Abstract
Direct observation of Leishmania parasites in tissue aspirates has shown low sensitivity for the detection of canine visceral leishmaniasis (VL). Therefore in the last quarter century immunoenzymatic tests have been developed to improve diagnosis. The purpose of this study was to develop a fast recombinant K28 antigen, naked-eye qualitative enzyme-linked immunosorbent assay (VL Ql-ELISA) and a quantitative version (VL Qt-ELISA), and to display it in a kit format, whose cutoff value (0.156) was selected as the most adequate one to differentiate reactive from nonreactive samples. Considering 167 cases and 300 controls, sensitivity was 91% for both assays and specificity was 100% and 98.7% in Ql-ELISA and Qt-ELISA, respectively. Positive predictive values were 100% and 97.4% for Ql-ELISA and Qt-ELISA, respectively, and negative predictive values were 95.2% for both ELISAs. Reagent stability, reliability studies, including periodic repetitions and retest of samples, cutoff selection, and comparison of rK28 ELISAs with rK39 immunochromatographic test, were the international criteria that supported the quality in both kits. The performance of both ELISA kits in this work confirmed their validity and emphasized their usefulness for low-to-medium complexity laboratories.
Introduction
Visceral leishmaniasis (VL), a severe infectious vector-borne disease, is caused by Leishmania infantum, an obligatory intracellular protozoon. In Latin America, autochthonous VL cases have been reported in Argentina, Bolivia, Brazil, Colombia, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, and Venezuela.1 In Argentina, 103 cases have been diagnosed since 2006 in Misiones, Corrientes, Santiago del Estero, and Salta; mortality rate was 9–10%,2 primarily due to late diagnosis and treatment.
In this region, VL is a zoonosis, with domestic dogs as the main reservoirs in the transmission cycle. The development of diagnostic tools to detect the disease in dogs is considered one of the keys for effective control measures of VL.3,4 The gold standard in VL diagnosis is the visualization of Leishmania amastigotes in tissue samples, such as bone marrow or lymph nodes and spleen aspirates, by means of smears, cultures, or histopathology.5 The detection of the parasite demands both long time devoted to the search, as well as operators who are skilled in this procedure. Rapid and more efficient serological tests have been developed and validated as a tool to supplement the microscopic diagnosis.6,7
Immunoserologic studies for VL in dogs, such as direct agglutination test (DAT),8 indirect immunofluorescence assay (IFA),4 enzyme-linked immunosorbent assay (ELISA),6,7 polymerase chain reaction,9 and immunochromatographic tests6,7 (ICTs) were adapted from assays validated in humans,10–13 with different levels of performance in canine VL.
A recombinant fusion protein with tandem repeats of k39 kinesin regions and K26/HASPb1 when used in ELISA was found to detect high levels of antibody responses in infected patients.14–16 A new-generation fusion antigen—named rK28—used for ELISA and for ICT has shown sensitivity and specificity values similar to or higher than those described for rK39. Hence, this antigen has been proposed as a new option, especially in regions where rK39 has shown low levels of sensitivity. Furthermore, the validity of this ELISA test in canine leishmaniasis has not been reported. Chagas disease is the most common disease in Argentina that cross-reacts with diagnostic tests for leishmaniasis in humans and dogs.17 This situation was a compelling factor driving the current study, aimed at finding more specific serological tools to diagnose leishmaniasis, without cross-reactions in subjects with Chagas disease. An easy-to-handle kit that does not demand specialized laboratory equipment to be used in the field is a realistic option for the control of VL in Latin America.
The goal of our study was to develop two ELISA protocols for canine VL using the recombinant K28 protein. This antigen was used in a conventional in-house ELISA protocol for equipped laboratories. Other protocol was an ELISA with a commercial substrate–coated plate that can be visually evaluated in the field. The latter involves two basic adaptations: 1) a dehydrated rK28 antigen–coated plate, which is stable at refrigerator temperature and 2) a diluted conjugate ready for use and suitable for mass distribution.
MATERIALS AND METHODS
Sample collection.
Positive and negative standard control sera: a total of 467 coded samples of sera from dogs of various ages and breeds were used. Aliquots of fresh samples and samples diluted in glycerol [9/10 glycerol–1/10 phosphate buffer 10× (pH 7.2–7.4)]18 stored at −40°C and −20°C were used for rK39 ICT and ELISA, respectively.
The criteria for VL cases (N = 167/467) used as sources of VL reactive sera were dogs that had lived in VL-endemic areas and that had parasitological evidence of L. infantum infection, detected either by culture or tissue microscopic observation. Ten of them came from Para and Minas Gerais, Brazil, both VL-endemic areas, whereas the remaining cases (N = 157) came from Posadas, Misiones, in Argentina. Control dogs were classified in three groups: group 1 was subdivided into subgroups A and B, according to geographical, epidemiological and clinical criteria, as follows (Table 1 ). Group 1: dogs living in the city of Buenos Aires (N = 121), a non-endemic area for VL, treated at the Zoonosis Institute “Dr. Luis Pasteur.” These included groups of (A) healthy dogs (N = 80) and (B) ill dogs (N = 41), affected by infectious and other diseases including distemper, brucellosis, bacterial infections, and noninfectious pathologies as neoplasm, anemias, renal failure, urolithiasis, Cushing syndrome, heart disease, vestibular syndrome, prolapsed organs, and inflammatory diseases; group 2: Trypanosoma cruzi-infected dogs (N = 108) from rural areas of Santiago del Estero and Chaco, both endemic Argentinean provinces for Chagas disease, which were diagnosed using two or three serological tests.19,20 Group 3 corresponds to T. cruzi-noninfected dogs living in Chaco (N = 71) in the same area as those dogs mentioned in group 2.
