Abstract
The first biomimetic enantioselective total synthesis of (−)-communesin F based on a late-stage heterodimerization and aminal exchange is described. Our synthesis features the expedient diazene–directed assembly of two advanced fragments to secure the congested C3a–C3a′ linkage in three steps, followed by a highly efficient biogenetically inspired aminal reorganization to access the heptacyclic communesin core in only two additional steps. Enantioselective syntheses of the two fragments were developed, with highlights including the catalytic asymmetric halocyclization and diastereoselective oxyamination reactions of tryptamine derivatives, a stereoselective sulfinimine allylation, and an efficient cyclotryptamine–C3a-sulfamate synthesis by either a new silver–promoted nucleophilic amination or a rhodium–catalyzed C–H amination protocol. The versatile synthesis of the fragments, their stereocontrolled assembly, and the efficient aminal–exchange as supported by in situ monitoring experiments, in addition to the final stage N1′-acylation of the communesin core provide a highly convergent synthesis of (−)-communesin F.
Introduction
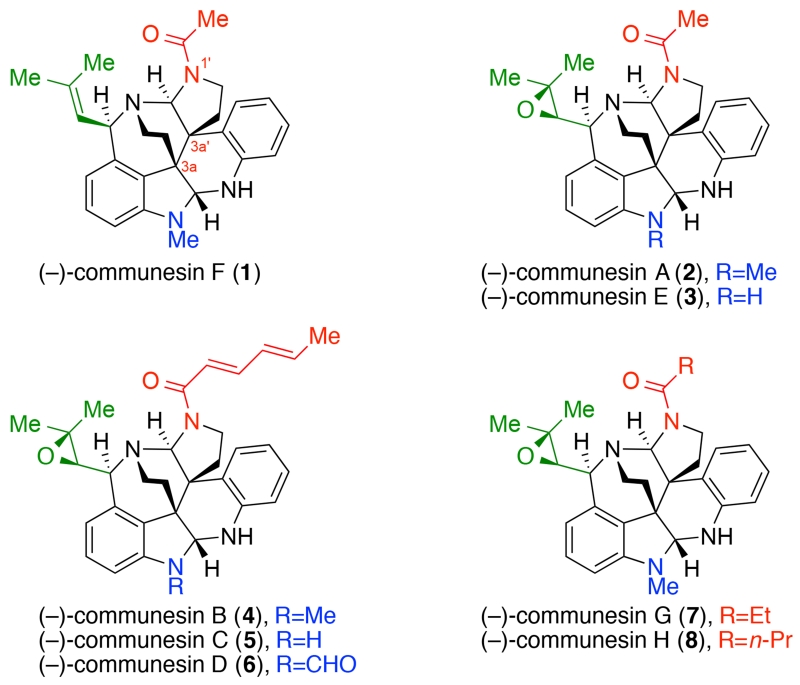
The communesin alkaloids are a family of structurally complex natural products isolated from various marine and terrestrial Penicillium fungi, which have been shown to possess insecticidal and antiproliferative activities as well as significant cytotoxicity against lymphocytic leukemia.1,2 The core structures of these alkaloids share a unique heptacyclic skeleton containing two aminals and at least five stereogenic centers, of which two are vicinal and quaternary (Figure 1). This exquisite structural complexity coupled with an array of interesting biological properties has prompted investigations directed towards the chemical synthesis of these alkaloids,2 culminating in innovative solutions for the total synthesis of (±)-communesin F (1) by Qin,3 Weinreb,4 and Funk,5 in addition to a formal synthesis by Stoltz.6 To date, Ma’s total synthesis of (−)-communesin F (1)7 remains the only enantioselective solution for this archetypical alkaloid. As an outgrowth of our investigations in the area of calycanthaceous alkaloids,8,9 we sought to develop a unified and convergent approach to the communesin alkaloids. We drew inspiration from a speculated biogenesis involving the stereocontrolled oxidative union of two dissimilar tryptamine derivatives followed by reorganization of a C3a–C3a′ linked heterodimer,10,11,12,13 reminiscent of the pathways leading to the related calycanthoids.12,14, While our biosynthetic considerations were purely hypothetical at the outset of this synthesis campaign, Houk, Garg, and Tang have recently disclosed15 incisive biosynthetic and computational studies that echo the key transformations developed in our synthesis. Herein, we present the shortest enantioselective total chemical synthesis of (−)-communesin F (1) with late-stage chemistry that fortuitously parallels the latest insights15b and hypotheses concerning the biogenesis of these alkaloids.
Figure 1.
The Structure of Communesin Alkaloids
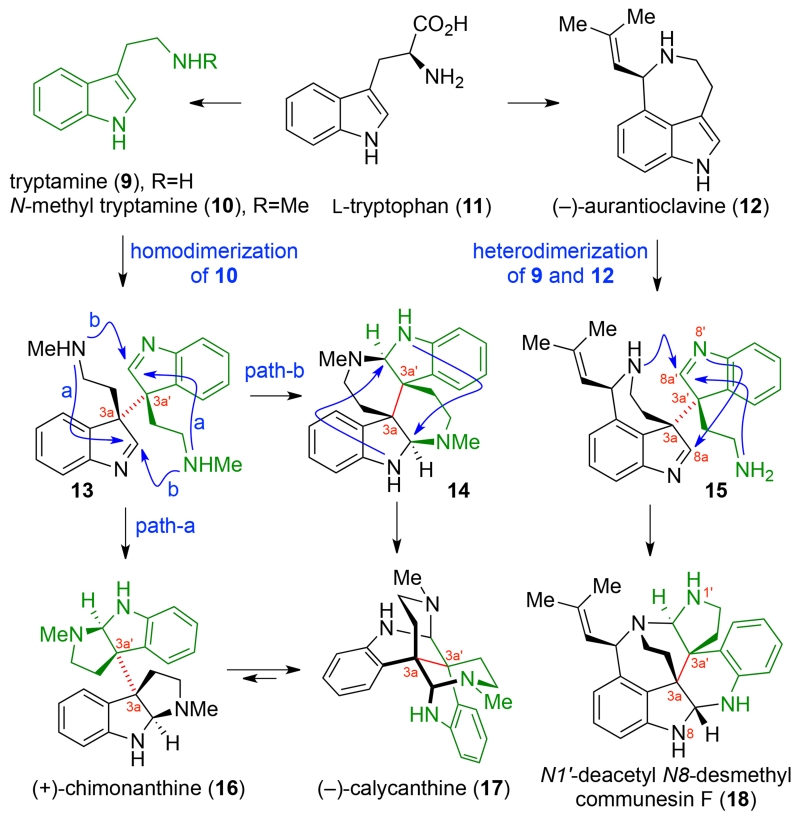
The expectation that the biosynthesis of calycanthaceous family of alkaloids14 has relevance to the biogenesis of the communesins stems from the structural similarity of these alkaloids and their precursors (Scheme 1). Woodward and Robinson independently proposed that the biogenesis of the calycanthoids is predicated on the oxidative homodimerization of N-methyl tryptamine (10) to form indolenine dimers, such as 13, which can give rise to five constitutional isomers upon reorganization of the two aminal functional groupings.10–11 In the illustrative case of indolenine dimer 13 (Scheme 1), amine cyclization via path-a results in (+)-chimonanthine (16), whereas an alternative cyclization via path-b, perhaps through the intermediacy of hexacycle 14, affords the isomeric alkaloid (−)-calycanthine (17). Indeed, the equilibration of (+)-chimonanthine (16) to (−)-calycanthine (17) under acidic aqueous conditions (16:17=15:85)8a,16 demonstrates the potential dynamic nature of these polycyclic structures. Anticipating a related biogenesis for the communesins, we expected the heterodimeric intermediate 15 to undergo a similar dynamic reorganization to afford heptacycle 18. Consistent with this hypothesis, an enzyme capable of the oxidative coupling of tryptamines and Penicillium fungal alkaloid (−)-aurantioclavine (12)17,18 has been identified that leads to the formation of the communesin core or an isomeric heptacycle depending on the tryptamine.15b We began our studies with the recognition that successful implementation of a biomimetic strategy for the efficient synthesis of these alkaloids requires the directed and stereocontrolled union of two dissimilar fragments followed by selective reorganization of a C3a–C3a′ linked heterodimer to a single constitutional isomer consistent with the communesin skeleton 18.
