Abstract
Background
Limited data are available on the use of noninvasive ventilation in patients with asthma exacerbations. The objective of this study was to characterize hospital patterns of noninvasive ventilation use in patients with asthma and to evaluate the association with the use of invasive mechanical ventilation and case fatality rate.
Methods
This cross-sectional study used an electronic medical record dataset, which includes comprehensive pharmacy and laboratory results from 58 hospitals. Data on 13,558 patients admitted from 2009 to 2012 were analyzed. Initial noninvasive ventilation (NIV) or invasive mechanical ventilation (IMV) was defined as the first ventilation method during hospitalization. Hospital-level risk-standardized rates of NIV among all admissions with asthma were calculated by using a hierarchical regression model. Hospitals were grouped into quartiles of NIV to compare the outcomes.
Results
Overall, 90.3% of patients with asthma were not ventilated, 4.0% were ventilated with NIV, and 5.7% were ventilated with IMV. Twenty-two (38%) hospitals did not use NIV for any included admissions. Hospital-level adjusted NIV rates varied considerably (range, 0.4-33.1; median, 5.2%). Hospitals in the highest quartile of NIV did not have lower IMV use (5.4% vs 5.7%), but they did have a small but significantly shorter length of stay. Higher NIV rates were not associated with lower risk-adjusted case fatality rates.
Conclusions
Large variation exists in hospital use of NIV for patients with an acute exacerbation of asthma. Higher hospital rates of NIV use does not seem to be associated with lower IMV rates. These results indicate a need to understand contextual and organizational factors contributing to this variability.
Key Words: asthma, CPAP, mechanical ventilation, noninvasive ventilation
Abbreviations: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; IMV, invasive mechanical ventilation; LAPS, Laboratory-based Acute Physiology Score; NIV, noninvasive ventilation; RS, risk-standardized
In the United States, there are approximately 500,000 annual hospitalizations for asthma exacerbation, and 4% are treated with invasive mechanical ventilation (IMV).1 Patients requiring IMV have in-hospital death rates ranging from 15% to 22%.2, 3 Mortality associated with asthma has changed little over the last decade because of a lack of significant advances in treatment.4
There is strong evidence that noninvasive mechanical ventilation (NIV) reduces the risk of intubation and mortality in patients with COPD exacerbations.5, 6, 7 The pathophysiology of acute respiratory failure in asthma is in some ways similar to that of COPD, providing reasons to believe that NIV could be successful in patients with acute asthma. However, data on the efficacy of NIV in asthma exacerbations are scarce. A systematic review concluded that NIV improves patients’ respiratory rate and lung function, while decreasing hospitalizations, but did not permit any conclusions regarding its effects on intubation or mortality.8 The guidelines from the Global Initiative for Asthma recognize the increase in NIV use in clinical practice but describe NIV use in patients with asthma exacerbation as controversial.9
One potential way to understand the impact of NIV on asthma outcomes would be to assess hospital-level utilization of NIV and whether hospitals that have higher use of NIV achieve better patient outcomes. The present article describes a large observational study that assessed the hospital patterns of mechanical ventilation for patients hospitalized with asthma exacerbations. The relationship between hospital use of NIV and hospital-level IMV and death rates was also analyzed.
Methods
Study Design and Setting
A cross-sectional analysis was performed by using Cerner’s Health Facts database, which contains electronic medical records from a geographically and structurally diverse sample of US hospitals. In 2012, a total of 125 hospitals were included in the database; the majority of hospitals were urban, 47% were teaching institutions, and 49% had < 200 beds. Details about the Health Facts database have been described previously10, 11, 12 (e-Appendix 1 provides more information about the database). We included patients aged ≥ 18 years admitted from January 2009 to December 2012 with a principal diagnosis of asthma (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM],13 codes 493.0x, 493.1x, 493.9x, 493.2x, and 493.8x). For coding purposes, a principal diagnosis of respiratory failure is assigned for patients with severe presentation of asthma or with respiratory failure complicating an exacerbation of asthma, including those who are treated with ventilation; we therefore also included patients with a principal diagnosis of acute respiratory failure (ICD-9-CM codes 518.81, 518.82, 518.84, and 518.4) and a secondary diagnosis of asthma. To ensure that patients were treated for an acute asthma exacerbation, the cohort was restricted to patients treated with short-acting bronchodilators and systemic steroids.
