Abstract
Soil-transmitted helminth (STH) infections and micronutrient deficiencies are closely related and often coexist among low-income populations. We studied the association between infections with specific STH species and micronutrient status in rural Vietnamese schoolchildren. Children (N = 510) aged 6–9 years were recruited from two primary schools. STH infections were determined in stool samples. Hemoglobin, ferritin, retinol, and zinc were measured in blood samples, as well as C-reactive protein to control for inflammation. Iodine excretion was measured in urine. Associations of single and multiple infections with Ascaris lumbricoides, Trichuris trichiura, and hookworm with micronutrient status (hemoglobin, plasma ferritin, retinol, zinc, and urinary iodine) were estimated by multiple regression analysis. Ascaris infections showed a specific and intensity-dependent negative association with vitamin A. Trichuris and hookworm infections were associated with lower hemoglobin concentration, but not with plasma ferritin. Trichuris-infected children had zinc deficiency less often than uninfected children. In conclusion, our study shows species-specific associations between STH infections and micronutrient status in children. The different life cycles of STH species might have specific effects on the absorption or loss of specific micronutrients. Tailor-made combinations of deworming and nutritional interventions may be needed to improve child health and nutrition.
INTRODUCTION
Soil-transmitted helminths (STHs) Ascaris lumbricoides, Trichuris trichiura, and hookworm and micronutrient deficiencies such as iron, zinc, and vitamin A pose major health issues in the developing world. Both STH infections and micronutrient deficiencies are highly prevalent in tropical countries and are strongly related to poverty.1,2 As school-age children are most often infected with STH and micronutrients are essential for growth and development, these children are particularly vulnerable to both conditions.
Various studies have reported associations between STH infections and micronutrient status in children.3,4 However, the reported associations differed between studies performed in different populations, harboring different STH species and measuring different markers of micronutrient status.5 Although STH species are often studied as a group, they represent distinct organisms with different life cycles and transmission patterns. Adult A. lumbricoides worms reside in the small intestine. They are the largest of the STH species, reaching up to 40 cm in length, and can obstruct the gut lumen in high-intensity infection.1 Ascaris feeds on intestinal content, not on host tissue.6 Trichuris trichiura inhabits the colon and feeds on the gut mucosa. Hookworm feeds on host blood in the upper small intestine and is therefore often indicated as an important cause of anemia.6 As the different STHs inhabit different parts of the gut and use distinct feeding mechanisms, they may have specific effects on absorption or loss of certain micronutrients. In addition, the extent of this effect may be dependent on the infection intensities of these intestinal worms. Using baseline data from a micronutrient fortification and deworming trial in rural Vietnamese schoolchildren,7 we examined the species-specific associations of STH infections with iron, hemoglobin, vitamin A, zinc, and iodine status.
METHODS
Study population.
The study was conducted in Hung Yen Province in northern Vietnam, a region with a humid, subtropical climate. Hung Yen Province is located just southeast from Hanoi, northern Vietnam, and while urbanization is on the rise, the province can be classified as poor and rural, with most families dependent on agriculture. Hung Yen Province was chosen because of the relative close distance to Hanoi, enabling daily transport of blood, urine, and stool samples, while the socioeconomic circumstances in Hung Yen are typical for most of northern Vietnam. A sample of 510 children (ages 6–9 years old) was randomly selected from a total of 642 children. Exclusion criteria were severe malnutrition, obesity, hemoglobin concentration below 80 g/L (referred to health center) chronic illness, congenital abnormalities, mental or severe physical handicap, or having received deworming treatment in the last 6 months. Baseline data were collected in January 2007. Written informed consent was obtained from parents or caregivers. Ethical approval was obtained from the Ethical Committee on Human Research of the National Institute of Nutrition (NIN), Hanoi, Vietnam, and the Human Ethics Committee of Mahidol University, Bangkok, Thailand. This study population has been described in detail elsewhere.7
Measurements.
