Abstract
Background
To determine the effect of the MUC5B promoter polymorphism (rs35705950) on the CT imaging appearance of pulmonary fibrosis.
Methods
High-resolution CT scans of 1,764 subjects were scored as part of a, genomewide association study with institutional review board approval; 1,491 of these had pulmonary fibrosis on CT scans and were included in the study. Two thoracic radiologists independently scored CT scans systematically. Discrepancies were resolved by a third thoracic radiologist. All patients were genotyped specifically for the rs35705950 single-nucleotide polymorphism (SNP). Two-tailed Fisher exact or χ2 tests and Student t tests or Mann-Whitney U tests were used to compare proportions and means, respectively.
Results
The major and minor alleles at the rs35705950 SNP are guanine (G) and thymine (T), respectively: 514 were homozygous for the major allele (G group), and 977 were heterozygous or homozygous for the minor allele (T group). The G group had a higher proportion than the T group with ground-glass opacity (62.1% vs 54.2%; P = .04). There was no significant difference between the G and T groups regarding presence of honeycombing. The T group showed a significantly higher subpleural axial distribution of fibrosis than did the G group (62.3% vs 42.2%; P < .0001). The T group showed a lower proportion of diagnoses inconsistent with usual interstitial pneumonitis (UIP; 20.3% compared with 30.5% for the G group) and a greater proportion of confident (probable UIP and UIP) UIP diagnoses (43.8% compared with 32.6% for the G group).
Conclusions
The MUC5B promoter polymorphism identifies a pattern of fibrosis that is different from other causes of fibrosis and may respond differently to potential therapies.
Key Words: CT imaging, idiopathic pulmonary fibrosis, MUC5B, rs35705950, usual interstitial pneumonitis
Abbreviations: FIP, familial interstitial fibrosis; G, guanine; IPF, idiopathic pulmonary fibrosis; SNP, single-nucleotide polymorphism; T, thymine; UIP, usual interstitial pneumonitis
There are many secondary causes of pulmonary fibrosis. However, only a minority of patients with predispositions or exposures actually develop pulmonary fibrosis. This has led many to theorize that genetic factors as well as inherent host susceptibility predispose some people to development of pulmonary fibrotic disease. Evidence suggests that genetic mutations (eg, surfactant protein C, surfactant protein A2, and telomerase) likely play an important role in the development of pulmonary fibrosis in a substantial minority of cases.1, 2, 3, 4 Seibold et al5 showed that the rs35705950 single-nucleotide polymorphism (SNP), a probable promoter site of an airway mucin gene (MUC5B), is associated with idiopathic pulmonary fibrosis (IPF) and familial pulmonary fibrosis. This finding has been validated in multiple separate study cohorts.6, 7, 8, 9, 10, 11, 12, 13 Other studies failed to show an association between the MUC5B promoter polymorphism and other causes of pulmonary fibrosis, suggesting that it may be specific to IPF.6, 8, 14
IPF is the most common subtype of the idiopathic interstitial pneumonias. Despite its relative frequency, the underlying cause of IPF is unknown. The typical findings of IPF on chest CT scans are that of usual interstitial pneumonitis (UIP): reticular abnormality with peripheral and basilar preponderance, presence of subpleural honeycombing, and absence of other features suggestive of an alternative diagnosis. There is little information about the CT imaging pattern of pulmonary disease in subjects with genetic causes of pulmonary fibrosis. A large study of 340 subjects evaluating the CT imaging pattern in familial interstitial fibrosis (FIP) showed that the imaging pattern in FIP was dissimilar from that of sporadic IPF/UIP.15 However, this study did not evaluate imaging findings relative to specific genetic mutations and likely included multiple heterogeneous genetic variations with similar phenotypes. To our knowledge, there is no study that has extensively evaluated the CT imaging appearance of pulmonary fibrosis relative to a specific genetic variation. The purpose of this study was to detail the CT imaging phenotype of pulmonary fibrosis regarding the MUC5B promoter site (rs35705950) polymorphism, which has been strongly associated with both IPF and familial pulmonary fibrosis.5, 6, 7, 8, 9, 10 The major allele at this SNP is guanine (G), and the minor allele is thymine (T). On the basis of previous studies that have shown that the T allele is associated with dominant expression of IPF, we hypothesized that the CT imaging patterns of those with the T allele (whether heterozygous or homozygous) at the rs35705950 SNP would be more consistent with a UIP pattern than would that of those with the G allele.
Materials and Methods
This case control study was approved by our institutional review board (NJH 1441A). Informed consent was obtained from all subjects.