Scheme 1.
Comparison of the Bond Formations in the Biogenesis of Structurally Related Dimeric Alkaloids
Results and Discussion
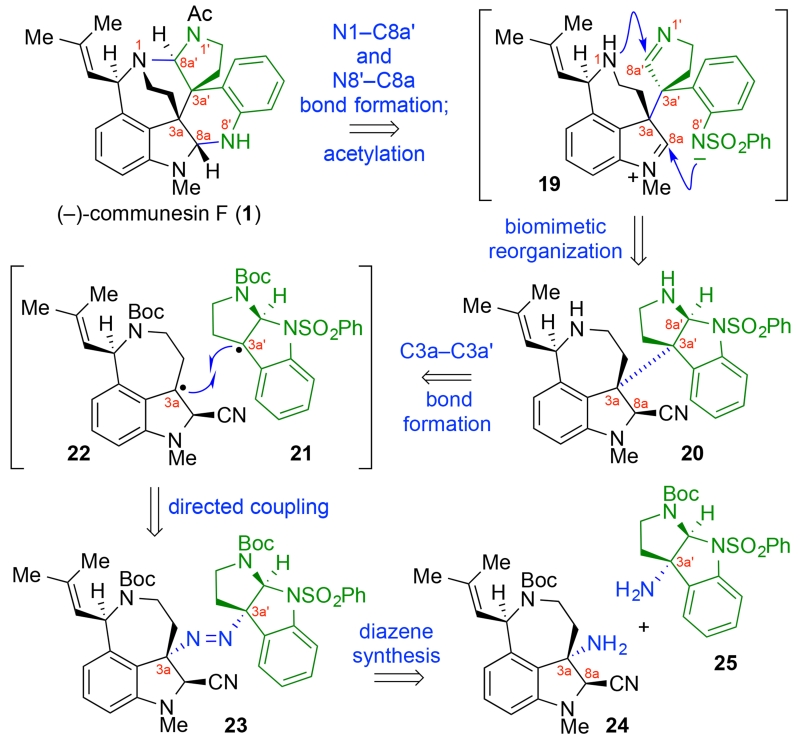
As illustrated in our retrosynthetic analysis of (−)-communesin F (1, Scheme 2), strict adherence to the central paradigm in the biogenesis of calycanthaceous alkaloids focused our design on the efficient assembly and reorganization of a key heterodimeric intermediate 20. Prompted by our strategy for directed heterodimerization of cyclotryptamines,19 we envisioned hexacycle 20 (Scheme 2) to serve as a surrogate for the hypothetical biosynthetic intermediate 15 (Scheme 1). We anticipated the N8′-sulfonamide would guide the opening of the C8a′-aminal to present the C8a′-imine for N1-addition. Furthermore, we projected the ionization of the C8a-nitrile would offer the C8a-imininium ion needed for aminal formation via N8′-addition. The challenging C3a–C3a′ linkage of heterodimer 20 required a directed and stereocontrolled union of a cyclotryptamine fragment 21 and aurantioclavine derivative 22 to simultaneously secure the two critical quaternary stereocenters. Our diazene-based strategy for directed complex fragment assembly19 provided the essential framework to explore this exciting and convergent approach to (−)-communesin F (1). While we were confident the C8a′-stereochemisry of the cyclotryptamine moiety would guide the desired C3a′-stereochemical outcome in this union, we were intrigued by the potential level of stereochemical control expected at C3a during carbon–carbon bond formation. We envisioned the synthesis of complex heterodimeric diazene 23 from tricyclic amines 24 and 25 as tryptamine–surrogates necessary for securing the C3a–C3a′ linkage (Scheme 2).
Scheme 2.
Biogenetically Inspired Retrosynthetic Analysis of (−)-Communesin F (1)
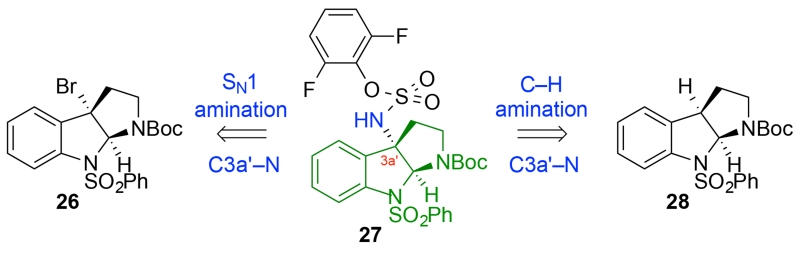
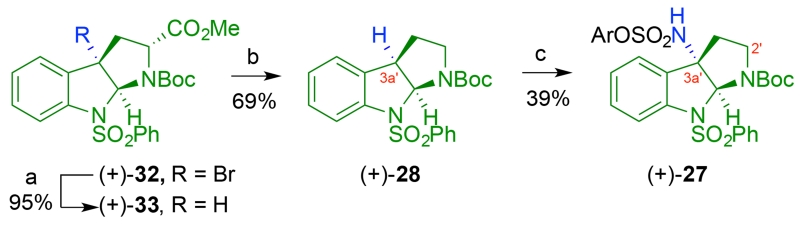
Our synthesis of (−)-communesin F (1) commenced with the preparation of the two key tricyclic amines 24 and 25 required for the assembly of critical diazene 23 (Scheme 2). We pursued two approaches to the synthesis of the C3a′-amino cyclotryptamine 25 and the corresponding sulfamate 27 (Scheme 3). In the first approach, motivated by efficient access to enantiomerically enriched C3a′-halocyclotryptamine derivatives,20a we envisioned a nucleophilic C3a′-amination (Scheme 4) reminiscent of our Friedel-Crafts strategy for C3a-derivatization developed in the context of our naseseazine alkaloid total synthesis.21 Our second approach to amine 25 relied on Du Bois amination22 of cyclotryptamine 28 to secure the sulfamate 27 (Scheme 5).19c
Scheme 3.
