Abstract
Background
Surfactant protein D (SP-D) is an essential component of the innate immune defense against pathogens within the airways. SP-D also regulates allergic inflammation and promotes the removal of apoptotic cells. SP-D dysregulation is evident in several pulmonary diseases. Our aim was to investigate whether airway and serum levels of SP-D are altered in treatment-resistant severe asthma.
Methods
SP-D concentrations were measured in matched serum and BAL samples collected from 10 healthy control subjects (HC) and 50 patients with asthma (22 with mild asthma [MA] and 28 with severe asthma [SA]). These samples were also evaluated by using Western blot analysis to investigate variations in SP-D size.
Results
SP-D levels in BAL samples were significantly lower in SA compared with HC and MA (P < .001) and inversely correlated with BAL eosinophil cationic protein concentrations in SA (P < .01). Serum SP-D was significantly increased in SA compared with HC and MA (P < .001), and BAL/serum ratios were significantly lower in SA compared with HC and MA (P < .001). Reduced SP-D levels in BAL samples, with concomitant increases in serum in SA, were associated with degraded fragments of SP-D in the serum and increased BAL neutrophil counts and lipopolysaccharide levels.
Conclusions
These findings suggest defective innate immunity within the airways in SA, as reflected by low BAL SP-D concentrations and altered bacterial presence with airway neutrophilia. Furthermore, BAL SP-D leakage into the serum in patients with SA may provide a peripheral blood biomarker, reflecting increased epithelial damage and/or epithelial permeability within the peripheral airways.
Key Words: asthma, biomarkers, bronchoalveolar lavage, eosinophilic inflammation, immunology asthma, immunology (lung), neutrophilic inflammation, severe asthma, surfactant protein D
Abbreviations: α-rfhSP-D, antirecombinant fragment human surfactant protein D; ECP, eosinophil cationic protein; IPF, idiopathic pulmonary fibrosis; IQR, interquartile range; LPS, lipopolysaccharide; SP-D, surfactant protein D
FOR EDITORIAL COMMENT SEE PAGE 1121
Asthma is a chronic airway disorder that is characterized pathologically by airway inflammation and structural tissue remodeling. Airway inflammation in asthma is typically eosinophilic, with elevated levels of type 2 cytokines such as IL-4, IL-5, and IL-13.1 Airway remodeling is characterized by structural airway changes associated with airway wall thickening, which is most evident in patients with severe asthma, and inversely correlates with lung function measures.2 In severe asthma, there may be neutrophilic as well as eosinophilic airway inflammation.3 We reported previously that neutrophilic asthma is linked to an altered bacterial profile within recovered BAL samples.4 Because surfactant protein D (SP-D) is an important component of innate immunity within the distal airways, we investigated the potential that dysregulation of airway SP-D is a feature of severe asthma.
SP-D has numerous actions whose deficiency could be detrimental in asthma. In the airways, SP-D binds to carbohydrates in a calcium-dependent manner, orchestrating pathogen aggregation and enhancing their phagocytosis.5 In addition, SP-D enhances their chemotactic, phagocytic, and oxidative properties and interacts with phagocytic cells, such as macrophages and neutrophils.6 SP-D also modifies allergic responses in that it reduces the activation of basophils,7 mast cells,8 and eosinophils9 while regulating the release of transforming growth factor-β,10 IL-10,11 and IL-1212 and reducing concentrations of IL-2.13 In allergen challenge models, exogenous SP-D inhibits allergen-induced T lymphocyte proliferation and hypersensitivity,12 inducing a shift from a type 2 to type 1 cytokine response, and decreases induced airway remodeling.9, 14 Conversely, SP-D-deficient mice exhibit an enhanced IL-13-dependent inflammatory allergic response within the airways.15
The aim of the present study was to investigate airway luminal and serum SP-D concentrations in patients with mild and severe asthma compared with healthy control subjects and to examine the structural integrity of SP-D in these study groups. We hypothesized that severe asthma may be associated with a deficiency in SP-D within the airways.
Subjects and Methods
Subjects
Volunteers aged 18 to 65 years (recruited from the Wessex Severe Asthma Cohort and a departmental database of volunteers) participated in the study, which had previously received ethics approval from Southampton and West Hampshire Research Ethics Committee A (reference numbers 05/Q1702/165 and 08/H0502/6). All subjects provided written informed consent. The healthy control subjects had no history of respiratory disease and no evidence of bronchial hyperreactivity to methacholine challenge. Volunteers with asthma all had a physician-confirmed diagnosis of the disease. In addition, subjects with mild asthma all tested positive on skin prick tests to house dust mite allergen extract (Dermatophagoides pteronyssinus), had abnormal airway reactivity to methacholine required to lower the FEV1 by 20% of < 8 mg/mL, were life-long nonsmokers, and only receiving (as required) short-acting β-agonist therapy. Patients with severe asthma were on step 4 (n = 12) or step 5 (n = 16) of the Global Initiative for Asthma guidelines therapy16; were poorly controlled, with a score ≥ 1.5 on the six-domain Asthma Control Questionnaire; and had not smoked for at least 1 year.
Bronchoscopic Airway Samples
Fiberoptic bronchoscopy was performed according to established guidelines.17 BAL was performed by instilling 6 × 20-mL aliquots of prewarmed normal saline into a subsegmental bronchus of the anterior segment of the right upper lobe followed by gentle suction. BAL fluid was filtered (BD Falcon Cell Strainer) and then centrifuged at 800g for 10 min at 4°C. Cell pellets were resuspended in phosphate-buffered saline for cytospins, and the supernatant was stored at –80°C for later analysis. Cells were stained by using a rapid Romanowsky stain (Raymond Lamb Ltd) to distinguish between macrophages, neutrophils, and eosinophils, and 400 cells were counted blind by using coded samples.
Serum Sampling
Venous blood was allowed to clot for 60 min and then centrifuged for 15 min at 1,500g at 4°C. The serum layer was removed and stored at –80°C for further analysis.