Demographic characteristics, such as age and sex, were determined by interviewing parents or caregivers of the children by trained research assistants. The day before the survey, children were given containers and detailed instructions for stool sample collection. The next day, laboratory technicians retrieved and preserved the samples. STH infections were determined on the day of collection in stool samples by Kato-Katz (duplicate 25 mg smears of one stool) at the Medicine Laboratory Technology Co. Ltd., Hanoi, Vietnam, and were expressed as eggs per gram of stool. Hemoglobin concentrations were measured in a 2-mL nonfasting blood sample, drawn in an EDTA vacutainer, by an electronic Coulter counter (Beckman Coulter, Brea, CA) at the Medicine Laboratory Technology co. For biochemical measurements of micronutrients, 4-mL blood samples were drawn in a heparinized trace element–free tube. All samples were transported in coolboxes with icepacks. Plasma ferritin concentration was measured by enzyme-linked immunosorbent assay (ELISA) according to the kit manufacturer's protocol (Ramco Laboratories, NY) at the NIN. Plasma retinol concentration was determined by reverse-phase high-performance liquid chromatography (LC-10 ADVP, Shimadzu, Kyoto, Japan) and plasma zinc was analyzed using a flame atomic absorption spectrophotometer (GBC, Avanta+, IL) using trace element–free procedures at NIN.8 Results were verified against reference materials for zinc (Merck, Darmstadt, Germany) and iodine (BDH Laboratory Supplies, PA). Plasma C-reactive protein (CRP) was measured by ELISA according to the kit manufacturer's protocol (Diagnostic Systems Laboratories, Webster, TX). Iodine concentration was measured in urine samples, using spectrophotometric methods and was performed at the Provincial Preventive Medicine Center, Thai Nguyen, Vietnam.9 For quality control, 10% of the samples were measured in duplicate for each biochemical parameter. The within-assay variability was < 6% for all parameters and between-assay variability was < 10%.
Definitions.
STH infection intensities were categorized according to World Health Organization references.6 Ascaris infections with < 5,000 eggs/gram feces (epg) were categorized as light, 5,000–50,000 epg as moderate, and > 50,000 epg as heavy. Trichuris infections below 1,000 epg were categorized as light, 1,000–10,000 epg as moderate, and > 10,000 as heavy. Hookworm infections below 2,000 epg were defined as light, 2,000–4,000 epg as moderate, and > 4,000 epg as heavy. Vitamin A deficiency was defined as plasma retinol below 0.70 μmol/L; marginal vitamin A status was defined as retinol below 1.05 μmol/L.10 Iron deficiency was defined as plasma ferritin below 15 μg/L and anemia as hemoglobin below 115 g/L.11 Zinc deficiency was defined as plasma zinc < 9.9 μmol/L and low urinary iodine excretion was defined as < 100 μg/L.12,13 CRP was considered elevated when concentrations exceeded 5 mg/L.14
Statistical analysis.
Statistical analyses were done using SPSS version 21 (IBM, New York, NY). Double and triple infections were combined into a “multiple STH infections” group. Single and multiple infections were compared with the uninfected group in all analyses. Associations with micronutrient concentrations were estimated by linear regression with the micronutrient concentration as dependent variable. Variables ferritin and iodine were log-transformed to achieve a Gaussian distribution. Associations with deficiencies of micronutrients (as dichotomous outcome) were analyzed by logistic regression. For estimations of associations between infection intensities and micronutrient status, intensities (light, moderate, and heavy) were analyzed as categorical independent variables. In all regression analyses, the primary independent variable, STH infection, was accompanied by covariates sex, age (in months), and elevated CRP (yes/no). Interaction between two STH species was studied by adding interaction terms to the full model including the two separate species. Statistical significance was defined as P < 0.05, whereas an interaction was regarded as significant if P < 0.10.
RESULTS
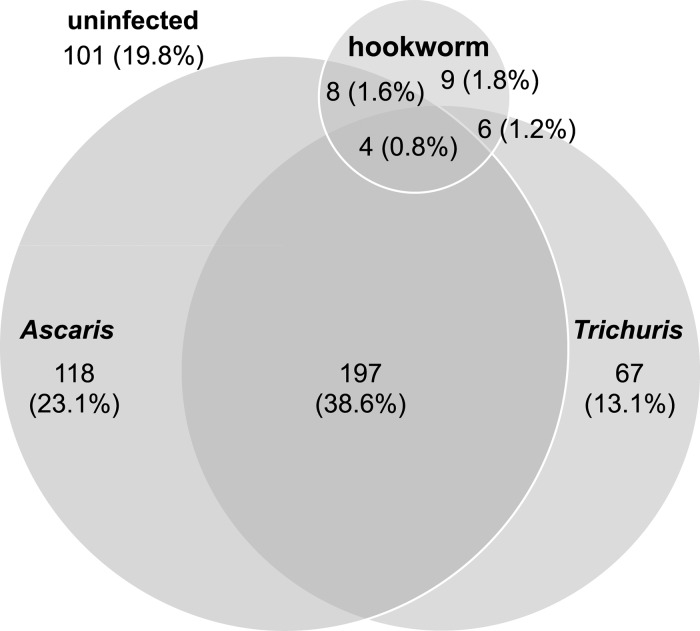
The STH infection prevalence was high (80.2%) in this study population (Table 1 ). Ascaris lumbricoides was the most common STH species (64.1%), followed by T. trichiura (53.7%) and hookworm (5.3%). Trichuris and hookworm infection intensities were generally light, whereas most Ascaris infections were of moderate intensity. Figure 1 shows the distribution of single, double, and triple STH infections among the Vietnamese schoolchildren. In this study population, children were most often found to be infected by both Ascaris and Trichuris (N = 197, 38.6%).