Strategies for Synthesis of Tricycle 27
Scheme 4.
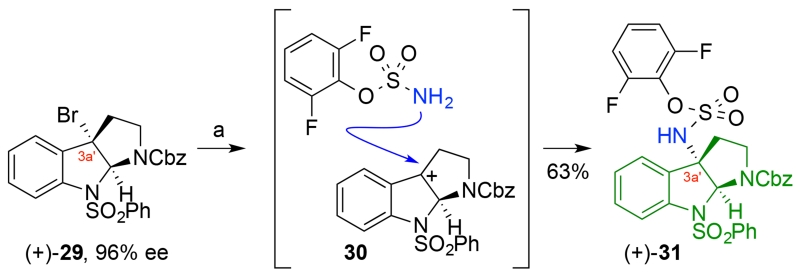
Concise Synthesis of Sulfamate (+)-31a
aReagents and conditions: (a) AgSbF6, 2,6-di-tert-butyl-4-methylpyridine, 2,6-difluorophenylsulfamate, CH2Cl2, 23 °C, 63%.
Scheme 5.
Gram-scale Synthesis of Sulfamate (+)-27a
aReagents and conditions: (a) (Me3Si)3SiH, Et3B, air, 23 °C, >99:1 dr; (b) (i) KOH (aq.), MeOH, CH2Cl2, 23 °C, (ii) N,N,N′,N′-tetramethylchloroformamidinium hexafluorophosphate, thiopyridine N-oxide, 4-(N,N-dimethylamino)pyridine, Et3N, THF; t-BuSH, hν, 23 °C; (c) Rh2(esp)2, H2NSO3Ar, PhI(OAc)2, Ph(CH3)2CCO2H, MgO, 5Å-MS, i-PrOAc, 23 °C; Ar=2,6-difluorobenzene.
Given the versatility of cyclotryptamine–sulfamates as precursors to the corresponding mixed sulfamides,19c we developed an efficient synthesis to access sulfamate (+)-31 and related derivatives starting with C3a′-bromocyclotryptamine (+)-29 (Scheme 4). Enantioselective bromocyclization20a of Nβ-Cbz-N1-benzenesulfonyltryptamine catalyzed by (S)-3,3′-bis(2,4,6-triisopropylphenyl)-1,1′-binaphthyl-2,2′-diyl hydrogenphosphate (TRIP)20b afforded C3a′-bromocyclotryptamine (+)-29 in 93% yield and 96% enantiomeric excess.23 Significantly, electrophilic activation21 of the tricyclic bromide (+)-29 in the presence of 2,6-difluorophenylsulfamate22 provided the desired sulfamate (+)-31 in 63% yield (Scheme 4). The use of 2,6-difluorophenylsulfamate as a nucleophile to trap an intermediate C3a′-electrophile 30 provides a new and expedient route for the directed synthesis of complex diazenes.19c While this new single-step synthesis of C3a′-sulfamates from the corresponding C3a′-bromides offers a concise solution to the desired precursors, its utility in conversion of the more acid sensitive tert-butyl carbamate substrate 26 to sulfamate 27 gave capricious and inferior outcomes (~50% yield).
Our alternate approach for the synthesis of tert-butylcarbamate derivative 27 relied on the C–H amination chemistry illustrated in Scheme 5. Mild reduction of bromocyclotryptophan (+)-32 provided the desired C3a′–H cyclotryptophan (+)-33 in 95% yield.23 Subsequent decarboxylation furnished cyclotryptamine (+)-28 in 69% yield. Under optimal conditions, a Rh-catalyzed C–H amination22 of cyclotryptamine (+)-28 afforded the desired sulfamate (+)-27 in 39% yield after recrystallization.24 This three-step sequence efficiently generated gram quantities of (+)-27 from the readily available bromocyclotryptophan (+)-32 as an activated form of C3a′-aminocyclotryptamine 25 (Scheme 2) that is ready for coupling with tricyclic amine 24 for diazene synthesis.
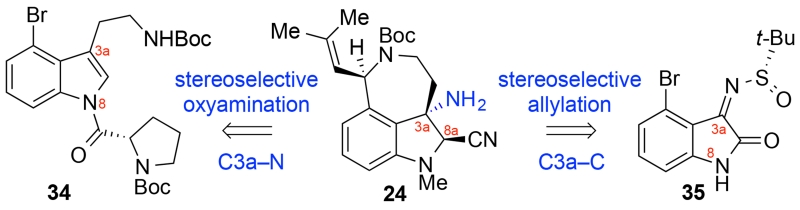
With an expedient synthesis of sulfamate (+)-27 available, we turned our attention to the synthesis of the tricyclic amine 24 (Scheme 6). While syntheses of aurantioclavine (12) have been reported,18 the synthesis of a derivative needed to mimic fragment 22, necessary for our biomimetic approach to (−)-communesin F (1), has not been described. As a result, we sought to develop an enantioselective synthesis of a tricycle reminiscent of alkaloid 12 that would allow for implementation of our synthetic strategy (Scheme 2). The tricyclic aminonitrile 24 offered the necessary C3a-amine for diazene synthesis and the C8a-aminonitrile to allow for mild generation of the corresponding C8a-iminium ion needed for aminal synthesis. We developed two strategies to access the key intermediate 24 as illustrated in Scheme 6. The first strategy involved tryptamine 34 as the substrate for the application of Yoon’s oxyamination chemistry,25 while the second strategy utilized tert-butyl sulfinimine 35 and Ellman’s asymmetric allylation26 of such substrates.27
Scheme 6.