Table 1.
Sensitivity and overall specificity for rK28 VL Qt-ELISA, rK28 naked-eye VL Ql-ELISA, and rK39 ICT
| Dog sera origin (N = 466) | rK28 VL ELISA | ICT | ||
|---|---|---|---|---|
| Qt-ELISA | Ql-ELISA | rK39 | ||
| Reactive sera/nonreactive sera (%) (95% CI) | ||||
| Cases (N = 167) | Sensitivity | |||
| Misiones Province, Argentina (n = 157); Pará and Minas Gerais states, Brazil (n = 10) (VL-endemic areas) VL-infected dogs | 152/167 (91.0) (85.6–94.9) | 152/167 (91.0) (85.6–94.9) | 131/151 (86.7) (80.3–91.7) | |
| Nonreactive sera/nonreactive sera (%) | ||||
| Controls (N = 300) | Group 1 | |||
| A) Buenos Aires city (non-endemic areas for VL and Chagas disease) healthy dogs, n = 80 | 80/80 (100) | 80/80 (100) | 80/80 (100) | |
| B) Buenos Aires city T. cruzi-noninfected dogs affected by other pathologies, n = 41 | 41/41 (100) | 41/41 (100) | 39/40 (97.5) | |
| Group 2 | ||||
| Chaco and Santiago del Estero provinces (endemic areas for Chagas disease) T. cruzi-infected dogs, n = 108 | 104/108 (96.3) | 108/108 (100) | 107/108 (99.1) | |
| Group 3 | ||||
| Chaco Province T. cruzi-noninfected dogs, n = 71 | 71/71 (100) | 71/71 (100) | 68/68 (100) | |
| Overall specificity, N = 300 (%) (95% CI) | 296/300 (98.7) (96.6–99.6) | 300/300 (100) (98.8–100.0) | 294/296 (99.3) (97.6–99.9) | |
ICT = immunochromatographic test; Ql-ELISA = qualitative enzyme-linked immunosorbent assay; Qt-ELISA = quantitative ELISA; VL = visceral leishmaniasis.
Laboratory procedures.
Trypanosoma cruzi serology in dogs was performed by using T. cruzi antigens in IFA, ELISA, and indirect hemaglutination assay to evaluate the infection status of dogs.19
VL serology in dogs was performed using rK39 ICT (Kalazar Detect Canine Rapid Test, lot no. JM 1062 and PM 1020 from Inbios International, Inc., Seattle, WA) with manufacturer's instructions to process 151/167 case sera samples and 296/300 VL noninfected control dog sera.
Two immunoenzymatic tests were used: a quantitative ELISA protocol (VL Qt-ELISA), using o-phenylenediamine (OPD) as substrate and a naked-eye qualitative ELISA (VL Ql-ELISA), using a commercial ABTS 2-component peroxidase substrate kit (KPL, Inc., Gaithersburg, MD). The results of both ELISA protocols were compared with those obtained with the rK39 ICT (Canine Kalazar Detect; Inbios International, Inc.). The recombinant K28 antigen (rK28)14–16 was supplied by the Infectious Disease Research Institute (Seattle, WA). The lyophilized sample of rK28 antigen was restored to 1 mL, in 10 aliquots and stored at −70°C. Plates were coated with 100 μL/well rK28 antigen in various dilutions (0.25–2.5 μg/mL) to choose the minimal titer to be used. It is always advisable to use the rK28 antigen in excess; in this case, 2.5 μg/mL was used to determine the title conjugate.18
Two reactive (high and low absorbance values) and two nonreactive sera were chosen as controls.
For the in-house quantitative OPD ELISA method, the procedure described above21 was used without significant changes: plates were washed 4× after each step, rK28 antigen–coated plates stored at −20°C were blocked with phosphate-buffered saline (PBS)–Tween 20 0.1% (w/v), bovine serum albumin (BSA) 1% (v/v), plus 1/400 PBS–Tween 20 (0.1%) and BSA (0.1%), and serum samples diluted in the same volume of glycerol were added; the plates thus prepared were shaken as usual for 1 hour; after that, peroxidase-conjugated AffiniPure Rabbit Anti-Dog IgG (H+L) (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) was added in dilutions of 1/50,000 in serum diluent buffer (PBS/0.1% BSA/0.1% Tween 20).
The cutoff point to determine reactivity was calculated by using 300 nonreactive VL control sera as follows: 1) the 99th percentile of negative sera, 2) mean value of the negative controls plus two standard deviations, and 3) the intersection point of the sensitivity and specificity curves for different cutoff values.
