Abstract
Histoplasmosis, caused by the dimorphic fungus Histoplasma capsulatum, is a disease of protean manifestations and of global distribution. Although increasingly reported in Asia, there are few reports from the Philippines. Here, we describe a case of microbiologically diagnosed histoplasmosis, probably acquired from the Philippines, in a returning traveler who presented with a right foot wound and papular rash. The final diagnosis was disseminated histoplasmosis with cutaneous and bone involvement, both unusual manifestations of the disease.
Introduction
Histoplasmosis, caused by the dimorphic fungus Histoplasma capsulatum, is a disease of protean manifestations, often mimicking those of tuberculosis (TB). Though initially thought to be primarily a North American occurrence, especially endemic to the Ohio and Mississippi river valleys, histoplasmosis has a global distribution. Indeed, cases have increasingly been reported in Central and South America, sub-Saharan Africa, Oceania, and Asia.1 In Asia, the lion's share of cases has been reported from Thailand2 and China3 with only sporadic cases from Taiwan, India, Malaysia, and Singapore.4,5 However, elevated rates of histoplasmin skin hypersensitivity on cohort screening, up to 86% in some areas,1 suggest that a large portion of the population is exposed to the fungus, raising the possibility that disease may be under-recognized. In the Philippines, despite a histoplasmin skin hypersensitivity rate of 26% in one study,6 only one microbiologically confirmed case of histoplasmosis presenting with diffuse papulonodular skin lesions has been described,7 whereas four other cases were presumptively diagnosed by histopathology alone.8–11 Here, we present a case of microbiologically diagnosed histoplasmosis, probably acquired from the Philippines, in a returning traveler who presented with a right foot wound and papular rash.
CASE
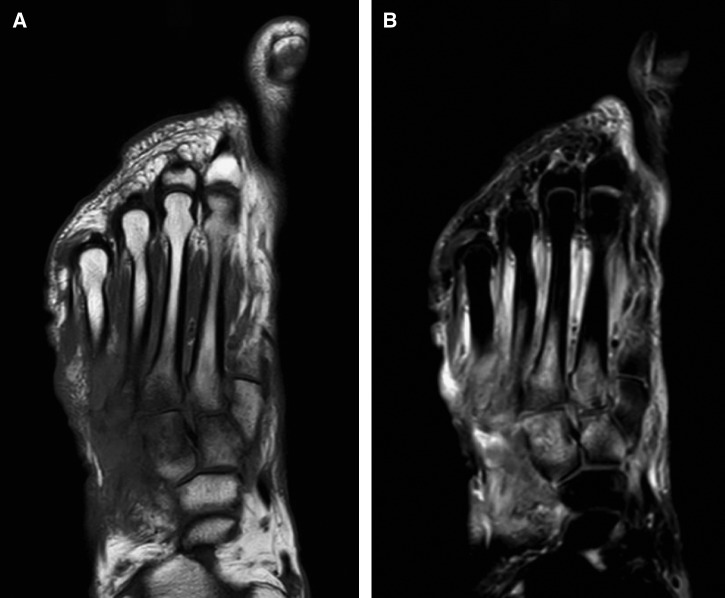
A 69-year-old Filipino male, with a history of pulmonary TB and multiple myeloma (MM) treated with an autologous bone marrow transplant (BMT) in 2011, presented to our hospital with a non-healing right foot wound. He first noticed the wound during his flight back to the United States from a 3-week vacation in the Philippines 5 months ago. During his sojourn, he resided in the Pangasinan Province (205 km north of Manila), visited the Metro Manila area, a public cemetery, and bathed in a river and hot springs of Laguna (104 km south of Manila). He denied trauma or exposure to excavations or landscaping. One week after his arrival to the United States, he developed fatigue and malaise. He also noticed increasing pain and fluctuant swelling of the right foot, which prompted a visit to the emergency department. A magnetic resonance image (MRI) of the foot revealed destructive changes of the second through fifth metatarsals and the first through third cuneiform bones, consistent with osteomyelitis (Figure 1). He subsequently underwent an intraoperative bone biopsy and debridement. Preliminary bacterial, fungal, and acid-fast stains were negative. He was discharged home with intravenous vancomycin and cefepime. A few days after discharge, he developed fever and a non-pruritic, papular rash over his face, chest, and extremities. Extensive periodic acid–Schiff positive yeast elements and granulomatous infiltrates were then identified on skin histopathology from outside-hospital biopsy specimen (biopsy specimen ultimately only contained skin, not bone), while fungal cultures grew mold. In light of these findings, he was admitted to an outside hospital but later transferred to our institution for treatment of fungal cellulitis and possible osteomyelitis.