Table 1.
Characteristics of the study population (N = 510)
| N (%) | |
|---|---|
| Male | 243 (47.6) |
| Age* | 7.5 ± 0.9 |
| Any STH infection | 409 (80.2) |
| Ascaris lumbricoides | 327 (64.1) |
| Light (< 5,000 epg) | 116 (22.7) |
| Moderate (5,000–50,000 epg) | 198 (38.8) |
| Heavy (> 50,000 epg) | 13 (2.5) |
| Trichuris trichiura | 274 (53.7) |
| Light (< 1,000 epg) | 241 (47.3) |
| Moderate (1,000–10,000 epg) | 33 (6.5) |
| Heavy (> 10,000 epg) | 0 (0.0) |
| Hookworm | 27 (5.3) |
| Light (< 2,000 epg) | 25 (4.9) |
| Moderate (2,000–4,000 epg) | 2 (0.4) |
| Heavy (> 4,000 epg) | 0 (0.0) |
| C-reactive protein > 5 mg/L | 78 (16.7) |
epg = eggs per gram; STH = soil-transmitted helminth.
Mean ± standard deviation.
Figure 1.
Distribution of single, double, and triple soil-transmitted helminth infections among the 510 Vietnamese schoolchildren in this study population.
The study population's micronutrient status (concentrations as well as prevalences of deficiency and marginal status) by STH infection is shown in Table 2. Ascaris single infections were significantly associated with lower plasma retinol (aB = −0.10 μmol/L, 95% confidence interval [CI] = −0.17, −0.02) and higher prevalence of marginal vitamin A status (adjusted odds ratio [aOR] = 2.31, 95% CI = 1.23, 4.31). Vitamin A deficiency occurred almost exclusively in Ascaris-infected children; only two out of 52 vitamin A deficient children did not harbor an Ascaris infection. Multiple infections were also significantly associated with all three measures of vitamin A status. This was most likely attributable to the Ascaris infection, as most cases of multiple infections included Ascaris and there was no significant interaction effect on plasma retinol between Ascaris and Trichuris (aB = −0.03 μmol/L, P = 0.51) or Ascaris and hookworm (aB = 0.00 μmol/L, P = 0.98).
Table 2.
Micronutrient concentrations and prevalence of (marginal) micronutrient deficiencies among uninfected children and children with single and multiple STH infections, respectively
| STH uninfected | Ascaris single infection | Trichuris single infection | Hookworm single infection | Multiple STH infections | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| N | 101 | 118 | 67 | 9 | 215 |
| Retinol, μmol/L† | 1.13 ± 0.21 | 1.03 ± 0.30* | 1.13 ± 0.2 | 1.08 ± 0.13 | 0.98 ± 0.26* |
| Marginal vitamin A status (< 1.05 μmol/L) | 28 (34.1) | 60 (56.6)* | 21 (33.3) | 1 (11.1) | 128 (61.5)* |
| Vitamin A deficiency (< 0.70 μmol/L) | 1 (1.2) | 17 (16.0)* | 0 (0.0) | 0 (0.0) | 34 (16.3)* |
| Zinc, μmol/L† | 8.30 ± 1.93 | 8.53 ± 2.14 | 8.71 ± 2.36 | 8.45 ± 2.60 | 8.67 ± 2.19 |
| Zinc deficiency (< 9.9 μmol/L) | 70 (86.4) | 79 (75.2) | 46 (73.0)* | 6 (66.7) | 159 (76.8) |
| Hemoglobin, g/L† | 122.00 ± 5.87 | 121.08 ± 7.32 | 116.88 ± 7.51* | 117.44 ± 8.41* | 118.13 ± 7.67* |
| Anemia (Hb < 115 g/L) | 12 (11.9) | 14 (11.9) | 30 (44.8)* | 3 (33.3) | 68 (31.6)* |
| Ferritin, μg/L‡ | 61.68 (43.09–82.73) | 63.99 (41.37–88.09) | 59.89 (47.00–75.48) | 69.21 (48.00–86.55) | 65.11 (42.53–84.38) |
| Iron deficiency (ferritin < 15 μg/L) | 0 (0.0) | 3 (2.9) | 0 (0.0) | 0 (0.0) | 1 (0.5) |
| Urinary iodine, μg/L‡ | 108 (77.00–167.00) | 112.50 (79–149) | 106 (83.00–174.00) | 96.00 (77.00–255.00) | 115.00 (77.00–167.00) |
| Low urinary iodine excretion (< 100 μg/L) | 46 (45.5) | 50 (42.4) | 27 (40.3) | 5 (55.6) | 91 (42.3) |
Hb = hemoglobin; STH = soil-transmitted helminth.