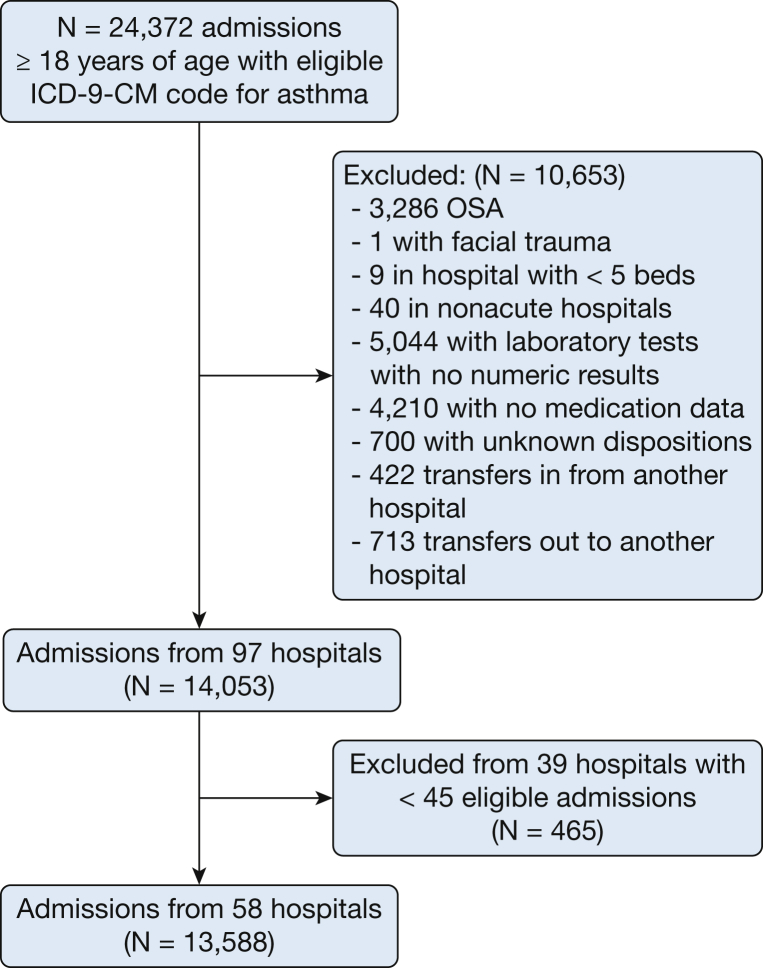
Patients without laboratory results were excluded because these variables were used to calculate the severity-of-illness score at admission. We also excluded the following: patients with no medication data, because treatment variables were used to define the cohort; patients with OSA because it is not possible to differentiate chronic use of NIV from treatment for acute respiratory failure; and patients who were transferred from or to another hospital.
To provide stable hospital rate estimates and avoid distortion of rates of NIV and IMV due to small sample sizes in the context of low use of NIV, we calculated that for a 5% rate of NIV use, 45 admissions would give a hospital a 90% chance of having at least one patient treated with NIV; we thus restricted the analysis to hospitals with ≥ 45 admissions. Data on characteristics of patients and hospitals excluded from analysis are provided in e-Table 1.
The institutional review board at Baystate Medical Center Committee determined that the project was not considered to be Human Subjects Research as defined by the Office of Human Research Protections (institutional review board project 431812-2).
Patient and Hospital Information
Patient data included sociodemographic characteristics, comorbidities, admission for asthma and use of NIV or IMV in the prior year, and coexistent pneumonia. Comorbidities were assessed by using the Healthcare Cost and Utilization software14 and the combined Charlson/Romano/Gagne comorbidities software.15 To assess the severity of asthma at admission, the Laboratory-based Acute Physiology Score (LAPS) was calculated; this score integrates information from 14 laboratory tests recorded at admission (including the arterial blood gas) into a single continuous variable. The LAPS is similar to other acuity scores that predict mortality and was internally and externally validated.16, 17, 18
Information on several hospital characteristics (including number of beds, teaching status, if the hospital serves a rural or urban community, and its geographical region) was collected.
NIV and IMV
The primary variable of interest was the initial ventilation, NIV or IMV, defined as the first method of ventilation used during hospitalization. ICD-9-CM procedure codes were used to define NIV and IMV exposure (ICD-9-CM code 93.90 for NIV; ICD-9-CM codes 96.04 and 96.70-96.72 for IMV). Of note, the ICD-9-CM codes do not differentiate between CPAP and bilevel pressure ventilation.
Outcomes
The primary study outcomes were hospital-level risk-standardized (RS) rates of initial NIV or initial IMV, in-patient case fatality rate, and length of stay (in hours). NIV failure was defined as IMV used after NIV; we also report on a combined outcome of NIV failure or death following NIV.
Statistical Analysis
Descriptive statistics included frequencies and percentiles for categorical factors, and means and percentile distributions for continuous factors. For each hospital, the rates of ventilation among all admissions with an asthma exacerbation were calculated.
To estimate hospital-level RS NIV rates, the statistical modeling techniques utilized by the Centers for Medicare & Medicaid Services for reported readmission and mortality rates were used.19, 20 We developed hierarchical generalized logistic models with hospital-level random effects to account for the clustering of patients within hospitals; we adjusted for patient characteristics, the LAPS score, and hospital characteristics as fixed effects. This approach simultaneously accounts for the variance in patient outcomes within and between hospitals. RS-NIV was calculated as the ratio between the hospital-predicted and hospital-expected NIV rate, multiplied by the overall unadjusted NIV rate for all admissions. Similar methods were used to calculate the hospital RS-IMV rate and the hospital RS-mortality rate. For the mortality outcome, to avoid survival bias, one random admission was selected if the patient had multiple admissions.
