Abstract
Humans are intermittently exposed to large variations in potassium intake, which range from periods of fasting to ingestion of potassium-rich meals. These fluctuations would abruptly alter plasma potassium concentration if not for rapid mechanisms, primarily in skeletal muscle and the liver, that buffer the changes in plasma potassium concentration by means of transcellular potassium redistribution and feedback control of renal potassium excretion. However, buffers have capacity limits, and even robust feedback control mechanisms require that the perturbation occur before feedback can initiate corrective action. In contrast, feedforward control mechanisms sense the effect of disturbances on the system’s homeostasis. This review highlights recent experimental insights into the participation of feedback and feedforward control mechanisms in potassium homeostasis. New data make clear that feedforward homeostatic responses activate when decreased potassium intake is sensed, even when plasma potassium concentration is still within the normal range and before frank hypokalemia ensues, in addition to the classic feedback activation of renal potassium conservation when plasma potassium concentration decreases. Given the clinical importance of dyskalemias in patients, these novel experimental paradigms invite renewed clinical inquiry into this important area.
Hypokalemia is a common disorder that can result from potassium redistribution between plasma and intracellular fluid (ICF), inadequate potassium intake, or excessive potassium excretion (1). When hypokalemia reflects true potassium depletion, the body activates several mechanisms, especially in the kidney, to conserve potassium (Figure 1). Although short periods of mild potassium depletion are typically well tolerated in healthy individuals, severe potassium depletion can result in glucose intolerance (2) and serious cardiac (3), renal (4), and neurologic (5) dysfunction, including death. Prolonged potassium depletion of even modest proportion can provoke or exacerbate kidney injury or hypertension (6, 7). Indeed, reduced potassium intake correlates directly with higher blood pressure in both normotensive and hypertensive individuals (8).
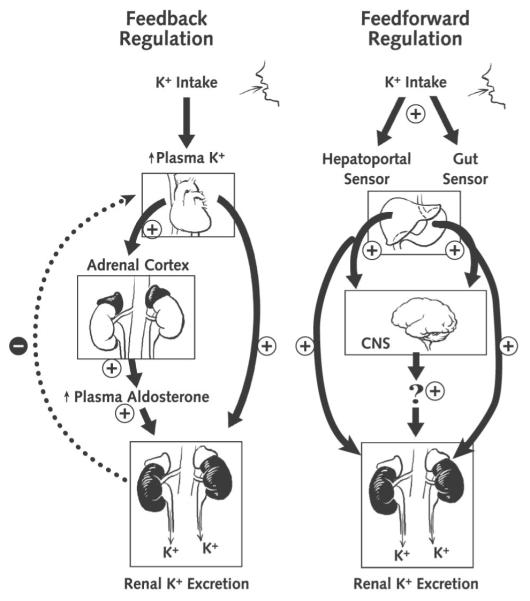
Figure 1. Integrated model of the regulation of body potassium balance.
CNS = central nervous system. Left. Classic mechanisms. Right. Additional putative mechanisms.
Normal Potassium Balance and Renal Potassium Excretion
To set the context, we first summarize key concepts of steady-state potassium handling. Total body potassium is roughly 55 mmol/kg of body weight, with 98% distributed to the ICF (primarily in muscle, the liver, and erythrocytes) and 2% in the extracellular fluid (1). Na,K-ATPase isoforms (see Glossary) actively pump potassium into the cell and maintain and restore the electrochemical gradient between the normal extracellular potassium concentration of 3.5 to 5.0 mmol/L and the intracellular potassium concentration of approximately 150 mmol/L, which is particularly important for normal functioning of excitable cells. β-Catecholamines, aldosterone, insulin, pH, and osmolality influence the transcellular potassium distribution and thus how well cells buffer changes in plasma potassium concentration (9). Physicians exploit this fact in the emergency management of severe hyperkalemia when they use insulin or β-catecholamines to drive plasma potassium into cells.