Significantly different from uninfected (P < 0.05).
Mean ± standard deviation.
Median (interquartile range).
Plasma zinc concentrations were higher in children with STH infection than in those uninfected, but this difference was not significant. Zinc deficiency was found to be significantly and negatively associated with Trichuris infection (aOR = 0.39, 95% CI = 0.16, −0.94), although the associations of zinc deficiency with Ascaris and multiple infections were also borderline significant (aOR = 0.46, 95% CI = 0.21, 1.01 and aOR = 0.49, 95% CI = 0.24, 1.01, respectively).
Hemoglobin concentrations were significantly lower in children with Trichuris (aB = −5.32 g/L, 95% CI = −7.46, −3.17), hookworm (aB = −6.42 g/L, 95% CI = −11.20, −1.64) and multiple infections (aB = −4.20 g/L, 95% CI = −6.02, −2.37) than in those uninfected. No interaction effect was observed between Trichuris and hookworm infections on hemoglobin (aB = 2.72 g/L, P = 0.36). Anemia occurred significantly more often in children with Trichuris (aOR = 11.74, 95% CI = 4.17, 33.04) and multiple (aOR = 4.91, 95% CI = 2.11, 11.39) infections. However, none of the STH infections were associated with iron status as measured by plasma ferritin concentrations. No associations between urinary iodine excretion and STH infections were observed.
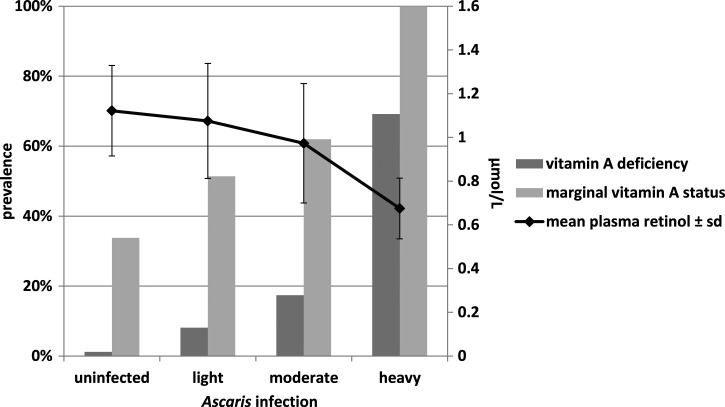
Ascaris infection intensity showed a dose-dependent association with plasma retinol concentrations (aB = −0.08 μmol/L, 95% CI = −0.1, −0.06) and with the prevalence of marginal vitamin A status (Wald 26.06, P < 0.001) (Figure 2). The same relationship was found for vitamin A deficiency (Wald 30.34, P < 0.001) (Figure 2). No intensity-dependent relationships were found for Trichuris or hookworm infections and hemoglobin concentration or for Trichuris infection and zinc deficiency.
Figure 2.
Mean plasma retinol and prevalences of marginal and deficient vitamin A status across Ascaris infection intensity categories.
DISCUSSION
This study in rural Vietnamese schoolchildren shows that associations between STH infections and micronutrient status are STH species specific. Ascaris single infections and multiple infections were significantly associated with lower retinol concentrations. Ascaris infection intensity showed an intensity-dependent relationship with plasma retinol and with prevalences of marginal vitamin A status and vitamin A deficiency.
