Abstract
Background
Patients with pulmonary arterial hypertension (PAH) are routinely instructed to avoid performing the Valsalva maneuver for fear of syncope or sudden cardiac death. The mechanism of this action has not been elucidated. We conducted a case-control trial of nine patients with PAH and 15 healthy control subjects to determine if systemic hemodynamic changes during the Valsalva maneuver in these patients invoke greater susceptibility to syncope than healthy control subjects. Metrics commonly employed in autonomic testing were used to assess the degree of autonomic failure.
Methods
Common Valsalva parameters, including adrenergic baroreflex sensitivity, pressure recovery time, systolic BP (SBP) recovery, diastolic BP (DBP) recovery, mean arterial pressure recovery, and the Valsalva ratio, were calculated. Mann-Whitney U tests were used to compare continuous variables. The primary end point was adrenergic baroreflex sensitivity.
Results
Patients with PAH had lower adrenergic baroreflex sensitivity (9.7 ± 4.6 mm Hg/s vs 18.8 ± 9.2 mm Hg/s; P = .005), longer pressure recovery time (3.6 ± 2.5 s vs 1.7 ± 0.8 s; P = .008), similar SBP recovery (–13 ± 11 mm Hg vs –12 ± 23 mm Hg; P = .640), less DBP recovery (–1 ± 12 mm Hg vs 13 ± 14 mmHg; P = .025), less mean arterial pressure recovery (–5 ± 11 mm Hg vs 5 ± 17 mm Hg; P = .048), and a decreased Valsalva ratio (1.25 ± 0.11 vs 1.60 ± 0.22; P < .001) compared with healthy control subjects.
Conclusions
Compared with healthy control subjects, patients with PAH are more susceptible to syncope during the Valsalva maneuver because of autonomic dysfunction causing cerebral hypoperfusion. These study patients with PAH exhibited a degree of susceptibility to syncope similar to a spectrum of patients with intermediate autonomic failure who typically experience a SBP drop of 10 to 30 mm Hg with standing.
Key Words: autonomic function, autonomic nervous, pulmonary arterial hypertension, syncope
Abbreviations: BRS-a, adrenergic baroreflex sensitivity; DBP, diastolic blood pressure; HC, healthy control; HR, heart rate; MAP, mean arterial pressure; MSNA, muscular sympathetic nerve activity; PAH, pulmonary arterial hypertension; PRT, pressure recovery time; SBP, systolic blood pressure; VM, Valsalva maneuver
Pulmonary arterial hypertension (PAH) is a significant problem worldwide, with a high degree of morbidity and mortality within a few years of diagnosis.1 Syncope in patients with PAH is thought to portend a poorer prognosis and is associated with sudden death.2, 3 The etiology of syncope in these cases has been associated with arrhythmias.3, 4 Patients with PAH are routinely counseled to avoid straining or other situations in which they would strain against a closed glottis, such as the Valsalva maneuver (VM), for fear of syncope.5 The contribution of systemic hypotension to syncope in this setting has not been explored. Opotowsky et al6 recently showed that a bedside VM may reliably differentiate between those patients with pulmonary hypertension with or without elevated pulmonary artery wedge pressure, and thus differentiate PAH from pulmonary hypertension due to left-sided heart failure. However, no studies have evaluated the hemodynamic changes in the systemic circulation during all four phases of VM in patients with PAH compared with normal subjects as they relate to BP and heart rate (HR).
We hypothesized that patients with PAH will have hemodynamic profiles consistent with a significantly higher vulnerability to presyncope or syncope compared with healthy control (HC) subjects. To test this hypothesis, a case-control study was conducted to evaluate the hemodynamic changes that occur during VM in patients with PAH and HC subjects. The study used validated Valsalva metrics to assess orthostatic hypotension in patients referred for evaluation of syncope.
Materials and Methods
Patient Population
Between August 2013 and December 2013, patients with PAH cared for by the Vanderbilt Pulmonary Hypertension Center and age-/BMI-matched HC subjects recruited from the Vanderbilt University Clinical Research Center volunteer database and local advertisements were enrolled in the study.7 Patients with PAH were diagnosed according to accepted international criteria, including a mean pulmonary arterial pressure > 25 mm Hg and pulmonary artery wedge pressure < 15 mm Hg.8 Subjects were excluded if they had a square root sign on their VM tracing or were classified as New York Heart Association functional class III or higher. Patients with a square root sign were excluded because it invalidates Valsalva metrics.9 Patients were neither included nor excluded based on history of syncope or BP ranges. HC subjects had no cardiovascular disease or other major illnesses, and none were current smokers. Pregnancy was excluded in female subjects on the basis of a urine or serum pregnancy test result.