Standardized requirements to validate a diagnostic kit for quantitative measures are as follows. The evaluation methodology for diagnostic tests was carried out according to Fegan22 and Banoo and others.23 Results of these tests should be reproducible, considering that 1) the same sample was processed several times by one operator (internal consistency, reliability), 2) the same sample was processed by two or more independent operators (interoperator reliability), 3) the same sample was processed by the same operator during successive days (periodic variability), and 4) the same sample was evaluated by two different assays (test–retest). In case 1, six VL-positive sera and five negative control sera were evaluated three times on the same plate and on the same day. In case 2, five positive and five negative control sera were evaluated by two operators on the same day. In case 3, the assay was carried out during three consecutive days, using the same number of control sera as in 2. In case 4, the results of 50 randomly selected case samples were compared with both rK28 ELISAs and the rK39 ICT for an expected concordance of 90% or higher.
In cases where results from the original and duplicate from each serum showed differences higher than 15%, ELISA was repeated twice, and the mean of those new four values was used.
For the naked-eye VL Ql-ELISA protocol, rK28 antigen–coated plates were blocked, washed 4× and stored at −20°C until used. Samples were diluted as described, and a 1/7,500 dilution of the same conjugate and ABTS Peroxidase Substrates (A+B) (KPL, Inc.) were used as recommended. Blue green–colored and non-colored wells corresponded to reactive and nonreactive sera, respectively. The time devoted to process one plate was 3 hours.
The most relevant steps in the development of the naked-eye VL Ql-ELISA kit were both the coating/dehydration of the antigen on the plate and a stable dilution of the conjugate. 1) The rK28 antigen was added to the flat-bottom plates, dehydrated overnight inside a laminar flow, packed within a metallic envelope, and stored at 4–8°C and tested monthly to determine its expiration date; and 2) a stable preservation solution containing a phosphate 0.1 M pH 7.5, BSA 1% buffer–thimmerosal 50 mg/mL was used to dilute the anti-dog IgG peroxidase to determine the best dilution (1/50 to1/300) that remains usable at 4–8°C for the longest period.
Concordance of results between VL Qt-ELISA, VL Ql-ELISA, and rK39 ICT was quantified according to the Youden's index.24
Data analysis.
Statistical analyses of sensitivity, specificity, and positive/negative predictive values for each diagnostic test were calculated according to Altman and Bland.25,26
For calculating predictive values, a 20% VL canine prevalence in Posadas was a necessary requirement to calculate those values during the study period, 2010–2012, (V. Fragueiro Frias, C. Romagosa, A. Sinagra, C. Luna, C.A. Pravia, V. Negri, R. Gacek, F.A. Infran, O. Almada, T. Rottoli, L. Esquivel, M. Quaglino, A.M. Ruiz, L. Tartaglino, and A. Riarte, unpublished data).
The t test for unpaired samples and the χ2 test were applied according to the type of analysis required. A P value ≤ 0.05 was considered significant to determine differences between samples. All statistical measures25,26 were performed using GraphPad Prism 5 (La Jolla, CA).
RESULTS
ELISA standardization.
Visceral leishmaniasis Qt-ELISA.
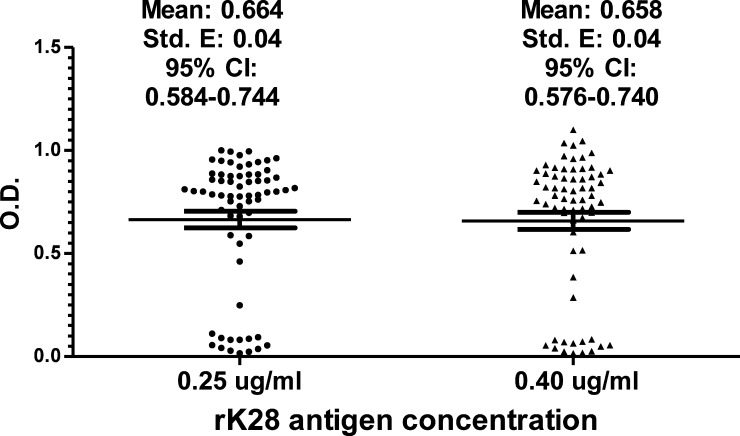
With rK28 antigen, used in excess (2.5 μg/mL), a titer of 1/50,000 of the peroxidase conjugate was chosen as the most adequate concentration for a quantified colorimetric reaction. Once this conjugate value had been selected, the minimal concentration was chosen for the antigen so that the test was positive for case samples. Two candidate antigen concentrations (0.25 and 0.40 μg/mL) used in the reaction showed appropriate results when testing VL case sera. To retest the concentration of rK28 antigen, 66 randomly selected case sera were used. Since no differences were observed in absorbance values (neither mean nor median values) for both concentrations, 0.25 μg/mL was selected (Figure 1). Three cutoff values—0.312, 0.156, and 0.100—were determined (according to Materials and Methods) and the value of 0.156 (the mean value plus two standard deviations), was selected as the best one to discriminate case versus control populations.