The correlations between the hospital RS-NIV rates and RS-IMV and RS-case-fatality rates were evaluated by using the Spearman correlation coefficient. We also grouped hospitals into quartiles of the initial RS-NIV rate and compared initial RS-IMV, RS-case-fatality rates, and RS-length of stay across quartiles of RS-NIV by using the Kruskal-Wallis test and Cuzick’s test for trend.21 Stata/MP version 13.1 for Windows (StataCorp LP) was used for statistical analyses.
Results
Hospital and Patient Characteristics
After applying patient-level exclusion criteria and restricting analysis to hospitals with < 45 eligible encounters, the cohort for analysis included 13,588 admissions from 58 hospitals (Fig 1) (e-Table 1 provides data about hospitals and patients excluded after applying the cut-off of minimum 45 admissions with asthma). All 58 hospitals were urban, 62% were teaching hospitals, and 50% had between 200 and 499 beds. The median volume of admissions with asthma per hospital over the study period was 325 (200-445). The median age of the study population was 52 years, 79.9% were female, 53.4% were white, and the median LAPS score was 26 (20-38). The median length of stay was 73 (44-120) hours, and 1.2% patients died during hospitalization.
Figure 1.
Study cohort flowchart.
Of all hospitalizations with an acute exacerbation of asthma, 90.3% were not ventilated, 546 (4.0%) were initially treated with NIV, and 774 (5.7%) were treated with IMV; only 4.9% of patients ventilated with NIV experienced NIV failure. Overall, 38.0% of patients treated with NIV and 73.0% of those treated with IMV had a primary diagnosis of acute respiratory failure and a secondary diagnosis of asthma. Among patients treated with NIV, the majority (79.1%) were admitted to the regular medical ward, 4.4% to a step-down unit, and 16.5% to the ICU. The in-hospital case-fatality rate was 0.3% in patients who were not ventilated, 2.6% in those treated with NIV, and 15.6% in those treated with IMV. Among the 27 patients with NIV failure (started on NIV and later intubated), in-hospital mortality was 21.4%; when defining NIV failure as intubation or death (n = 35), mortality was 40%.
The use of NIV as the first method of ventilation increased during the study period from 2.3% in 2009 to 4.8% in 2012.
Hospital Use of NIV and IMV
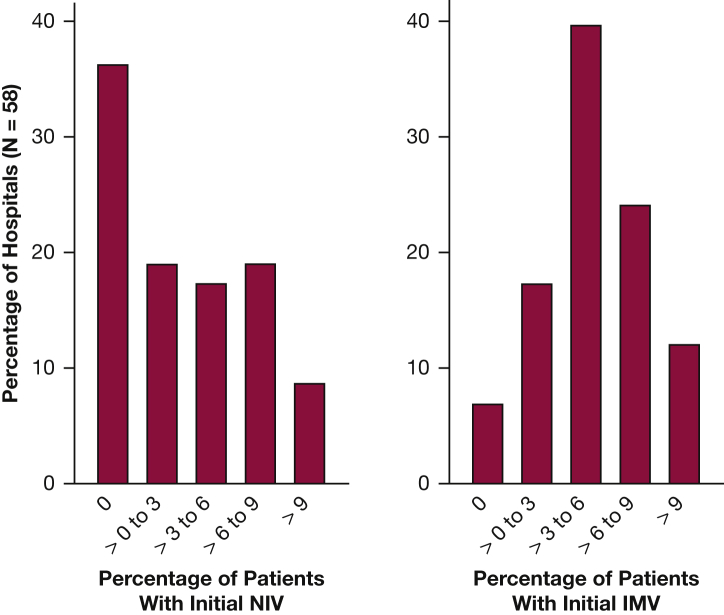
The unadjusted initial NIV hospital rates ranged from a minimum of 0% to a maximum of 16.3%, with an unweighted mean of 3.4%. Overall, 36% (n = 21) of the hospitals did not use NIV in any admission with asthma during the study period. The initial IMV rate varied between 0% and 14.5%, with a mean of 5.0% (Fig 2).
Figure 2.
Distribution of observed initial NIV and IMV rates among hospitals included in the analysis. IMV = invasive mechanical ventilation; NIV = noninvasive ventilation.
Hospital initial RS-NIV rates ranged from 0.4% to 33.1% with a median of 5.2%, and hospital initial RS-IMV rates ranged from 3.5% to 10.1% with a median of 5.6%. The likelihood ratio tests comparing the hierarchical models predicting initial NIV or initial IMV versus a logistic regression model that did not use hospitals as random effects were significant (P < .001), indicating that there was significant variation at the hospital level in the use of either ventilation type. No significant difference was observed among hospital characteristics and either RS-NIV or RS-IMV. Using the variance F test, there was more variation in RS-NIV overall compared with RS-IMV (P < .001) (Table 1). Table 2 illustrates that patient demographic characteristics and LAPS scores did not differ according to hospital quartiles of NIV rates. Patients admitted to hospitals with higher rates of NIV use were more likely to have received NIV during an admission to the same hospital in the year prior. We found no difference in the initial admission venue (ICU, step-down unit, or general ward) for patients treated with NIV.