The Vanderbilt University institutional review board approved this study (study no. 9401), and each subject gave his or her written informed consent for participation. Study investigations were performed at the Elliot V. Newman Clinical Research Center at Vanderbilt University. Pulmonary hypertension medications were not held prior to testing.
PAH Patient Data
Nine patients with PAH were enrolled in the study, and their pertinent clinical information, including right heart catheterization (RHC) and transthoracic echocardiogram (TTE) data, are listed in Table 1. RHC and TTE data were that most proximate to the Valsalva study. In eight of nine subjects, RHC was performed within 4 weeks of the study; the exception was subject 5, whose most proximate RHC was performed 8 months later. TTE was performed within 6 months of the Valsalva study for seven of nine subjects; the exceptions were subjects 2 and 3, who underwent TTE 7 and 10 months prior, respectively. These values are only meant to provide insight into the PAH patient population that was studied. Because neither TTE nor RHC was part of the study protocol, their significance in this setting is unclear, and statistical analyses were not performed on these data.
Table 1.
Data From Patients With PAH
| Subject No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Age, y | 35 | 41 | 36 | 42 | 36 | 36 | 51 | 36 | 38 |
| Sex | Female | Female | Female | Female | Male | Female | Female | Male | Female |
| PAH classification | Heritable PAH | Idiopathic PAH | Heritable PAH | Heritable PAH | Heritable PAH | Heritable PAH | Idiopathic PAH | Heritable PAH | Idiopathic PAH |
| Age at diagnosis, y | 32 | 32 | 33 | 39 | 26 | 34 | 45 | 32 | 36 |
| 6-min walk test, m | 408 | 405 | 318 | 360 | 472 | n/a | 408 | n/a | 403 |
| SBP, mm Hg | 125 | 96 | 140 | 88 | 83 | 145 | 118 | 96 | 134 |
| BNP, ng/L | 194 | NA | NA | 1,149 | 312 | 322 | <100 | 222 | 8,227 |
| Clinical history of syncope | Yes | No | No | Yes | Yes | No | Yes | Yes | No |
| Clinical history of presyncope | Yes | No | Yes | Yes | Yes | No | Yes | Yes | No |
| RA pressure, mm Hg | 8 | 1 | 1 | 20 | 5 | 14 | 6 | 11 | 11 |
| Mean PAP pressure, mm Hg | 47 | 44 | 80 | 62 | 77 | 73 | 44 | 64 | 51 |
| Pulmonary vascular resistance (Woods units) | 10.4 | 5.2 | 28.7 | 12.8 | 29.5 | 18.7 | 6.7 | 13 | 13.3 |
| Cardiac index, L/min/m2 | 2.15 | 3.4 | 1.1 | 1.9 | 2.2 | 1.8 | 2.8 | 1.6 | 1.99 |
| Mean PCWP, mm Hg | 5 | 7 | 11 | 10 | 12 | 13 | 8 | 10 | 3 |
| AVT responsiveness | No | Yes | No | No | No | No | No | No | Yes |
| Right ventricular function | Moderate impairment | Normal | Normal | Mild impairment | Moderate impairment | Normal | Normal | Moderate impairment | Normal |
| PAH medications | Epoprostenol; sildenafil | Amlodipine | Ambrisentan; tadalafil | Epoprostenol | Epoprostenol; tadalafil | Epoprostenol: tadalafil | Epoprostenol; tadalafil | Epoprostenol; tadalafil | Amlodipine |
AVT = acute vasodilator responsiveness; BNP = brain natriuretic peptide; NA = not available; PAH = pulmonary arterial hypertension; PAP = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; RA = right atrial; RHC = right heart catheterization; SBP = systolic blood pressure.
Data Acquisition During VM
Systolic BP (SBP) and diastolic BP (DBP) were measured continuously by using the finger volume clamp method (Nexfin; BMEYE) and intermittently with an automated oscillometric brachial cuff (Vital-Guard 450C, Ivy Biomedical Systems). HR was determined by using continuous ECG monitoring (Vital-Guard 450C, Ivy Biomedical Systems). ECG and BP data were digitalized with 14-bit resolution at a 500- and 1,000-Hz sample frequency by using a WINDAQ data acquisition system (DI720; DATAQ) and processed off-line by using custom software in PV-Wave language (PV-Wave; Visual Numerics Inc) written by one of the study authors (A. D.).