Study Population
A cohort of subjects (self-reported white) with pulmonary fibrosis was recruited from multiple sources including National Jewish Health, the Lung Tissue Research Consortium, Vanderbilt University, University of California San Francisco, the InterMune-supported IPF γ-interferon l and pirfenidone trials, and a cohort of known families with FIP from 1999 to 2010. A total of 1,914 subjects were genotyped from this group. CT scans of the chest were available for review in 1,764 subjects. In a prior study, we reported on 201 of the 1,764 subjects included in the current study.16 The prior report only included subjects with pathologic correlation; the current study expands on this by having a much larger subject number and includes new evaluation of the CT imaging pattern of pulmonary fibrosis relative to the MUC5B promoter site polymorphism. Of the 1,764 subjects, 1,491 had evidence of pulmonary fibrosis on chest CT scans and were included in our study.
CT Imaging Evaluation
Two thoracic radiologists (J. C. and A. C.; approximately 6-8 years of experience in chest imaging) scored the chest CT scans independently. Discrepancies were resolved by a third chest radiologist (D. L.; 23 years of experience in chest imaging). All readers were blinded to histopathologic, clinical, and genotypic data. CT scans were scored for pulmonary fibrosis, honeycombing, and ground-glass opacity as defined by the Fleischner glossary of terms. Pulmonary fibrosis was considered present if there was reticular abnormality and/or subpleural irregularity or traction bronchiectasis with or without honeycombing. Preponderance of disease distribution was scored in both the zonal (upper, middle, lower, diffuse) and axial (peribronchovascular, peripheral, diffuse) planes when possible. Presence or absence of pulmonary fibrosis, honeycombing, and ground-glass opacity was scored on a three-point scale (none, probable, or definite). Percentage lung involvement regarding pulmonary fibrosis, honeycombing, and ground-glass opacity was scored to the nearest 10%.
Readers were allowed to select any diagnosis or combination of diagnoses including the whole spectrum of the idiopathic interstitial pneumonias, hypersensitivity pneumonitis, asbestosis, silicosis, sarcoidosis, obliterative bronchiolitis, and cellular bronchiolitis with level of confidence. If a single diagnosis was scored as definite, then no other diagnoses were scored. Confidence of diagnosis specific to UIP was scored as inconsistent with UIP, indeterminate UIP, probable UIP, or UIP depending on the radiologists’ opinion of the likelihood of the diagnosis based on imaging findings (Figure 1, Figure 2, Figure 3, Figure 4).16, 17, 18, 19 A UIP pattern was defined as basilar and peripheral preponderant fibrosis with honeycombing and absence of features to suggest another alternative diagnosis. Probable UIP was defined as basilar and peripheral preponderant fibrosis with little or no honeycombing and absence of features to suggest another alternative diagnosis. Inconsistent with UIP was defined according to current guidelines in IPF diagnosis.20 Indeterminate UIP was defined as pulmonary fibrosis with imaging features not sufficiently specific to reach a level of diagnosis that was definite, probable, or inconsistent with UIP; these were cases in which the CT imaging pattern was intermediate between that of probable UIP and inconsistent with UIP (eg, mild to moderate degree of air trapping, mild ground-glass opacity slightly more prominent than reticulation) or the axial or zonal distribution was diffuse (which is not addressed in current guidelines). The vast majority of indeterminate UIP and probable UIP CT imaging cases in this study would be categorized as possible UIP on CT scans according to current guidelines.20
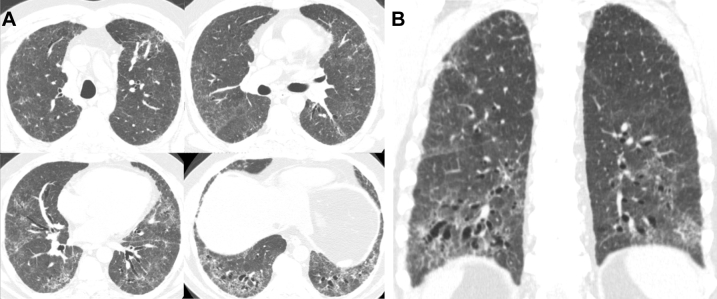
Figure 1.
A-B, Multiple axial (A) and coronal (B) images from chest CT imaging in a 62-year-old man with GG genotype at the rs35705950 single-nucleotide polymorphism show basilar and peribronchovascular predominant pulmonary fibrosis characterized by ground-glass opacity, reticulation, and traction bronchiectasis without subpleural honeycombing. The CT scans were scored as inconsistent with usual interstitial pneumonitis because of the peribronchovascular predominance.