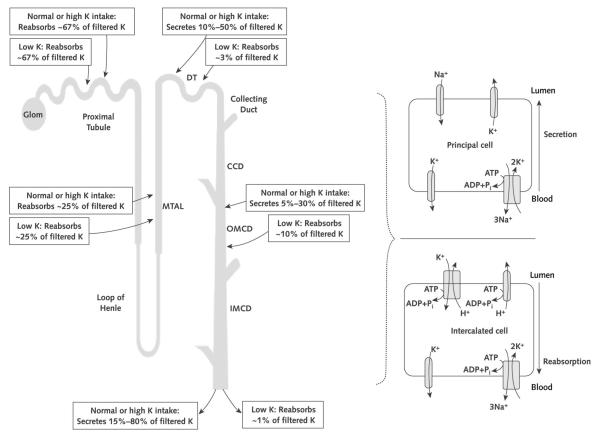
Normal persons who consume a typical Western diet ingest approximately 70 mmol of potassium per day (10). The intestine absorbs virtually all of the ingested potassium and delivers it to the liver for processing by means of the hepatoportal circulation. Minimal amounts of potassium are excreted in the feces. Renal potassium excretion, the principal defense against chronic potassium imbalances, depends on free filtration at the glomerulus, extensive proximal tubule reabsorption, and a highly regulated secretory process of the distal convoluted tubule and segments of the collecting duct in the cortex and outer medulla (the cortical collecting duct and the outer medullary collecting duct, respectively) (Figure 2). The cortical collecting duct and outer medullary collecting duct consist of at least 2 very different cell types, termed principal cells and intercalated cells (Figure 2). Principal cells, which comprise approximately 70% to 75% of collecting duct cells, mediate sodium reabsorption and potassium secretion and are targets for angiotensin II (11, 12), aldosterone, aldosterone receptor antagonists, and potassium-sparing diuretics (Figure 2). Principal cells exploit the electrochemical gradient established by sodium entry into the cell through a sodium channel at the luminal membrane (the molecular target of amiloride) and the basolateral membrane Na,K-ATPase to drive potassium secretion through 2 classes of luminal membrane potassium channels (13). One of these, the renal outer medullary potassium (known among renal physiologists as “ROMK”) channels, secrete potassium under normal tubular fluid flow conditions and are inserted into or internalized from the luminal membrane, depending on the demand for potassium secretion. The other class of potassium channels are the “big” conductance channels (known as “BK” channels), which are relatively inactive under normal conditions but exhibit increased activity during high tubular flow or high-potassium conditions (13). Factors that regulate principal cell potassium secretion include previous potassium intake; intracellular potassium level; sodium delivery to the cells; urine flow rate; and hormones, such as aldosterone and β-catecholamines (14). The other collecting duct cell type, intercalated cells, mediate acid–base transport but upregulate expression of luminal H,K-ATPases (see Glossary) during potassium depletion to enhance potassium reabsorption (1) (Figure 2).
Figure 2. Segmental handling of potassium excretion along the nephron and collecting duct cell types under normal conditions or conditions of potassium excess or deficiency.
We present a simplified model of potassium handling by collecting duct cell types. Principal cells in the collecting duct are responsible for secretion of excess potassium in the circulation into the tubule lumen and thus into the urine. This secretion is accomplished by luminal membrane potassium channels responding primarily to the electrochemical gradient for potassium generated by the combined actions of the basolateral membrane Na,K-ATPase and a luminal membrane sodium channel (the target of the potassium-sparing diuretic amiloride). In states of potassium depletion, potassium secretion by the principal cells is inhibited and the luminal membrane H,K-ATPase is activated in the intercalated cells to reclaim the potassium that remains in the tubular fluid, thereby limiting urinary potassium wasting. ADP = adenosine diphosphate; ATP = adenosine triphosphate; CCD = cortical collecting duct; DT = distal tubule; Glom = glomerulus; IMCD = inner medullary collecting duct; MTAL = medullary thick ascending limb of Henle loop; OMCD = outer medullary collecting duct; Pi = inorganic phosphate.
To summarize, mammalian cells require a steep concentration gradient of potassium between ICF and extracellular fluid to function properly, which requires primary active transport by Na,K-ATPase. The kidney excretes sufficient amounts of potassium to maintain total body homeostasis. Although the proximal nephron reabsorbs the bulk of the potassium filtered at the glomerulus, the collecting duct fine-tunes potassium excretion and is subject to several regulatory influences.
Feedback Control of Potassium Balance
The use of thermostats to adjust heating or cooling is a common example of feedback control, a control mechanism in a homeostatic system that uses the consequences or outputs of a process to “feed back” and regulate the process itself. The thermostat detects the “error” (for example, the room is too hot) and signals for the air conditioner to provide cool air. Once the room reaches the temperature set at the thermostat (the room becomes cool enough), the air conditioner turns off. This example of feedback control also applies to potassium homeostasis. Although feedback control of potassium balance has been recognized for decades, only recently have some of its secrets been discovered.