Valsalva Maneuver
Baseline HR, SBP, and DBP were obtained just prior to initiation of VM. Patients were asked to maintain an expiratory pressure of at least 40 mm Hg for 15 s. VM (Fig 1) can be divided into four phases.10 Phase 1 and phase 2 are the “strain phases” of VM, with a Valsalva-induced reduction in cardiac venous return and relative hypotension. Phase 3 and phase 4 are the “recovery phases” of VM, when cardiac venous return normalizes to baseline levels. Phase 1 includes the period from the onset of VM until the SBP peak. Phase 2 lasts from the end of phase 1 until the release of the VM. Phase 4 begins after the nadir SBP of phase 3 and lasts until the end of the overshoot of SBP (if present), or when SBP returns to baseline if no SBP overshoot in phase 4 is present. DBP was recorded immediately preceding the relevant SBP.
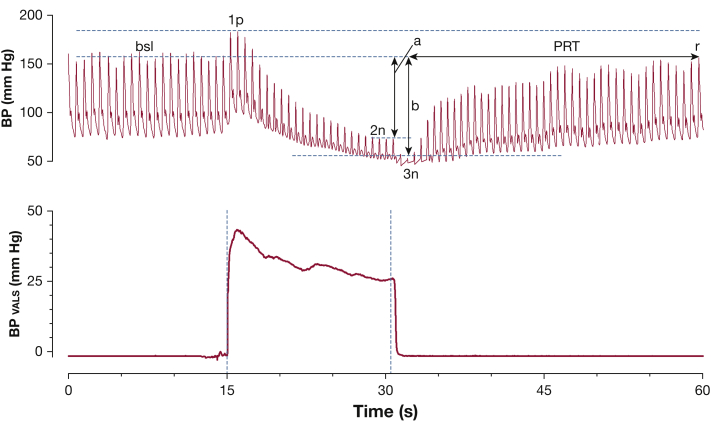
Figure 1.
Schematic parameterization of the Valsalva maneuver. Valsalva maneuver tracing from a patient with autonomic failure is shown, with BP displayed on top and expiratory Valsalva pressure on the bottom. Annotations include the bsl systolic BP (SBP) prior to the Valsalva maneuver, the peak SBP during Valsalva 1p, 2n, and 3n. Arrow a represents the SBP difference between bsl and end of phase 2, or the total BP recovery; arrow b represents the SBP difference between bsl and 3n. PRT represents the time between the BP nadir of Valsalva release until BP recovery back to bsl at point r. The adrenergic baroreflex sensitivity is equal to the BP difference represented by arrow b divided by the PRT. 1p = phase 1; 2n = the nadir of Valsalva phase 2; 3n = the nadir of phase 3; bsl = baseline; PRT = pressure recovery time.
Various Valsalva metrics (Table 2) have been reported as useful in characterizing the severity of sympathetic nervous system dysfunction and were measured in this study. These include total BP recovery9 (Fig 1), pressure recovery time (PRT),11 and the adrenergic baroreflex sensitivity index (BRS-a).12 Total BP recovery is defined as the change in BP from baseline until the end of phase 2, and it can be further divided into systolic, diastolic, and mean arterial pressure (MAP) components. PRT is defined as the amount of time required for the SBP to recover from the nadir of phase 3 to the baseline SBP level in phase 4.11 BRS-a is defined as the BP drop between baseline and the nadir of phase 3 divided by the PRT.12 The lowest SBP during phase 3 was used to calculate the starting point for the PRT and BRS-a. Other recorded Valsalva parameters included baseline HR, maximum HR during VM, and the Valsalva ratio, which is defined as the maximum HR during VM divided by the lowest HR within 30 s of the HR peak.
Table 2.
Valsalva Metrics
| Metric | Abbreviation | Definition | Significance |
|---|---|---|---|
| Adrenergic baroreflex sensitivity, mm Hg/s | BRS-a | Drop in BP between baseline and phase 3, divided by time to recover from phase 3 BP back to baseline | Adrenergic function |
| Pressure recovery time, s | PRT | Time to recover from phase 3 BP back to baseline | Adrenergic function |
| Total BP recovery, mm Hg | Total SBP recovery Total DBP recovery Total MAP recovery |
BP difference between baseline and end of phase 2 | Adrenergic function |
| Valsalva ratio | … | Peak HR achieved during Valsalva maneuver divided by lowest HR within 30 s of peak HR | Cardiovagal function |
Metrics listed here are commonly used to assess autonomic health and stratify severity of orthostatic hypotension. BRS-a = adrenergic baroreflex sensitivity; DBP = diastolic BP; HR = heart rate; MAP = mean arterial pressure; PRT = pressure recovery time. See Table 1 legend for expansion of other abbreviation.