Table 1.
RS Initial Ventilation Rates According to Characteristics of Hospitals Included in the Analysis
| Overall | No. of Hospitals |
% RS-Initial NIV,a Median [IQR] |
% RS-Initial IMV,a Median [IQR] |
|---|---|---|---|
| Hospital size | |||
| < 199 beds | 18 | 4.4 [1.3-9.9] | 5.5 [4.9-6.6] |
| 200-499 beds | 29 | 4.0 [1.1-16.1] | 5.7 [4.7-6.5] |
| ≥ 500 beds | 11 | 8.7 [0.7-8.9] | 5.6 [4.2-6.9] |
| Teaching status | |||
| Teaching hospital | 36 | 4.3 [1.3-15.8] | 5.6 [4.6-6.7] |
| Nonteaching hospital | 22 | 6.2 [1.1-13.2] | 5.7 [4.9-6.6] |
| Region | |||
| Midwest | 11 | 8.8 [0.9-18.1] | 5.7 [4.9-6.8] |
| Northeast | 21 | 4.0 [1.3-15.6] | 5.5 [4.7-6.6] |
| South | 21 | 4.6 [1.3-9.9] | 5.2 [4.9-6.6] |
| West | 5 | 5.8 [1.3-9.1] | 6.0 [5.7-6.3] |
IMV = invasive mechanical ventilation; IQR = interquartile range; NIV = noninvasive ventilation; RS = risk-standardized.
Refers to the first method of ventilation during hospitalization.
Table 2.
Patient Characteristics According to Quartiles of RS Initial NIV
| Characteristic | Quartiles of Hospital RS Initial NIVa |
P Value for Trend |
|||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Age (median, IQR), y | 56 [54-58] | 54 [51-60] | 53 [49-55] | 55 [51-58] | .23 |
| Female | 74 [70-76] | 74 [69-78] | 73 [68-75] | 74 [72-76] | .87 |
| Black | 13 [6-43] | 24 [12-45] | 18 [5-55] | 13 [4-30] | .67 |
| Patients with no admissions at 1 y | 68 [66-71] | 74 [67-78] | 67 [62-74] | 69 [62-70] | .50 |
| Congestive heart failure | 15 [11-16] | 12 [4-13] | 9 [6-11] | 12 [7-15] | .07 |
| Valvular disease | 4 [3-5] | 3 [2-5] | 3 [2-4] | 3 [2-6] | .64 |
| Peripheral vascular disease | 3 [1-3] | 2 [1-4] | 2 [1-3] | 2 [1-3] | .33 |
| Neurologic disorders | 6 [4-7] | 6 [3-8] | 5 [3-7] | 5 [4-7] | .43 |
| Chronic pulmonary disease | 13 [8-16] | 10 [5-19] | 8 [5-13] | 13 [9-17] | .79 |
| Diabetes with no chronic complications | 23 [20-29] | 22 [20-26] | 21 [19-22] | 20 [15-23] | .07 |
| Renal failure | 6 [5-7] | 7 [2-9] | 4 [3-5] | 7 [1-8] | .27 |
| Obesity | 16 [13-22] | 17 [11-22] | 17 [12-20] | 17 [13-22] | .86 |
| Deficiency anemia | 11 [8-15] | 9 [4-11] | 8 [5-9] | 10 [8-13] | .38 |
| Depression | 10 [5-18] | 12 [3-17] | 15 [9-18] | 9 [8-23] | .58 |
| Hypertension | 45 [34-56] | 47 [32-56] | 44 [34-54] | 45 [26-56] | .59 |
| Prior IMV within 12 mo | 2 [1-4] | 1 [0-2] | 4 [0-5] | 2 [1-5] | .96 |
| Prior NIV within 12 mo | 0 [0-0] | 0 [0-1] | 3 [2-6] | 5 [4-7] | < .001 |
| Pneumonia present on admission | 11 [9-15] | 11 [6-13] | 9 [7-14] | 8 [6-10] | .07 |
| LAPS | 30 [29-31] | 30 [28-33] | 30 [29-32] | 32 [29-34] | .13 |
| pH | 7.36 [7.34-7.37] | 7.38 [7.32-7.38] | 7.36 [7.33-7.40] | 7.37 [7.34-7.39] | .57 |
| Paco2, mm Hg | 46 [44-52] | 46 [44-53] | 46 [44-49] | 49 [44-51] | .98 |
| Pao2, mm Hg | 133 [106-161] | 122 [109-128] | 128 [114-183] | 132 [108-148] | .89 |
| Oxygen saturation, % | 96 [96-98] | 97 [96-97] | 96 [95-97] | 96 [95-97] | .05 |
| Prior IMV within 12 mo | 2 [1-4] | 1 [0-2] | 4 [0-5] | 2 [1-5] | .96 |
| Prior NIV within 12 mo | 0 [0-0] | 0 [0-1] | 3 [2-6] | 5 [4-7] | < .001 |
| Admission to ICU | 5 [1-11] | 4 [1-8] | 6 [0-11] | 5 [0-11] | .74 |
| Admission to ICU or step-down unit | 7 [1-11] | 4 [1-8] | 6 [0-17] | 5 [0-16] | .75 |
Numbers in each row express the median and IQR. LAPS = Laboratory-based Acute Physiology Score. See Table 1 legend for expansion of other abbreviations.