Figure 1.
(A) Right foot magnetic resonance image T1-weighted images show replacement of normal fatty signal in areas of second through fifth metatarsals and first through third cuneiform bones. (B) T2-weighted sequences show bone marrow edema in corresponding areas, consistent with osteomyelitis.
Past medical history was significant for relapsed MM after autologous BMT, currently on maintenance treatment with thalidomide and dexamethasone (4 mg orally, daily) since 2011, chronic anemia and thrombocytopenia secondary to MM and pulmonary TB treated in the 1970s in the Philippines. He was born and raised in the Philippines, then immigrated to Connecticut in the 1990s, where he lives with his wife and daughter. He reported brief travel to the midwest and southeast United States in the 1990s.
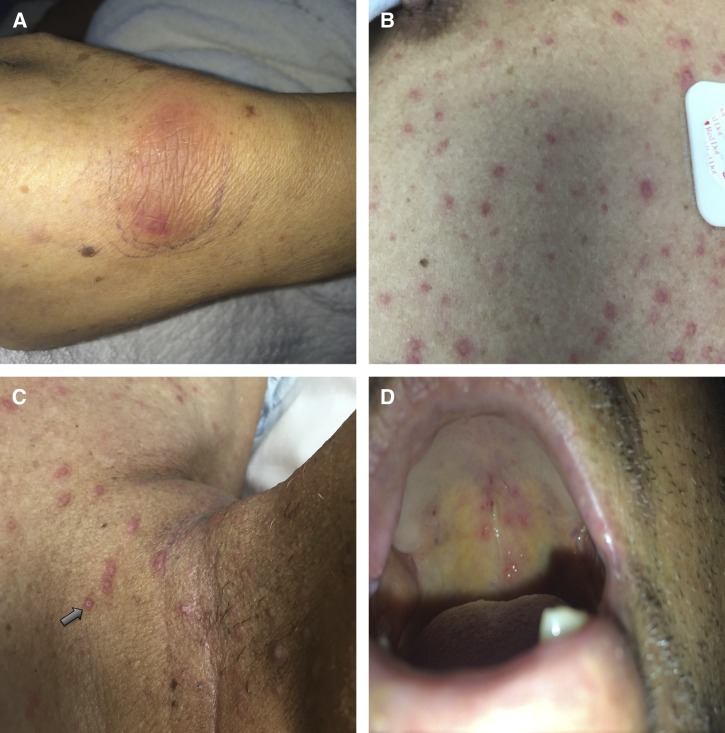
On physical exam at presentation to our institution, temperature was 36.6°C, blood pressure was 111/63 mmHg, pulse was 78 beats per minute, respiratory rate was 18 breaths per minute, and oxygen saturation was 99% while breathing ambient air. Significant findings on exam included a 3-cm oblong skin defect over the dorsal mid-foot with a clean base and exposed tendons, a non-tender ecchymotic lesion over the dorsal left wrist with intact range of motion, and multiple scattered papular lesions on the patient's face, soft palate, neck, chest, and back. Some of these lesions were umbilicated (Figure 2).
Figure 2.
Physical exam findings: (A) oblong nodular rash over left wrist, (B) scattered papular skin lesions, (C) some skin lesions were umbilicated (arrow), and (D) palatal lesions.