In response to a high-potassium meal that includes glucose, pancreatic insulin secretion activates skeletal muscle and liver Na,K-ATPase, which pumps potassium from the plasma to the ICF of these cells. This mechanism minimizes the postprandial increase in plasma potassium concentration (15). With muscle activity, potassium is released into the plasma and filtered at the glomerulus. To maintain balance, the amount of potassium consumed in the meal (minus the small amount lost in the feces) is secreted into the urine. When potassium consumption increases plasma potassium concentration enough, it triggers aldosterone synthesis and release from the adrenals, which stimulates the activity and synthesis of Na,K-ATPase and luminal potassium channels in collecting duct principal cells to secrete the excess potassium (16) (Figures 1 and 2). Aldosterone also enhances potassium secretion in the distal colon (17), which can be especially important when renal function is compromised.
Conversely, if potassium intake is very low or its output is very high, plasma potassium concentration decreases and feedback regulation redistributes potassium from ICF to plasma and minimizes renal potassium excretion. Skeletal muscle becomes insulin-resistant to potassium (but not glucose) uptake even before plasma potassium concentration decreases, which blunts the shift of potassium from plasma into the cell (18). After hypokalemia ensues, the expression of skeletal muscle Na,K-ATPase α2 isoform decreases, which allows a net potassium “leak” from ICF to the plasma (19). The low plasma potassium concentration suppresses adrenal aldosterone release so that the kidney can reclaim all but about 1% of the filtered potassium (Figure 2).
This renal potassium conservation involves downregulation of potassium secretion by means of the ROMK channels in cortical collecting duct principal cells. Chronic potassium depletion activates a renal NADPH oxidase (a relative of the enzyme that produces the “respiratory burst” in neutrophils) that produces reactive oxygen species to signal for the ROMK channels to be internalized and thus incompetent to conduct membrane transport of potassium (20, 21). In addition, an H,K-ATPase (a relative of the proton pump active in the gastric mucosa) is activated in outer medullary collecting duct intercalated cells to reclaim any remaining filtered potassium into the plasma. Figure 2 summarizes the responses of the different nephron segments to accommodate changes in potassium intake.
Feedforward Control of Potassium Balance
Feedforward control refers to a pathway in a homeostatic system that responds to a signal in the environment in a predetermined manner, without responding to how the system subsequently reacts (that is, without responding to feedback). The most famous example of feedforward control is the conditioned salivation of Pavlov’s dogs in anticipation of food (22). Pavlov implanted small stomach pouches in dogs to measure salivation. He and his assistants would ring bells to call the dogs to their food and measure the dogs’ increase in salivation. After several repetitions of this experiment, the dogs increased their salivation when they heard the bell, without any food being presented. The dogs had been trained to “sense” the impending arrival of a meal and prepare for it, in a physiologic sense, by increasing the production of saliva.
Physiologists studying potassium homeostasis were surprised to discover that even minor changes in dietary potassium intake, insufficient to change plasma concentrations of either potassium (23) or aldosterone (24) and therefore too minor to activate feedback control, evoked rapid changes in renal potassium excretion through feedforward mechanisms. Twenty-five years ago, Rabinowitz and colleagues (25) challenged feedback regulation as the sole mechanism for compensatory renal potassium excretion and proposed a feedforward kaliuretic reflex, in which potassium sensors in the splanchnic vascular bed (the gut, portal vein, or liver) detect local changes in potassium concentration resulting from potassium ingestion and signal the kidney to alter potassium excretion to restore balance (Figure 1). These investigators showed that sheep that ingested a potassium-rich meal over 1 hour rapidly and appropriately increased renal potassium excretion, even though plasma concentrations of potassium and aldosterone did not increase sufficiently to account for the ensuing kaliuresis. Subsequent studies in adrenalectomized rats proved that this rapid urinary potassium excretion was not mediated by aldosterone, because doses of aldosterone much higher than the physiologic range were required (26, 27). Studies in normal humans undergoing water diuresis corroborated these findings by showing that ingestion of potassium salts promoted urinary potassium excretion within 20 minutes, before any increase in plasma concentrations of potassium or aldosterone (23), which means that it was not mediated through classical feedback regulation.