Statistical Analyses
The BRS-a was the primary end point for the study because it correlates with muscle sympathetic nerve discharges produced by VM and provides a noninvasive index to quantify total peripheral resistance in patients with adrenergic failure.12 Secondary end points for this study included PRT, total BP recovery, and the Valsalva ratio. Mann-Whitney U tests were used to compare the continuous variables between the PAH and the HC groups. Fisher exact tests were used to analyze categorical data due to the small study population. Data are presented as mean ± SD, and P values ≤ .05 were considered statistically significant. Statistical analyses were performed by using SPSS for Windows version 19 (IBM SPSS Statistics, IBM Corporation, Armonk, New York).
Results
Demographic Characteristics
Nine patients with PAH and 15 HC subjects were included in the study. There was no difference in age between the PAH and HC groups (39 ± 5 years vs 40 ± 7 years; P = .907) or BMI (33.7 ± 5 kg/m2 vs 29.4 ± 7 kg/m2; P = .180) (Table 3). Height (P = .788) and weight (P = .170) did not differ between groups. Sex distribution was comparable between groups (P = .210). All of the patients with PAH were white, whereas nine of 15 (60%) of the patients in the HC group were white (P = .052).
Table 3.
Demographic Characteristics and Baseline Hemodynamics
| Variable | PAH (n = 9) | Healthy Control Subjects (n = 15) | P Value |
|---|---|---|---|
| Male sexa | 2/9 (22%) | 8/15 (54%) | .210 |
| Age, y | 39 ± 5 | 40 ± 7 | .904 |
| Weight, kg | 95 ± 16 | 85 ± 27 | .135 |
| Height, cm | 168 ± 12 | 169 ± 11 | .590 |
| BMI, kg/m2 | 33 ± 5 | 29 ± 7 | .180 |
| Race (White)a | 9/9 (100%) | 9/15 (60%) | .052 |
| Heart rate, beats/min | 88 ± 17 | 71 ± 12 | .010b |
| SBP, mm Hg | 116 ± 24 | 133 ± 21 | .138 |
| DBP, mm Hg | 68 ± 14 | 75 ± 16 | .347 |
Data are presented as mean ± SD unless indicated otherwise. Continuous data were analyzed with Mann-Whitney U tests comparing patients with PAH vs healthy control groups. See Table 1 and 2 legends for expansion of abbreviations.
Categorical data were analyzed by using the Fisher exact test.
P < .05.
Supine HR, SBP, and DBP
The baseline supine HR was much higher in the PAH group compared with the HC group (88 ± 17 beats/min vs 71 ± 12 beats/min; P = .010) (Table 3). The baseline SBP (116 ± 24 mm Hg vs 133 ± 21 mm Hg; P = .138) and DBP (68 ± 14 mm Hg vs 75 ± 16 mm Hg; P = .347) were similar between groups.
VM Metrics
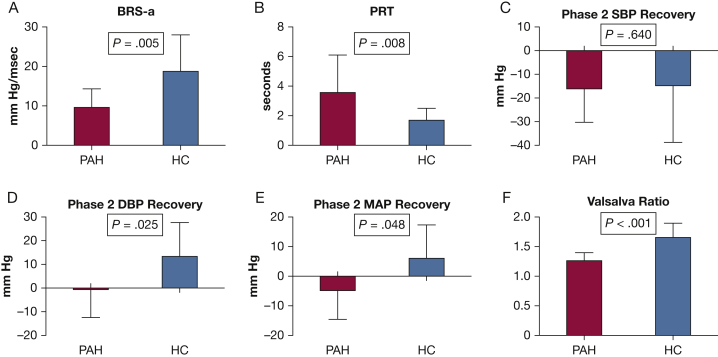
The primary end point, BRS-a, was significantly lower in patients with PAH compared with HC subjects (9.7 ± 4.6 mm Hg/s vs 18.8 ± 9.2 mm Hg/s; P = .005) (Fig 2). PRT was also longer in the PAH population compared with the HC group (3.6 ± 2.5 s vs 1.7 ± 0.8 s; P = .008) (Table 4). Total MAP recovery was significantly less in the PAH group (–5 ± 11 s vs 5 ± 17 s; P = .048). Total SBP recovery was similar between the PAH and HC groups (–13 ± 11 mm Hg vs –12 ± 23 mm Hg; P = .640), but total DBP recovery was considerably greater in the HC group (–1 ± 12 mm Hg vs 13 ± 14 mm Hg; P = .025). Figure 3 displays the typical VM tracings of an HC subject and a patient with PAH.