Associations have been reported between Ascaris and xerophthalmia (dry eyes), a consequence of vitamin A deficiency.15,16 However, epidemiological studies addressing associations between Ascaris infection and vitamin A (retinol) itself have shown conflicting results.17–22 A recent study conducted in Kenya reported higher prevalence of vitamin A deficiency (defined as low retinol-binding protein) in Ascaris-infected preschool children. In accordance with our findings, moderate intensity infections had a stronger association with vitamin A deficiency than light infections did.23
Although we cannot infer causality from our cross-sectional data, we speculate that Ascaris infection might impair vitamin A absorption from the diet. Vitamin A is absorbed after emulsification with fatty acids and bile salts in the jejunum.24 Ascaris may interfere with this process as the adult worms reside in and can obstruct the jejunum. If so, this interference with absorption may extend to other fat-soluble vitamins D, E, and K. In the 1970s, small clinical studies have reported negative effects of Ascaris infection on fat and vitamin A absorption.25,26 However, a 1993 study could not confirm this theory.27 In addition, vitamin A deficiency may predispose to Ascaris infection by impairing gut mucosal immunity and integrity.28,29 However, this would increase susceptibility to STH infections in general, not specifically to Ascaris. An observational prospective study in Panama reported less Ascaris reinfection in children receiving vitamin A supplements.30 However, two randomized placebo-controlled trials found no protective effect of vitamin A supplementation on Ascaris infection.31,32 In the children in this study, both Trichuris and Ascaris reinfection rates were significantly decreased by the combination of multi-micronutrient fortified biscuits and albendazole treatment (P for interaction < 0.05).7 Only for Ascaris, significantly lower egg counts were observed after consumption of fortified biscuits combined with albendazole treatment, compared with albendazole alone.33
Hemoglobin concentrations were lower in Trichuris, hookworm, and in children infected with multiple STH species than in those uninfected. Surprisingly, these infections were not associated with ferritin concentrations. Plasma ferritin reflects the amount of intracellular iron stores. During inflammation, iron is redistributed in the body by intracellular sequestering, reducing its availability to pathogens. This phenomenon has been termed nutritional immunity, as it can reduce susceptibility to infections.34 In this study, only CRP was available as an indicator for inflammatory status, perhaps leading to incomplete adjustment of ferritin concentrations during inflammation. Ideally alpha-1-acid glycoprotein (AGP) concentration should have been included as well. Ferritin can still be increased in the convalescent phase of the acute phase response after CRP concentrations have already decreased, while AGP is still elevated.35 Furthermore, the light intensity of Trichuris and hookworm infections in this study population may have precluded a measurable decrease in plasma ferritin (and possibly other markers of micronutrient status). Alternatively, the observed anemia may not have been because of iron deficiency but a deficiency in folate or vitamin B12, which were not measured in this study.36
The observed positive association between zinc and STH infections was surprising. Like vitamin A, zinc is absorbed in the jejunum. As Trichuris is found lower in the gastrointestinal tract, this species might not interfere with zinc absorption. Nevertheless, this does not explain higher zinc concentrations in infected children. Zinc concentrations are affected by inflammation also, with lower zinc concentrations during the acute phase response.14 Perhaps the helminths have given rise to anti-inflammatory responses, leading to higher zinc concentrations in the infected children.37 As with our ferritin results, we were not able to completely adjust for inflammation because AGP data were not available.14
Our study has strengths and limitations. All three common STH infections were prevalent in this population, which meant the number of children with single infections by either Ascaris or Trichuris and with multiple infections were large enough to provide reasonable estimates for these groups separately. Also, the presence of different infection intensities, especially for Ascaris, allowed us to study dose-response relationships and may render our results more generalizable than studies with only light infection intensities or clinical cases. A limitation is the absence of AGP measurements, which would have allowed for better adjustment of micronutrient measurements for inflammation.14 Furthermore, our study does not include assessment of micronutrient intake, which could have confounded the associations between STH infections and micronutrient status. However, if the differences in micronutrient status between STH infected and uninfected children were because of differences in intake, an overall effect on susceptibility to infection would be more likely and the observed species-specific associations would be unexpected. Another possible source of confounding are coinfections by protozoa such as Giardia, which have also been implicated as cause of vitamin A deficiency and malnutrition in general.25,38,39 A limitation of the Kato Katz technique for diagnosis of STH is that light infections may remain undiagnosed, especially for hookworm.40 Thus, the light intensity of Trichuris and hookworm infections in this population may have led to an underestimation of prevalence of these species. The use of single stool samples further reduces the sensitivity of STH diagnosis.41 Therefore, possible misclassification may constrain the validity of our findings.