Refers to the first method of ventilation during hospitalization.
Relationship Between Hospital RS Initial NIV Rates and Outcomes
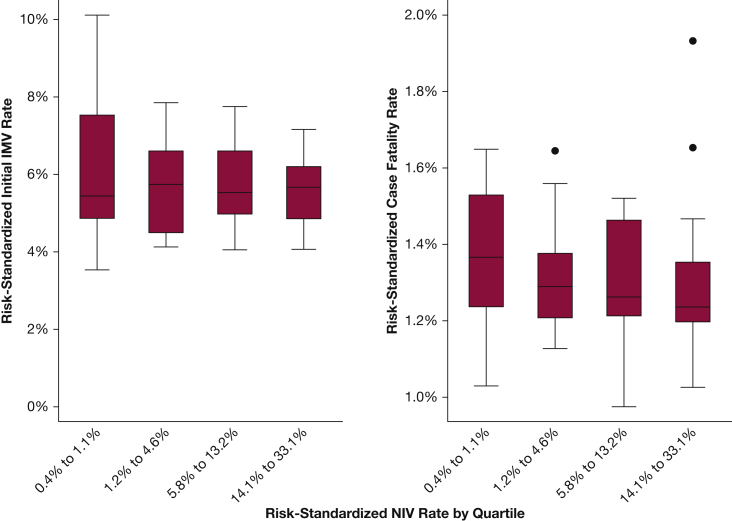
There was no significant correlation between the hospital initial RS-NIV rate and the hospital RS-initial IMV rate (rho = –0.10; P = .45). There was a modest, nonsignificant negative correlation with RS-NIV and the RS-case-fatality rate (rho = –022; P = .09) (Fig 3).
Figure 3.
Correlation between risk-standardized initial NIV and risk-standardized initial IMV rate and in-hospital case fatality rate. See Figure 2 legend for expansion of abbreviations.
Outcomes stratified according to RS-NIV quartiles are shown in Table 3 and e-Figure 1. There was no clear pattern of RS-IMV use across RS-NIV quartiles, and hospitals in the highest quartile of RS-NIV did not have lower RS-IMV use (median quartile 4 vs quartile 1, 5.4% vs 5.7%; P = .60). Hospitals with higher use of NIV did have lower adjusted length of stay (P value for trend = .01). There was no statistically significant decline in RS-case-fatality across RS-NIV quartiles (test for trend, P = .10).
Table 3.
Hospital RS Rates of IMV, Case-Fatality Rate, and LOS According to Quartiles of RS Initial NIV
| Characteristic | RS Initial NIV |
P Value (Kruskal-Wallis Test) |
P Value for Trend |
|||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
| RS-initial NIV,a % | 0.6 [0.5-0.7] | 1.5 [1.3-2.5] | 8.8 [7.8-9.1] | 18.1 [16.1-22.6] | NA | NA |
| RS- initial IMV,a % | 5.4 [4.9-7.5] | 5.7 [4.5-6.6] | 5.5 [5.0-6.6] | 5.7 [4.9-6.2] | .95 | .58 |
| RS-total IMV, % | 5.6 [5.0-7.5] | 5.9 [4.6-6.8] | 5.7 [5.2-6.7] | 5.9 [5.2-6.7] | .99 | .83 |
| RS-case fatality rate, % | 1.4 [1.2-1.5] | 1.3 [1.2-1.4] | 1.3 [1.2-1.5] | 1.2 [1.2-1.4] | .41 | .10 |
| RS-LOS, h | 102 [98-113] | 106 [95-113] | 91 [84-95] | 94 [78-112] | .02 | .01 |
Numbers in each row express the median and IQR. The Kruskal-Wallis test was used to compare all 4 quartiles. It tests if the 4 quartiles are the same (it ranks the values rather than computes a mean) versus the hypothesis that at least one is different. The P value test for trend assessed all 4 quartiles as well. LOS = length of stay; NA = not applicable. See Table 1 legend for expansion of other abbreviations.
Refers to the first method of ventilation during hospitalization.