Figure 1.
Optical densities (ODs) obtained from 66 randomly selected serum case samples by rK28 quantitative visceral leishmaniasis enzyme-linked immunosorbent assay showed no differences by using 0.25 and 0.40 μg/mL rK28 antigen concentrations. Horizontal lines represent mean and 95% confidence interval [CI].
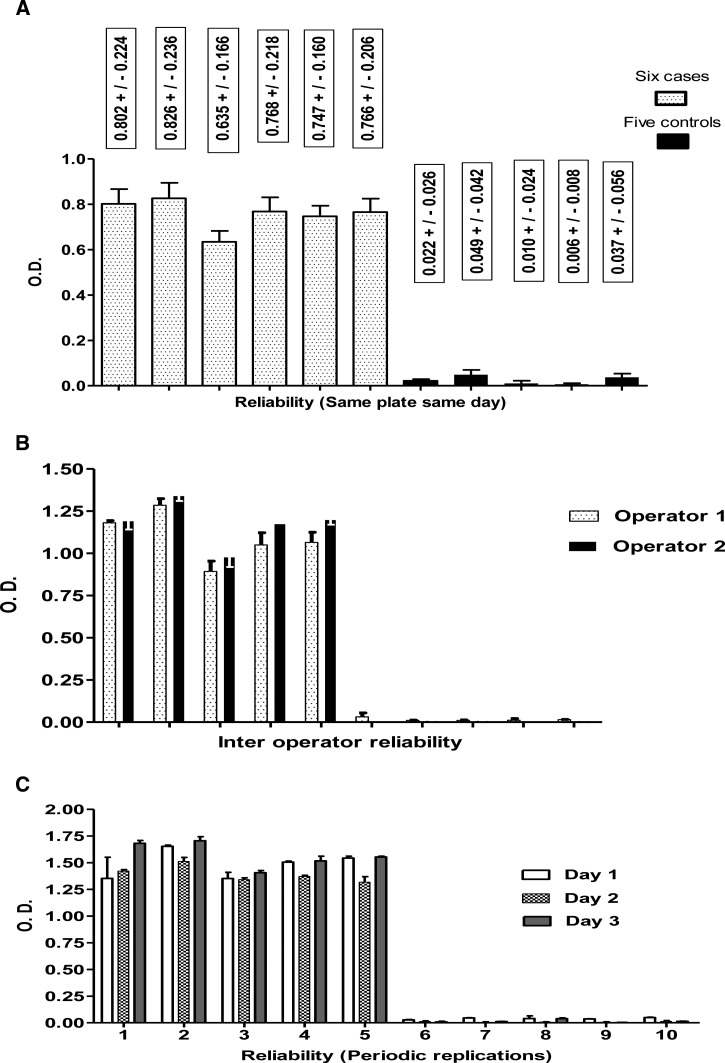
Reliability studies for VL Qt-ELISA showed no differences in systematic repetitions of samples on the same day (Figure 2A), interoperator repetitions (Figure 2B) and periodic repetitions of samples (Figure 2C). The test–retest of 50 case samples showed a concordance of 96% (48/50) between rK39 ICT and VL Qt-ELISA and of 94% (47/50) between rK39 ICT and VL Ql-ELISA (data not shown).
Figure 2.
Standardized requirements to validate a diagnostic kit. Reliability of rK28 quantitative visceral leishmaniasis enzyme-linked immunosorbent assay by means of (A) serial replications (confidence interval 95%), (B) interoperator replications, and (C) periodic replications.
Visceral leishmaniasis Ql-ELISA.
The 1/7,500 conjugate dilution was selected as the minimum concentration that showed a visibly clear discrimination between reactive and nonreactive control sera 10 minutes after the addition of ABTS Peroxidase Substrates (KPL, Inc.), for both 0.25 and 0.40 μg/mL rK28 antigen concentrations.
A 1/50 diluted conjugate remained stable for 120 days at 4–8°C since no significant differences were observed when peroxidase-conjugated anti-dog IgG was prepared at a 1/7,500 titer to be used in the assay from a pure batch and a refrigerated 1/50 dilution.