Figure 2.
A-F, Summary of Valsalva metrics between groups. Summary Valsalva data are presented for PAH and HC subjects. (A) BRS-a, (B) PRT, (C) total SBP recovery, (D) total DBP recovery, (E) total MAP recovery, and (F) Valsalva ratio. Data are presented as mean ± SEM. Student t tests were used to generate P values. BRS-a = adrenergic baroreflex sensitivity; DBP = diastolic blood pressure; HC = healthy control; MAP = mean arterial pressure; PAH = pulmonary arterial hypertension. See Figure 1 legend for expansion of other abbreviation.
Table 4.
Valsalva Maneuver Metrics
| Valsalva Metric | PAH (n = 9) | Healthy Control Subjects (n = 15) | P Value |
|---|---|---|---|
| BRS-a, mm Hg/s | 9.7 ± 4.6 | 18.8 ± 9.2 | .005a |
| Pressure recovery time, s | 3.6 ± 2.5 | 1.7 ± 0.8 | .008a |
| Total SBP recovery, mm Hg | –13 ± 11 | –12 ± 23 | .640 |
| Total DBP recovery, mm Hg | –1 ± 12 | 13 ± 14 | .025a |
| Total MAP recovery, mm Hg | –5 ± 11 | 5 ± 17 | .048a |
| Valsalva ratio | 1.25 ± 0.11 | 1.60 ± 0.22 | <.001a |
| Phase 3 HR, beats/min | 89 ± 13 | 93 ± 17 | .640 |
| Baseline-phase 3N HR change, beats/min | 0.8 ± 17 | 22 ± 12 | .004a |
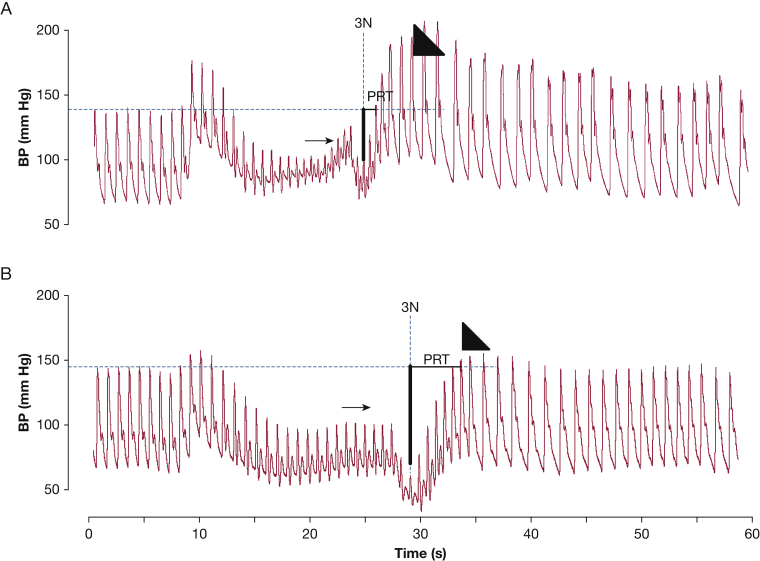
Figure 3.
A and B, Representative Valsalva maneuver tracings. Continuous BP tracings of an (A) HC subject and (B) a patient with PAH. Small arrow indicates phase 2 recovery; large arrowhead indicates phase 4 overshoot. There is a blunted phase 2 recovery of BP and no phase 4 overshoot with a prolonged PRT in panel B, a pattern that is commonly observed in patients with autonomic dysfunction. 3N = point at which Valsalva is released when BP is at its lowest. See Figure 1 and 2 legends for expansion of other abbreviations.
The Valsalva ratio was significantly lower in the PAH group (1.25 ± 0.11 vs 1.60 ± 0.22; P < .001). Phase 3 HR was similar between groups (89 ± 13 beats/min vs 93 ± 17 beats/min; P = .640). However, given a higher baseline HR in the PAH group, the change in HR was significantly less in the PAH group compared with the HC group (0.8 ± 17.3 beats/min vs 21.6 ± 12.1 beats/min; P = .004).