Strategies for Synthesis of Tricycle 24
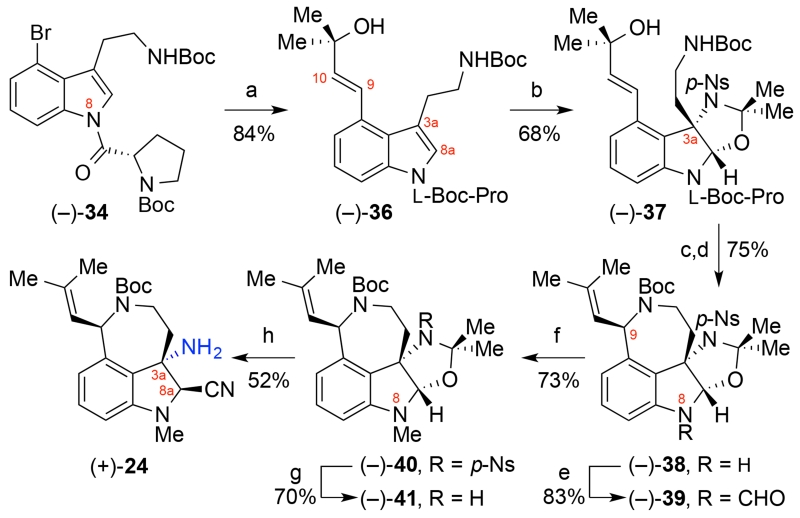
The oxyamination25 route to aminonitrile 24 commenced with a Mizoroki-Heck reaction of bromoindole (−)-3423 with 1,1-dimethylallyl alcohol to provide allylic alcohol (−)-36. Despite early reservations regarding possible competing C9-C10-oxyamination25b-f of vinyl indole (−)-36 in place of the desired C3a-C8a-oxyamination, we observed higher levels of diastereoselection28 for the oxyamination of the more advanced substrate (−)-36 (Scheme 7). The use of stoichiometric copper(II) chloride facilitated the reaction and gave oxazoline (−)-37 in 68% yield (89:11 dr). Treatment of alcohol (−)-37 with bis(acetonitrile)dichloropalladium(II) in acetonitrile to form the desired azepane (85% yield)23 followed by removal of the chiral auxiliary (88% yield) provided the desired indoline (−)-38. The formylation of indoline (−)-38 to give formamide (−)-39 (83% yield) followed by mild reduction with sodium borohydride in the presence of trifluoroacetic acid gave the desired N-methylindoline (−)-40 (73% yield).29 Exposure of sulfonamide (−)-40 to thiophenol and potassium carbonate led to removal of the para-nitrobenzenesulfonyl group and the isolation of the stable oxazolidine (−)-41 in 70% yield. Given the propensity of oxazolidine (−)-41 and aminonitrile (+)-24 toward elimination of the C3a-amino group under strongly acidic or basic conditions, we developed mild hydrolysis conditions to allow for cyanation of a transient C8a-hemiaminal leading to aminonitrile (+)-24 in 52% yield in addition to the C8a-epimer (26%). While this approach provides flexibility for the late-stage introduction of various N8-substituents and establishes the C3a-configuration, the challenge in unraveling the oxazolidine substructure prompted our investigation of an alternate route to aminonitrile (+)-24 (Scheme 6) involving C3a–C bond formation.
Scheme 7.
Oxyamination Approach to Tricycle (+)-24a
aReagents and conditions: (a) 1,1-dimethylallyl alcohol, Pd(OAc)2, P(o-tol)3, Et3N, MeCN, 95 °C; (b) 3,3-dimethyl-2-(p-nitrobenzenesulfonyl)-1,2-oxaziridine, CuCl2, n-Bu4NCl, CHCl3, 21 °C, 89:11 dr; (c) PdCl2(MeCN)2, MeCN, 82 °C; (d) (i) i-Bu2AlH, THF, 0 °C, (ii) 1,8-diazabicyclo[5.4.0]undec-7-ene, MeOH, 21 °C; (e) Ac2O, HCO2H, pyridine, CH2Cl2, 21 °C; (f) NaBH4, TFA, THF, 0 °C; (g) PhSH, K2CO3, DMF, 50 °C; (h) Me3SiCN, (F3C)2CHOH, H2O, 21 °C; p-Ns = para-nitrobenzenesulfonyl.
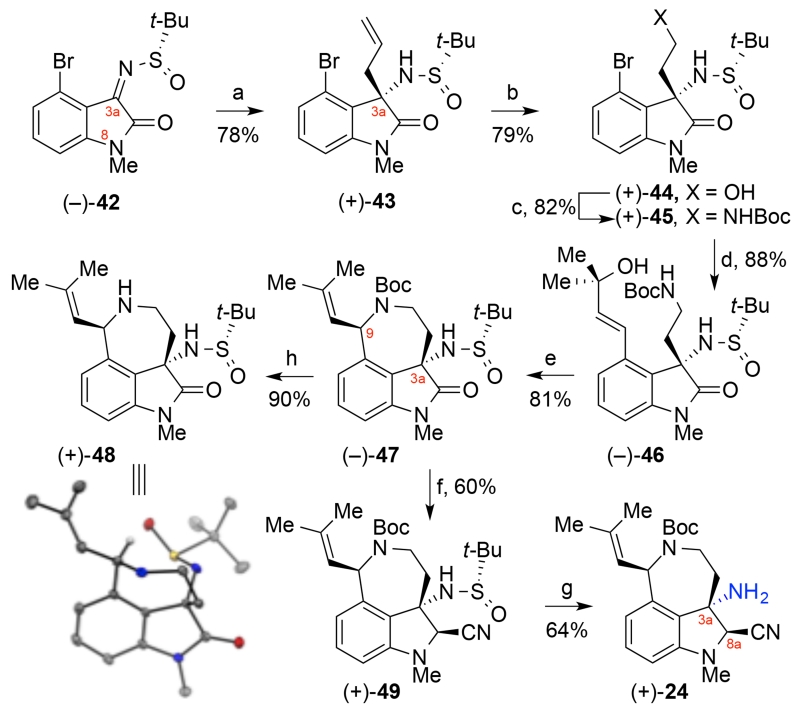
Our alternative synthesis of aminonitrile (+)-24 began with the diastereoselective allylation26 of N8-methyl sulfinimine (−)-42 (Scheme 8) to provide allyl oxindole (+)-43 in 78% yield and with excellent diastereopurity after trituration of the crude addition product with hexane (>98:2 dr).23 In contrast to our first approach to aminonitrile (+)-24, the placement of the chiral auxiliary on the C3a-substituent enabled our use of the N8-methyl variant of sulfinimine 35 (Scheme 6).23 Ozonolysis of alkene (+)-43 followed by a reductive work-up afforded the primary alcohol (+)-44 in 79% yield. The alcohol (+)-44 was then converted to tert-butyl carbamate (+)-45 in 82% yield via a Mitsunobu displacement and subsequent in situ desulfonylation. The allylic alcohol needed for synthesis of the azepane substructure was introduced via a Stille vinylation to furnish allylic alcohol (−)-46 in 88% yield.30 A palladium-catalyzed allylic amination provided azepane (−)-47 in 81% yield as a single diastereomer. The stereochemistry at C3a and C9 of azepane (−)-47 was confirmed unambiguously through analysis of the crystal structure of the corresponding amine (+)-48 (Scheme 8). We then focused on development of conditions for mild and efficient conversion of oxindole (−)-47 to the desired aminonitrile (+)-24. Partial reduction of oxindole (−)-47 with lithium borohydride afforded a mixture of C8a-hemiaminal diastereomers that were too labile for isolation. Direct treatment of the crude hemiaminal with trimethylsilyl cyanide in hexafluoroisopropanol31 furnished the desired aminonitrile (+)-49 in 60% yield32 and the easily separable minor C8a-epimer (30%).23 Methanolysis of the tert-butyl sulfinamide (+)-49 provided the desired amino-azepane (+)-24 in 64% yield.33 The C8a-aminonitrile proved to be an ideal trigger for late stage hemiaminal formation34 while providing adequate stability for the implementation of an efficient fragment assembly. We anticipate future adaptation of this robust synthetic route to other N8-variants of azepane (+)-24 via judicious N8-substitution of sulfinimine 35.