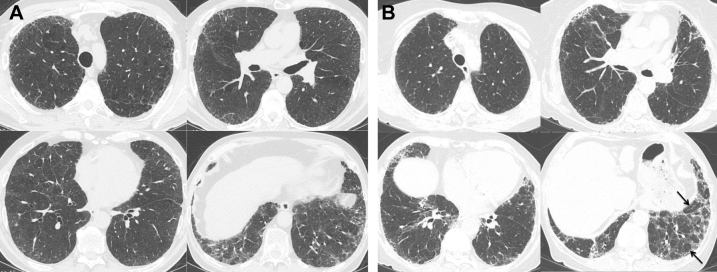
Figure 2.
A, Multiple axial images from chest CT imaging in a 57-year-old man with GT genotype at the rs35705950 single-nucleotide polymorphism (SNP) show mild basilar and peripheral predominant pulmonary fibrosis characterized by ground-glass opacity, reticulation, and traction bronchiectasis without subpleural honeycombing. These CT scans were scored as indeterminate usual interstitial pneumonitis (UIP) given the low level of confidence in making a UIP diagnosis. B, Multiple axial images from chest CT imaging in a 62-year-old woman with GG genotype at the rs35705950 SNP show mild peripheral and basilar predominant reticulation and traction bronchiectasis without subpleural honeycombing. There is moderate degree of mosaic attenuation (arrows) in the basilar left lower lobe and mild mosaic attenuation in the right lower lobe, shown to represent air trapping on expiratory images (not shown). These CT scans were scored as indeterminate UIP given the borderline degree of air trapping.
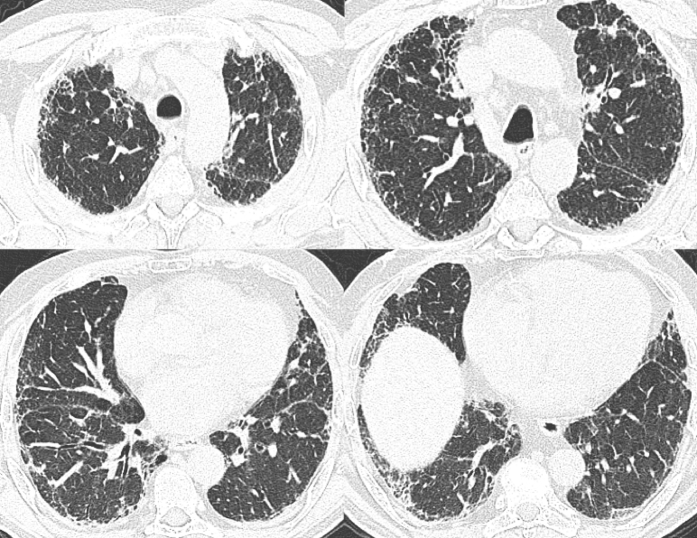
Figure 3.
Multiple axial images from chest CT imaging in a 55-year-old man with GT genotype at the rs35705950 single-nucleotide polymorphism show mild peripheral predominant pulmonary fibrosis characterized by reticulation and traction bronchiectasis without subpleural honeycombing. These CT scans were scored as probable usual interstitial pneumonitis given the absence of definite subpleural honeycombing.
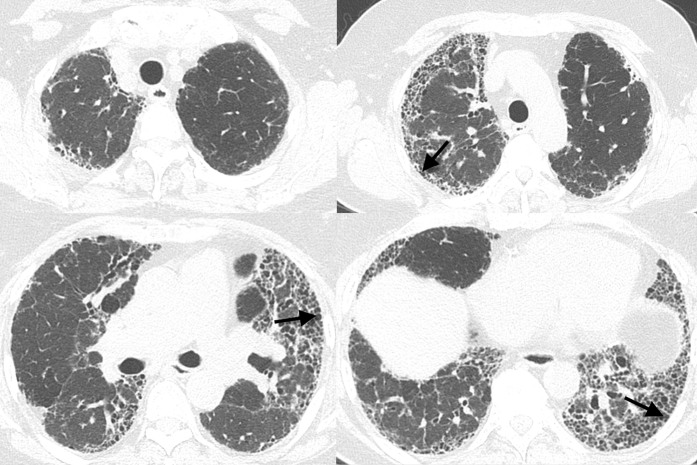
Figure 4.
Multiple axial and coronal images from chest CT imaging in a 72-year-old man with GT genotype at the rs35705950 single-nucleotide polymorphism show basilar and peripheral predominant pulmonary fibrosis characterized by reticulation and traction bronchiectasis with subpleural honeycombing (arrows) without other findings to suggest an alternative diagnosis to usual interstitial pneumonitis (UIP). The CT scans were scored as UIP.