Discussion
We used standardized, comprehensive assessments of the Valsalva response to determine the detailed hemodynamic profile of patients with PAH compared with HC subjects. Our results suggest that patients with PAH may be more prone to syncope than HC subjects due to systemic and cerebral hypoperfusion during and after performance of VM. In particular, patients with PAH have several noticeable differences that suggest an inherent susceptibility to syncopal episodes compared with healthy subjects, including: (1) a blunted chronotropic response following the release phase, (2) a greater decrease in MAP, particularly DBP, during the strain phases, and (3) an impaired recovery of BP following the release phase of VM. Each of these abnormalities may contribute to syncope via cerebral hypoperfusion, and they offer an alternative explanation to cardiac arrhythmias as a possible cause of syncope.
During the strain phases of VM (phases 1 and 2), venous return progressively diminishes until the beginning of phase 3, when the Valsalva is released. Consequently, BP will also drop during these phases unless the adrenergic portion of the sympathetic nervous system intervenes to cause peripheral vasoconstriction.10 Autonomic testing using VM revolves around the recovery of BP as a surrogate marker of adrenergic activity and sympathetic nervous system health as it affects vascular responsiveness.11 This effect is seen beyond phase 2 recovery (Fig 3), as the residual effects of systemic vasoconstriction translate into an exaggerated recovery in BP that is also seen during phase 4 in normal subjects.10 Total BP recovery was developed to objectively quantify adrenergic activity in phase 2, whereas BRS-a and PRT were developed to objectively quantify adrenergic activity in phase 4. Figure 3 highlights the difference in BP recovery, both in phase 2 and phase 4, between a representative tracing from each study group. In fact, the absence of phase 2 recovery and a blunted phase 4 overshoot, both of which are present in this patient with PAH, resemble Valsalva tracings commonly seen in individuals with autonomic failure (Fig 1).9
Under normal circumstances, HR will peak during phases 2 or 3 of VM when venous return and BP are at their lowest as part of the cardiovagal reflex to maintain cardiac output.13 Failure to mount a chronotropic response during VM is strong evidence for cardiovagal dysfunction and is best measured with the Valsalva ratio.14 Normal Valsalva ratios ranged from 1.44 to 2.71 in a previous study of healthy 30- to 49-year-old individuals.15 The Valsalva ratio in the PAH group (1.25) was not only less than the lower limit of normal but is actually less than the 2.5th percentile (1.36) in age-matched control subjects.
Previous studies have shown that HR variability is diminished in patients with PAH, which suggests cardiovagal insufficiency14, 16; these reports are consistent with our Valsalva ratio in the PAH group. Also noteworthy is the high resting HR seen in patients with PAH, which makes further HR augmentation more difficult compared with the HC subjects with lower resting HR. Nevertheless, the Valsalva ratio and the blunted HR response following the release phase of VM in patients with PAH provide a tenable explanation for syncope in this population.
A prolonged PRT, a reduced BRS-a, and impaired total DBP and MAP recovery after the strain phase are all findings in VM indices that have previously been shown to accurately grade severity of adrenergic failure and orthostatic hypotension symptoms.9, 11, 12 Compared with HC subjects, the PAH population exhibited evidence of significantly greater adrenergic dysfunction and hypotension in all of these indices. The BRS-a in the PAH group (9.7 ± 4.6 mm Hg/s) was comparable to that reported in a spectrum of patients with intermediate adrenergic failure and borderline orthostatic hypotension (defined as a drop in SBP between 10 and 30 mm Hg with standing as noted in a previously published study).12 Although these indices were not originally developed to assess for hypotension in patients with PAH, there currently are no means to objectively quantify propensity to experience syncope or presyncope in these patients. These VM indices may provide a novel approach to quantify the degree of adrenergic dysfunction and susceptibility to syncope these patients experience.
Our findings are somewhat inconsistent with previous PAH studies of muscle sympathetic nerve activity (MSNA), the gold standard measurement of sympathetic activity.16, 17, 18, 19 Several studies dating back to 2004 have documented increased MSNA in patients with PAH at baseline.16, 17, 18, 20 However, because BRS-a was decreased in the PAH group, our findings suggest MSNA may actually be decreased during performance of the VM in PAH patients. One reason may be that MSNA is actually elevated during the VM, and vasodilators, such as pulmonary hypertension medications, are responsible for this apparent MSNA/BRS-a mismatch.