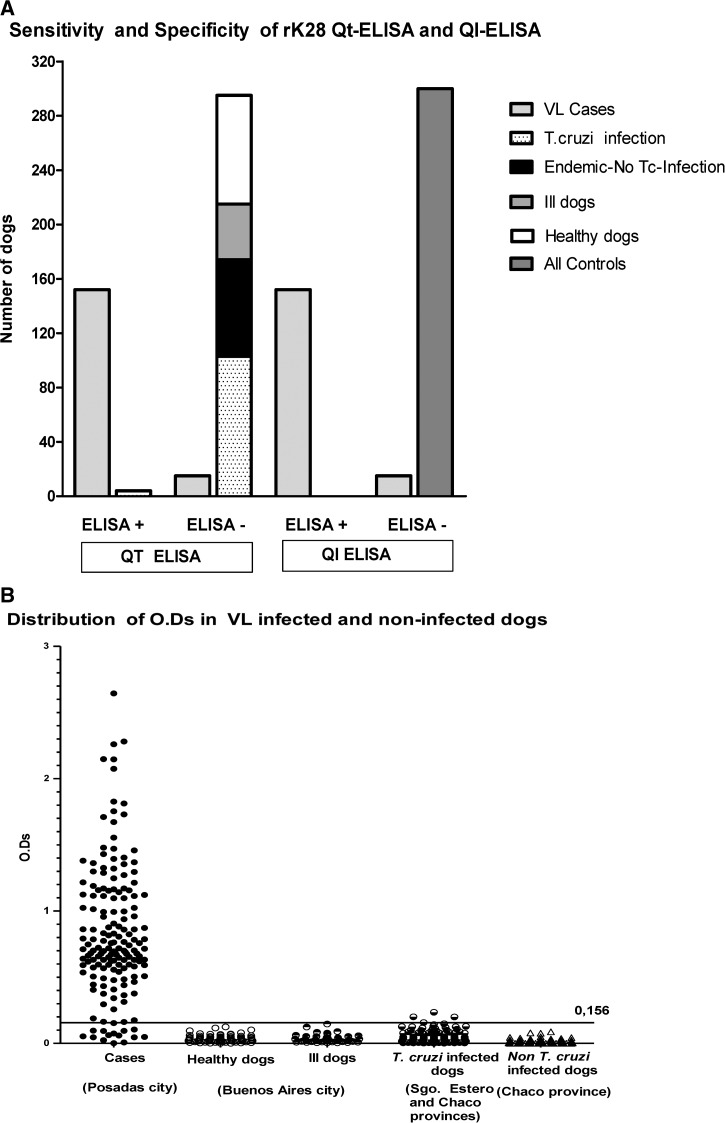
The case–control design that compares VL Qt-ELISA and VL Ql-ELISA data is represented in Figure 3. True positive cases and negative controls, as well as false positive and false negative data are represented in Figure 3A. The optical density dispersion of cases and controls can be clearly visualized in Figure 3B.
Figure 3.
rK28 visceral leishmaniasis quantitative (VL) quantitative enzyme-linked immunosorbent assay (Qt-ELISA) and rK28 naked-eye qualitative ELISA (VL Ql-ELISA) by using case samples (167) and controls (300). (A) Comparative data between Qt-ELISA and Ql-ELISA. False positive and negative results were observed in Qt-ELISA. Only false-negative results were observed in Ql-ELISA. (B) Case–control design. Cutoff was calculated by three methods and only one (0.156) is shown. Trypanosoma cruzi-infected dogs came from Argentinian endemic Chaco and Santiago del Estero provinces.
Table 1 shows the confidence intervals for sensitivity and specificity in both VL Qt-/Ql-ELISAs in comparison with rK39 ICT. A sensitivity of 91.0% (152/167) for case samples was obtained for both ELISAs and 86.7% (131/151) for rK39 ICT. The specificity of samples from healthy and ill dogs from the non-endemic area and from T. cruzi-noninfected dogs from the endemic area was 100% for both ELISAs, and 97.5% (39/40) for rK39 ICT, because a dog from a non-endemic area was affected by a condition unrelated to Chagas (brain neoplasm). The specificity of samples from T. cruzi-infected dogs was 96.3%, 100%, and 99.1%, respectively for Qt-ELISA, Ql-ELISA, and rK39 ICT, respectively. Overall specificity levels of 98.7% (296/300), 100% (300/300), and 99.3% (294/296) were obtained for Qt-ELISA, Ql-ELISA, and rK39 ICT, respectively.
Stability of plates coated with rK28 antigen stored at −20°C for VL Qt-ELISA or dehydrated and stored at 4–8°C for VL Ql-ELISA has been demonstrated for 1 year.
Positive predictive values of 97.4%, 100%, and 98.5% were obtained for Qt-ELISA, Ql-ELISA, and rK39 ICT, respectively, and the corresponding negative predictive values were 95.2%, 95.2%, and 93.6%. In sera samples from T. cruzi-infected dogs, positive/negative predictive values were 97.4%/87.4%, 100%/87.8%, and 99.2%/84.2% for Qt-ELISA, Ql-ELISA, and rK39 ICT, respectively.
The reproducibility values corresponding to Youden's indexes for Qt-ELISA, Ql-ELISA, and rK39 ICT were 0.90, 0.91, and 0.86, respectively.
DISCUSSION
The serological diagnosis of infectious diseases is key to carry out a differential diagnosis for leishmaniasis, to study susceptible populations, and to evaluate clinical treatments, all essential factors for surveillance of infectious diseases. Serology for VL and Chagas disease has become an irreplaceable ally for control in vector-borne diseases, due to the low sensitivity when it comes to detecting the respective parasites in tissues.
Among various options for canine VL serology, the immunoenzymatic assays using Leishmania crude,7,27 chimeric,28 rK39,29 or rK2830 antigens have offered a number of serological tools. In this study, we presented two kits, a VL Qt-ELISA, a conventional OPD version ELISA, and an innovative naked-eye VL Ql-ELISA to diagnose canines, discriminating VL infected from noninfected dogs, the most important reservoirs in domestic cycle of Leishmania. The sensitivity attained was 91% for both versions, and specificity was 100% for naked-eye VL Ql-ELISA kit, which represents an increase in specificity compared to the quantitative kit; they have shown a good performance, both for field research and for VL canine diagnosis.
