Abstract
Two parallel outbreaks of Brucella melitensis infection occurred in 2014 in two geographical areas in Israel. In two medical centers in northern Israel and one medical center in Jerusalem, 102 patients (58 children, 47 adults) were diagnosed with brucellosis. Most patients (N = 76, 72%) were Muslim Arabs, 28 (27%) were Druze, and one was Jewish. The source of infection was often traced to cheese from the Palestinian Authority. Biovar-1 was evident in 98% in northern Israel but only in 42% in Jerusalem. Most common manifestations were fever (82%) and osteoarticular symptoms (49%). The major differences between the geographic areas were ethnicity and duration until diagnosis. Compared with adults, children had higher rates of hospitalization (93% versus 64%, P = 0.001), osteoarticular symptoms (60% versus 36%, P = 0.05), elevated alanine aminotransferase (12% versus 0%, P = 0.01), and lower C-reactive protein (2.28 ± 2.08 versus 5.57 ± 6.3l mg/dL, P = 0.001). Two unrelated brucellosis outbreaks occurred in 2014 in two different geographic areas of Israel and were limited to sections of the Arab and Druze populations. Most of the demographic and clinical aspects of patients were not affected by geographic variability. Clinical and laboratory differences were found between children and adults emphasizing the nonuniformity of the disease in different age groups. Effective control of unpasteurized dairy foods, health education programs, and improved regional cooperation are required to control brucellosis in Israel.
Introduction
Brucellosis is uncommon in Israel except for areas inhabited by rural Arab population where it is an ongoing, albeit contained, public health problem.1–3 Most of human brucellosis cases in Israel are caused by ingestion of unpasteurized dairy products from infected animals.3 The incidence of brucellosis ranges from 0.2/100,000 in the Jewish population to 7/100,000 among Arabs.3 In 2014, the number of human brucellosis cases was almost doubled compared with 2013 (603 versus 336 cases, respectively) and previous years and was about 10 times higher compared with 2013 in Jerusalem and northern Israel areas.4 In addition to the cases occurring yearly in the Negev endemic area, primarily among Bedouins, two parallel outbreaks of brucellosis occurred in 2014 in the area of Jerusalem and in two districts in northern Israel: the Acre and Yezreel Valley districts.
The aims of this article was to describe the unique characteristics of these outbreaks, compare between the outbreaks occurring in parallel in two distinct geographical areas, and characterize brucellosis in pediatric versus adult population.
METHODS
Clinical, epidemiological, and laboratory data were collected from the charts of patients diagnosed with brucellosis during 2014: children in the Galilee Medical Center (GMC), Nahariya; and children and adults in Emek Medical Center (Emek), Afula, both in northern Israel; and children and adults in Shaare Zedek Medical Center (SZMC), Jerusalem. Data were compared between the different geographical areas and between adult and pediatric patients. A diagnosis of brucellosis was made based on a positive blood culture or a positive serology test with a titer of 1:160 or greater within 2 weeks of clinical disease onset. Microsoft Excel 2007 (Microsoft, Redwood, WA) was used for data collection. Statistical analysis was done using Excel 2007 and WINPEPI.5 Qualitative variables were compared using the χ2 test. Quantitative variables were compared by the t test.
The isolates were identified at the genus level by conventional microbiological methods and biotyped as previously described based on requirement of CO2 for growth, urease activity, production of H2S, susceptibility to penicillin and streptomycin, growth on fuchsin and thionin dyes, Tb and Iz phage typing, and agglutination pattern with monospecific anti-A and anti-M sera.6 Brucella strains were typed according to procedures described elsewhere, as well as controls.
The study was approved by the institutional review boards and local ethics committees of GMC, Emek, and SZMC.
Results
Patients' demographic and clinical characteristics.
During the study period, 58 children and 47 adults were diagnosed with brucellosis (total number of patients = 105). Of them, 75 (72%) were Muslim Arabs, 28 (27%) were Druze (all from GMC), and only one patient was Jewish (1%). Forty eight (46%) of the patients were males. The average ages of children and adult patients were 10 years (range = 2–17 years) and 43 years (range = 18–80 years), respectively.