In conclusion, our study demonstrates STH species-specific associations with micronutrient status in schoolchildren. Moreover, this association was clearly related to infection intensity for Ascaris and vitamin A status. Hence, both prevalence and intensity of specific helminth infection species in a population may predispose to specific nutritional deficiencies. If so, then some micronutrient deficiencies, such as vitamin A deficiency, are best combated by a combination of deworming and nutritional interventions. Tailor-made public health strategies according to the STH species and micronutrient deficiencies present in a specific population may need to be designed to improve child health and nutrition in the most effective way.
Footnotes
Financial support: This study was supported by the Neys-van Hoogstraten Foundation, The Netherlands, and the Ellison Medical Foundation.
Authors' addresses: Brechje de Gier and Margot van de Bor, Department of Health and Life Sciences, Athena Institute, Vrije Universiteit (VU University) Amsterdam, The Netherlands, E-mails: b.de.gier@vu.nl and margot.vande.bor@vu.nl. Tran Thuy Nga, National Institute of Nutrition, Hanoi, Vietnam, E-mail: thuynga1997@gmail.com. Pattanee Winichagoon, Institute of Nutrition, Mahidol University, Salaya, Thailand, E-mail: pattanee.win@mahidol.ac.th. Marjoleine A. Dijkhuizen, Department of Nutrition, Exercise and Sports, University of Copenhagen, Copenhagen, Denmark, E-mail: madijkhuizen@gmail.com. Nguyen Cong Khan, Ministry of Health, Hanoi, Vietnam, E-mail: dr_nguyen_cong_khan@yahoo.com. Maiza Campos Ponce, Section Infectious Diseases, Department of Health Sciences, Vrije Universiteit (VU University) Amsterdam, The Netherlands, E-mail: m.camposponce@vu.nl. Katja Polman, Department of Biomedical Sciences, Institute of Tropical Medicine, Antwerp, Belgium and Department of Health Sciences, Vrije Universiteit (VU University) Amsterdam, The Netherlands, E-mail: kpolman@itg.be. Frank T. Wieringa, UMR-204 NutriPass IRD-UM-SupAgro, Institut de Recherche pour le Développement, Montpellier, France, E-mail: franck.wieringa@ird.fr.
References
- 1.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed T, Hossain M, Sanin KI. Global burden of maternal and child undernutrition and micronutrient deficiencies. Ann Nutr Metab. 2012;61((Suppl 1)):8–17. doi: 10.1159/000345165. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed A, Al-Mekhlafi HM, Al-Adhroey AH, Ithoi I, Abdulsalam AM, Surin J. The nutritional impacts of soil-transmitted helminths infections among Orang Asli schoolchildren in rural Malaysia. Parasit Vectors. 2012;5:119. doi: 10.1186/1756-3305-5-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crompton DW, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 5.de Gier B, Campos Ponce M, van de Bor M, Doak CM, Polman K. Helminth infections and micronutrients in school-age children: a systematic review and meta-analysis. Am J Clin Nutr. 2014;99:1499–1509. doi: 10.3945/ajcn.113.069955. [DOI] [PubMed] [Google Scholar]
- 6.Hall A, Hewitt G, Tuffrey V, de Silva N. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern Child Nutr. 2008;4((Suppl 1)):118–236. doi: 10.1111/j.1740-8709.2007.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nga TT, Winichagoon P, Dijkhuizen MA, Khan NC, Wasantwisut E, Furr H, Wieringa FT. Multi-micronutrient-fortified biscuits decreased prevalence of anemia and improved micronutrient status and effectiveness of deworming in rural Vietnamese school children. J Nutr. 2009;139:1013–1021. doi: 10.3945/jn.108.099754. [DOI] [PubMed] [Google Scholar]
- 8.Valentine AR, Tanumihardjo SA. Adjustments to the modified relative dose response (MRDR) test for assessment of vitamin A status minimize the blood volume used in piglets. J Nutr. 2004;134:1186–1192. doi: 10.1093/jn/134.5.1186. [DOI] [PubMed] [Google Scholar]