In Latin America, including Argentina, there is an eco-epidemiologic overlapping of endemic areas between Chagas disease and VL.31 Thus, a great amount of sera from T. cruzi infected and noninfected dogs from endemic and non-endemic provinces was used to determine the overall specificity in both kits. Most sera from T. cruzi-infected dogs were nonreactive to either ELISA. Only four sera from Santiago del Estero and Chaco showed cross-reactivity and achieved a specificity of 96.3% and 100% for Qt-ELISA and Ql-ELISA, respectively, in the T. cruzi-infected dog group. In spite of this feature, the overall specificity of rK28 Qt- and Ql-ELISAs showed a great performance (98.7% and 100%, respectively).
Regarding adaptation of ELISAs for humans to the canine diagnosis, those tests did not guarantee their usefulness in canine sera. In this study, an ELISA tool used for humans was standardized for dogs. A number of methodological pillars sustained our data: 1) we selected the best cutoff point to enhance specificity without decreasing sensitivity; 2) the naked-eye VL Ql-ELISA was supported by the antigen performance, the standardization of the reagents, and the reliability of the assay, by means of the quantitative version of OPD; and 3) high positive and negative predictive values for both ELISAs have indicated their strength, demonstrating VL infection when serum is reactive and absence of infection when it is nonreactive, suitable for field studies.
In dogs, rK39 ICT has proven effective in the VL diagnosis (Inbios International, Inc.) showing a variable specificity of 77.8–100%. Low yield with the same antigen was observed with VL dipstick test (Rapydtest, Wokingham, United Kingdom).7 In this study, although rK39 ICT showed a good efficiency, sensitivity was higher in Qt- and Ql-ELISAs.
In the last 15 years, the usefulness of novel synthetic fusion proteins,14 as well as developments in molecular biology10 in human diagnosis, have triggered advances and a great deal of knowledge, with more effective immunochromatographic tools, so that the use of these techniques has strengthened VL control, especially in massive screening studies,11 inducing very significant progress in surveillance, diagnosis, and epidemiology in human VL all over the world. Serologic assays confined to human diagnosis, such as IFA. ELISA based on crude or recombinant antigens, DAT, and freeze-dried DAT, are nowadays also being used for canine diagnosis.6 The sensitivity/specificity values, the comparison between techniques,8 and the detection of cross-reactions and reliability8 often drew an incomplete quality profile in the VL canine diagnosis. In this article, we made an attempt to improve that profile. Reagent stability, adjusted intra-/interoperator variability, cutoff selection, and comparison with ICT tools were the international criteria that supported the quality in both kits.32 The low cross-reactivity (less than 4%) with T. cruzi-infected dog sera suggested the potential good performance of the kits in the VL epidemiological area.
This powerful current phase II study has used near 500 canine sera samples. The usefulness of these tests in areas that are distant from big cities could contribute to the diagnosis and knowledge on VL prevalence in dogs in endemic areas.7,8,29
The naked-eye VL Ql-ELISA version presented here does not required skilled personnel or complex laboratory equipment. Good performance and effectiveness in the diagnosis of VL canine reservoir are based on an easy, user-friendly, sensitive, and specific assay for medium-complexity laboratories.
ACKNOWLEDGMENTS
We are grateful to Laura Von Steiger and Federico Infran from the IMUSA, Posadas City (Misiones, Argentina), for contributing in the storage of sera and to all IMUSA personnel who collaborated with technical support. We also thank Ana María De Rissio and Gabriela García for diagnostic and clinical advices. We also want to thank to IDRI for the generous provision of the rK28 antigen.
Footnotes
Financial support: This project received support from OPS/OMS (PAHO-2010-05-0016) (SGP0924) and Focanlis (ANLIS) 2009.
Authors' addresses: Marta Alicia Lauricella, Cristina Graciela Maidana, Victoria Fragueiro Frias, Vanesa Negri, Ruben Benedetti, Angel J. Sinagra, Concepcion Luna, Susana Laucella, and Adelina R. Riarte, Instituto Nacional, de Parasitología “Dr. Mario Fatala Chaben,” ANLIS, Buenos Aires, Argentina, E-mails: mlauri_2000@yahoo.es, cgmaidana_1999@yahoo.com, vickyfragueiro@hotmail.com, vanesanegri@hotmail.com, rubenedetti@yahoo.com, ajsinagra@yahoo.com.ar, caluna00@yahoo.com, slaucella@yahoo.com, and ariarte@yahoo.com. Carlo M. Romagosa and Lilian Tartaglino, Secretaría de Calidad de Vida, Municipalidad de Posadas, Misiones, Argentina, E-mails: carlomroma@yahoo.com.ar and lilitartaglino@hotmail.com. Steven G. Reed, Infectious Disease Research Institute, Seattle, WA, E-mail: sreed@idri.org.