SP-D Enzyme-Linked Immunosorbent Assay
Antibodies were raised in rabbits against a recombinant fragment of SP-D (neck/head), which is considered the functional domain of the protein. Briefly, SP-D was assayed in 96-well microtiter plates (Nunc labware products; MaxiSorp 96 well plates) coated with rabbit antirecombinant fragment human SP-D (α-rfhSP-D) at a 1:1,000 dilution as previously described and detected with biotinylated-α-rfhSP-D. Native human SP-D (0-500 ng/mL) was used as a standard (full methods are given in e-Appendix 1).18
SP-D Western Blotting
The same antibody, as described earlier, was used to detect “functional” SP-D in patient samples. A total of 20 μL of neat BAL or serum (100 μL of serum incubated with 20 μL of StrataClean Resin, in 500 μL of phosphate-buffered saline [Agilent Technologies, Inc] with calcium chloride 2 mM) was incubated for 30 min with rotation at room temperature. Samples were then spun at 1,300g and reduced according to the manufacturer’s instructions (NuPAGE, Life Technologies). Proteins were resolved by 12% (w/v) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (NuPAGE). Degradation of endogenous native human SP-D in BAL was visualized by immunoblotting of polyvinylidene difluoride membranes (iBlot, Life Technologies) using α-rfhSP-D antibodies.
Enzyme-Linked Immunosorbent Assay for Measures of Inflammation
Assays to measure BAL concentrations of myeloperoxidase (MPO, detection range 1.6-100 ng/mL) and neutrophil elastase (NE, detection range 0.4-25 ng/mL) were from Hycult Biotech. Eosinophilic cationic protein (ECP, detection range 0.125-40 ng/mL) assays were from Medical and Biological Laboratories. And IL-8 (detection range, 1-1,000 pg/mL) was from R&D systems. All assays were used according to manufacturer's instructions.
Measurement of BAL Lipopolysaccharide