The pulmonary hypertension medications these patients were taking at the time of testing (Table 2) (endothelin receptor antagonists, phosphodiesterase inhibitors, and IV prostaglandins) have been shown to cause decreased systemic vascular resistance and hypotension.21, 22, 23 These findings would suggest that the hypotensive systemic effects of these medications are profound because they are able to cause excessive systemic vasodilatation despite an increased MSNA state. It would also suggest that these medications, via their actions on systemic BP and systemic vascular resistance, may be responsible for, or at least contribute to, the increased MSNA state as well as the VM results found in patients with PAH.
Although the present study provides insights into the potential mechanisms of syncope among those with PAH, it does have limitations. First, it is notable that subjects with severe functional impairment due to PAH were not included in this study due to the perceived risks of performing VM in these patients. However, although their hemodynamic measures at diagnosis demonstrate severe pulmonary vascular disease (Table 2), their functional classification likely represents right ventricular adaptation to load stress rather than a reduced severity of PAH. The exclusion of more functionally limited patients makes some of the differences between patients with PAH and HC subjects difficult to put into full context. For example, although a PRT difference of 2 s (3.6 s vs 1.7 s) was statistically significant, it is unclear if this finding is clinically significant. Inclusion of more functionally limited patients with PAH may reveal more profound and interesting findings. Another limitation in the study is the lack of MSNA testing concurrent with performance of VM. Although BRS-a has previously been validated against MSNA, there is no substitution for the gold standard test to determine if, in fact, MSNA was elevated during VM.16
It is notable that two of the patients with PAH were vasodilator responsive according to accepted criteria, which could have confounded the results; however, separate analysis of the data with the exclusion of these two subjects resulted in no changes in the data and conclusions (data not shown). Finally, the number of patients with PAH in this study was small and only constitutes those with idiopathic and heritable PAH. Therefore, our study findings may not be applicable to patients with other forms of PAH. However, despite this limited number, statistically significant differences were found in most VM metrics tested.
Conclusions
Both changes in SBP and HR during VM contributed to greater susceptibility to syncope in patients with PAH versus HC subjects. This finding was determined by using standard Valsalva metrics commonly used to evaluate syncope in patients with autonomic failure, which include BRS-a, PRT, total BP recovery, and the Valsalva ratio. In the PAH group, all of these metrics exhibited statistically significant trends toward greater autonomic dysfunction compared with the HC group, making it the most likely etiology behind this pattern of hemodynamic change predisposing to cerebral hypoperfusion. Furthermore, this propensity for syncope during VM supports the recommendation that patients with PAH avoid straining.
Acknowledgments
Author contributions: P. L. M. is the guarantor of the paper and confirms that the study objectives and procedures are honestly disclosed. Moreover, he has reviewed study execution data and confirms that procedures were followed to an extent that convinces all authors that the results are valid and generalizable to a population similar to that enrolled in this study. P. L. M., S. R. J., E. D. A., and V. N. contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript. B. K. B., I. B., A. D., S. Y. P., J. E. L., A. R. H., I. V. R., and D. R. contributed substantially to the collection, analysis, and interpretation of the data in addition to critical review of the intellectual content of the manuscript. S. R. R. and E. D. A. co-supervised work on this project.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: A. R. H. has received grant money from United Therapeutics and the National Institutes of Health and consulting fees from Bayer, United Therapeutics, Actelion, and Pfizer. S. R. J. has served as a consultant to Lundbeck Pharmaceuticals, GE Healthcare, and Medtronic. None declared (P. L. M., V. N., B. K. B., I. B., A. D., S. Y. P., J. E. L., I. M. R., D. R., E. D. A.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This study was funded by the National Institutes of Health [Grants P01 HL 108800 and K23 HL 098743].