Genotyping Assay
All subjects were genotyped for the MUC5B promoter polymorphism (rs35705950). MUC5B SNP genotypes were determined using TaqMan genotyping (Life Technologies) as reported in a previous publication.5
Statistical Analysis
We used χ2 tests to test for any association between groups. A two-tailed Fisher exact test was used when cell counts were expected to be less than 5, and Monte Carlo estimates were used for statistical analysis for tables exceeding a 2 × 2 configuration. Two-tailed Student t tests and Mann-Whitney U tests were used to compare means. A P value < .05 was considered statistically significant for all tests. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
The major and minor alleles at the rs35705950 SNP are G and T, respectively. There were 514 GG (G group) and 977 GT or TT (T group) subjects. The heterozygous GT group and the homozygous TT group were combined based on the dominant allele model.21
Demographic Characteristics
The study subject demographic characteristics are detailed in Table 1. Demographic characteristics between the G and T groups were similar except that the T group was older than the G group, though this difference was quantitatively small. The average ± SD age, percentage female, and percentage with smoking history in the G group were 63.3 ± 10.3 years, 34%, and 66%, respectively. The average age, percentage female, and percentage with smoking history in the T group were 67.0 ± 8.0 years, 32%, and 67%, respectively.
Table 1.
Basic Demographic Characteristics of Subjects Relative to the rs35705950 Single Nucleotide Polymorphism
| Subjecta Characteristic by MUC5B Genotype | GG (n = 514) | GT/TT (n = 977) |
|---|---|---|
| Sex (female), No. (%) | 176 (34) | 313 (32) |
| Age at CT scan, y, mean ± SDb | 63.3 ± 10.3 | 67.0 ± 8.0 |
| Smoking History (Ever), No. (%) | 302 (58.8) | 595 (60.9) |
| Ethnicity, No. (%) | ||
| Hispanic | 7 (1) | 10 (1) |
| Non-Hispanic | 386 (75) | 709 (73) |
| Unknown | 121 (24) | 258 (26) |
G = guanine; T = thymine.
Self-identified as white with radiologic evidence of fibrosis and available genotypes.
Student t test P < .001.
Summary of Radiographic Findings
In those in whom honeycombing could be confidently scored by two or more readers, 48.8% (682 of 1,398) had honeycombing. In those in whom ground-glass opacity could be confidently scored by two or more readers, 56.9% (575 of 1,011) had ground-glass opacity. The average percentage of involved lung among all subjects for total lung fibrosis, honeycombing, and ground-glass opacity were 22.5% ± 10.1%, 5.3% ± 6.2%, and 19.1% ± 10.4%, respectively. The majority of subjects in whom a confident zonal (61.0% [910 of 1,491]) and axial (76.1% [1,135 of 1,491]) distribution of fibrosis could be determined demonstrated pulmonary fibrosis predominant in the lower (57.9%) and peripheral (55.2%) lung. In terms of UIP diagnosis, 23.8% (328 of 1,377) were scored as not UIP, 36.2% (499 of 1,377) as indeterminate UIP, 19.6% (270 of 1,377) as probable UIP, and 20.3% (280 of 1,377) as UIP. Using current guidelines in which the probable and indeterminate UIP CT imaging patterns are combined into a single possible UIP CT imaging pattern, the proportion of UIP CT imaging diagnoses would be as follows: 23.8% (328 of 1,377) not UIP, 55.8% (769 of 1,377) possible UIP, and 20.3% (280 of 1,377) UIP. In 114 of the 1,491 subjects, the CT scans were considered inadequate for UIP diagnostic assessment.
Findings of Pulmonary Fibrosis in Relation to rs35705950
The percentage of subjects with honeycombing (Table 2) was not significantly different between the G and T groups (49.0% [237 of 484] vs 48.7% [445 of 914], respectively). There was a higher percentage of subjects in the G group than in the T group who had ground-glass opacity (62.1% [213 of 343] vs 54.2% [362 of 668], respectively; P = .04) (Table 3). The G and T groups did not differ significantly in average percentage of lung involvement for total lung fibrosis, honeycombing, and ground-glass opacity (Mann-Whitney U test P values of .24, .98, and .49, respectively).
Table 2.
Presence of Honeycombing Relative to the rs35705950 Single Nucleotide Polymorphism
| MUC5B Genotype | Honeycombing, No. (%) |
||
|---|---|---|---|
| No | Probable | Definite | |
| GG | 247 (51) | 55 (11) | 182 (38) |
| GT/TT | 469 (51) | 110 (12) | 335 (37) |
χ2P = .90. See Table 1 legend for expansion of abbreviations.