References
- 1.Arias J, Beltran F, Desjeux P, Walton B. Epidemiología y Control de la Leishmaniasis en las Américas, por País o Territorio. Cuaderno Técnico No. 44. Washington, DC: Organización Panamericana de la Salud; 1996. pp. 1–52. [Google Scholar]
- 2.Gould IT, Perner MS, Santini MS, Saavedra SB, Bezzi G, Maglianese MI, Antman JG, Gutiérrez JA, Salomón OD. Leishmaniasis visceral en la Argentina. Notificación y Situación vectorial (2006–2012) Medicina (Buenos Aires) 2013;73:104–110. [PubMed] [Google Scholar]
- 3.Palatnik-de-Sousa CB, Batista-de-Melo LM, Borja-Cabrera GP, Palatnik M, Lavor CC. Improving methods for epidemiological control of canine visceral leishmaniasis based on a mathematical model. Impact on the incidence of the canine and human disease. An Acad Bras Cienc. 2004;76:583–593. doi: 10.1590/s0001-37652004000300012. [DOI] [PubMed] [Google Scholar]
- 4.Alves WA, Bevilacqua PD. Quality of diagnosis of canine visceral leishmaniasis in epidemiological surveys: an epidemic in Belo Horizonte, Minas Gerais, Brasil, 1993–1997. Cad Saúde Pública. 2004;20:259–265. doi: 10.1590/s0102-311x2004000100043. [DOI] [PubMed] [Google Scholar]
- 5.Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 6.Romero GAS, Boelaert M. Control of visceral leishmaniasis in Latin America—a systematic review. PLoS Negl Trop Dis. 2010;4:1–17. doi: 10.1371/journal.pntd.0000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reithinger R, Quinnell RJ, Alexander B, Davies CR. Rapid detection of Leishmania infantum infection in dogs: comparative study using an immune chromatographic dipstick test, enzyme-linked immunosorbent assay, and PCR. J Clin Microbiol. 2002;40:2352–2356. doi: 10.1128/JCM.40.7.2352-2356.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Castro Ferreira E, de Lana M, Carneiro M, Barbosa Reis A, Vieira Paes D, da Silva ES, Schallig H, Ferreira Gontijo C. Comparison of serological assays for the diagnosis of canine visceral leishmaniasis in animals presenting different clinical manifestations. Vet Parasitol. 2007;146:235–241. doi: 10.1016/j.vetpar.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Reithinger R, Lambson BE, Barker DC, Davies CR. Use of PCR to detect Leishmania (Viannia) spp. in dog blood and bone marrow. J Clin Microbiol. 2000;38:748–751. doi: 10.1128/jcm.38.2.748-751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reithinger R, Dujardin JC. Molecular diagnosis of leishmaniasis: current status and future applications. J Clin Microbiol. 2007;45:21–25. doi: 10.1128/JCM.02029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diro E, Techane Y, Tefera T, Assefa Y, Kebede T, Genetu A, Kebede Y, Tesfaye A, Ergicho B, Gebre-Yohannes A, Mengistu G, Engers H, Asseffa A, Desjeux P, Boelaert M, Hailu A. Field evaluation of FD-DAT, rK39 dipstick and KATEX (urine latex agglutination) for diagnosis of visceral leishmaniasis in northwest Ethiopia. Trans R Soc Trop Med Hyg. 2007;101:908–914. doi: 10.1016/j.trstmh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Sundar S, Singh RK, Bimal SK, Gidwani K, Mishra A, Maurya R, Singh SK, Manandhar KD, Boelaert M, Rai M. Comparative evaluation of parasitology and serological tests in the diagnosis of visceral leishmaniasis in India: a phase III diagnostic accuracy study. Trop Med Int Health. 2007;12:284–289. doi: 10.1111/j.1365-3156.2006.01775.x. [DOI] [PubMed] [Google Scholar]
- 13.Boelaert M, El-Safi S, Hailu A, Mukhtar M, Rijal S, Sundar S, Wasunna MA, Aseffa A, Mbui J, Menten J, Desjeux P, Peeling RW. Diagnostic tests for kala-azar: a multi-centre study of the freeze-dried DAT, rK39 strip test and KAtex in east Africa and the Indian subcontinent. Trans R Soc Trop Med Hyg. 2008;102:32–40. doi: 10.1016/j.trstmh.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Pattabhi S, Whittle J, Mohamath R, El-Safi S, Moulton GG, Guderian JA, Colombara D, Abdoon AO, Mukhtar MM, Mondal D, Esfandiari J, Kumar S, Chun P, Reed SG, Bhatia A. Design, development and evaluation of rK28-based point of care tests for improving rapid diagnosis of visceral leishmaniasis. PLoS Negl Trop Dis. 2010;4:1–11. doi: 10.1371/journal.pntd.0000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alce TM, Gokool S, McGhie D, Stäger S, Smith DF. Expression of hydrophilic surface proteins in infective stages of Leishmania donovani. Mol Biochem Parasitol. 1999;102:191–196. doi: 10.1016/s0166-6851(99)00074-2. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia A, Daifalla NS, Jen S, Badaro R, Reed SG, Skeiky YA. Cloning, characterization and serological evaluation of K9 and K26: two related hydrophilic antigens of Leishmania chagasi. Mol Biochem Parasitol. 1999;102:249–261. doi: 10.1016/s0166-6851(99)00098-5. [DOI] [PubMed] [Google Scholar]