Comparing mean values in the highest quartile of RS-NIV use versus the lowest, there was an absolute difference in total ventilation of 19.5%, implying that approximately an additional 20 of 100 patients underwent ventilation in these hospitals. Moreover, there was a substantial, nonlinear increase in the RS-total rate of initial ventilation. The rate in the highest quartile of RS-NIV was 3.8 times the rate in the lowest quartile and 1.8 times the rate of the third highest quartile.
Discussion
In this large observational study of > 13,000 admissions with an asthma exacerbation, we found a wide variation in the use of NIV among a diverse group of hospitals in the United States. Twelve hospitals (21%) had RS NIV rates < 1.0%, whereas 14 (24%) had RS rates > 15%. Notably, we observed that hospitals with higher rates of NIV did not have lower rates of IMV, and their overall rates of ventilation (NIV and IMV) were higher. This finding suggests that NIV was not being used in place of IMV but rather that hospitals were expanding the use of ventilatory assistance and lowered the threshold for initiating NIV. There was no association between hospital initial RS-NIV rates and hospital RS-case-fatality rate, although the P value for trend was borderline nonsignificant and mortality was rare, making it hard to detect significant variations.
Despite weak evidence on efficacy, there was an increased use of NIV in patients hospitalized with asthma exacerbation. A study using data from the Nationwide Inpatient Sample from 2000 to 2008 found that the utilization of NIV in admissions with asthma had increased from 0.3% to 1.9% (a fivefold increase).22 Our study conducted at the hospital level showed further increases in the NIV use to 2011. The growing use of NIV in patients with asthma should be understood in the context of the rapid increase in NIV use in patients with acute respiratory failure of any etiology, although strong evidence for its efficacy exist only for a few conditions.23 In addition, physicians may initiate NIV during an asthma exacerbation, recognizing the resemblances with a COPD exacerbation.
The large variation across hospitals in the use of NIV in patients with an asthma exacerbation was not explained by hospital characteristics or the hospitals’ case mix, suggesting that the source of variation may be at the institution and/or provider level. Variation in practices of NIV use across hospitals has been reported in COPD and in acute decompensation of heart failure.24, 25 A large study of NIV use among patients hospitalized with COPD between 2009 and 2011 found that the interquartile range of initial NIV was 10.2% to 19.4% versus 2.3% to 6.4% for initial IMV among all patients hospitalized with COPD.24 In contrast to our study, hospitals with greater use of NIV for COPD had lower rates of IMV. In the absence of strong evidence regarding the comparative effectiveness of NIV use versus other ventilation strategies (including usual care, high-flow oxygen, and IMV), the likelihood of a patient with asthma being treated with NIV seems primarily dependent on the hospital to which the patient is admitted.
One possible reason for the variability in institutional and/or provider practices is the limited evidence regarding the efficacy of NIV in asthma and the uncertainty of the guidelines on NIV use. Single-center studies that have described NIV use in asthma included a heterogeneous population from moderate asthma attacks to status asthmaticus,26, 27 and they reported a large range of use of NIV.28, 29, 30 Hospital-level factors (including hospital use of NIV for conditions that have stronger evidenced-based recommendations such as COPD or acute pulmonary edema, presence of respiratory therapists, and availability of NIV devices) may all influence the use of NIV in asthma. Greater familiarity and comfort with NIV at certain hospitals, high rates of use in COPD or other respiratory conditions, and the appropriate infrastructure may explain our findings that the hospital in which the patient was treated had a significant association with the type of ventilation received. Supporting this hypothesis is the fact that patients admitted at hospitals with higher rates of NIV use were more likely to have received NIV in a prior admission.
Death is rare in the large majority of patients hospitalized with an asthma exacerbation, but those who are intubated and mechanically ventilated have a high death rate, ranging from 16% to 22%.2, 3 In the present study, there was no significant survival benefit for hospitals with a higher use of NIV, which is understandable given the lack of decrease in the use of IMV. NIV could have been used inappropriately in this not well-defined population; it is possible that those patients who quickly underwent intubation should not have received NIV initially but also that some patients could have fared well without any NIV. Because the use of IMV in patients with asthma is associated with increased morbidity and mortality,2, 3 and that there is a rationale for the use of NIV in asthma,30 randomized controlled trials should explore if there is a benefit of using NIV in selected patients with asthma.
Our study had several strengths. The multihospital electronic health record dataset contains laboratory results and medications, and we calculated a severity illness score that included results of arterial blood gas measurements to adjust for differences in patient case mix. Only patients treated with bronchodilators and steroids were studied to increase the likelihood of including only patients with at least moderate exacerbation of asthma. We restricted the analysis to hospitals with a minimum of 45 admissions to obtain more stable estimates of hospital rates, and we calculated hospital RS-outcome rates accounting for variance in patient outcomes within and between hospitals.