Scheme 8.
Sulfinimine Allylation Approach to Tricycle (+)-24a
aReagents and conditions: (a) allylMgBr, MgBr2, CH2Cl2, −78 °C, >98:2 dr; (b) O3, MeOH, −78 °C; NaBH4, −78 → 23 °C; (c) o-NsNHBoc, diisopropyl azodicarboxylate, polystyrene–PPh3, THF, 50 °C; PhSH, Cs2CO3, 50 °C; (d) Me2C(OH)CH=CHSn(n-Bu)3, PdCl2(PPh3)2, PhMe, THF, 110 °C; (e) PdCl2(MeCN)2, MeCN, 80 °C; (f) (i) LiBH4, MeOH, THF, 0 → 23 °C; (ii) Me3SiCN, (F3C)2CHOH, 0 °C; (g) HCl, dioxane, MeOH, 23 °C; (h) Sc(OTf)3, F3CCH2OH, 23 °C; o-Ns = ortho-nitrobenzenesulfonyl. ORTEP representation of amine (+)-48: thermal ellipsoids drawn at 50% probability.23
After developing versatile syntheses of both essential fragments, we next examined the union of azepane (+)-24 and cyclotryptamine (+)-27 to introduce the critical C3a–C3a′ bond. Dissolution of the two fragments in tetrahydrofuran in the presence of 4-(N,N-dimethylamino)pyridine afforded sulfamide (+)-50 in 80% yield on gram-scale (Scheme 9).23 Consistent with our prior observation, the oxidation of sterically shielded sulfamides containing electron-rich arenes, such as the N-methyl aniline substructure of sulfamide (+)-50, suffers from competitive arene-halogenation. After extensive experimentation, we discovered the unique ability of tertiary N-chloroamides to effect chemoselective oxidation of sulfamide (+)-50 to the corresponding diazene (Scheme 9) without competitive arenehalogenation. Exposure of sulfamide (+)-50 to N-chloro-N-methylbenzamide (6 equiv) in conjunction with polystyrene-bound 2-tert-butylimino-2-diethylamino-1,3-dimethyl-perhydro-1,3,2-diazaphosphorine (BEMP) in methanol provided the desired diazene (+)-23 in 57% yield.35 Photoexcitation and expulsion of dinitrogen from a thin film of diazene (+)-23, followed by radical combination of the resulting cyclotryptamine 21 and azepane 22 (Scheme 2), afforded the desired heterodimer (+)-51 in 39% yield as a single diastereomer.36 While the stereochemical outcome at C3a′ of heterodimer (+)-51 is anticipated,19 the remarkable diastereoselection at C3a is notable and likely due to the confluence of a rapid radical combination step and the additional stereoinduction imposed by the C8a-nitrile.23,37 Importantly, our diazene-based strategy for directed complex fragment assembly allowed for the stereoselective construction of the critical C3a–C3a′ linkage, securing the corresponding vicinal quaternary stereocenters.
Scheme 9.
Directed Synthesis of Heterodimer (+)-51a
aReagents and conditions: (a) 4-(N,N-dimethylamino)pyridine, THF, 23 °C; (b) polystyrene–2-t-butylimino-2-diethylamino-1,3-dimethyl-perhydro-1,3,2-diazaphos-phorine, N-chloro-N-methylbenzamide, MeOH, 23 °C; (c) hν (350 nm), 25 °C.