- 9.Gnat D, Dunn AD, Chaker S, Delange F, Vertongen F, Dunn JT. Fast colorimetric method for measuring urinary iodine. Clin Chem. 2003;49:186–188. doi: 10.1373/49.1.186. [DOI] [PubMed] [Google Scholar]
- 10.de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr. 2002;132:2895S–2901S. doi: 10.1093/jn/132.9.2895S. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . In: Iron Deficiency Anaemia: Assessment, Prevention and Control: A Guide for Programme Managers. World Health Organization, editor. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 12.International Zinc Nutrition Consultative Group (IZiNCG) Assessment of the risk of zinc deficiency in populations and options for its control. In: Hotz C, Brown KH, editors. Food and Nutrition Bulletin. Tokyo, Japan: United Nations University Press; 2004. [PubMed] [Google Scholar]
- 13.Laurberg P. Iodine. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, editors. Modern Nutrition in Health and Disease. Baltimore, MD: Lippincott Williams and Wilkins; 2014. pp. 217–224. [Google Scholar]
- 14.Thurnham DI. Interactions between nutrition and immune function: using inflammation biomarkers to interpret micronutrient status. Proc Nutr Soc. 2014;73:1–8. doi: 10.1017/S0029665113003662. [DOI] [PubMed] [Google Scholar]
- 15.Pal R, Sagar V. Antecedent risk factors of xerophthalmia among Indian rural preschool children. Eye Contact Lens. 2008;34:106–108. doi: 10.1097/ICL.0b013e3181379fd7. [DOI] [PubMed] [Google Scholar]
- 16.Curtale F, Pokhrel RP, Tilden RL, Higashi G. Intestinal helminths and xerophthalmia in Nepal. A case-control study. J Trop Pediatr. 1995;41:334–337. doi: 10.1093/tropej/41.6.334. [DOI] [PubMed] [Google Scholar]
- 17.Muniz-Junqueira MI, Queiroz EF. Relationship between protein-energy malnutrition, vitamin A, and parasitoses in living in Brasilia. Rev Soc Bras Med Trop. 2002;35:133–141. doi: 10.1590/s0037-86822002000200002. [DOI] [PubMed] [Google Scholar]
- 18.Friis H, Mwaniki D, Omondi B, Muniu E, Magnussen P, Geissler W, Thiong'o F, Michaelsen KF. Serum retinol concentrations and Schistosoma mansoni, intestinal helminths, and malarial parasitemia: a cross-sectional study in Kenyan preschool and primary school children. Am J Clin Nutr. 1997;66:665–671. doi: 10.1093/ajcn/66.3.665. [DOI] [PubMed] [Google Scholar]
- 19.Persson V, Ahmed F, Gebre-Medhin M, Greiner T. Relationships between vitamin A, iron status and helminthiasis in Bangladeshi school children. Public Health Nutr. 2000;3:83–89. doi: 10.1017/s1368980000000100. [DOI] [PubMed] [Google Scholar]
- 20.Kongsbak K, Wahed MA, Friis H, Thilsted SH. Acute-phase protein levels, diarrhoea, Trichuris trichiura and maternal education are predictors of serum retinol: a cross-sectional study of children in a Dhaka slum, Bangladesh. Br J Nutr. 2006;96:725–734. [PubMed] [Google Scholar]
- 21.Rai SK, Nakanishi M, Upadhyay MP, Hirai K, Ohno Y, Ono K, Uga S, Shrestha HG, Matsumura T. Effect of intestinal helminth infection on retinol and beta-carotene status among rural Nepalese. Nutr Res. 2000;20:15–23. [Google Scholar]
- 22.Taren DL, Nesheim MC, Crompton DWT, Holland CV, Barbeau I, Rivera G, Sanjur D, Tiffany J, Tucker K. Contributions of ascariasis to poor nutritional-status in children from Chiriqui Province, Republic of Panama. Parasitology. 1987;95:603–613. doi: 10.1017/s0031182000058029. [DOI] [PubMed] [Google Scholar]
- 23.Suchdev PS, Davis SM, Bartoces M, Ruth LJ, Worrell CM, Kanyi H, Odero K, Wiegand RE, Njenga SM, Montgomery JM, Fox LM. Soil-transmitted helminth infection and nutritional status among urban slum children in Kenya. Am J Trop Med Hyg. 2014;90:299–305. doi: 10.4269/ajtmh.13-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross AC. Vitamin A. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, editors. Modern Nutrition in Health and Disease. Baltimore, MD: Lippincott Williams and Wilkins; 2014. [Google Scholar]