The results of this study need to be interpreted in the context of several limitations. First, ICD-9-CM diagnostic codes were used for selecting patients with asthma. To identify patients with asthma, we included the principal diagnosis of asthma and the principal diagnosis of acute respiratory failure with a secondary diagnosis of asthma. Although we reduced the chance of misclassification of the diagnosis by only including patients treated with steroids and bronchodilators, our classification strategy has not yet been validated. Second, to assess ventilation type, ICD-9-CM procedure codes were used, and there may be variation in coding across hospitals. We have validated the NIV procedure codes by retrospective chart review of 200 patients with acute respiratory failure from 2010 to 2011. The ICD-9-CM codes had a sensitivity of 86% (95% CI, 81-92) and a specificity of 92% (95% CI, 84-98).31 Third, the ICD-9-CM procedure codes do not differentiate the mode of NIV used (CPAP or bilevel pressure ventilation). Fourth, although our regression models adjusted for illness severity, the potential of unmeasured patient and hospital-level confounders remain. Nevertheless, the hospital-level analysis is less likely to be affected by confounding by indication, and patient case mix differences between hospitals would have to be large to change the results. Lastly, the hospitals included in the Cerner Health Facts dataset tended to be larger teaching hospitals in an urban environment that invested in an electronic medical system between 2009 and 2012. Thus, our results may not be applicable to all hospitals in the United States and particularly to those with low number of admissions for asthma.
Conclusions
We found a wide variation in the hospital use of NIV for patients with an acute exacerbation of asthma; the increase in NIV use in this population does not seem to be associated with a reduction in IMV use. These results indicate a need to understand contextual and organizational factors contributing to this variability.
Acknowledgments
Author contributions: M. S. S., P. K. L., P. S. P., J. S. S., T. L., and N. S. H. conceived and designed the study. M. S. S. acquired the data used in the analysis; M. S. S., P. K. L., T. L., J. S. S., B. H. N., A. P., D. M. K., and N. S. S. were involved in the analysis and interpretation of the data. M. S. S. drafted the manuscript. P. K. L., P. S. P., T. L., J. S. S., B. H. N., R. J. G., P. S. P., A. P., D. M. K., and N. S. H. reviewed and contributed to revisions prior to submission.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: N. S. H. has received grants from Breathe Technologies and Fisher Paykel and has served as a medical advisory board member for ResMed Inc. and as a consultant for Phillips Respironics, Fisher Paykel, and Vapotherm. None declared (M. S. S., B. H. N., A. P., P. S. P., T. L., J. S. S., R. J. G., D. M. K., and P. K. L.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figure, and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: Funding support was providing through the National Heart, Lung, and Blood Institute of the National Institutes of Health by the National Center for Research Resources [Grant 1K01HL114631-01A1 to Dr Stefan].
Supplementary Data
References
- 1.Krishnan V., Diette G.B., Rand C.S. Mortality in patients hospitalized for asthma exacerbations in the United States. Am J Respir Crit Care Med. 2006;174(6):633–638. doi: 10.1164/rccm.200601-007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afessa B., Morales I., Cury J.D. Clinical course and outcome of patients admitted to an ICU for status asthmaticus. Chest. 2001;120(5):1616–1621. doi: 10.1378/chest.120.5.1616. [DOI] [PubMed] [Google Scholar]
- 3.Gupta D., Nath A., Agarwal R., Behera D. A prospective randomized controlled trial on the efficacy of noninvasive ventilation in severe acute asthma. Respir Care. 2010;55(5):536–543. [PubMed] [Google Scholar]
- 4.Akinbami L.J., Moorman J.E., Bailey C. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012;(94):1–8. [PubMed] [Google Scholar]
- 5.Brochard L., Mancebo J., Wysocki M. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333(13):817–822. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 6.Carlucci A., Richard J.C., Wysocki M., Lepage E., Brochard L. Noninvasive versus conventional mechanical ventilation. An epidemiologic survey. Am J Respir Crit Care Med. 2001;163(4):874–880. doi: 10.1164/ajrccm.163.4.2006027. [DOI] [PubMed] [Google Scholar]
- 7.Ram F.S., Lightowler J.V., Wedzicha J.A. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003;(1):CD004104. doi: 10.1002/14651858.CD004104. [DOI] [PubMed] [Google Scholar]
- 8.Lim W.J., Mohammed Akram R., Carson K.V. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2012;12:CD004360. doi: 10.1002/14651858.CD004360.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global strategy for asthma management and prevention, 2014. Global Initiative for Asthma website. http://www.ginasthma.org/local/uploads/files/GINA_Report_2015_Aug11.pdf. Accessed May, 20, 2015.