Table 3.
Presence of Ground-glass Opacity Relative to the rs35705950 Single Nucleotide Polymorphism
| MUC5B Genotype | Ground-glass Opacity, No. (%) |
||
|---|---|---|---|
| No | Probable | Definite | |
| GG | 130 (38) | 39 (11) | 174 (51) |
| GT/TT | 306 (46) | 73 (11) | 289 (43) |
χ2P = .04. See Table 1 legend for expansion of abbreviations.
Predominant Zonal and Axial Distribution of Pulmonary Fibrosis in Relation to rs35705950
The proportions of predominant zonal and axial distribution of pulmonary fibrosis are summarized in Table 4. There was no statistical difference in the zonal distribution of pulmonary fibrosis relative to the MUC5B promoter site polymorphism. However, the G group showed a lower proportion of subpleural (G, 42.2% [168 of 398] vs T, 62.3% [459 of 737]) and a higher proportion of peribronchovascular (G, 26.1% [104 of 398] vs T, 12.5% [92 of 737]) pulmonary fibrosis than did the T group (P < .0001).
Table 4.
Zonal and Axial Distribution of Pulmonary Fibrosis Relative to the rs35705950 Single Nucleotide Polymorphism
| MUC5B Genotype | Zonal Distribution (Consensus), No. (%)a |
Axial Distribution (Consensus), No. (%)b |
|||||
|---|---|---|---|---|---|---|---|
| Diffuse | Lower | Middle | Upper | Diffuse | Peribronchovascular | Subpleural | |
| GG | 119 (36) | 184 (56) | 8 (2) | 18 (5) | 126 (32) | 104 (26) | 168 (42) |
| GT/TT | 200 (34) | 343 (59) | 15 (3) | 23 (4) | 186 (25) | 92 (12) | 459 (62) |
Only high confidence distribution data was included in the study. See Table 1 legend for expansion of abbreviations.
Not statistically different.
χ2P < .0001.
UIP Diagnosis Relative to rs35705950
The proportion of CT imaging UIP diagnoses relative to the rs35705950 SNP is summarized in Table 5 according to the CT imaging scoring system in the current study methodology and that in current guidelines. There was a statistically significant difference between the proportion of CT imaging UIP diagnoses (P < .0001). The G group showed a higher proportion of diagnoses inconsistent with UIP (30.5% compared with 20.3% for the T group) and a lower proportion of confident (probable UIP and UIP) UIP diagnoses (32.6% compared with 43.8% for the T group).
Table 5.
Proportion of UIP Diagnoses on Chest CT Scans Relative to the rs35705950 Single Nucleotide Polymorphism
| CT Imaging Scoring System and MUC5B Genotype | UIP, No. (%) |
|||
|---|---|---|---|---|
| Inconsistent With UIP | Indeterminate for UIP | Probable UIP | UIP | |
| Current study | ||||
| GG | 145 (31) | 175 (37) | 79 (17) | 76 (16) |
| GT/TT | 183 (20) | 324 (36) | 191 (21) | 204 (23) |
| Possible UIP | ||||
|---|---|---|---|---|
| Current guidelines | ||||
| GG | 145 (31) | … | 254 (54) | 76 (16) |
| GT/TT | 183 (20) | … | 515 (57) | 204 (23) |
In a small subset of subjects, concordant diagnosis could not be established and were excluded from this portion of the analysis. χ2P < .0001. UIP = usual interstitial pneumonitis. See Table 1 legend for expansion of other abbreviations.
Discussion
There is growing interest in the relationship of the rs35705950 SNP variant and pulmonary fibrosis; however, to our knowledge, no studies have systematically reported the chest CT imaging manifestations relative to this SNP.5, 7, 8, 10, 14, 22 Multiple separate studies have shown a strong association between IPF and the T minor allele.6, 7, 8, 9, 10, 11, 12, 13 The odds ratios for pulmonary fibrosis among subjects who were heterozygous and those who were homozygous for the T minor allele of this SNP were 9.0 (95% CI, 6.2-13.1) and 21.8 (95% CI, 5.1-93.5) in the original description.5 Our results showed significant differences in the pattern of pulmonary fibrosis on CT scans relative to the rs35705950 SNP, with the T group showing a lower proportion of ground-glass opacity, higher proportion of subpleural distribution, and higher proportion of confident UIP diagnoses.