What is the potassium sensor in feedforward regulation, and where is it located in the body? Studies in rats done in 2 of the reviewers’ laboratories (28) demonstrated that a gut sensor detects potassium intake during a meal and triggers a signal to the kidneys to increase potassium excretion. An intragastric potassium infusion given with a meal to unfed animals led to greater renal clearance of plasma potassium than did the same intragastric infusion given without the meal or given by systemic infusion of potassium (28). The fact that plasma potassium clearance was enhanced in response to a potassium load only when food was present in the stomach provides strong evidence that the sensor in this feedforward pathway is in the gut. Whether mechanical factors associated with the bulk of food (such as stretching of the stomach) play a role in the gut potassium sensing, and how such mechanical sensors might initiate the kaliuretic reflex signal, remain to be established. A hepatoportal potassium sensor may also participate in the kaliuretic reflex. Morita and colleagues (29) found that intraportal infusion of potassium chloride in animals yielded greater urinary potassium excretion than did an intravenous infusion. Severing the periarterial hepatic nervous plexus attenuated the kaliuresis. Furthermore, an acute potassium chloride infusion directly into the hepatoportal circulation stimulated hepatic afferent nerve activity and increased urinary potassium excretion in the absence of changes in plasma potassium concentration (29). Bumetanide, a clinically used loop diuretic that inhibits cation/chloride cotransporters (including the Na+, K+, 2Cl− cotransporter NKCC1 [see Glossary]), ablated the activity of this potassium sensor. This suggests that the hepatoportal Na+, K+, 2Cl− cotransporter, or a related bumetanide-sensitive cotransporter, sensed an increase in portal venous potassium and activated the periarterial hepatic nerve complex, which sent a neurally mediated signal transmission to the kidneys with consequent kaliuresis (30). Consistent with these studies, the NKCC1 knockout mouse (which lacks the NKCC1 cotransporter and therefore lacks this feedforward mechanism) exhibits hyperkalemia after ingesting a potassium load and inappropriately low urinary potassium excretion (31, 32).
We have not fully determined the molecular events involved in the kaliuretic reflex. However, several precedents for feedforward regulation exist: the related Na+, K+, 2Cl− cotransporter isoform NKCC2 (see Glossary) senses NaCl delivery to the macula densa segment of the glomerulus and signals for afferent arteriolar constriction at the glomerulus (which decreases glomerular filtration) (33); several tissues express Ca2+-sensing receptors (34); glucose sensors operate in the portal vein (35); and diverse subpopulations of afferent nerves coordinate reflex control of the local mechanical and chemical environment in the liver (36), gut mucosa, muscle, and mesentery (37). These examples of sensors in other feedforward mechanisms make it plausible that potassium sensors govern feedforward regulation of potassium in the skeletal muscle and kidney.
Feedforward regulation in these tissues also occurs with chronic restriction of potassium intake, again even before hypokalemia occurs. As noted, when dietary potassium restriction is prolonged and severe enough to cause hypokalemia, Na,K-ATPase pumps become less numerous and less active in the skeletal muscle to protect against further decrements in muscle potassium. In contrast, animals chronically fed a moderately potassium-restricted diet that did not produce hypokalemia maintained normal muscle potassium levels and normal amounts and activity of Na,K-ATPase pumps in their membranes. However, potassium-conserving mechanisms were activated: Insulin-stimulated clearance of potassium from the plasma was blunted (evidence of insulin resistance to cellular potassium uptake) (38), and renal potassium excretion was reduced to 20% of that of control participants (indicating potassium conservation). The decrease in renal potassium excretion was apparently the result of enhanced phosphorylation and consequent internalization of ROMK channels from the plasma membrane (rendering them inaccessible to conduct potassium secretion) in the collecting duct principal cells (38). These results indicate that even before the plasma potassium concentration begins to decrease, feedforward activation of potassium-conserving mechanisms acts to maintain plasma potassium concentration. The error signals that incite and maintain these responses are unknown but may be related to gastric or hepatoportal sensing of potassium intake.