The Limulus Amebocyte Lysate (Thermo Scientific Pierce; detection range, 0.1-1.0 EU/mL) Chromogenic Endotoxin Quantitation Kit was used to measure lipopolysaccharide (LPS) levels in BAL samples.
Statistical Analysis
SPSS version 21 (IBM SPSS Statistics, IBM Corp) was used for statistical analysis of the data. Data that were not parametrically distributed (measures of SP-D, inflammation, and LPS) were analyzed by using the Kruskal-Wallis test for between-group comparisons, with Mann-Whitney testing between pairs of groups as appropriate. For normally distributed data, a one-way analysis of variance test was initially used to determine differences between groups, with an unpaired t test used for further analyses. Linear regression analysis was performed to investigate biological relationships. A P value ≤ .05 was considered to indicate statistical significance.
Results
Patient Demographic Characteristics
The 10 healthy control subjects (eight women, two men; FEV1 % predicted [group mean ± SD], 107.4 ± 7.1) had significantly better lung function than either the 22 patients with mild asthma (15 women, seven men; FEV1 % predicted, 91.8 ± 13.3 [P < .01]) or 28 patients with severe asthma (20 women, eight men; FEV1 % predicted, 70.0 ± 24.8 [P < .001]). Data on characteristics for all participants are shown in e-Table 1.
Measures of Inflammation
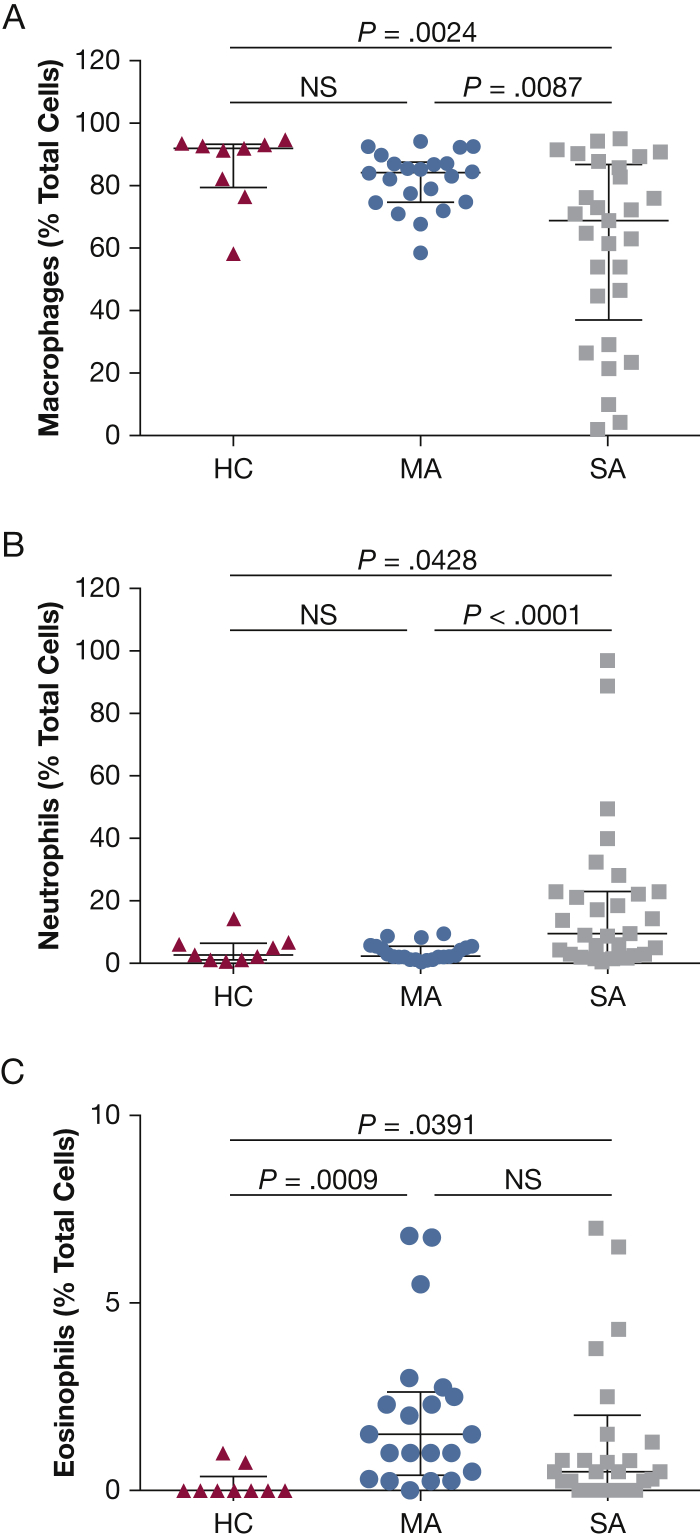
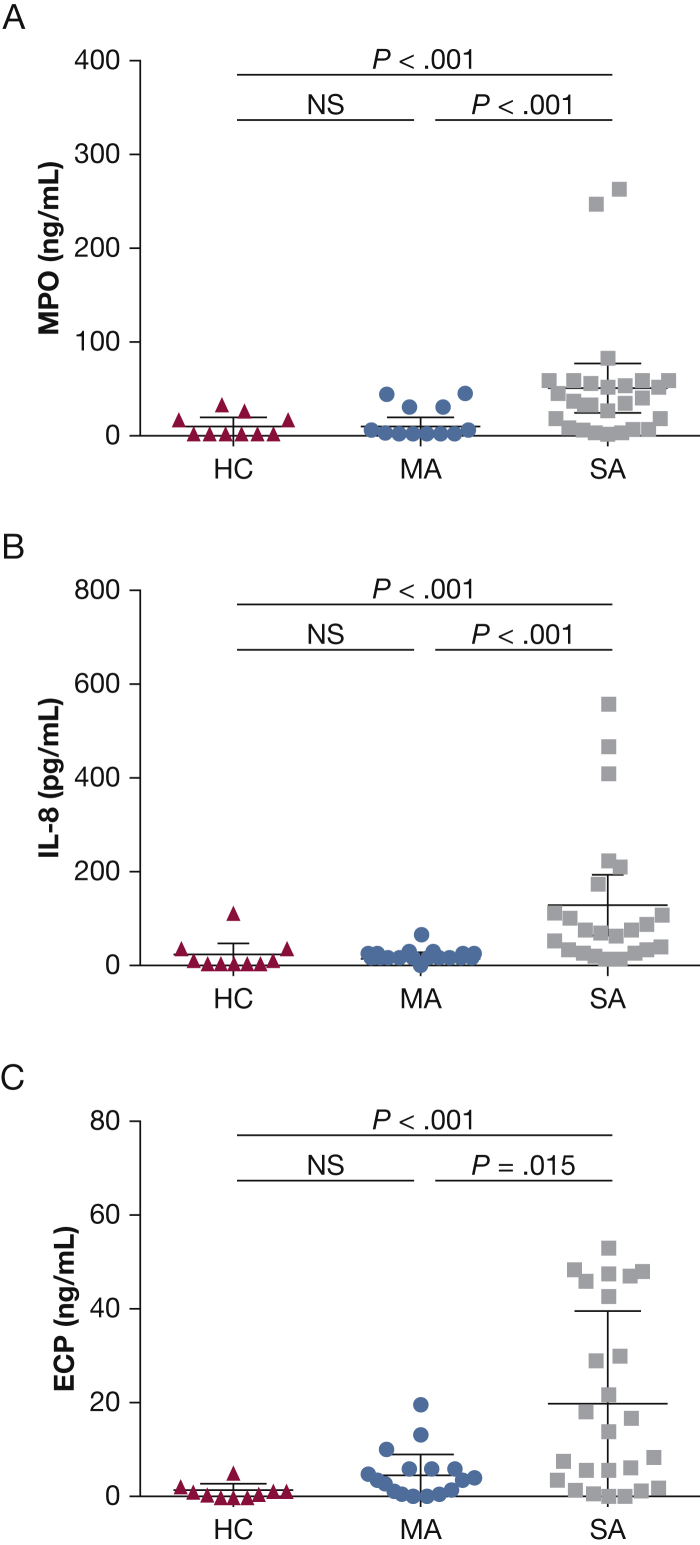
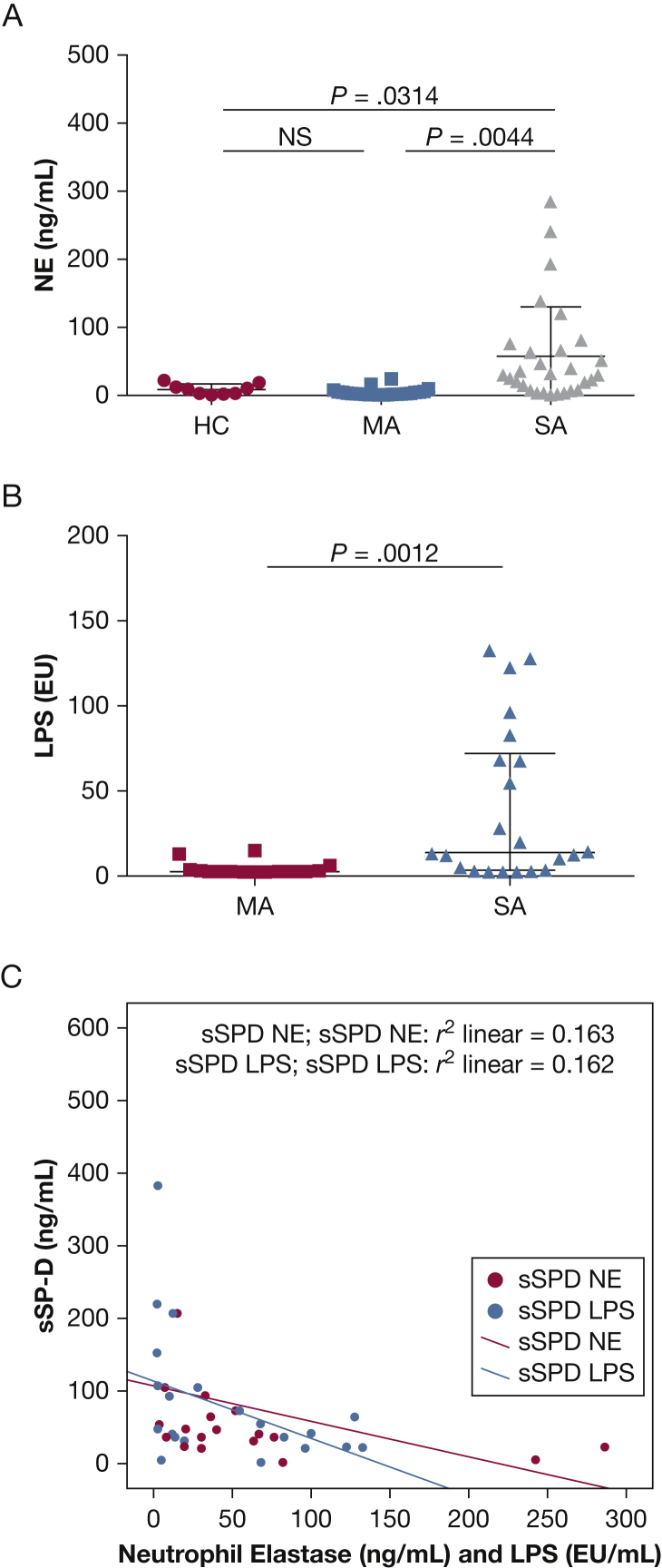
BAL cytospin differential cell counts were significantly altered in patients with asthma compared with the healthy control subjects. Both the mild and severe asthma groups had increased BAL eosinophil counts (group mean ± SD %) compared with healthy control subjects: severe asthma, 3.1% ± 7.1%; mild asthma, 2.6% ± 3.2%; and healthy control, 0.1% ± 0.3% (P < .01). In