In UIP, there is peripheral and basilar predominant pulmonary fibrosis on CT scans in approximately 90% to 100% and 70% to 75% of cases, respectively.17, 19, 23, 24, 25, 26 In our study, the T group more often demonstrated the typical axial distribution of UIP than did the major allele group; this likely led to a higher percentage of UIP and probable UIP scores in these subjects than in those in the G group. We theorize that subjects who carry the minor T allele at the rs35705950 SNP, and then develop pulmonary fibrosis, are more likely to develop the typical imaging manifestations of UIP/IPF. This is supported by the strong association between IPF and the MUC5B polymorphism in previous studies.6, 7, 8, 9, 10, 11, 12, 13 If this is true, this SNP would be an important marker in those at risk for pulmonary fibrosis.
The complex relationship between CT imaging appearance with genetics and survival has not yet been adequately studied. Although the UIP pattern of pulmonary fibrosis usually portends poor prognosis, other nonimaging findings can augment survival in patients with pulmonary fibrosis. Previous studies have explored the advantage of combining nonimaging data with chest CT scans to predict patient prognosis in the setting of pulmonary fibrosis.17, 27 Flaherty et al17 showed that subjects with histologic UIP without a confident high-resolution CT imaging diagnosis of UIP had better survival than did subjects with histologic UIP and a confident high-resolution CT imaging diagnosis of UIP. Park et al27 found that subjects with collagen vascular disease-related UIP had superior survival relative to those with idiopathic UIP, showing that the setting in which pulmonary fibrosis occurs is still pertinent regardless of imaging findings.
The rs35705950 SNP may be another promising biomarker in predicting patient prognosis and possibly optimal treatment. A 2013 study showed that the minor allele variant at the MUC5B promoter site polymorphism is associated with increased rather than decreased survival among those with IPF.7 The adjusted hazard ratio for those who were homozygous for the minor allele (TT) was 0.15 to 0.23 relative to those who were homozygous for the major allele (GG), and the adjusted hazard ratio for those who were heterozygous was 0.39 to 0.48. In the general population, the minor allele appears to predispose individuals to development of pulmonary fibrosis and has been most strongly associated with IPF—the most fatal form of pulmonary fibrosis.8, 14, 22 The mechanism behind this seemingly contradictory juxtaposition of associations has not yet been studied but may be related to improved innate immune host defense.28, 29 In our study, the T group had a lower prevalence of ground-glass opacity. Ground-glass opacity is a relatively nonspecific CT imaging finding; however, in the setting of pulmonary fibrosis, superimposed diffuse alveolar damage representing acute exacerbation of pulmonary fibrosis (associated with poor prognosis) is a consideration.30, 31 A study evaluating the synergy of combining MUC5B promoter polymorphism and CT imaging data in survival prognostication would be the obvious next logical study to pursue.17, 32, 33, 34
The G group had a CT imaging pattern more often inconsistent with UIP and a higher rate of peribronchovascular distribution (suggestive of nonspecific interstitial pneumonia) than did the T group.20 It is likely that a greater proportion of the G group had a secondary form of pulmonary fibrosis rather than IPF. Stock et al8 showed a lack of an association between pulmonary fibrosis in subjects with scleroderma (usually nonspecific interstitial pneumonia) and sarcoidosis with the rs35705950 SNP, implying that this variant likely does not constitute a shared fibrotic mechanism across all fibrotic lung diseases. Instead, the authors suggested that the rs35705950 SNP variant is associated with an IPF-specific pathway, differing from fibrotic lung disease related to underlying immunologic/inflammatory causes. Peljto et al14 also found no association between interstitial lung disease in subjects with scleroderma and the rs35705950 SNP.
Our study had some limitations. We did not attempt to compare imaging scoring with the true gold standard in diagnosis of interstitial lung disease—multidisciplinary radiology/pathology/clinical correlation. However, the accuracy of high-resolution CT imaging in UIP diagnosis is 80% to 90% when a trained radiologist chooses UIP as the first choice diagnosis.19, 24, 25 When a confident diagnosis of UIP is made at high-resolution CT imaging (peripheral and basilar predominant pulmonary fibrosis with subpleural honeycombing and no other features to suggest an alternative diagnosis), the accuracy increases to 90% to 100%. Therefore, the divergence between the percentage of confident UIP diagnoses based on CT scans between the G and T groups should not be discounted. Furthermore, the purpose of this study was not to assess the true diagnostic proportion of patients with the MUC5B promoter site polymorphism but rather to show that patients with different genotypes relative to this SNP have different imaging phenotypes. We also did not assess any underlying genetic mutations other than the specified MUC5B promoter site polymorphism, which may have augmented the CT imaging phenotype. However, the MUC5B promoter polymorphism is the strongest genetic risk factor for pulmonary fibrosis and currently the only one that has been consistently replicated.