- 10.Amin A.P., Salisbury A.C., McCullough P.A. Trends in the incidence of acute kidney injury in patients hospitalized with acute myocardial infarction. Arch Intern Med. 2012;172(3):246–253. doi: 10.1001/archinternmed.2011.1202. [DOI] [PubMed] [Google Scholar]
- 11.Kosiborod M. Blood glucose and its prognostic implications in patients hospitalised with acute myocardial infarction. Diab Vasc Dis Res. 2008;5(4):269–275. doi: 10.3132/dvdr.2008.039. [DOI] [PubMed] [Google Scholar]
- 12.Kosiborod M., Inzucchi S.E., Krumholz H.M. Glucose normalization and outcomes in patients with acute myocardial infarction. Arch Intern Med. 2009;169(5):438–446. doi: 10.1001/archinternmed.2008.593. [DOI] [PubMed] [Google Scholar]
- 13.ICD-9-CM coding and reporting official guidelines. American Hospital Association, American Medical Record Association, Health Care Financing Administration, National Center for Health Statistics. J Am Med Rec Assoc. 1990;61(10) suppl 1-17. [PubMed] [Google Scholar]
- 14.Overview of the national (nationwide) inpatient sample (NIS). Heathcare Cost and Utilization Project (HCUP) website. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed December 6, 2011.
- 15.Gagne J.J., Glynn R.J., Avorn J., Levin R., Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2010;64(7):749–759. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escobar G.J., Gardner M.N., Greene J.D., Draper D., Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51(5):446–453. doi: 10.1097/MLR.0b013e3182881c8e. [DOI] [PubMed] [Google Scholar]
- 17.Escobar G.J., Greene J.D., Scheirer P., Gardner M.N., Draper D., Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232–239. doi: 10.1097/MLR.0b013e3181589bb6. [DOI] [PubMed] [Google Scholar]
- 18.van Walraven C., Escobar G.J., Greene J.D., Forster A.J. The Kaiser Permanente inpatient risk adjustment methodology was valid in an external patient population. J Clin Epidemiol. 2010;63(7):798–803. doi: 10.1016/j.jclinepi.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Krumholz H.M., Keenan P.S., Brush J.E., Jr. Standards for measures used for public reporting of efficiency in health care: a scientific statement from the American Heart Association Interdisciplinary Council on Quality of Care and Outcomes Research and the American College of Cardiology Foundation. Circulation. 2008;118(18):1885–1893. doi: 10.1161/CIRCULATIONAHA.108.190500. [DOI] [PubMed] [Google Scholar]
- 20.Krumholz H.M., Wang Y., Mattera J.A. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with an acute myocardial infarction. Circulation. 2006;113(13):1683–1692. doi: 10.1161/CIRCULATIONAHA.105.611186. [DOI] [PubMed] [Google Scholar]
- 21.Cuzick J.A. Wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 22.Nanchal R., Kumar G., Majumdar T. Utilization of mechanical ventilation for asthma exacerbations: analysis of a national database. Respir Care. 2014;59(5):644–653. doi: 10.4187/respcare.02505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walkey A.J., Wiener R.S. Utilization of non-invasive ventilation in patients with acute respiratory failure from 2000-2009: a population-based study. Am J Respir Crit Care Med. 2012;185:A6488. [Google Scholar]
- 24.Lindenauer P.K., Stefan M.S., Shieh M.S., Pekow P.S., Rothberg M.B., Hill N.S. Hospital patterns of mechanical ventilation for patients with exacerbations of COPD. Ann Am Thorac Soc. 2015;12(3):402–409. doi: 10.1513/AnnalsATS.201407-293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarni V.T., Kim N., Dai Y. Hospital variation in noninvasive positive pressure ventilation for acute decompensated heart failure. Circ Heart Fail. 2014;7(3):427–433. doi: 10.1161/CIRCHEARTFAILURE.113.000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scala R. Noninvasive ventilation in severe acute asthma? Still far from the truth. Respir Care. 2010;55(5):630–637. [PubMed] [Google Scholar]
- 27.Diehl J.L., Guérot E. Non-invasive ventilation in severe asthma attacks. Minerva Anestesiol. 2013;79(8):926–933. [PubMed] [Google Scholar]
- 28.Fernández M.M., Villagrá A., Blanch L., Fernández R. Non-invasive mechanical ventilation in status asthmaticus. Intensive Care Med. 2001;27(3):486–492. doi: 10.1007/s001340100853. [DOI] [PubMed] [Google Scholar]
- 29.Meduri G.U., Cook T.R., Turner R.E., Cohen M., Leeper K.V. Noninvasive positive pressure ventilation in status asthmaticus. Chest. 1996;110(3):767–774. doi: 10.1378/chest.110.3.767. [DOI] [PubMed] [Google Scholar]
- 30.Murase K., Tomii K., Chin K. The use of non-invasive ventilation for life-threatening asthma attacks: changes in the need for intubation. Respirology. 2010;15(4):714–720. doi: 10.1111/j.1440-1843.2010.01766.x. [DOI] [PubMed] [Google Scholar]
- 31.Stefan MS, Nathanson BH, Higgins T, Steingrub JS, Lagu T, Lindenauer PK. Validity of noninvasive and invasive ventilation billing and procedure codes in patients with acute respiratory failure. Paper presented at: American Thoracic Society International Conference; May 16-21, 2014; San Diego, CA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.