Metabolic Control of Potassium Balance
Intense exercise or ischemia significantly alters potassium homeostasis. The 2 situations share similarities, which include elevated extracellular fluid potassium concentration caused by an increase in potassium efflux relative to influx and an increased ratio of intracellular adenosine monophosphate to adenosine triphosphate. Homeostasis is restored in both cases by net potassium uptake into the cells through mediators that have not been clearly identified.
A good candidate mediator for this net potassium uptake is cellular adenosine monophosphate–activated protein kinase. This enzyme, which activates when it detects an increase in the cellular ratio of adenosine monophosphate to adenosine triphosphate, mediates increased glucose transport in exercising muscles to replete cellular adenosine triphosphate (39). Indeed, 2 reviewers established that chemical activation of adenosine monophosphate–activated protein kinase in conscious rats acutely decreased plasma potassium concentration without increasing urinary potassium excretion, which indicates that it shifted potassium to the ICF (40). In support of this concept, mutant mice with inactive muscle adenosine monophosphate–activated protein kinase exhibited impaired potassium uptake into the cells in response to an activator of adenosine monophosphate–activated protein kinase. Whether active potassium uptake by cells or depressed potassium efflux from cells mediates this potassium redistribution remains to be determined, but this novel mechanism—activated by metabolic stress—operates in parallel to the potassium uptake pathway stimulated by insulin (40).
Clinical Implications
The data we reviewed indicate that plasma potassium concentration is a relatively insensitive marker for the integrated homeostatic responses that are activated by changes in potassium intake or renal excretion. These responses occur while plasma potassium concentration is still within the normal range and before frank hypokalemia and potassium depletion ensue. Studies in rats that were fed a modestly potassium-restricted diet (38) indicate that feedforward adjustments to low-potassium diets occur in both muscle and kidney, operate independently of changes in plasma potassium concentration, and can persist in the long term without hypokalemia. The classic paradigm for diagnosis and treatment of hypokalemia (1) distinguishes only between transcellular potassium shifts and alterations in total body potassium due to altered intake or feedback regulation of potassium excretion. Feedforward regulation of potassium buffering and renal potassium excretion changes this paradigm, which may change the treatment of some clinical conditions.
The metabolic syndrome and type 2 diabetes mellitus are both associated with insulin resistance of muscle, fat, and liver cells. Recent animal studies (18) demonstrating that skeletal muscle becomes insulin-resistant to cellular potassium (but not glucose) uptake even before plasma potassium concentration decreases may lead to new strategies to optimize potassium homeostasis—and thus, to a degree, modulate cardiovascular risk—in these patients. Moreover, the finding that adenosine monophosphate–activated protein kinase activation promotes not only cellular glucose uptake (a key mechanism of action of the antidiabetic drugs metformin and thiazolidinediones) but also potassium uptake in experimental animals may drive clinical investigation to consider expanded uses of these or closely related drugs. For example, serious hyperkalemia can occur in patients with heart failure who receive spironolactone (41). In hyperkalemic spironolactone-treated rats, pharmacologic stimulation of adenosine monophosphate–activated protein kinase with 5-aminoimidazole-4-carboxamide ribonucleoside quickly restored plasma potassium concentration to baseline (40). This observation suggests that co-administering such agents with medications that can increase plasma potassium concentration—such as spironolac-tone—might be clinically useful. On a cautionary note, infusing 5-aminoimidazole-4-carboxamide ribonucleoside into potassium-depleted hypokalemic rats induced profound hypokalemia (40). This observation suggests that metformin, a commonly used adenosine monophosphate–activated protein kinase activator, could provoke dangerous hypokalemia in patients susceptible to potassium depletion from taking potassium-wasting diuretics or fasting (2). We need to explore these clinical scenarios.
Enteral feeding is an increasingly common and generally safe practice in acutely ill patients and those who chronically cannot eat. Feedforward regulation of renal potassium excretion by the putative gut or hepatoportal sensors may help to ensure the safety of potassium replacement by the enteral route. The growing use of enteral feeding tubes in patients who chronically cannot eat offers an excellent investigative opportunity to identify the optimal route of enteral potassium administration in these patients and to confirm the existence of the putative gut potassium sensor in humans. When we understand the afferent and efferent mechanisms that respond to potassium intake by means of the hepatoportal and gut potassium sensors, we may find new targets for treating hypokalemia.