In conclusion, variation at the rs35705950 SNP is associated with different phenotypes of pulmonary fibrosis on chest CT scans. The T group was more likely to demonstrate a subpleural predominant pattern of pulmonary fibrosis and a UIP pattern on chest CT scans. The G group more often demonstrated a CT imaging pattern inconsistent with UIP. This difference in CT imaging appearance across this SNP suggests that integration of imaging findings and genetic data may be valuable biomarkers in patients with interstitial lung disease and highlights the need for more research in the genetics of interstitial lung disease. The minor allele at this SNP has already been shown to be a valuable biomarker in predicting superior patient survival. The next step would be a study evaluating the synergy of this SNP combined with CT imaging findings in predicting patient outcome. Future work in a larger cohort with imaging, histologic examination, genetic data, and outcome measures (such as survival and response to treatment) should be pursued.
Acknowledgments
Author contributions: J. H. C. takes responsibility for (is the guarantor of) the content of the manuscript, including the data and analysis. J. H. C., A. L. P., and D. A. L. designed the study. J. L. T. coordinated the clinical evaluations. J. H. C., A. C., and D. A. L. performed radiological phenotyping of study subjects. D. F. M. managed the database. A. L. P. and T. E. F. analyzed data. A. L. P. created tables. J. H. C., A. L. P., M. I. S., D. A. S., and D. A. L. provided advice on design and interpretation of results. J. H. C., A. L. P., D. A. S., and D. A. L. performed literature review. J. H. C., A. L. P., M. I. S., D. A. S., and D. A. L. wrote and edited the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. I. S. has participated in an industry advisory board for Roche-Genentech, Boehringer Ingelheim. None declared (J. H. C., A. L. P., A. C., J. L. T., D. F. M., B. H. R., T. E. F., D. A. S., D. A. L.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
Dr Chung is currently at The University of Chicago Medicine (Chicago, IL).
FUNDING/SUPPORT: This research was funded by R01 HL097163 (PI D. A. Schwartz), 1I01BX001534 (D. A. Schwartz), P01 HL092870 (PD D. A. Schwartz), and R01 HL095393 (PI D.A. Schwartz).
References
- 1.Steele M.P., Speer M.C., Loyd J.E. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172(9):1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nogee L.M., Dunbar A.E., 3rd, Wert S.E., Askin F., Hamvas A., Whitsett J.A. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344(8):573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Kuan P.J., Xing C. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84(1):52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armanios M.Y., Chen J.J., Cogan J.D. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356(13):1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 5.Seibold M.A., Wise A.L., Speer M.C. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borie R., Crestani B., Dieude P. The MUC5B variant is associated with idiopathic pulmonary fibrosis but not with systemic sclerosis interstitial lung disease in the European Caucasian population. PLoS One. 2013;8(8):e70621. doi: 10.1371/journal.pone.0070621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peljto A.L., Zhang Y., Fingerlin T.E. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA. 2013;309(21):2232–2239. doi: 10.1001/jama.2013.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stock C.J., Sato H., Fonseca C. Mucin 5B promoter polymorphism is associated with idiopathic pulmonary fibrosis but not with development of lung fibrosis in systemic sclerosis or sarcoidosis. Thorax. 2013;68(5):436–441. doi: 10.1136/thoraxjnl-2012-201786. [DOI] [PubMed] [Google Scholar]
- 9.Fingerlin T.E., Murphy E., Zhang W. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45(6):613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Noth I., Garcia J.G., Kaminski N. A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med. 2011;364(16):1576–1577. doi: 10.1056/NEJMc1013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horimasu Y., Ohshimo S., Bonella F. MUC5B promoter polymorphism in Japanese patients with idiopathic pulmonary fibrosis. Respirology. 2015;20(3):439–444. doi: 10.1111/resp.12466. [DOI] [PubMed] [Google Scholar]