addition, patients with severe asthma had significantly increased BAL neutrophil counts (16.1% ± 19.1%) compared with patients with mild asthma (3.7% ± 2.7%) and with healthy control subjects (5.0% ± 6.3%; P < .05), with a resultant decreased BAL macrophage percentage (severe, 63.6% ± 7.4%; mild, 82.7% ± 9.3%; and healthy control, 86.0% ± 12.1% [P < .01] (Fig 1). BAL concentrations of myeloperoxidase, IL-8, and ECP were increased in patients with severe asthma compared with patients with mild asthma (P < .02) and healthy control subjects (P < .001) (e-Table 2, Fig 2). Neutrophil elastase levels were also significantly increased in BAL specimens from patients with severe asthma compared with specimens from healthy control subjects (P = .0314) and patients with mild asthma (P = .0044).
Figure 1.
A-C, BAL inflammatory cell counts. Percentages of total cell count for (A) macrophages, (B) neutrophils, and (C) eosinophils from HC (n = 10), MA (n = 22), and SA (n = 28) subjects. HC = healthy control; MA = mild asthma; NS = not significant; SA = severe asthma.
Figure 2.
A-C, BAL inflammatory mediator concentrations. Concentrations in BAL of (A) MPO, (B) IL-8, and (C) ECP from HC (n = 10), MA (n = 22), and SA (n = 28) subjects. ECP = eosinophil cationic protein; MPO = myeloperoxidase. See Figure 1 legend for expansion of other abbreviations.
SP-D Measures in Matched BAL and Serum Samples
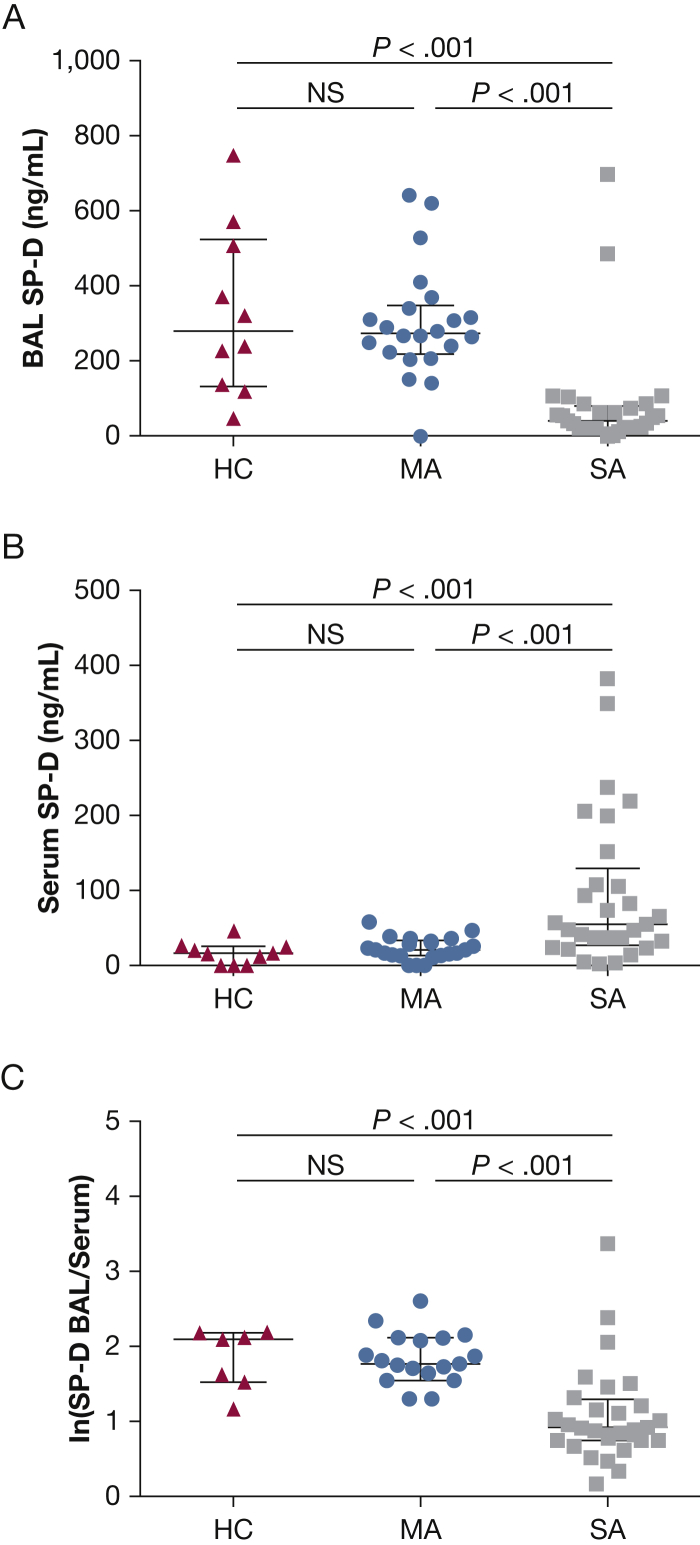
SP-D concentrations in BAL specimens from healthy control subjects and patients with mild asthma were not significantly different (median interquartile range (IQR), 282 [134-526] ng/mL vs 268 [212 to 355] ng/mL, respectively) (Fig 3A). BAL SP-D concentrations from patients with severe asthma were significantly decreased (median [IQR], 42 [21 to 81] ng/mL) vs both healthy control subjects (P < .005) and patients with mild asthma (P < .005). Conversely, patients with severe asthma had significantly increased SP-D concentrations in serum (median [IQR], 55 [28-130] ng/mL) compared with both healthy control subjects (median [IQR], 16 [0-25] ng/mL; P < .005) and patients with mild asthma (median [IQR], 19 [11 to 32] ng/mL; P < .001) (Fig 3B). To integrate these measures from the two biological compartments, the log ratio of BAL/serum SP-D concentrations was calculated (Fig 3C). There was no difference between the BAL/serum SP-D log ratio in healthy control subjects (median [IQR], 1.5 [0-2.1]) or patients with mild asthma (median [IQR], 1.8 [1.7-2.1]); this ratio in patients with severe asthma (median [IQR], 1.3 [0.8-1.4]) was significantly different from the healthy control subjects (P < .001) and the patients with mild asthma (P < .001).
Figure 3.
A-C, Surfactant protein D (SP-D) concentrations, in matched (A) BAL and (B) serum samples from HC (n = 10), MA (n = 22), and SA (n = 28) subjects as well as the (C) log ratio BAL:serum SP-D concentrations for the same groups. ln = natural logarithm. See Figure 1 legend for expansion of other abbreviations.
SP-D Relationships in Severe Asthma
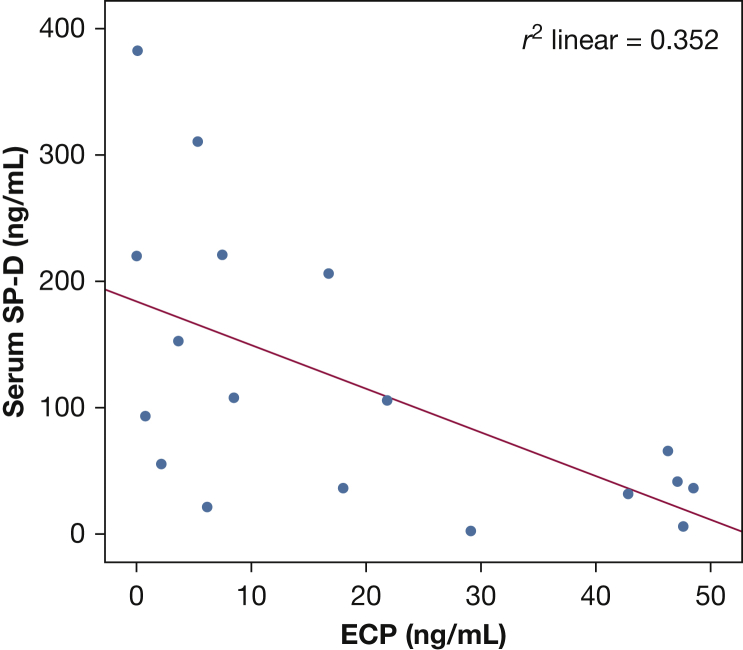
In patients with severe asthma, there was no significant impact on SP-D concentrations of age (e-Table 3), history of smoking, oral steroid use (e-Table 1), inhaled steroid concentration, or atopy. There was, however, a significant relationship to inflammation, in that serum SP-D concentrations correlated with BAL ECP (Fig 4) in those with severe asthma (Spearman’s ρ, P < .01; r2 = 0.352).
Figure 4.
Correlation between serum SP-D and ECP concentrations in patients with severe asthma (Spearman ρ, r2 = 0.352; P < .05). See Figure 2, Figure 3 legends for expansion of other abbreviations.