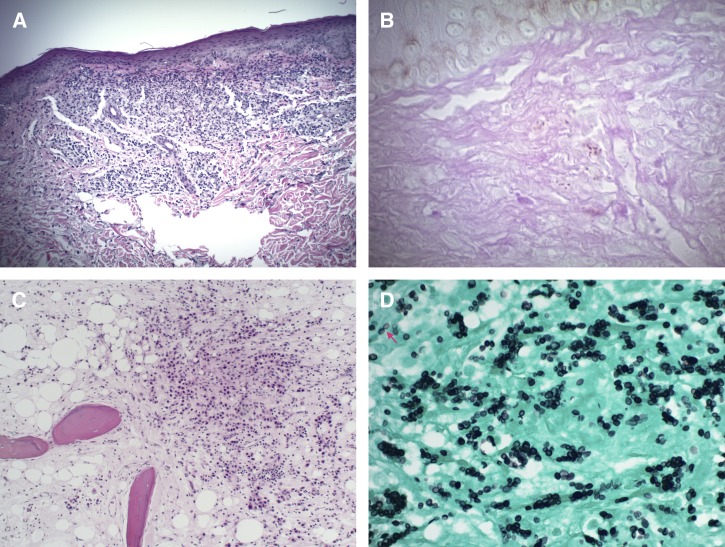
Laboratory analysis was notable for a mildly elevated creatinine (1.3 mg/dL), anemia (11.8 g/dL), and thrombocytopenia (108 × 103/μL). Bacterial and fungal blood cultures showed no growth. Serologic testing for human immunodeficiency virus (HIV) was negative. Computed tomography scan of the chest was negative for focal consolidations, nodules, or adenopathy. There was no evidence of intracranial pathology on MRI of the brain with gadolinium. The patient was empirically treated with liposomal amphotericin B (5 mg/kg daily) to cover the most likely dimorphic fungal diseases. The papular skin lesion on his chest and the right foot metatarsal bones were biopsied. In both cases, histopathology revealed abundant intra- and extracellular budding yeast elements and acute or chronic necrotizing inflammation (Figure 3). Bone changes were consistent with chronic osteomyelitis. The outside-hospital fungal culture was then confirmed as H. capsulatum by fungal internal transcribed spacer region gene sequencing. A Histoplasma urine antigen test (MiraVista Diagnostics, Indianapolis, IN) was > 39 ng/mL. Because of worsening anemia during his hospital stay (hemoglobin nadir of 6.5 g/dL), there was a concern for possible fungal bone marrow infiltration, for which a bone marrow aspirate and biopsy was performed. These found no evidence of fungal invasion.
Figure 3.
Skin and bone pathology. (A) Low-power (original magnification ×100) examination of a hematoxylin and eosin (H&E)–stained skin biopsy specimen shows granulomatous inflammation of the dermis. (B) High-power magnification (original magnification ×400) of the same specimen stained by periodic acid–Schiff stain shows intracellular yeast forms within the dermis. (C) Low-power examination of an H&E-stained bone biopsy specimen demonstrates a predominantly neutrophilic infiltrate within bone architecture. (D) High-power magnification of the bone specimen stained by the Gomori methenamine silver stain reveals numerous intracellular and extracellular yeast cells, some of which exhibit narrow-based budding (arrow).
The final diagnosis was disseminated histoplasmosis with cutaneous and bone involvement. The patient was treated with liposomal amphotericin B (5 mg/kg daily) for 5 weeks and successfully transitioned to lifelong itraconazole (200 mg twice daily) with drug therapeutic monitoring. On follow-up, skin lesions resolved and right foot wound healed completely and creatinine was at baseline. Histoplasma urine antigen decreased to 1.40 ng/mL 5 months after discharge.
DISCUSSION
The current case describes an immunocompromised Filipino patient who presented with disseminated histoplasmosis 5 months after returning to the United States from a trip to the Philippines. The onset of signs and symptoms immediately after trip and significant exposure to waterways during the trip are suggestive of de novo acquisition of infection while in the Philippines. However, it is also plausible the patient may have developed reactivation of latent histoplasmosis, acquired during his visit to the midwest United States in the 1990s. Reactivation leading to disseminated disease can occur in patients many years after residing in endemic areas and is most common in immunocompromised hosts,12 such as the patient described here. In this case, the patient developed cutaneous and bone involvement, both unusual manifestations of the disease.