Conclusion
Given the importance of keeping the plasma potassium concentration within a narrow range, it is not surprising that distinct but integrated mechanisms of feedback and feedforward modes have evolved that act on skeletal muscle and the liver and kidneys to regulate potassium balance. These dual mechanisms provide the exquisite and efficient control needed to maintain and restore potassium homeostasis in response to acute or chronic changes in body potassium levels. Although humans clearly have a feedforward kaliuretic reflex, the existence and location of the potassium sensors remain to be established. Moreover, we do not know the specific renal effector mechanisms downstream of these potassium sensors in animals or in humans. Despite the remaining gap between the experimental physiology of these pathways in animals and clinical practice in humans, these new paradigms of body potassium metabolism provide exciting new insights that may eventually guide diagnosis and treatment.
Acknowledgments
Grant Support: By National Institutes of Health grants R01 DK47981 and R01 DK075065 (Dr. Kone), R01 DK49750 and the Department of Veterans Affairs (Dr. Wingo), R01 DK34316 (Dr. McDonough), and R21 DK080233 (Dr. Youn).
Glossary
- Na,K-ATPase
Plasma membrane protein that pumps 3 sodium ions out of cell and 2 potassium ions into the cell.
- H,K-ATPase
Plasma membrane protein that pumps 1 hydrogen ion out of cell and 1 potassium ion into the cell.
- NKCC1
Isoform 1 of a plasma membrane protein that cotransports 1 sodium ion, 1 potassium ion, and 2 chloride ions into the cell.
- NKCC2
Isoform 2 of a plasma membrane protein that cotransports 1 sodium ion, 1 potassium ion, and 2 chloride ions into the cell.
Footnotes
Potential Financial Conflicts of Interest: None disclosed.
Current author addresses are available at www.annals.org.
References
- 1.Kone BC. Hypokalemia. In: Hamm LL, Dubose TD, editors. Acid–Base and Electrolyte Disorders: A Companion to Brenner and Rector’s The Kidney. Saunders; Philadelphia: 2002. pp. 381–94. [Google Scholar]
- 2.Zillich AJ, Garg J, Basu S, Bakris GL, Carter BL. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension. 2006;48:219–24. doi: 10.1161/01.HYP.0000231552.10054.aa. [PMID: 16801488] [DOI] [PubMed] [Google Scholar]
- 3.Gheeraert PJ, De Buyzere ML, Taeymans YM, Gillebert TC, Henriques JP, De Backer G, et al. Risk factors for primary ventricular fibrillation during acute myocardial infarction: a systematic review and meta-analysis. Eur Heart J. 2006;27:2499–510. doi: 10.1093/eurheartj/ehl218. [PMID: 16952926] [DOI] [PubMed] [Google Scholar]
- 4.Reungjui S, Roncal CA, Sato W, Glushakova OY, Croker BP, Suga S, et al. Hypokalemic nephropathy is associated with impaired angiogenesis. J Am Soc Nephrol. 2008;19:125–34. doi: 10.1681/ASN.2007030261. [PMID: 18178802] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riggs JE. Neurologic manifestations of electrolyte disturbances. Neurol Clin. 2002;20:227–39. vii. doi: 10.1016/s0733-8619(03)00060-4. [PMID: 11754308] [DOI] [PubMed] [Google Scholar]
- 6.Khosla N, Hogan D. Mineralocorticoid hypertension and hypokalemia. Semin Nephrol. 2006;26:434–40. doi: 10.1016/j.semnephrol.2006.10.004. [PMID: 17275580] [DOI] [PubMed] [Google Scholar]
- 7.Krishna GG, Kapoor SC. Potassium depletion exacerbates essential hypertension. Ann Intern Med. 1991;115:77–83. doi: 10.7326/0003-4819-115-2-77. [PMID: 2058867] [DOI] [PubMed] [Google Scholar]
- 8.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA. 1997;277:1624–32. doi: 10.1001/jama.1997.03540440058033. [PMID: 9168293] [DOI] [PubMed] [Google Scholar]
- 9.Gennari FJ. Hypokalemia. N Engl J Med. 1998;339:451–8. doi: 10.1056/NEJM199808133390707. [PMID: 9700180] [DOI] [PubMed] [Google Scholar]