SP-D Integrity in BAL and Serum
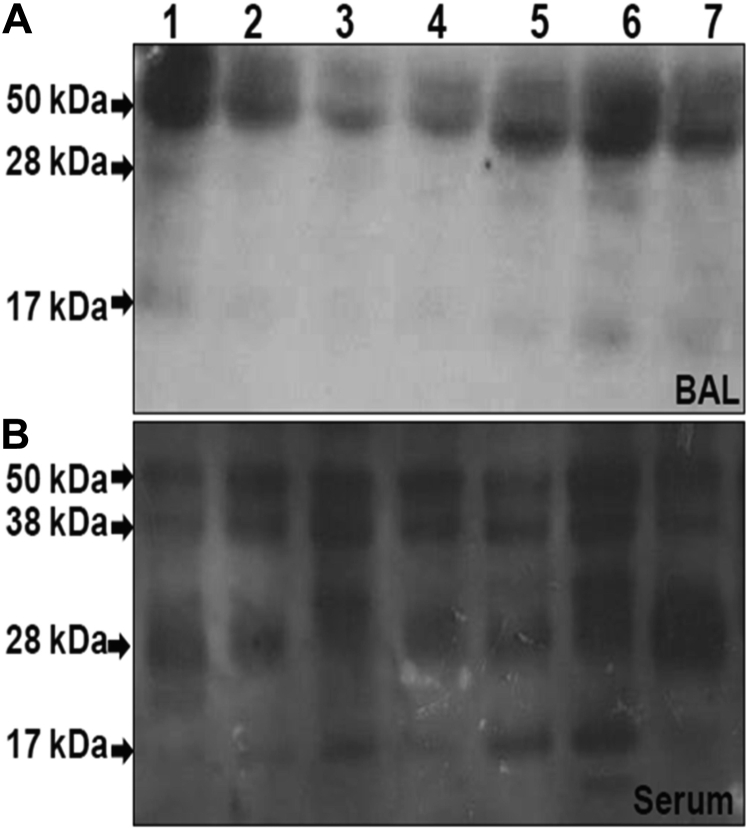
The structural integrity of SP-D was assessed by using Western blot analysis. In BAL specimens, there was no evidence of SP-D breakdown in either the healthy control subjects or those with mild asthma (e-Fig 1) who exhibited a single band at 50 kDa under reducing conditions with no detectable SP-D in serum (data not shown). In contrast, in the group with severe asthma, in addition to the 50-kDa band, there were faint bands detected in the BAL samples at 28 and 17 kDa (Fig 5A) and strong bands in serum at 38, 28, and 17 kDa (Fig 5B).
Figure 5.
A and B, Western blots showing the structural integrity of SP-D. In patients 1 through 7 with SA, matched patient (A) BAL and (B) serum samples. See Figure 1 legend for expansion of abbreviation.
Lipopolysaccharide
LPS levels were increased in patients with severe asthma compared with those with mild asthma (P = .0012). Both BAL neutrophil elastase (Spearman’s ρ, r2 = 0.163) and LPS (Spearman’s ρ, r2 = 0.162) produced weak but significant inverse relationships with serum SP-D concentrations (P < .05) (Fig 6C).
Figure 6.
A-C, BAL NE and LPS levels and relationship to sSP-D. (A) NE levels in BAL from HC (n = 10), MA (n = 22), and SA (n = 28) subjects. (B) LPS (EU/mL) concentrations in BAL from MA (n = 16) and SA (n = 19). (C) Linear regression plot of sSP-D against both BAL NE and BAL LPS identifying significant inverse correlations (Spearman’s ρ, r2 = 0.163 and r2 = 0.162, respectively; both, P < .05). LPS = lipopolysaccharide; NE = neutrophil elastase; sSP-D = serum surfactant protein D. See Figure 1 legend for expansion of other abbreviations.
Discussion
In the present study, we reported reduced concentrations of SP-D in BAL samples of patients with severe asthma, with an associated increase in serum concentrations. Furthermore, the SP-D concentrations in patients with severe asthma have 50-kD forms but also fragmented bands at 17 and 38 kD in BAL specimens and 17, 28, and 30 kD bands in serum.18 These findings are all distinct from those in healthy subjects and also from patients with mild nonsteroid-treated asthma, suggestive of their particular relevance to the severe asthma population. All patients with severe asthma in the present study had treatment-resistant disease; that is, despite treatment at steps 4 and 5 of the Global Initiative for Asthma guidelines, these patients still had inadequate disease control, as reflected by a high Asthma Control Questionnaire score. All had experienced at least one disease exacerbation within the last year, although none occurred within 8 weeks of their bronchoscopy, and they were in a stable phase of their disease. To the best of our knowledge, this study is the first to investigate the relationship between asthma and SP-D concentration in patients with such severe disease.
Severe treatment-resistant asthma is more complex than mild asthma, in that it is not purely a type 2-oriented disease. We thus identified that, in contrast to those patients with mild asthma who had increased BAL eosinophil counts, patients with severe asthma had an increase in both eosinophil and neutrophil counts within their distal airways. Neutrophil serine proteinases can cleave SP-D within its carbohydrate recognition domain region and render it functionally inactive.18, 19 Furthermore, bacterial proteases have been shown to cleave SP-D, rendering the molecule incapable of binding and aggregating lung pathogens.20 Thus, in severe asthma, neutrophilic airway inflammation, and possibly alterations in bacterial colonization, may both contribute to SP-D degradation and impaired function.
In COPD-reduced BAL specimens, SP-D concentrations are associated with increased concentrations of SP-D in the serum.21 This finding has been interpreted as reflecting altered epithelial and/or endothelial permeability within the distal airways and alveoli, allowing leakage of SP-D from the airways into the serum. This interpretation is supported by studies of the effects of cigarette smoking. Exposure to cigarette smoke reduces BAL SP-D concentrations and increases serum SP-D concentrations while increasing airway SP-D messenger RNA, indicating that the reduced airway SP-D is not due to reduced synthesis but due to enhanced leakage into the serum.22 Furthermore, acute lung injury in animals, which induces airway inflammation, enhances spillage of SP-D molecules into the systemic circulation and increases serum SP-D concentrations.23 The increase in serum SP-D concentration in COPD is associated with a risk of exacerbation and is reduced by high-dose oral steroid therapy.24 Glucocorticoids upregulate the expression of surfactant proteins, including SP-D, both in vivo and in vitro.25 The influence of steroids on reducing concentrations of serum SP-D is thus likely to be indirect, related to an antiinflammatory effect reducing the airway leakage. It is therefore unlikely that the altered SP-D dynamics in the patients with severe asthma in this study can be explained by their steroid therapy.