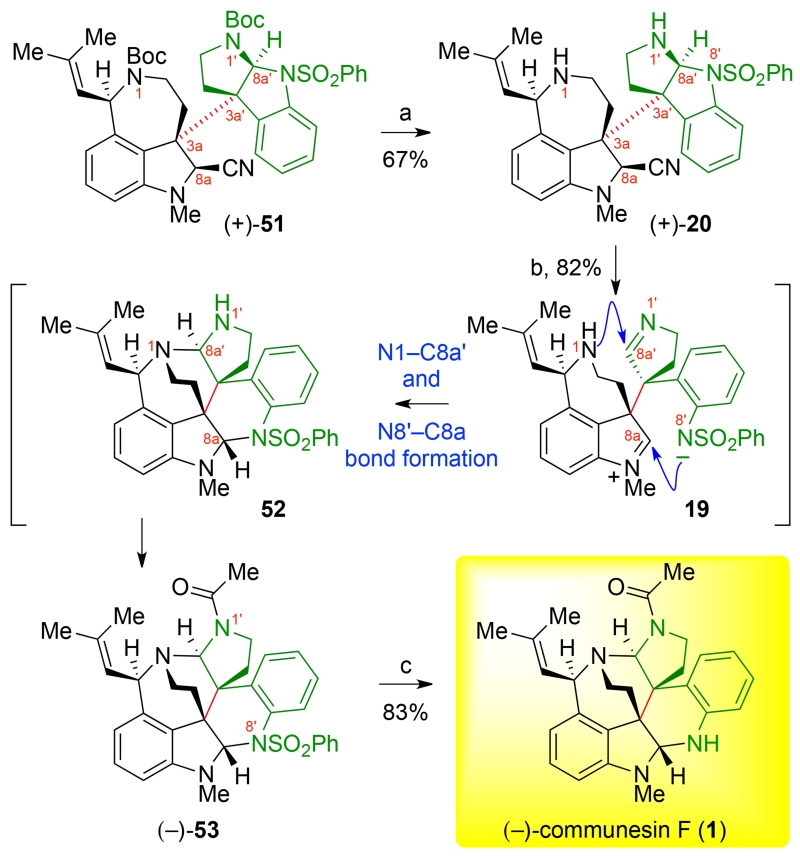
With the successful union of the tricyclic–azepane and the cyclotryptamine fragments at hand, we turned our attention to the development of a biogenetically inspired aminal reorganization of heterodimer (+)-51 to access the heptacyclic communesin core. Informed by experience in rearrangement of the calycanthaceous alkaloids,8,14 we recognized the significance in the judicious choice of reaction conditions for the planned transformation due to the sensitive nature of the C3a–C3a′ linkage. Furthermore, we anticipated that an appropriate sequence of amine unveiling would maximize efficiency for the desired aminal exchange. We projected that unveiling the N1- and N1′-amines of heterodimer (+)-51 would allow opening of the C8a′ aminal with the benzenesulfonamide as the leaving group, thus allowing rapid trapping of the C8a′-imine of intermediate 19 en route to heptacycle 52. Treatment of heterodimer (+)-51 with scandium trifluoromethanesulfonate in trifluoroethanol provided the desired heterodimer (+)-20 by selective removal of the tert-butyl carbamates while preserving the sensitive C8a-aminonitrile (Scheme 10). The electron-withdrawing N8′-sulfonamide enabled our examination of basic conditions to selectively open the cyclotryptamine substructure. In the event, treatment of heterodimer (+)-20 with lithium tert-butoxide in methanol provided clean and complete conversion to the desired heptacyclic structure 52 within 1 h at 50° C as observed by in situ 1H-NMR spectroscopy.23 Significantly, only the desired heptacycle 52 was formed in preference to other constitutional isomers.15b Methanol was found to be an excellent solvent38 for this transformation, likely due to its ability to stabilize reactive intermediates as the corresponding O-alkyl-hemiaminals. Although intermediate 52 could be observed by in situ 1H NMR spectroscopy,23 this compound did not show sufficient stability for isolation. We suspect this may be due to the sensitive nature of the C8a′-aminal of heptacycle 52, which upon reversible opening to the C8a′-imine increases the lability of the C3a–C3a′ bond. As a testimony to the sensitivity of the C3a–C3a′ linkage of heterodimer (+)-20, simple heating of a derivative (C8a-OMe instead of C8a-CN) in acetonitrile-d3 at 80 °C predominantly led to fragmentation.39 Indeed, treatment of the basic solution of heptacycle 52 with pyridinium p-toluenesulfonate to quench the alkoxides, followed by addition of acetic anhydride afforded the N1′-acetyl derivative (−)-53 in 82% overall yield.23 A final-step unveiling of the N8′-amine was accomplished by treatment of (−)-53 with sodium amalgam to provide (−)-communesin F (1) in 83% yield. All 1H and 13C NMR data as well as optical rotation (observed [α]D24 = −249, c = 0.13, CHCl3; literature [α]D20 = −264, c = 0.34, CHCl3),1c,23 for our synthetic (−)-communesin F (1) were in agreement with literature data.
Scheme 10.
Synthesis of (−)-Communesin F (1) via a Biogenetically Inspired Final Stage Reorganizationa
aReagents and conditions: (a) Sc(OTf)3, F3CCH2OH, 23 °C; (b) t-BuOLi, MeOH, 50 °C; dry PPTS, Ac2O, 23 °C; (c) Na(Hg), NaH2PO4, THF, MeOH, 23 °C.
Conclusion
A highly convergent enantioselective total synthesis of (−)-communesin F (1) with late-stage chemistry that parallels the latest insights15 and hypotheses concerning the biogenesis of these alkaloids is described. Our expedient synthesis involves the union of fragments (+)-24 and (+)-27 to provide complex sulfamide (+)-50 on gram-scale. This advanced intermediate is converted to alkaloid (−)-1 in only five additional steps (Schemes 9 and 10) which include the application of our diazene–directed fragment assembly strategy to secure the congested C3a–C3a′ linkage, and a guided biomimetic rearrangement to selectively provide the heptacyclic core of these alkaloids. Highlights of our synthesis include an efficient cyclotryptamine–C3a-sulfamate synthesis by either a new silver–promoted nucleophilic amination or rhodium–catalyzed C–H amination protocol, application of catalytic asymmetric halocyclization and diastereoselective oxyamination reactions in complex settings, a stereoselective sulfinimine allylation, and efficient assembly and utility of a richly functional diazene for complex fragment coupling. The successful implementation of this synthetic strategy and the versatile synthesis of the fragments, along with a final stage acylation of the communesin core provide a foundation for a unified synthetic route to access structurally related complex alkaloids and derivatives.
Supplementary Material
ACKNOWLEDGMENT
We are grateful for financial support from NIH-NIGMS (GM089732) and NSF (CHE1212527). S. P. L. acknowledges a Ruth L. Kirschstein NRSA postdoctoral fellowship (F32GM097776). M. P. thanks the Natural Sciences and Engineering Research Council of Canada (NSERC) for a PGS D3 Scholarship. We thank Dr. Peter Müller, Dr. Justin Kim, and Mr. Brandon M. Nelson for X-ray crystal structure analysis of (+)-48, and thank Ms. Petra Lindovska, Dr. François Brucelle, and Mr. Jonathan V. Truong for helpful discussions.
Footnotes
ASSOCIATED CONTENT
The Supporting Information is available free of charge on the ACS Publications website. Experimental procedures, spectroscopic data, crystal structure of (+)-48 (CIF), and copies of NMR spectra (PDF).
The authors declare no competing financial interests.
REFERENCES
- (1).Numata A, Takahashi C, Ito Y, Takada T, Kawai K, Usami Y, Matsumura E, Imachi M, Ito T, Hasegawa T. Tetrahedron Lett. 1993;34:2355. Jadulco R, Edrada RA, Ebel R, Berg A, Schaumann K, Wray V, Steube P, Proksch P. J. Nat. Prod. 2004;67:78. doi: 10.1021/np030271y. Hayashi H, Matsumoto H, Akiyama K. Biosci. Biotechnol. Biochem. 2004;68:753. doi: 10.1271/bbb.68.753. Andersen B, Smedsgaard J, Frisvad JC. J. Agric. Food Chem. 2004;52:2421. doi: 10.1021/jf035406k. Dalsgaard PW, Blunt JW, Munro MGH, Frisvad JC, Christophersen C. J. Nat. Prod. 2005;68:258. doi: 10.1021/np049646l. For the structurally related perophoramidine, see Verbitski SM, Mayne CL, Davis RA, Concepcion GP, Ireland CM. J. Org. Chem. 2002;67:7124. doi: 10.1021/jo026012f.