In all Jerusalem patients with a history of ingestion of unpasteurized dairy products (N = 16, 59%), the infection was traced to homemade cheese from the Palestinian Authority (PA). Overall duration of symptoms before diagnosis was 16.9 ± 17.2 days. Most patients had fever on admission (82%), and the most common symptoms were osteoarticular (49%), constitutional symptoms such as fatigue and weakness (36%), headache (19%), and gastrointestinal symptoms (15%). Localizing symptoms also included epididymo-orchitis (3%) and seizures (1%). Three patients (3%) were asymptomatic and were diagnosed based on screening serology tests for brucellosis in an epidemiologic investigation of the local health authorities.
Seventy percent (63/90) of blood cultures were positive and all were identified as Brucella melitensis. The Rose Bengal screening test was positive in 97% of patients (99/102). Two pregnant women were diagnosed during second trimester, were treated with trimethoprim–sulfamethoxazole (TMP-SMX) and rifampin, and gave birth to a healthy term infant. Three patients had complications related to brucellosis: one had neurobrucellosis with status epilepticus, one had endocarditis, and one had a splenic infarct. The treatment regimens included mainly doxycycline + gentamicin, doxycycline + rifampin, and TMP-SMX + gentamicin. Doxycycline + rifampin or TMP-SMX was given for a total of at least 6 weeks; gentamicin was given for 5–14 days. Only two patients (2%) had major drug-related adverse events (thrombocytopenia induced by TMP-SMX and rifampin). Eighty percent of patients were hospitalized. All patients recovered and none had a relapse so far.
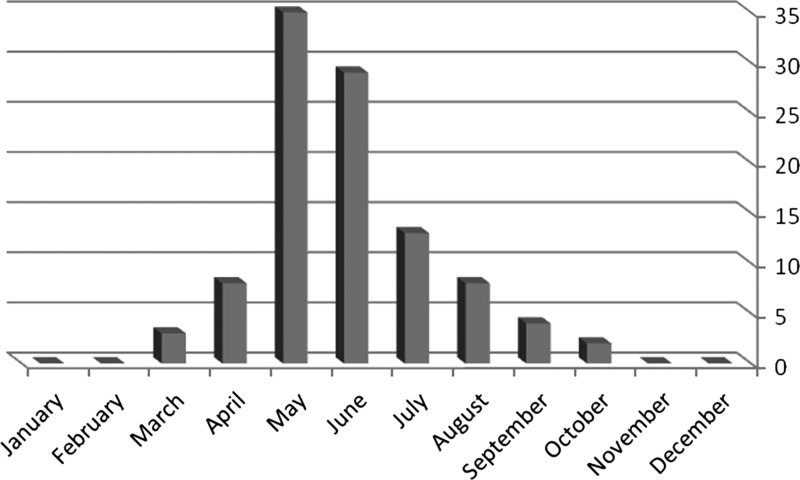
The occurrence of human brucellosis had a seasonality pattern: the majority of patients were diagnosed from May to July (Figure 1). Of blood cultures, 50 (81%) were identified as biovar 1, 11 (18%) as biovar 2, and one as biovar 3. In one positive blood culture, the biovar was unavailable. Patients with biovar 1 had longer symptoms before hospital admission (21.8 ± 10.6 versus 12.4 ± 16.8 days, [P = 0.02]).
Figure 1.
Number of patients with brucellosis by month in two geographical areas in Israel in 2014.
Jerusalem versus northern Israel.
Demographic, clinical, and laboratory characteristics of patients with brucellosis in the two geographic areas are presented in Table 1. There were no major differences between the populations in most of the demographic and clinical variables except for Brucella biovars in blood cultures. Biovar 1 was isolated in all but one of the blood cultures from patients in northern Israel, while in Jerusalem biovars 1 and 2 were equally represented. One patient was found to have biovar 3. Most of the patients in GMC were of Druze origin. The length of time until diagnosis was significantly longer in Jerusalem patients, and their blood levels of lactate dehydrogenase were significantly higher.