In severe asthma, the reduced distal airway SP-D concentrations may exacerbate the disease process. Although patients with both mild and severe asthma had increased eosinophil percentages within their BAL samples (compared with healthy airways), only those with severe asthma had increased BAL ECP concentrations. SP-D interacts through its carbohydrate recognition domain with the Fc-gamma II receptor on eosinophils and has been shown to inhibit eosinophil degranulation.26 Reduced airway SP-D concentrations will thus increase the impact of airway eosinophilic inflammation and provide an explanation for the significant inverse correlation between serum SP-D and BAL ECP and the significantly higher ECP concentrations in patients with severe asthma. Altered airway concentrations of SP-D could also leave the airways susceptible to opportunistic airway infection due to impaired bacterial clearance and enhance the IL-13-dependent allergic airway response.9, 15 An altered airway microbiome has been described in severe asthma, linked to neutrophilic airway inflammation and poorer lung function4; both of these features were evident in patients with severe asthma included in the present study. Furthermore, the elevated levels of LPS present in the BAL sample of those with severe asthma, compared with those with milder asthma, is consistent with the presence of increased gram-negative bacteria within the airways. The altered innate immune profile, as reflected by defective and/or deficient SP-D, provides a potential explanation for this finding. The SP-D antibody used in these studies recognizes the carbohydrate region of SP-D, the region responsible for interactions with pathogens and immune mediators, as such the enzyme-linked immunosorbent assay measures reflect functional SP-D (e-Appendix 1). Thus, the altered innate immune profile, as reflected by defective and deficient SP-D concentrations, provides a potential explanation for this occurrence.
Patients with severe asthma offer a history of disease exacerbation within the last year. Exacerbations of asthma are mainly triggered by viral infections, such as rhinovirus and influenza A viruses. SP-D has been shown to directly inhibit viral activity and modulate subsequent innate and adaptive immunity and inflammation.27 Consistent with these findings, genetic mutations in SP-D that result in a reduced ability to form dodecamers or multimerise are associated with severe respiratory syncytial infections in children.28 As SP-D enhances allergen removal and modulates allergic inflammation,29 as well as aids in viral eradication,30 both allergen and viral responses may be more severe in treatment-resistant asthma. It is thus likely that the reduced SP-D levels in the airways is a significant contributor to the disease progression in these patients with severe asthma characterized by decreased airflow and increased inflammatory mediators in the airway. It raises the possibility that recombinant SP-D administration, which has been shown to restore the type 1-type 2 cytokine balance, may offer a therapeutic approach for patients with severe asthma unresponsive to standard therapy.31 Serum SP-D concentrations may also provide a peripheral blood biomarker reflecting airway inflammation in severe asthma and be used to monitor the effects of novel therapies, especially those that attempt to alter airway permeability.24, 25
Conclusions
We found that SP-D concentrations in BAL samples declined and serum concentrations increased in patients with treatment-resistant severe asthma. Because reduced BAL concentrations of SP-D are reflected by elevated serum SP-D concentrations, measures of serum SP-D may serve as a potential biomarker of airway events underlying treatment-resistant asthma. Furthermore, we identified that the structural integrity of SP-D in the BAL and serum samples of patients with severe asthma is compromised. Because SP-D is a component of the innate immune defense within the airway (contributing to pathogen clearance and resolution of inflammation), SP-D deficiency in severe asthma may have relevance to disease persistence. As such, airway replacement could offer a potential therapy option for treatment-resistant asthma.
Acknowledgments
Author contributions: R.-M. A. M. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. R.-M. A. M., C. L. G., L. C. L., C. B., H. W. C., and P. H. H. contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: C. L. G. has received research funding for lectures from Boeringher Ingelheim and AstraZeneca. H. W. C. holds authorship on granted patents for treatment of lung diseases using SP-D (patent numbers WO03035679A, EP1440083A2, WO03035679A2, US20040259, WO03035679A3, US20040259201A1, and JP2005522988T2). None declared (R.-M. A. M., L. C. L., C. B., and P. H. H.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: The authors are grateful to the staff of the Southampton National Institute of Health Research Respiratory Biomedical Research Unit and the staff of the Southampton Centre for Biomedical Research for their support for the conduct of this study and for their professional care of the volunteers and patients involved in the study.
Additional information: The e-Appendix, e-Tables, and e-Figure can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was funded by the following grants: Wessex Severe Asthma Cohort Medical Research Council grant [code G0800649] and A Life Course Approach to Investigating Asthma Pathogenesis and Progression Medical Research Council grant [code G0900453].
Supplementary Data
References
- 1.Schuijs M.J., Willart M.A., Hammad H., Lambrecht B.N. Cytokine targets in airway inflammation. Curr Opin Pharmacol. 2013;3(3):351–361. doi: 10.1016/j.coph.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Niimi A., Matsumoto H., Amitani R. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. Am J Respir Crit Care Med. 2000;162(4 pt 1):1518–1523. doi: 10.1164/ajrccm.162.4.9909044. [DOI] [PubMed] [Google Scholar]
- 3.Moore W.C., Hastie A.T., Li X. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133(6):1557–1563.e5. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green B.J., Wiriyachaiporn S., Grainge C. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PloS One. 2014;9(6):e100645. doi: 10.1371/journal.pone.0100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reid K.B. Interactions of surfactant protein D with pathogens, allergens and phagocytes. Biochim Biophys Acta. 1998;1408(2-3):290–295. doi: 10.1016/s0925-4439(98)00074-x. [DOI] [PubMed] [Google Scholar]