- (2).For a recent review, see Trost BM, Osipov M. Chem. Eur. J. 2015;21:16318. doi: 10.1002/chem.201501735.
- (3).Yang J, Wu H, Shen Y, Qin Y. J. Am. Chem. Soc. 2007;129:13794. doi: 10.1021/ja075705g. [DOI] [PubMed] [Google Scholar]
- (4).(a) Liu P, Seo JH, Weinreb SM. Angew. Chem. Int. Ed. 2010;49:2000. doi: 10.1002/anie.200906818. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Seio JH, Artman GD, III, Weinreb SM. J. Org. Chem. 2006;71:8891. doi: 10.1021/jo061660a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Belmar J, Funk RL. J. Am. Chem. Soc. 2012;134:16941. doi: 10.1021/ja307277w. (b) For synthesis of structurally related (±)-perophoramidine, see Fuchs JR, Funk RL. J. Am. Chem. Soc. 2004;126:5068. doi: 10.1021/ja049569g.
- (6).(a) Han S-J, Vogt F, Krishnan S, May JA, Gatti M, Virgil SC, Stoltz BM. Org. Lett. 2014;16:3316. doi: 10.1021/ol5013263. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Han S-J, Vogt F, May JA, Krishnan S, Gatti M, Virgil SC, Stoltz BM. J. Org. Chem. 2015;80:528. doi: 10.1021/jo502534g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Zuo Z, Xie W, Ma D. J. Am. Chem. Soc. 2010;132:13226. doi: 10.1021/ja106739g. For enantioselective syntheses of (−)-1 and (−)-2, see Zuo Z, Ma D. Angew. Chem. Int. Ed. 2011;50:12008. doi: 10.1002/anie.201106205.
- (8).(a) Movassaghi M, Schmidt MA. Angew. Chem. Int. Ed. 2007;46:3725. doi: 10.1002/anie.200700705. [DOI] [PubMed] [Google Scholar]; (b) Schmidt MA, Movassaghi M. Synlett. 2008;3:313. [Google Scholar]
- (9).Kim J, Movassaghi M. Acc. Chem. Res. 2015;48:1159. doi: 10.1021/ar500454v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Woodward RB, Yang NC, Katz TJ, Clark VM, Harley-Mason J, Ingleby RFJ, Sheppard N. Proc. Chem. Soc. 1960:76. [Google Scholar]
- (11).Robinson R, Teuber HJ. Chem. Ind. 1954;27:783. [Google Scholar]
- (12).Wigley LJ, Mantle PG, Perry DA. Phytochemistry. 2006;67:561. doi: 10.1016/j.phytochem.2005.10.011. [DOI] [PubMed] [Google Scholar]
- (13).(a) May JA, Zeidan RK, Stoltz BM. Tetrahedron Lett. 2003;44:1203. [Google Scholar]; (b) May JA, Stoltz BM. Tetrahedron. 2006;62:5262. [Google Scholar]
- (14).(a) Cordell GA, Saxton JE. In: The Alkaloids: Chemistry and Physiology. Manske RHF, Rodrigo RGA, editors. Vol. 20. Academic Press; New York: 1981. pp. 3–295. [Google Scholar]; (b) Hino T, Nakagawa M. In: The Alkaloids: Chemistry and Pharmacology. Brossi A, editor. Vol. 34. Academic Press; New York: 1989. pp. 1–75. [Google Scholar]; (c) Crich D, Banerjee A. Acc. Chem. Res. 2007;40:151. doi: 10.1021/ar050175j. [DOI] [PubMed] [Google Scholar]; (d) Steven A, Overman LE. Angew. Chem. Int. Ed. 2007;46:5488. doi: 10.1002/anie.200700612. [DOI] [PubMed] [Google Scholar]; (e) Xu J-B, Cheng K-J. Molecules. 2015;20:6715. doi: 10.3390/molecules20046715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).(a) Lin H-C, Chiou G, Chooi Y-H, McMahon TC, Xu W, Garg NK, Tang Y. Angew. Chem. Int. Ed. 2015;54:3004. doi: 10.1002/anie.201411297. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lin H-C, McMahon TC, Patel A, Corsello M, Simon A, Xu W, Zhao M, Houk KN, Garg NK, Tang Y. J. Am. Chem. Soc. 2016;138:4002. doi: 10.1021/jacs.6b01413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).(a) Hendrickson JB, Göschke R, Rees R. Tetrahedron. 1964;20:565. [Google Scholar]; (b) Hall ES, McCapra F, Scott AI. Tetrahedron. 1967;23:4131. doi: 10.1016/s0040-4020(01)97925-6. [DOI] [PubMed] [Google Scholar]; (c) Guéritte-Voegelein F, Sévenet T, Pusset J, Adeline MT, Gillet B, Beloeil JC, Guenard D, Potier P. J. Nat. Prod. 1992;55:923. doi: 10.1021/np50085a012. [DOI] [PubMed] [Google Scholar]; (d) Lebsack AD, Link JT, Overman LE, Stearns BA. J. Am Chem. Soc. 2002;124:9008. doi: 10.1021/ja0267425. [DOI] [PubMed] [Google Scholar]
- (17).Kozlovskii AG, Soloveva TF, Sakharobskii VG, Adanin VM. Dokl. Akad. Nauk SSSR. 1981;260:230. [PubMed] [Google Scholar]
- (18).For syntheses of (±)-aurantioclavine, see: Yamada F, Makita Y, Suzuki T, Somei M. Chem. Pharm. Bull. 1985;33:2162. Hegedus LS, Toro JL, Miles WH, Harrington PJ. J. Org. Chem. 1987;52:3319. Somei M, Yamada F. Heterocycles. 2007;74:943. Yamada K, Namerikawa Y, Haruyama T, Miwa Y, Yanada R, Ishikura M. Eur. J. Org. Chem. 2009:5752. For syntheses of (−)-aurantioclavine, see Krishnan S, Bagdanoff JT, Ebner DC, Ramtohul YK, Tambar UK, Stoltz BM. J. Am. Chem. Soc. 2008;130:13745. doi: 10.1021/ja804738b. Brak K, Ellman JA. Org. Lett. 2010;12:2004. doi: 10.1021/ol100470g. Xu ZR, Hu WM, Liu QA, Zhang LH, Jia YX. J. Org. Chem. 2010;75:7626. doi: 10.1021/jo101506c. Behenna DC, Krishnan S, Stoltz BM. Tetrahedron Lett. 2011;52:2152. Suetsugu S, Nishiguchi H, Tsukano C, Takemoto Y. Org. Lett. 2014;16:996. doi: 10.1021/ol4037314.