Table 1.
Demographic, clinical, and laboratory characteristics of patients with brucellosis in two geographical areas in Israel
| Characteristic | Northern Israel (N = 78) | Jerusalem area (N = 27) | Total (N = 105) | P value |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 35 (45) | 13 (48) | 48 (46) | 0.84 |
| Female | 43 (55) | 14 (52) | 57 (54) | |
| Ethnicity, n (%) | ||||
| Arab | 50 (64) | 26 (96) | 76 (72) | 0.11 |
| Druze | 28 (36) | 0 (0) | 28 (27) | 0.002 |
| Jewish | 0 (0) | 1 (4) | 1 (1) | |
| Osteoarticular manifestations, n (%) | 40 (51) | 12 (44) | 52 (49) | 0.75 |
| Fever at presentation, n (%) | 64 (82) | 22 (82) | 86 (82) | 1 |
| Days to diagnosis (mean ± SD) | 12.7 ± 13.8 | 24.8 ± 23.4 | 16.9 ± 17.2 | 0.004 |
| Patients hospitalized, n (%) | 62 (79) | 22 (81) | 84 (79) | 1 |
| Hospitalization days (mean ± SD) | 6 ± 4 | 5.9 ± 3.8 | 6 ± 3.9 | 0.89 |
| ALT, U/L > twice upper limit, n (%) | 4 (5) | 3 (11) | 7 (7) | 0.29 |
| LDH, U/L (mean ± SD) | 609 ± 354 | 962 ± 452 | 763 ± 434 | 0.001 |
| WBC/μL (mean ± SD) | 6.35 ± 2.51 | 5.51 ± 1.93 | 6.14 ± 2.39 | 0.11 |
| CRP, mg/dL (mean ± SD) | 3.45 ± 4.84 | 4.52 ± 4.27 | 3.71 ± 4.70 | 0.37 |
| Positive blood culture, n (%) | 45/63 (71) | 18/27 (67) | 63/90 (70) | 0.78 |
| Biovar in blood culture, n (%) | ||||
| 1 | 42 (98) | 8 (42) | 50 (81) | 0.03 |
| 2 | 1 (2) | 10 (53) | 11 (17) | |
| 3 | 0 (0) | 1 (5) | 1 (2) | |
| Average time to positive blood culture (days) (mean ± SD) | 3.08 ± 1.35 | 2.83 ± 0.82 | 3 ± 1.17 | 0.46 |
ALT = alanine aminotransferase; CRP = C-reactive protein; LDH = lactate dehydrogenase; SD = standard deviation; WBC = white blood cells.
Children versus adults.
Demographic, clinical, and laboratory characteristics of children and adults with brucellosis are presented in Table 2. Children had a higher rate of osteoarticular symptoms. They also had higher rates of elevated liver enzymes and lower C-reactive protein (CRP) values compared with adults. The rate of positive blood cultures did not differ significantly, but the time until blood cultures deemed positive by the automated system was shorter in children. Children had higher rates of hospitalization and were hospitalized for longer durations.
Table 2.