- 10.Elliott P, Dyer A, Stamler R. The INTERSALT study: results for 24 hour sodium and potassium, by age and sex. INTERSALT Co-operative Research Group. J Hum Hypertens. 1989;3:323–30. [PMID: 2810328] [PubMed] [Google Scholar]
- 11.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–5. doi: 10.1097/01.asn.0000013292.78621.fd. [PMID: 11960999] [DOI] [PubMed] [Google Scholar]
- 12.Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem. 2007;282:6455–62. doi: 10.1074/jbc.M607477200. [PMID: 17194699] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sansom SC, Welling PA. Two channels for one job. Kidney Int. 2007;72:529–30. doi: 10.1038/sj.ki.5002438. [PMID: 17713560] [DOI] [PubMed] [Google Scholar]
- 14.Field MJ, Stanton BA, Giebisch GH. Differential acute effects of aldosterone, dexamethasone, and hyperkalemia on distal tubular potassium secretion in the rat kidney. J Clin Invest. 1984;74:1792–802. doi: 10.1172/JCI111598. [PMID: 6501571] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Felig P, Ferrannini E, Wahren J. Effect of graded doses of insulin on splanchnic and peripheral potassium metabolism in man. Am J Physiol. 1980;238:E421–7. doi: 10.1152/ajpendo.1980.238.5.E421. [PMID: 6990783] [DOI] [PubMed] [Google Scholar]
- 16.Wang W. Regulation of renal K transport by dietary K intake. Annu Rev Physiol. 2004;66:547–69. doi: 10.1146/annurev.physiol.66.032102.112025. [PMID: 14977413] [DOI] [PubMed] [Google Scholar]
- 17.Giebisch G, Krapf R, Wagner C. Renal and extrarenal regulation of potassium. Kidney Int. 2007;72:397–410. doi: 10.1038/sj.ki.5002288. [PMID: 17568786] [DOI] [PubMed] [Google Scholar]
- 18.McDonough AA, Youn JH. Role of muscle in regulating extracellular potassium. Semin Nephrol. 2005;25:335–42. doi: 10.1016/j.semnephrol.2005.03.009. [PMID: 16139689] [DOI] [PubMed] [Google Scholar]
- 19.McDonough AA, Thompson CB, Youn JH. Skeletal muscle regulates extracellular potassium. Am J Physiol Renal Physiol. 2002;282:F967–74. doi: 10.1152/ajprenal.00360.2001. [PMID: 11997312] [DOI] [PubMed] [Google Scholar]
- 20.Babilonia E, Lin D, Zhang Y, Wei Y, Yue P, Wang WH. Role of gp91phox-containing NADPH oxidase in mediating the effect of K restriction on ROMK channels and renal K excretion. J Am Soc Nephrol. 2007;18:2037–45. doi: 10.1681/ASN.2006121333. [PMID: 17538186] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Lin DH, Wang ZJ, Jin Y, Yang B, Wang WH. K restriction inhibits protein phosphatase 2B (PP2B) and suppression of PP2B decreases ROMK channel activity in the CCD. Am J Physiol Cell Physiol. 2008;294:C765–73. doi: 10.1152/ajpcell.00528.2007. [PMID: 18184875] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavlov IP, Anrep GV. Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. Oxford Univ Pr; London: 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calò L, Borsatti A, Favaro S, Rabinowitz L. Kaliuresis in normal subjects following oral potassium citrate intake without increased plasma potassium concentration. Nephron. 1995;69:253–8. doi: 10.1159/000188466. [PMID: 7753258] [DOI] [PubMed] [Google Scholar]
- 24.Rabinowitz L, Denham SC, Gunther RA. Aldosterone and postprandial renal excretion of sodium and potassium in sheep. Am J Physiol. 1977;233:F213–6. doi: 10.1152/ajprenal.1977.233.3.F213. [PMID: 910916] [DOI] [PubMed] [Google Scholar]
- 25.Rabinowitz L, Green DM, Sarason RL, Yamauchi H. Homeostatic potassium excretion in fed and fasted sheep. Am J Physiol. 1988;254:R357–80. doi: 10.1152/ajpregu.1988.254.2.R357. [PMID: 3344840] [DOI] [PubMed] [Google Scholar]
- 26.Campen TJ, Vaughn DA, Fanestil DD. Mineralo- and glucocorticoid effects on renal excretion of electrolytes. Pflugers Arch. 1983;399:93–101. doi: 10.1007/BF00663903. [PMID:6647008] [DOI] [PubMed] [Google Scholar]