- 17.Gürtler RE. Sustainability of vector control strategies in the Gran Chaco Region: current challenges and possible approaches. Mem Inst Oswaldo Cruz. 2009;104((Suppl 1)):52–59. doi: 10.1590/s0074-02762009000900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson RH. Validation of serological assays for diagnosis of infectious diseases. Rev Sci Tech. 1998;17:507–526. doi: 10.20506/rst.17.2.1119. [DOI] [PubMed] [Google Scholar]
- 19.Lauricella MA, Castañera MB, Gürtler RE, Segura EL. Immunodiagnosis of Trypanosoma cruzi (Chagas' disease) infection in naturally infected dogs. Mem Inst Oswaldo Cruz. 1998;93:501–507. doi: 10.1590/s0074-02761998000400016. [DOI] [PubMed] [Google Scholar]
- 20.Gurevitz JM, Ceballos LA, Gaspe MS, Alvarado-Otegui JA, Enriquez GF, Kitron U, Gürtler RE. Factors affecting infestation by Triatoma infestans in a rural area of the humid Chaco in Argentina: a multi-model inference approach. PLoS Negl Trop Dis. 2011;5:1–13. doi: 10.1371/journal.pntd.0001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinnell RJ, Courtenay O, Garcez L, Dye C. The epidemiology of canine leishmaniasis: transmission rates estimated from a cohort study in Amazonian Brazil. Parasitology. 1997;115:143–156. doi: 10.1017/s0031182097001200. [DOI] [PubMed] [Google Scholar]
- 22.Fegan DF. Walker P, Subasinghe R. DNA-Based Molecular Diagnostic Techniques: Research Needs for Standardization and Validation of the Detection of Aquatic Animal Pathogens and Diseases. Rome, Italy: FAO; 2000. Evaluation of diagnostic tests: the epidemiological approach; pp. 30–37. FAO Fisheries Technical Paper 395. [Google Scholar]
- 23.Banoo S, Bell D, Bossuyt P, Herring A, Mabey D, Poole F, Smith PG, Sriram N, Wongsrichanalai CH, Linke R, O'Brien R, Perkins M, Cunningham J, Matsoso P, Nathanson CM, Olliaro P, Peeling RW, Ramsay A. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol. 2010;6:S17–S29. [PubMed] [Google Scholar]
- 24.Taner T, Antony J. The assessment of quality in medical diagnostic tests: a comparison of ROC/Jouden and Taguchi methods. Int J Health Care Qual Assur Inc Leadersh Health Serv. 2000;13:300–307. doi: 10.1108/09526860010378744. [DOI] [PubMed] [Google Scholar]
- 25.Altman DG, Bland JM. Diagnostic tests 1: sensitivity and specificity. BMJ. 1994;308:1552. doi: 10.1136/bmj.308.6943.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altman DG, Bland JM. Diagnostic tests 2: predictive values. BMJ. 1994;309:102. doi: 10.1136/bmj.309.6947.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosário EY, Genaro O, França-Silva JC, da Costa RT, Mayrink W, Reis AB, Carneiro M. Evaluation of enzyme-linked immunosorbent assay using crude Leishmania and recombinant antigens as a diagnostic marker for canine visceral leishmaniasis. Mem Inst Oswaldo Cruz. 2005;100:197–203. doi: 10.1590/s0074-02762005000200015. [DOI] [PubMed] [Google Scholar]
- 28.Aguirre S, Silber AM, Brito MEF, Ribone ME, Lagier CM, Marcipar IS. Design, construction, and evaluation of a specific chimeric antigen to diagnose Chagasic infection. J Clin Microbiol. 2006;44:3768–3774. doi: 10.1128/JCM.01043-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinnell RJ, Carlson C, Reithinger R, Garcez LM, Courtenay O. Evaluation of rK39 rapid diagnostic tests for canine visceral leishmaniasis. Longitudinal study and meta-analysis. PLoS Negl Trop Dis. 2013;7:e1992. doi: 10.1371/journal.pntd.0001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimaldi G, Jr, Teva A, Ferreira AL, dos Santos CB, de Souza Pinto I, de Azevedo CT, Falqueto A. Evaluation of a novel chromatographic immunoassay based on Dual-Path Platform technology (DPP® CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2012;106:54–59. doi: 10.1016/j.trstmh.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Vega Benedetti AF, Cimino RO, Cajal PS, Juarez M del V, Villalpando CA, Gil JF, Marcipar IS, Krolewiecki AJ, Nasser JR. Performance of different Trypanosoma cruzi antigens in the diagnosis of Chagas disease in patients with American cutaneous leishmaniasis from a co-endemic region in Argentina. Trop Med Int Health. 2013;18:1103–1109. doi: 10.1111/tmi.12144. [DOI] [PubMed] [Google Scholar]
- 32.Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:1–13. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]