- 6.Kishore U., Greenhough T.J., Waters P. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43(9):1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Singh M., Madan T., Waters P. Protective effects of a recombinant fragment of human surfactant protein D in a murine model of pulmonary hypersensitivity induced by dust mite allergens. Immunol Lett. 2003;86(3):299–307. doi: 10.1016/s0165-2478(03)00033-6. [DOI] [PubMed] [Google Scholar]
- 8.Deb R., Shakib F., Reid K. Major house dust mite allergens Dermatophagoides pteronyssinus 1 and Dermatophagoides farinae 1 degrade and inactivate lung surfactant proteins A and D. J Biol Chem. 2007;282(51):36808–36819. doi: 10.1074/jbc.M702336200. [DOI] [PubMed] [Google Scholar]
- 9.Qaseem A.S., Sonar S., Mahajan L. Linking surfactant protein SP-D and IL-13: implications in asthma and allergy. Mol Immunol. 2013;54(1):98–107. doi: 10.1016/j.molimm.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa H., Ledford J.G., Mukherjee S. Surfactant protein D attenuates sub-epithelial fibrosis in allergic airways disease through TGF-beta. Respir Res. 2014;15:143. doi: 10.1186/s12931-014-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda K., Miyahara N., Rha Y.H. Surfactant protein D regulates airway function and allergic inflammation through modulation of macrophage function. Am J Respir Crit Care Med. 2003;168(7):783–789. doi: 10.1164/rccm.200304-548OC. [DOI] [PubMed] [Google Scholar]
- 12.Strong P., Townsend P., Mackay R., Reid K.B., Clark H.W. A recombinant fragment of human SP-D reduces allergic responses in mice sensitized to house dust mite allergens. Clin Exp Immunol. 2003;134(2):181–187. doi: 10.1046/j.1365-2249.2003.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borron P.J., Crouch E.C., Lewis J.F. Recombinant rat surfactant-associated protein D inhibits human T lymphocyte proliferation and IL-2 production. J Immunol. 1998;161(9):4599–4603. [PubMed] [Google Scholar]
- 14.Hohlfeld J.M., Erpenbeck V.J., Krug N. Surfactant proteins SP-A and SP-D as modulators of the allergic inflammation in asthma. Pathobiology. 2002;70(5):287–292. doi: 10.1159/000070744. [DOI] [PubMed] [Google Scholar]
- 15.Haczku A., Cao Y., Vass G. IL-4 and IL-13 form a negative feedback circuit with surfactant protein-D in the allergic airway response. J Immunol. 2006;176(6):3557–3565. doi: 10.4049/jimmunol.176.6.3557. [DOI] [PubMed] [Google Scholar]
- 16.Reddy A.P., Gupta M.R. Management of asthma: the current US and European guidelines. Adv Exp Med Biol. 2014;795:81–103. doi: 10.1007/978-1-4614-8603-9_6. [DOI] [PubMed] [Google Scholar]
- 17.Guidelines for fiberoptic bronchoscopy in adults American Thoracic Society. Medical Section of the American Lung Association. Am Rev of Respir Dis. 1987;136(4):1066. doi: 10.1164/ajrccm/136.4.1066. [DOI] [PubMed] [Google Scholar]
- 18.Duvoix A., Mackay R.M., Henderson N. Physiological concentration of calcium inhibits elastase-induced cleavage of a functional recombinant fragment of surfactant protein D. Immunobiology. 2011;216(1-2):72–79. doi: 10.1016/j.imbio.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Cooley J., McDonald B., Accurso F.J., Crouch E.C., Remold-O'Donnell E. Patterns of neutrophil serine protease-dependent cleavage of surfactant protein D in inflammatory lung disease. J Leukoc Biol. 2008;83(4):946–955. doi: 10.1189/jlb.1007684. [DOI] [PubMed] [Google Scholar]
- 20.Alcorn J.F., Wright J.R. Degradation of pulmonary surfactant protein D by Pseudomonas aeruginosa elastase abrogates innate immune function. J Biol Chem. 2004;279(29):30871–30879. doi: 10.1074/jbc.M400796200. [DOI] [PubMed] [Google Scholar]
- 21.Winkler C., Atochina-Vasserman E.N., Holz O. Comprehensive characterisation of pulmonary and serum surfactant protein D in COPD. Respiratory Res. 2011;12:29. doi: 10.1186/1465-9921-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sin D.D., Pahlavan P.S., Man S.F. Surfactant protein D: a lung specific biomarker in COPD? Ther Adv Respir Dis. 2008;2(2):65–74. doi: 10.1177/1753465808088903. [DOI] [PubMed] [Google Scholar]
- 23.Gaunsbaek M.Q., Rasmussen K.J., Beers M.F., Atochina-Vasserman E.N., Hansen S. Lung surfactant protein D (SP-D) response and regulation during acute and chronic lung injury. Lung. 2013;191(3):295–303. doi: 10.1007/s00408-013-9452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lomas D.A., Silverman E.K., Edwards L.D. Serum surfactant protein D is steroid sensitive and associated with exacerbations of COPD. Eur Respir J. 2009;34(1):95–102. doi: 10.1183/09031936.00156508. [DOI] [PubMed] [Google Scholar]
- 25.Sims M.W., Tal-Singer R.M., Kierstein S. Chronic obstructive pulmonary disease and inhaled steroids alter surfactant protein D (SP-D) levels: a cross-sectional study. Respir Res. 2008;9:13. doi: 10.1186/1465-9921-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Bredow C., Hartl D., Schmid K. Surfactant protein D regulates chemotaxis and degranulation of human eosinophils. Clin Exp Allergy. 2006;36(12):1566–1574. doi: 10.1111/j.1365-2222.2006.02598.x. [DOI] [PubMed] [Google Scholar]
- 27.Hartshorn K.L. Role of surfactant protein A and D (SP-A and SP-D) in human antiviral host defense. Front Biosci (Schol Ed) 2010;2:527–546. doi: 10.2741/s83. [DOI] [PubMed] [Google Scholar]
- 28.Lahti M., Lofgren J., Marttila R. Surfactant protein D gene polymorphism associated with severe respiratory syncytial virus infection. Pediatr Res. 2002;51(6):696–699. doi: 10.1203/00006450-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Hawgood S., Brown C., Edmondson J. Pulmonary collectins modulate strain-specific influenza a virus infection and host responses. J Virol. 2004;78(16):8565–8572. doi: 10.1128/JVI.78.16.8565-8572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LeVine A.M., Elliott J., Whitsett J.A. Surfactant protein-d enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am J Respir Cell Mol Biol. 2004;31(2):193–199. doi: 10.1165/rcmb.2003-0107OC. [DOI] [PubMed] [Google Scholar]
- 31.Kishore U., Bernal A.L., Kamran M.F. Surfactant proteins SP-A and SP-D in human health and disease. Arch Immunol Ther Exp (Warsz) 2005;53(5):399–417. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.