Demographic, clinical, and laboratory characteristics of children vs. adults with brucellosis in two geographical areas in Israel
| Characteristic | Adults (N = 47) | Children (N = 58) | Total (N = 105) | P value |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 19 (40) | 29 (50) | 48 (46) | 0.56 |
| Female | 28 (60) | 29 (50) | 57 (54) | |
| Osteoarticular manifestations, n (%) | 17 (36) | 35 (60) | 52 (49) | 0.05 |
| Hepatomegaly and/or splenomegaly, n (%) | 11 (35) | 9 (16) | 20 (23) | 0.06 |
| Fever at presentation, n (%) | 36 (77) | 50 (86) | 86 (82) | 0.66 |
| Days to diagnosis (mean ± SD) | 20.7 ± 20.7 | 14.2 ± 13.5 | 16.9 ± 17.2 | 0.07 |
| Patients hospitalized, n (%) | 30 (64) | 54 (93) | 84 (80) | 0.001 |
| Hospitalization days (median ± SD) | 5 ± 4.17 | 7 ± 3.4.3 | 6 ± 3.93 | 0.001 |
| ALT, U/L > twice upper limit, n (%) | (0) 0 | 7 (12) | 7 (7) | 0.02 |
| LDH, U/L (mean ± SD) | 799 ± 377 | 722 ± 494 | 763 ± 434 | 0.49 |
| WBC/μL (mean ± SD) | 5.76 ± 2.09 | 6.43 ± 2.59 | 6.14 ± 2.39 | 0.15 |
| CRP, mg/dL (mean ± SD) | 5.57 ± 6.3 | 2.28 ± 2.08 | 3.71 ± 4.70 | 0.001 |
| Positive blood culture (%) | 82 | 62 | 70 | 0.29 |
| Average time to positive blood culture (days) (mean ± SD) | 3.56 ± 0.9 | 2.56 ± 1.16 | 3 ± 1.17 | 0.008 |
ALT = alanine aminotransferase; CRP = C-reactive protein; LDH = lactate dehydrogenase; SD = standard deviation; WBC = white blood cells.
DISCUSSION
Human brucellosis in Israel is caused by ingestion of unpasteurized dairy products and is mostly limited to areas inhabited by rural Arab population.3 In 2014, the number of human brucellosis cases in Israel was almost doubled compared with 2013 and previous years.4 In addition to the cases occurring yearly in the Negev endemic area, two parallel outbreaks of B. melitensis infections occurred in the area of Jerusalem and in northern Israel.
This increase in brucellosis cases can be attributed to several factors. All cases but one in our cohort occurred in Arabs and Druze (one Jewish patient bought unpasteurized cheese from Bedouins). The Israeli Ministry of Health traced some of the cases to dairy products made of goat milk from herds in the PA. Documents from the PA Ministry of Agriculture obtained by Ma'an Palestinian news agency showed that the gradual increase in infections since 2011 is directly linked to a shortage in Brucella vaccines provided by the PA to farmers. Recent data from the PA's Ministry of Agriculture reports on the rise of brucellosis due to shortage of Brucella vaccines.7 The high incidence rate of brucellosis in the Arab population could also be attributed, at least partially, to lack of awareness regarding the modes of transmission and prevention methods of the disease.
We found that all cases in our study occurred between March and October. Several factors can explain this pattern. A traditional Arab dish in the summer months is made of watermelon with homemade goat cheese. Many of our patients reported eating it in the weeks preceding infection. Door to door sale of unpasteurized dairy foods is a common practice in some sectors of the rural Arab population, especially during spring and summer. These habits and unvaccinated family-owned small herds of sheep and goats are problematic to control by the Ministries of Health and Agriculture, the ministries responsible for the regulation of food products in Israel. Among small family-owned flocks in Israel, the parturition period is mainly from December through March and is followed in the March–July months by the production of milk and dairy products. These months coincide with the peak of brucellosis incidence as previously reported in Israel3 and other places.8–10
We did not find major differences in clinical presentation between brucellosis patients in the two areas in Israel despite the differences in Brucella biovars. This can be explained by the relatively small geographic size of Israel and by the uniformity of the diagnostic methods for Brucella. One exception was the length of symptoms, which was longer in patients from Jerusalem. Differences of access to medical services of various populations or a lower threshold of suspicion in the northern area, where brucellosis is more prevalent yearly, can explain this observation. Another possibility is a more indolent course of disease caused by biovar 2, which was more prevalent in Jerusalem.