- 27.Stanton B, Pan L, Deetjen H, Guckian V, Giebisch G. Independent effects of aldosterone and potassium on induction of potassium adaptation in rat kidney. J Clin Invest. 1987;79:198–206. doi: 10.1172/JCI112783. [PMID: 3793923] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee FN, Oh G, McDonough AA, Youn JH. Evidence for gut factor in K+ homeostasis. Am J Physiol Renal Physiol. 2007;293:F541–7. doi: 10.1152/ajprenal.00427.2006. [PMID: 17522262] [DOI] [PubMed] [Google Scholar]
- 29.Morita H, Fujiki N, Miyahara T, Lee K, Tanaka K. Hepatoportal bumetanide-sensitive K(+)-sensor mechanism controls urinary K(+) excretion. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1134–9. doi: 10.1152/ajpregu.2000.278.5.R1134. [PMID: 10801279] [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiya Y, Nakashima S, Banno Y, Suzuki Y, Morita H. Effect of high-NaCl or high-KCl diet on hepatic Na+- and K+-receptor sensitivity and NKCC1 expression in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R591–6. doi: 10.1152/ajpregu.00559.2003. [PMID: 14656769] [DOI] [PubMed] [Google Scholar]
- 31.Meyer JW, Flagella M, Sutliff RL, Lorenz JN, Nieman ML, Weber CS, et al. Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na(+)-K(+)-2Cl(−) cotransporter. Am J Physiol Heart Circ Physiol. 2002;283:H1846–55. doi: 10.1152/ajpheart.00083.2002. [PMID: 12384462] [DOI] [PubMed] [Google Scholar]
- 32.Wall SM, Knepper MA, Hassell KA, Fischer MP, Shodeinde A, Shin W, et al. Hypotension in NKCC1 null mice: role of the kidneys. Am J Physiol Renal Physiol. 2006;290:F409–16. doi: 10.1152/ajprenal.00309.2005. [PMID: 16159893] [DOI] [PubMed] [Google Scholar]
- 33.Schnermann J, Briggs JP. Tubuloglomerular feedback: mechanistic insights from gene-manipulated mice. Kidney Int. 2008;74:418–26. doi: 10.1038/ki.2008.145. [PMID: 18418352] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–80. doi: 10.1038/366575a0. [PMID: 8255296] [DOI] [PubMed] [Google Scholar]
- 35.Hevener AL, Bergman RN, Donovan CM. Hypoglycemic detection does not occur in the hepatic artery or liver: findings consistent with a portal vein glucosensor locus. Diabetes. 2001;50:399–403. doi: 10.2337/diabetes.50.2.399. [PMID: 11272153] [DOI] [PubMed] [Google Scholar]
- 36.Uno K, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Imai J, et al. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science. 2006;312:1656–9. doi: 10.1126/science.1126010. [PMID: 16778057] [DOI] [PubMed] [Google Scholar]
- 37.Grundy D. Sensory signals from the gastrointestinal tract. J Pediatr Gastroenterol Nutr. 2005;41(Suppl 1):S7–9. doi: 10.1097/01.scs.0000180286.58988.cf. [PMID: 16131978] [DOI] [PubMed] [Google Scholar]
- 38.Chen P, Guzman JP, Leong PK, Yang LE, Perianayagam A, Babilonia E, et al. Modest dietary K+ restriction provokes insulin resistance of cellular K+ uptake and phosphorylation of renal outer medulla K+ channel without fall in plasma K+ concentration. Am J Physiol Cell Physiol. 2006;290:C1355–63. doi: 10.1152/ajpcell.00501.2005. [PMID: 16354756] [DOI] [PubMed] [Google Scholar]
- 39.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–41. doi: 10.1161/01.RES.0000256090.42690.05. [PMID: 17307971] [DOI] [PubMed] [Google Scholar]
- 40.Zheng D, Perianayagam A, Lee DH, Brannan MD, Yang LE, Tellalian D, et al. AMPK activation with AICAR provokes an acute fall in plasma potassium. Am J Physiol Cell Physiol. 2008;294:C126–35. doi: 10.1152/ajpcell.00464.2007. [PMID: 18003746] [DOI] [PubMed] [Google Scholar]
- 41.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–51. doi: 10.1056/NEJMoa040135. [PMID: 15295047] [DOI] [PubMed] [Google Scholar]