- 12.Noth I., Zhang Y., Ma S.F. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1(4):309–317. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei R., Li C., Zhang M. Association between MUC5B and TERT polymorphisms and different interstitial lung disease phenotypes. Transl Res. 2014;163(5):494–502. doi: 10.1016/j.trsl.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peljto A.L., Steele M.P., Fingerlin T.E. The pulmonary fibrosis-associated MUC5B promoter polymorphism does not influence the development of interstitial pneumonia in systemic sclerosis. Chest. 2012;142(6):1584–1588. doi: 10.1378/chest.12-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H.Y., Seo J.B., Steele M.P. High-resolution CT scan findings in familial interstitial pneumonia do not conform to those of idiopathic interstitial pneumonia. Chest. 2012;142(6):1577–1583. doi: 10.1378/chest.11-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung J.H., Chawla A., Peljto A.L. CT scan findings of probable usual interstitial pneumonitis have a high predictive value for histologic usual interstitial pneumonitis. Chest. 2015;147(2):450–459. doi: 10.1378/chest.14-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flaherty K.R., Thwaite E.L., Kazerooni E.A. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax. 2003;58(2):143–148. doi: 10.1136/thorax.58.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumikawa H., Johkoh T., Fujimoto K. Pathologically proved nonspecific interstitial pneumonia: CT pattern analysis as compared with usual interstitial pneumonia CT pattern. Radiology. 2014;272(2):549–556. doi: 10.1148/radiol.14130853. [DOI] [PubMed] [Google Scholar]
- 19.Silva C.I., Muller N.L., Lynch D.A. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology. 2008;246(1):288–297. doi: 10.1148/radiol.2453061881. [DOI] [PubMed] [Google Scholar]
- 20.Raghu G., Collard H.R., Egan J.J. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marney A., Lane K.B., Phillips J.A., 3rd, Riley D.J., Loyd J.E. Idiopathic pulmonary fibrosis can be an autosomal dominant trait in some families. Chest. 2001;120(1 suppl):56S. doi: 10.1378/chest.120.1_suppl.s56. [DOI] [PubMed] [Google Scholar]
- 22.Hunninghake G.M., Hatabu H., Okajima Y. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368(23):2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumikawa H., Johkoh T., Ichikado K. Usual interstitial pneumonia and chronic idiopathic interstitial pneumonia: analysis of CT appearance in 92 patients. Radiology. 2006;241(1):258–266. doi: 10.1148/radiol.2411050928. [DOI] [PubMed] [Google Scholar]
- 24.Hunninghake G.W., Lynch D.A., Galvin J.R. Radiologic findings are strongly associated with a pathologic diagnosis of usual interstitial pneumonia. Chest. 2003;124(4):1215–1223. doi: 10.1378/chest.124.4.1215. [DOI] [PubMed] [Google Scholar]
- 25.Tsubamoto M., Muller N.L., Johkoh T. Pathologic subgroups of nonspecific interstitial pneumonia: differential diagnosis from other idiopathic interstitial pneumonias on high-resolution computed tomography. J Comput Assist Tomogr. 2005;29(6):793–800. doi: 10.1097/01.rct.0000182853.90520.84. [DOI] [PubMed] [Google Scholar]
- 26.Johkoh T., Muller N.L., Cartier Y. Idiopathic interstitial pneumonias: diagnostic accuracy of thin-section CT in 129 patients. Radiology. 1999;211(2):555–560. doi: 10.1148/radiology.211.2.r99ma01555. [DOI] [PubMed] [Google Scholar]
- 27.Park J.H., Kim D.S., Park I.N. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med. 2007;175(7):705–711. doi: 10.1164/rccm.200607-912OC. [DOI] [PubMed] [Google Scholar]
- 28.Molyneaux P.L., Cox M.J., Willis-Owen S.A. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190(8):906–913. doi: 10.1164/rccm.201403-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy M.G., Livraghi-Butrico A., Fletcher A.A. Muc5b is required for airway defence. Nature. 2014;505(7483):412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akira M., Kozuka T., Yamamoto S., Sakatani M. Computed tomography findings in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(4):372–378. doi: 10.1164/rccm.200709-1365OC. [DOI] [PubMed] [Google Scholar]
- 31.Bjoraker J.A., Ryu J.H., Edwin M.K. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157(1):199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 32.Sumikawa H., Johkoh T., Colby T.V. Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am J Respir Crit Care Med. 2008;177(4):433–439. doi: 10.1164/rccm.200611-1696OC. [DOI] [PubMed] [Google Scholar]
- 33.Flaherty K.R., Toews G.B., Travis W.D. Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J. 2002;19(2):275–283. doi: 10.1183/09031936.02.00182002. [DOI] [PubMed] [Google Scholar]
- 34.Best A.C., Meng J., Lynch A.M. Idiopathic pulmonary fibrosis: physiologic tests, quantitative CT indexes, and CT visual scores as predictors of mortality. Radiology. 2008;246(3):935–940. doi: 10.1148/radiol.2463062200. [DOI] [PubMed] [Google Scholar]