References
- 1.McGoon M.D., Benza R.L., Escribano-Subias P. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62(25 Suppl):D51–D59. doi: 10.1016/j.jacc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Rubin L.J., Badesch D.B. Evaluation and management of the patient with pulmonary arterial hypertension. Ann Intern Med. 2005;143(4):282–292. doi: 10.7326/0003-4819-143-4-200508160-00009. [DOI] [PubMed] [Google Scholar]
- 3.James T.N. On the cause of syncope and sudden death in primary pulmonary hypertension. Ann Intern Med. 1962;56:252–264. doi: 10.7326/0003-4819-56-2-252. [DOI] [PubMed] [Google Scholar]
- 4.Rajdev A., Garan H., Biviano A. Arrhythmias in pulmonary arterial hypertension. Prog Cardiovasc Dis. 2012;55(2):180–186. doi: 10.1016/j.pcad.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin V.V., Archer S.L., Badesch D.B. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 6.Opotowsky A.R., Ojeda J., Rogers F. Blood pressure response to the Valsalva maneuver. A simple bedside test to determine the hemodynamic basis of pulmonary hypertension. J Am Coll Cardiol. 2010;56(16):1352–1353. doi: 10.1016/j.jacc.2010.03.095. [DOI] [PubMed] [Google Scholar]
- 7.Harris P.A., Scott K.W., Lebo L. ResearchMatch: a national registry to recruit volunteers for clinical research. Acad Med. 2012;87(1):66–73. doi: 10.1097/ACM.0b013e31823ab7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoeper M.M., Bogaard H.J., Condliffe R. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 9.Novak P. Assessment of sympathetic index from the Valsalva maneuver. Neurology. 2011;76(23):2010–2016. doi: 10.1212/WNL.0b013e31821e5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox I.J., Crowley W.P., Jr., Grace J.B. Effects of the Valsalva maneuver on blood flow in the thoracic aorta in man. J Appl Physiol. 1966;21(5):1553–1560. doi: 10.1152/jappl.1966.21.5.1553. [DOI] [PubMed] [Google Scholar]
- 11.Vogel E.R., Sandroni P., Low P.A. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology. 2005;65(10):1533–1537. doi: 10.1212/01.wnl.0000184504.13173.ef. [DOI] [PubMed] [Google Scholar]
- 12.Schrezenmaier C., Singer W., Swift N.M. Adrenergic and vagal baroreflex sensitivity in autonomic failure. Arch Neurol. 2007;64(3):381–386. doi: 10.1001/archneur.64.3.381. [DOI] [PubMed] [Google Scholar]
- 13.Korner P.I., Tonkin A.M., Uther J.B. Reflex and mechanical circulatory effects of graded Valsalva maneuvers in normal man. J Appl Physiol. 1976;40(3):434–440. doi: 10.1152/jappl.1976.40.3.434. [DOI] [PubMed] [Google Scholar]
- 14.Folino A.F., Bobbo F., Schiraldi C. Ventricular arrhythmias and autonomic profile in patients with primary pulmonary hypertension. Lung. 2003;181(6):321–328. doi: 10.1007/s00408-003-1034-x. [DOI] [PubMed] [Google Scholar]
- 15.Low P.A., Denq J.C., Opfer-Gehrking T.L. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997;20(12):1561–1568. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.McGowan C.L., Swiston J.S., Notarius C.F. Discordance between microneurographic and heart-rate spectral indices of sympathetic activity in pulmonary arterial hypertension. Heart. 2009;95(9):754–758. doi: 10.1136/hrt.2008.157115. [DOI] [PubMed] [Google Scholar]
- 17.Ciarka A., Doan V., Velez-Roa S. Prognostic significance of sympathetic nervous system activation in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;181(11):1269–1275. doi: 10.1164/rccm.200912-1856OC. [DOI] [PubMed] [Google Scholar]
- 18.Mak S., Witte K.K., Al-Hesayen A. Cardiac sympathetic activation in patients with pulmonary arterial hypertension. Am J Physiol Regul Integr Comp Physiol. 2012;302(10):R1153–R1157. doi: 10.1152/ajpregu.00652.2011. [DOI] [PubMed] [Google Scholar]
- 19.Low P.A. Testing the autonomic nervous system. Semin Neurol. 2003;23(4):407–421. doi: 10.1055/s-2004-817725. [DOI] [PubMed] [Google Scholar]
- 20.Velez-Roa S., Ciarka A., Najem B. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation. 2004;110(10):1308–1312. doi: 10.1161/01.CIR.0000140724.90898.D3. [DOI] [PubMed] [Google Scholar]
- 21.Jackson G., Benjamin N., Jackson N. Effects of sildenafil citrate on human hemodynamics. Am J Cardiol. 1999;83(5A):13c–20c. doi: 10.1016/s0002-9149(99)00043-0. [DOI] [PubMed] [Google Scholar]
- 22.Williamson D.J., Wallman L.L., Jones R. Hemodynamic effects of bosentan, an endothelin receptor antagonist, in patients with pulmonary hypertension. Circulation. 2000;102(4):411–418. doi: 10.1161/01.cir.102.4.411. [DOI] [PubMed] [Google Scholar]
- 23.Packer M. Vasodilator therapy for primary pulmonary hypertension. Limitations and hazards. Ann Intern Med. 1985;103(2):258–270. doi: 10.7326/0003-4819-103-2-258. [DOI] [PubMed] [Google Scholar]