3201/eid1006.030644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree M, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 25.Kilpatrick AM, Gluzberg Y, Burgett J, Daszak P. Quantitative risk assessment of the pathways by which West Nile virus could reach Hawaii. EcoHealth. 2004;1:205–209. [Google Scholar]
- 26.Nemeth NM, Bosco-Lauth AM, Sciulli RH, Gose RB, Nagata MT, Bowen RA. Serosurveillance for Japanese encephalitis and West Nile viruses in resident birds in Hawai'i. J Wildl Dis. 2010;46:659–664. doi: 10.7589/0090-3558-46.2.659. [DOI] [PubMed] [Google Scholar]
- 27.Hardy JW. Feral exotic birds in southern California. Wilson Bull. 1973;85:506–512. [Google Scholar]
- 28.Cox GW. Alien Species in North America and Hawaii: Impacts on Natural Ecosystems. Washington, DC: Island Press; 1999. [Google Scholar]
- 29.Venkat H, Krow-Lucal E, Hennessey M, Jones J, Adams L, Fischer M, Sylvester T, Levy C, Smith K, Plante L, Komatsu K, Erin Staples J, Hills S. Concurrent outbreaks of St. Louis encephalitis virus and West Nile virus disease—Arizona, 2015. MMWR. 2015;64:1349. doi: 10.15585/mmwr.mm6448a5. [DOI] [PubMed] [Google Scholar]
- 30.Franklin RP, Kinde H, Jay MT, Kramer LD, Green E, Chiles RE, Ostlund E, Husted S, Smith J, Parker MD. Eastern equine encephalomyelitis virus infection in a horse from California. Emerg Infect Dis. 2002;8:283–288. doi: 10.3201/eid0803.010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brault AC, Powers AM, Chavez CL, Lopez RN, Cachón MF, Gutierrez LF, Kang W, Tesh RB, Shope RE, Weaver SC. Genetic and antigenic diversity among eastern equine encephalitis viruses from North, Central, and South America. Am J Trop Med Hyg. 1999;61:579–586. doi: 10.4269/ajtmh.1999.61.579. [DOI] [PubMed] [Google Scholar]
- 32.McLean RG, Ubico SR. Arboviruses in birds. In: Thomas NJ, Hunter DB, Atkinson CT, editors. Infectious Diseases of Wild Birds. Ames, IA: Blackwell Publishing; 2008. pp. 17–62. [Google Scholar]
- 33.Holden P, Hayes RO, Mitchell CJ, Francy DB, Lazuick JS, Hughes TB. House sparrows, Passer domesticus (L.), as hosts of arboviruses in Hale County, Texas: I. Field studies, 1965–1969. Am J Trop Med Hyg. 1973;22:244–253. doi: 10.4269/ajtmh.1973.22.244. [DOI] [PubMed] [Google Scholar]
- 34.Huyvaert KP, Moore AT, Panella NA, Edwards EA, Bomberger, Brown M, Komar N, Brown CR. Experimental inoculation of house sparrows (Passer domesticus) with Buggy Creek virus. J Wildl Dis. 2008;44:331–340. doi: 10.7589/0090-3558-44.2.331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.