- (19).(a) Movassaghi M, Ahmad OK, Lathrop SP. J. Am. Chem. Soc. 2011;133:13002. doi: 10.1021/ja2057852. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lathrop SP, Kim J, Movassaghi M. Chimia. 2012;66:389. doi: 10.2533/chimia.2012.389. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lathrop SP, Movassaghi M. Chem. Sci. 2014;5:333. doi: 10.1039/C3SC52451E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Xie X, Jiang G, Liu H, Hu J, Pan X, Zhang H, Wan X, Lai Y, Ma D. Angew. Chem. Int. Ed. 2013;52:12924. doi: 10.1002/anie.201306774. For TRIP catalyst, see Hoffmann S, Seayad AM, List B. Angew. Chem. Int. Ed. 2005;44:7424. doi: 10.1002/anie.200503062.
- (21).(a) Kim J, Movassaghi M. J. Am. Chem. Soc. 2011;133:14940. doi: 10.1021/ja206743v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Boyer N, Movassaghi M. Chem. Sci. 2012;3:1798. doi: 10.1039/C2SC20270K. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Adams TC, Payette JN, Cheah JH, Movassaghi M. Org. Lett. 2015;17:4268. doi: 10.1021/acs.orglett.5b02059. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Loach RP, Fenton OS, Movassaghi M. J. Am. Chem. Soc. 2016;138:1057. doi: 10.1021/jacs.5b12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Roizen JL, Zalatan DN, Du Bois J. Angew. Chem. Int. Ed. 2013;52:11343. doi: 10.1002/anie.201304238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).See Supporting Information for details.
- (24).The regioisomeric C2′-amination side product was observed (C3a′:C2′ = 5.4:1) prior to purification.
- (25).For oxyamination of indoles, see Benkovics T, Guzei IA, Yoon TP. Angew. Chem. Int. Ed. 2010;49:9153. doi: 10.1002/anie.201004635. For oxyamination of styrenes, see: Michaelis DJ, Williamson KS, Yoon TP. Tetrahedron. 2009;65:5118. doi: 10.1016/j.tet.2009.03.012. DePorter SM, Jacobsen AC, Partridge KM, Williamson KS, Yoon TP. Tetrahedron Lett. 2010;51:5223. doi: 10.1016/j.tetlet.2010.08.015. Williamson KS, Yoon TP. J. Am. Chem. Soc. 2010;132:4570. doi: 10.1021/ja1013536. Williamson KS, Yoon TP. J. Am. Chem. Soc. 2012;134:12370. doi: 10.1021/ja3046684. Uraguchi D, Tsutsumi R, Ooi T. J. Am. Chem. Soc. 2013;135:8161. doi: 10.1021/ja403491j.
- (26).(a) Cogan DA, Liu G, Ellman JA. Tetrahedron. 1999;55:8883. [Google Scholar]; (b) Robak MT, Herbage MA, Ellman JA. Chem. Rev. 2010;110:3600. doi: 10.1021/cr900382t. [DOI] [PubMed] [Google Scholar]
- (27).(a) Davis FA, Zhou P, Chen B-C. Chem. Soc. Rev. 1998;27:13. [Google Scholar]; (b) Zhou P, Chen B-C, Davis FA. Tetrahedron. 2004;60:8003. [Google Scholar]; (c) Senanayake CH, Krishnamurthy D, Lu Z-H, Han Z, Gallou I. Aldrichim. Acta. 2005;38:93. [Google Scholar]; (d) Davis FA. J. Org. Chem. 2006;71:8993. doi: 10.1021/jo061027p. [DOI] [PubMed] [Google Scholar]; (e) Morton D, Stockman RA. Tetrahedron. 2006;62:8869. [Google Scholar]
- (28).For comparison, the oxyamination of the corresponding l-(3,5-di-tert-butylphenylcarbamoyl)-Pro derivatives of substrates (−)-34 and (−)-36 gave the desired product in 27% (53:47 dr) and 79% (73:27 dr) yield, respectively.
- (29).Methylation of indoline (−)-38 with Meerwein’s reagent or its reductive amination with formalin and NaBH3CN resulted in the desired N-methylindoline (−)-40 in 15% and 21% yield, respectively.
- (30).Likely due to the greater steric constraints imposed by the fully substituted tetrahedral C3a in bromide (+)-45, related Heck protocols described in prior syntheses of alkaloid 1 were unsuccessful.
- (31).Herzon SB, Myers AG. J. Am. Chem. Soc. 2005;127:5342. doi: 10.1021/ja0510616. [DOI] [PubMed] [Google Scholar]
- (32).The C8a-configuration of the major diastereomer was determined by nOe analysis of a derivative.
- (33).Exposure of the C8a-epimer to the same conditions resulted in only 22% yield of the desired epimeric aminonitrile.
- (34).For representative examples of related use of aminonitriles in synthesis, see: Overman LE, Jacobsen EJ. Tetrahedron Lett. 1982;23:2741. Ksander G, Bold G, Lattmann R, Lehmann C, Fruh T, Xiang Y-B, Inomata K, Buser HP, Schreiber J, Zass E, Eschenmoser A. Helv. Chim. Acta. 1987;70:1115. Stork GS. Pure Appl. Chem. 1989;61:439. Corey EJ, Gin DY, Kania RS. J. Am. Chem. Soc. 1996;118:9202. Myers AG, Kung DW. J. Am. Chem. Soc. 1999;121:10828.
- (35).Use of N-chlorosuccinimide (6 equiv) as the oxidant in conjunction with N,N-dimethylaniline (1 equiv) as electrophilic chlorine scavenger gave only 37% yield of diazene (+)-23, in addition to recovered (+)-50 (17%), and a tricyclic cyanoindole side product (25%) arising from E2-elimination of C3a-sulfamide.
- (36).The formation of the tricyclic cyanoindole (~47%) and a C3a′-H cyclotryptamine 28 (~51%) as disproportionation products is reflective of the significant steric barrier for C3a–C3a′ bond formation and competitive C8a-H abstraction.
- (37).Calculations suggest the C8a-CN provides 3.8 kcal/mol preference for the desired C3a stereochemical outcome.
- (38).Use of tetrahydrofuran-d8 as solvent led to a less efficient aminal reorganization and afforded product (−)-53 in 52% yield.
- (39).Experiment conducted over 13 h in the absence of acid or base promoter. We reason that reversible C8a-iminium ion formation in the absence of the desired N8′-nucleophile increases the lability of C3a–C3a′ bond.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.