In comparing children with adults, we found clinical and laboratory differences. Children had higher rates of osteoarticular manifestations, mainly arthralgia. Sacroiliac joint involvement was seen only in children, in contrary to previous reports describing higher rates of sacroiliitis in adults.11 We also noted hepatomegaly or splenomegaly to be uncommon in children, as opposed to previous reports, possibly stemming from more rapid diagnosis nowadays because of higher index of suspicion in outbreak situation and improvement in continuous surveillance incubators for blood cultures.12,13 In contrast to the past, when blood cultures had to be incubated for several weeks to grow the organism, the much improved incubators today provided a direct diagnosis in 70% of our patients, doing so in 2–4 days. Regarding laboratory differences, children had higher rates of abnormal liver enzymes and lower CRP, which can be attributed to the relative larger size of the reticuloendothelial system in children and the lower rate of underlying medical conditions, respectively (2% versus 36% in adults).1,14
Of our patients, 80% were hospitalized with no differences between the two geographical areas. However, over 90% of children were admitted as opposed to 64% of adults. Children were also hospitalized for a longer duration. These hospitalization rates were higher than in previous reports, including studies from Israel,1,2,14 and can be related to the increased concerns about complications in the setting of an epidemic and to the well-established superiority of aminoglycoside-containing regimens (given intravenously in hospitals to avoid repeated intramuscular injections) in decreasing relapse. Higher rates of osteoarticular manifestations in children may also account for longer hospitalizations. In addition, doxycycline + rifampin, an oral antibiotic regimen, cannot be given to children younger than 8 years, necessitating admissions of young children.
Our study has some potential limitations. There may be differences between the three medical centers, which might have influenced the decisions of the treating physicians regarding admission of patients, length of hospitalizations, blood tests ordered, and follow-up. Genetic variability between the populations treated might also influence (e.g., most of the patients treated in GMC were Druze). The results of this study might not reflect the entire spectrum of Brucella infections, as there might have been patients who were treated as outpatients or not diagnosed. Since the study was conducted retrospectively, some data were missing for some of the study patients, thus limited the power of the analysis for those variables. Nevertheless, we described here current and unique data of two outbreaks of brucellosis occurring in a developed country in variable geographic areas and age groups.
CONCLUSIONS
Brucellosis remains a public health problem in Israel. Two unrelated brucellosis outbreaks occurred in 2014 in two different geographic areas of Israel and were limited to sections of the Arab and Druze populations.
Most of the demographic and clinical aspects of patients were not affected by geographic variability and by Brucella biovar differences. However, clinical and laboratory differences were found between children and adults, mainly in the rates of osteoarticular symptoms and hepatosplenomagely, blood levels of liver enzymes and CRP, time to positive blood cultures, and rate and length of hospitalization. These differences should be brought in mind especially when diagnosis of brucellosis is contemplated in adults and children.
More and most important conclusion is that health education programs in Israel should be improved, and a more tight cooperation with the PA including herd vaccination should be implemented, to better control and prevent brucellosis in Israel and the PA.
ACKNOWLEDGMENTS
We are grateful to Edelstein Cahanov Hanna for her help in preparing the case report form and data collection.
Footnotes
Authors' addresses: Orli Megged, Pediatric Department, Shaare Zedek Medical Center, Jerusalem, Israel, E-mail: orlimegged@yahoo.com. Bibiana Chazan, Infectious Diseases Unit, Emek Medical Center, Afula, Israel, E-mail: chazan_b@clalit.org.il. Atef Ganem, Clinical Microbiology Laboratory, Western Galilee Medical Center, Nahariya, Israel, E-mail: atefG@gmc.gov.il. Abeer Ayoub and Daniel Glikman, Pediatric Department, Western Galilee Medical Center, Nahariya, Israel, E-mails: abeersa@hotmail.com and dannyg@gmc.gov.il. Anna Yanovskay, Waheeb Sakran, and Dan Miron, Pediatric Department, Emek Medical Center, Afula, Israel, E-mails: anna_fe@clalit.org.il, sakran_w@clalit.org.il, and miron_da@clalit.org.il. Ahuva Dror-Cohen, Immunology and Serology Laboratory, Shaare Zedek Medical Center, Jerusalem, Israel, E-mail: ahuvadc@walla.com. Yoram Keness, Microbiology Laboratory, Emek Medical Center, Afula, Israel, E-mail: keness@clalit.org.il. Svetlana Berdenstein, Brucellosis Lab, OIE, FAO Reference Laboratory, Kimron Veterinary Institute, Bet Dagan, Israel, E-mail: svetab@moag.gov.il.