Cutaneous manifestations of histoplasmosis are usually the result of hematogenous dissemination to the skin. Though much less common than with other endemic fungal diseases, skin lesions secondary to histoplasmosis have been reported in up to 25% of acquired immune deficiency syndrome (AIDS) patients.13 Skin lesions are also more common with South American histoplasmosis,14 possibly because of underlying genetic differences in fungal strains. In contrast, cutaneous lesions are infrequent in cases of histoplasmosis in Asia (6.6% in a study form China),3 but notably, both the patient described here and the previously reported case from the Philippines presented with disseminated papular lesions, some of which were umbilicated lesions. Skin lesions in histoplasmosis can be easily confused with those of penicilliosis, an endemic fungal infection in southeast Asia, as well as cryptococcal skin lesions, common in patients with AIDS. On histopathology, both Penicillium marneffei and H. capsulatum appear as small intra- or extracellular yeast, surrounded by granulomatous inflammation; however, narrow-based budding is suggestive of the former whereas a transverse septum and binary fission are consistent with the latter. Histoplasma capsulatum can be differentiated from Cryptococcus neoformans using a mucicarmine stain, which selectively highlights the cryptococcal capsule. Blastomycosis and coccidioidomycosis often produce skin manifestations but are exceedingly rare in Asia.15
Osteomyelitis due to H. capsulatum var. capsulatum has previously been described,16,17 but is an infrequent manifestation. Bone involvement can be due to hematogenous seeding or from direct inoculation through injury. In a retrospective study including 78 patients with disseminated histoplasmosis, there were no microbiologically or histopathologically confirmed cases of osteomyelitis.18 On the other hand, in African histoplasmosis, caused by H. capsulatum var. duboisii, osteolytic bone lesions are very common, affecting more than 50% of patients.19 Consensus guidelines on the treatment of histoplasmosis do not describe treatment of osteomyelitis in particular,20 but recommendations for disseminated histoplasmosis probably encompass bone involvement. Induction therapy with amphotericin B is recommended for moderate to severe disease followed by long-term treatment with itraconazole. The patient described in this report responded very well to the aforementioned treatment regimen. However, the literature on treating histoplasmosis osteomyelitis is very sparse. Zerbe and others described the case of a child with interferon-gamma receptor 1 deficiency, who was diagnosed with Histoplasma osteomyelitis. The patient was treated with multiple courses of amphotericin B but had multiple recurrences linked to his underlying immunodeficiency.21 Additional and more systematic data are needed to better characterize the optimal treatment of this rare manifestation.
In the Philippines, unlike other parts of Asia, there are few reports of histoplasmosis.22 However, the prevalence of histoplasmin skin hypersensitivity in a recent study from this country was 26%.6 Since histoplasmin skin hypersensitivity is a measure of cellular immunity that develops only in persons exposed to the fungus, it is plausible that histoplasmosis in the Philippines is much more common than reported. Underlying pathogen or host genetic factors that lead to a greater proportion of milder or subclinical cases may account for this phenomenon. In coccidioidomycosis, being of Filipino descent has been associated with a greater risk for dissemination,23 suggesting that host factors can indeed play a role in the manifestation of endemic fungal infections. Alternatively, histoplasmosis may be misdiagnosed as other chronic granulomatous diseases such as TB or dimorphic fungal infections or simply underreported in the literature.15 As the number of patients on life-prolonging immunosuppressive therapies and with HIV/AIDS increases globally, a greater number of patients will be at risk for overt manifestations of histoplasmosis. Increased availability of nucleic acid amplification tests and urine or serum Histoplasma antigen testing in the future may help clarify the true incidence of the disease, especially when the diagnosis is in question.
This case highlights histoplasmosis as a truly global disease with variable manifestations. In a patient returning from Asia, with compatible clinical signs and symptoms and especially if underlying immunosuppression is present, histoplasmosis should remain in the differential diagnosis regardless of reported incidence in a particular country.
Footnotes
Authors' addresses: Marwan M. Azar and Maricar F. Malinis, Section of Infectious Diseases, Department of Internal Medicine, Yale University, New Haven, CT, E-mails: marwan.azar@yale.edu and maricar.malinis@yale.edu.