- 25.Mahalanabis D, Simpson TW, Chakraborty ML, Ganguli C, Bhattacharjee AK, Mukherjee KL. Malabsorption of water miscible vitamin A in children with giardiasis and ascariasis. Am J Clin Nutr. 1979;32:313–318. doi: 10.1093/ajcn/32.2.313. [DOI] [PubMed] [Google Scholar]
- 26.Tripathy K, Duque E, Bolanos O, Lotero H, Mayoral LG. Malabsorption syndrome in ascariasis. Am J Clin Nutr. 1972;25:1276–1281. doi: 10.1093/ajcn/25.11.1276. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed F, Mohiduzzaman M, Jackson AA. Vitamin A absorption in children with ascariasis. Br J Nutr. 1993;69:817–825. doi: 10.1079/bjn19930082. [DOI] [PubMed] [Google Scholar]
- 28.McCullough FS, Northrop-Clewes CA, Thurnham DI. The effect of vitamin A on epithelial integrity. Proc Nutr Soc. 1999;58:289–293. doi: 10.1017/s0029665199000403. [DOI] [PubMed] [Google Scholar]
- 29.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Payne LG, Koski KG, Ortega-Barria E, Scott ME. Benefit of vitamin A supplementation on Ascaris reinfection is less evident in stunted children. J Nutr. 2007;137:1455–1459. doi: 10.1093/jn/137.6.1455. [DOI] [PubMed] [Google Scholar]
- 31.Al-Mekhlafi HM, Anuar TS, Al-Zabedi EM, Al-Maktari MT, Mahdy MA, Ahmed A, Sallam AA, Abdullah WA, Moktar N, Surin J. Does vitamin A supplementation protect schoolchildren from acquiring soil-transmitted helminthiasis? A randomized controlled trial. Parasit Vectors. 2014;7:367. doi: 10.1186/1756-3305-7-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long KZ, Rosado JL, Montoya Y, de Lourdes Solano M, Hertzmark E, DuPont HL, Santos JI. Effect of vitamin A and zinc supplementation on gastrointestinal parasitic infections among Mexican children. Pediatrics. 2007;120:e846–e855. doi: 10.1542/peds.2006-2187. [DOI] [PubMed] [Google Scholar]
- 33.Nga TT, Winichagoon P, Dijkhuizen MA, Khan NC, Wasantwisut E, Wieringa FT. Decreased parasite load and improved cognitive outcomes caused by deworming and consumption of multi-micronutrient fortified biscuits in rural Vietnamese schoolchildren. Am J Trop Med Hyg. 2011;85:333–340. doi: 10.4269/ajtmh.2011.10-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg ED. Nutritional immunity. Host's attempt to withhold iron from microbial invaders. JAMA. 1975;231:39–41. doi: 10.1001/jama.231.1.39. [DOI] [PubMed] [Google Scholar]
- 35.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr. 2010;92:546–555. doi: 10.3945/ajcn.2010.29284. [DOI] [PubMed] [Google Scholar]
- 36.Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet. 2011;378:2123–2135. doi: 10.1016/S0140-6736(10)62304-5. [DOI] [PubMed] [Google Scholar]
- 37.Finlay CM, Walsh KP, Mills KH. Induction of regulatory cells by helminth parasites: exploitation for the treatment of inflammatory diseases. Immunol Rev. 2014;259:206–230. doi: 10.1111/imr.12164. [DOI] [PubMed] [Google Scholar]
- 38.Quihui-Cota L, Astiazaran-Garcia H, Valencia ME, Morales-Figueroa GG, Lopez-Mata MA, Vazquez Ortiz F. Impact of Giardia intestinalis on vitamin a status in schoolchildren from northwest Mexico. Int J Vitam Nutr Res. 2008;78:51–56. doi: 10.1024/0300-9831.78.2.51. [DOI] [PubMed] [Google Scholar]
- 39.Verhagen LM, Incani RN, Franco CR, Ugarte A, Cadenas Y, Sierra Ruiz CI, Hermans PW, Hoek D, Campos Ponce M, de Waard JH, Pinelli E. High malnutrition rate in Venezuelan Yanomami compared to Warao Amerindians and Creoles: significant associations with intestinal parasites and anemia. PLoS One. 2013;8:e77581. doi: 10.1371/journal.pone.0077581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikolay B, Brooker SJ, Pullan RL. Sensitivity of diagnostic tests for human soil-transmitted helminth infections: a meta-analysis in the absence of a true gold standard. Int J Parasitol. 2014;44:765–774. doi: 10.1016/j.ijpara.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, Rollinson D, Marti H, Utzinger J. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis. 2008;2:e331. doi: 10.1371/journal.pntd.0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]