References
- 1.Gottesman G, Vanunu D, Maayan MC, Lang R, Uziel Y, Sagi H, Wolach B. Childhood brucellosis in Israel. Pediatr Infect Dis J. 1996;15:610–615. doi: 10.1097/00006454-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Fruchtman Y, Segev RW, Golan AA, Dalem Y, Tailakh MA, Novak V, Peled N, Craiu M, Leibovitz E. Epidemiological, diagnostic, clinical, and therapeutic aspects of Brucella bacteremia in children in southern Israel: a 7-year retrospective study 2005–2011. Vector Borne Zoonotic Dis. 2015;15:195–201. doi: 10.1089/vbz.2014.1726. [DOI] [PubMed] [Google Scholar]
- 3.Anis E, Leventhal A, Grotto I, Gandacu D, Warshavsky B, Shimshony A, Israeli A. Recent trends in human brucellosis in Israel. Isr Med Assoc J. 2011;13:359–362. [PubMed] [Google Scholar]
- 4.Ministry of Health, Israel Brucellosis Epidemic in Northern Israel. 2014. http://www.health.gov.il/NewsAndEvents/SpokemanMesseges/Pages/06072014_1.aspx Available at. (Hebrew). Accessed December 24, 2015.
- 5.Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011;8:1. doi: 10.1186/1742-5573-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alton GG, Jones Lois M, Angus RD, Verger JM. Techniques for the Brucellosis Laboratory. 22nd edition. Paris, France: Institut National de la Recherché Agronomique; 1988. [Google Scholar]
- 7.Ma'an News Agency Brucellosis on the Rise as PA Fails to Provide Sheep Vaccines. 2015. http://www.maannews.com/Content.aspx?id=759561 Available at. Accessed December 24, 2015.
- 8.Minas M, Minas A, Gourgulianis K, Stournara A. Epidemiological and clinical aspects of human brucellosis in central Greece. Jpn J Infect Dis. 2007;60:362–366. [PubMed] [Google Scholar]
- 9.Al-Ballaa SR, Al-Balla SR, Al-Aska A, Kambal A, Al-Hedaithy MA. Seasonal variation of culture positive brucellosis at a major teaching hospital. Ann Saudi Med. 1994;14:12–15. doi: 10.5144/0256-4947.1994.12. [DOI] [PubMed] [Google Scholar]
- 10.Dajani YF, Masoud AA, Barakat HF. Epidemiology and diagnosis of human brucellosis in Jordan. J Trop Med Hyg. 1989;92:209–214. [PubMed] [Google Scholar]
- 11.Sanaei Dashti A, Karimi A. Skeletal involvement of Brucella melitensis in children: a systematic review. Iran J Med Sci. 2013;38:286–292. [PMC free article] [PubMed] [Google Scholar]
- 12.Akhvlediani T, Clark DV, Chubabria G, Zenaishvili O, Hepburn MJ. The changing pattern of human brucellosis: clinical manifestations, epidemiology, and treatment outcomes over three decades in Georgia. BMC Infect Dis. 2010;10:346–354. doi: 10.1186/1471-2334-10-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young EJ. Brucella species. In: Long SS, Pickering LK, Prober CG, editors. Principals and Practice of Pediatric Infectious Diseases. 4th edition. Churchill Livingstone: Elsevier Saunders; 2012. pp. 861–865. [Google Scholar]
- 14.Logan LK, Jacobs NM, McAuley JB, Weinstein RA, Anderson EJ. A multicenter retrospective study of childhood brucellosis in Chicago, Illinois from 1986 to 2008. Int J Infect Dis. 2011;15:e812–e817. doi: 10.1016/j.ijid.2011.08.002. [DOI] [PubMed] [Google Scholar]