References
- 1.Bahr NC, Antinori S, Wheat LJ, Sarosi GA. Histoplasmosis infections worldwide: thinking outside of the Ohio River valley. Curr Trop Med Rep. 2015;2:70–80. doi: 10.1007/s40475-015-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norkaew T, Ohno H, Sriburee P, Tanabe K, Tharavichitkul P, Takarn P, Puengchan T, Bumrungsri S, Miyazaki Y. Detection of environmental sources of Histoplasma capsulatum in Chiang Mai, Thailand, by nested PCR. Mycopathologia. 2013;176:395–402. doi: 10.1007/s11046-013-9701-9. [DOI] [PubMed] [Google Scholar]
- 3.Pan B, Chen M, Pan W, Liao W. Histoplasmosis: a new endemic fungal infection in China? Review and analysis of cases. Mycoses. 2013;56:212–221. doi: 10.1111/myc.12029. [DOI] [PubMed] [Google Scholar]
- 4.Randhawa HS. Occurrence of histoplasmosis in Asia. Mycopathol Mycol Appl. 1970;41:75–89. doi: 10.1007/BF02051485. [DOI] [PubMed] [Google Scholar]
- 5.Sethi KK, Passi S. Histoplasmosis in India. A critical review. Mycopathol Mycol Appl. 1970;40:369–374. doi: 10.1007/BF02051792. [DOI] [PubMed] [Google Scholar]
- 6.Bulmer AC, Bulmer GS. Incidence of histoplasmin hypersensitivity in the Philippines. Mycopathologia. 2001;149:69–71. doi: 10.1023/a:1007277602576. [DOI] [PubMed] [Google Scholar]
- 7.Navarro EE, Tupasi TE, Verallo VM, Romero RC, Tuazon CU. Disseminated histoplasmosis with unusual cutaneous lesions in a patient from the Philippines. Am J Trop Med Hyg. 1992;46:141–145. doi: 10.4269/ajtmh.1992.46.141. [DOI] [PubMed] [Google Scholar]
- 8.Palencia RJ, Aniceto EG, Pena AC. Fever of unknown origin with hepatomegaly due to Histoplasma capsulatum: case report. Phil J Microbiol Infect Dis. 1990;19:63–67. [Google Scholar]
- 9.Mendoza S. Histoplasmosis. Report of a case. Monthly Bull Bur Health. 1947;23:33–40. [Google Scholar]
- 10.Natividad-Santos E. A case of progressive disseminated histoplasmosis. Chest Dis. 1974;9:15–19. [Google Scholar]
- 11.Hilda L-K. Disseminated histoplasmosis in a two month old Filipino infant: a preliminary report. Phil J Microbiol Infect Dis. 1981;10:124–130. [Google Scholar]
- 12.Wheat LJ, Slama TG, Norton JA, Kohler RB, Eitzen HE, French ML, Sathapatayavongs B. Risk factors for disseminated or fatal histoplasmosis. Analysis of a large urban outbreak. Ann Intern Med. 1982;96:159–163. doi: 10.7326/0003-4819-96-2-159. [DOI] [PubMed] [Google Scholar]
- 13.Eidbo J, Sanchez RL, Tschen JA, Ellner KM. Cutaneous manifestations of histoplasmosis in the acquired immune deficiency syndrome. Am J Surg Pathol. 1993;17:110–116. doi: 10.1097/00000478-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Karimi K, Wheat LJ, Connolly P, Cloud G, Hajjeh R, Wheat E, Alves K, Lacaz Cd Cda S, Keath E. Differences in histoplasmosis in patients with acquired immunodeficiency syndrome in the United States and Brazil. J Infect Dis. 2002;186:1655–1660. doi: 10.1086/345724. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarti A, Slavin MA. Endemic fungal infections in the Asia-Pacific region. Med Mycol. 2011;49:337–344. doi: 10.3109/13693786.2010.551426. [DOI] [PubMed] [Google Scholar]
- 16.Huang L, Wu Y, Miao X. Localized Histoplasma capsulatum osteomyelitis of the fibula in an immunocompetent teenage boy: a case report. BMC Infect Dis. 2013;13:132. doi: 10.1186/1471-2334-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCabe MP, Heck RK. Histoplasma osteomyelitis simulating giant-cell tumor of the distal part of the radius: a case report. J Bone Joint Surg Am. 2010;92:708–714. doi: 10.2106/JBJS.H.01507. [DOI] [PubMed] [Google Scholar]
- 18.Assi MA, Sandid MS, Baddour LM, Roberts GD, Walker RC. Systemic histoplasmosis: a 15-year retrospective institutional review of 111 patients. Medicine (Baltimore) 2007;86:162–169. doi: 10.1097/md.0b013e3180679130. [DOI] [PubMed] [Google Scholar]
- 19.Williams AO, Lawson EA, Lucas AO. African histoplasmosis due to Histoplasma duboisii. Arch Pathol. 1971;92:306–318. [PubMed] [Google Scholar]
- 20.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, Kauffman CA, Infectious Diseases Society of America Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 21.Zerbe CS, Holland SM. Disseminated histoplasmosis in persons with interferon-gamma receptor 1 deficiency. Clin Infect Dis. 2005;41:e38–e41. doi: 10.1086/432120. [DOI] [PubMed] [Google Scholar]
- 22.Wang TL, Cheah JS, Holmberg K. Case report and review of disseminated histoplasmosis in south-east Asia: clinical and epidemiological implications. Trop Med Int Health. 1996;1:35–42. doi: 10.1046/j.1365-3156.1996.d01-10.x. [DOI] [PubMed] [Google Scholar]
- 23.Rosenstein NE, Emery KW, Werner SB, Kao A, Johnson R, Rogers D, Vugia D, Reingold A, Talbot R, Plikaytis BD, Perkins BA, Hajjeh RA. Risk factors for severe pulmonary and disseminated coccidioidomycosis: Kern County, California, 1995–1996. Clin Infect Dis. 2001;32:708–715. doi: 10.1086/319203. [DOI] [PubMed] [Google Scholar]