Abstract
Background
Previous studies reported an association of diabetes mellitus (DM) with TB susceptibility. Many studies were retrospective, had weak diagnostic criteria for DM, and did not assess other comorbidities. The Effects of Diabetes on Tuberculosis Severity (EDOTS) study is addressing these limitations with a longitudinal comparison of patients with TB who are classified as diabetic or normoglycemic according to World Health Organization criteria. We report interim findings after enrolling 159 of a planned 300 subjects.
Methods
A cohort study of patients with TB in South India with DM or normoglycemia defined by oral glucose tolerance test (OGTT) and fasting glucose. Glycohemoglobin (HbA1c), serum creatinine, lipids, and 25-hydroxyvitamin D were measured at enrollment. Patients were monitored monthly during TB treatment, and HbA1c measurement was repeated after 3 months.
Results
Of 209 eligible patients, 113 (54.1%) were classified as diabetic, 44 (21.0%) with impaired glucose tolerance, and 52 (24.9%) as normoglycemic. More patients with diabetes were detected by OGTT than by HbA1c. Diabetes was a newly received diagnosis for 37 (32.7%) in the DM group, and their median HbA1c (6.8%) was significantly lower than in those with previously diagnosed DM (HbA1c, 10.4%). Among 129 patients monitored for 3 months, HbA1c declined in all groups, with the greatest difference in patients with a newly received diagnosis of DM.
Conclusions
Early EDOTS study results reveal a strikingly high prevalence of glycemic disorders in South Indian patients with pulmonary TB and unexpected heterogeneity within the patient population with diabetes and TB. This glycemic control heterogeneity has implications for the TB-DM interaction and the interpretation of TB studies relying exclusively on HbA1c to define diabetic status.
Key Words: chest imaging, diabetes, global medicine, tuberculosis
Abbreviations: DM, diabetes mellitus; DOTS, directly observed therapy, short course; EDOTS, Effects of Diabetes on Tuberculosis Severity Study; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c, or glycohemoglobin; KDM, known diabetic; NDM, new diabetic; NG, normoglycemic; OGTT, oral glucose tolerance test; TU, TB unit; WHO, World Health Organization
An association of diabetes mellitus (DM) with increased TB severity is well described,1 but most data come from retrospective cross-sectional studies, some using imprecise criteria to define DM. Given the rising prevalence of DM in countries with a high TB burden, a better understanding of the effects that DM has on the natural history of TB is needed.2, 3 Diabetes has been associated with increased odds for positive sputum smear results, cavitation, delayed sputum conversion, and recurrent TB, all of which increase transmission and hamper the goal of TB elimination.4, 5 Estimates for TB risk and for adverse outcomes have varied widely between individual studies.4, 6 This might reflect population-specific differences in the TB-DM interaction, but methodological differences and the retrospective nature of many reports contribute to uncertainty.
On that background we initiated Effects of Diabetes on Tuberculosis Severity (EDOTS), a longitudinal cohort study assessing TB severity at presentation, treatment response, and recurrence in patients with new smear-positive results with or without DM. We plan to recruit a total of 300 patients with TB in Chennai, India, with an equal number of patients with diabetes and participants who are not diabetic. Here we report an interim analysis of 209 screened and 153 enrolled subjects, of whom 129 received TB treatment for at least 3 months. Only 24.9% of screened patients with TB were euglycemic and there was unanticipated heterogeneity in patients with TB-DM, which may be important for disease treatment and the design and interpretation of future clinical studies.
Materials and Methods
Study Population
This study was approved by the Ethics Committee of the Prof. M. Viswanathan Diabetes Research Center (ECR/51/INST/TN/2013/MVDRC/01). Informed consent was obtained from all participants.
Ten participating clinics (TB units; TUs) notified the study team of patients with acid-fast bacilli and smear-positive results, and hence suspected of having TB. Patients re-treated for TB were not reported since this was an exclusion criterion. Patients qualified for screening if they were 25 to 60 years of age (inclusion criterion) and met exclusion criteria of having had prior incident TB disease, had received > 7 days of treatment for the current TB episode, had taken more than seven doses of a fluoroquinolone within the past 30 days, were pregnant or nursing, were seropositive for HIV, or were receiving immunosuppressive therapy. Eligible candidates reporting a prior history of DM were confirmed by glycohemoglobin (hemoglobin A1c, or HbA1c) testing or antidiabetic drug treatment and classified as known diabetic (KDM) (Fig 1). Those with no history of DM were screened by fasting plasma glucose (FPG) test and oral glucose tolerance test (OGTT) (75-g glucose challenge). Glycemic status based on plasma glucose 2 h post-challenge was determined according to World Health Organization (WHO) criteria: DM (≥ 200 mg/dL), impaired glucose tolerance (140 to 199 mg/dL), normoglycemia (< 140 mg/dL).7 One patient with diabetes and with a borderline OGTT result received a diagnosis on the basis of an FPG test result ≥ 126 mg/dL. Patients with OGTT or FPG test results in the diabetic range were classified as new diabetic (NDM). Patients with normal OGTT and FPG test results were classified as normoglycemic (NG) while those with impaired glucose tolerance were classified as prediabetic and excluded. Consenting KDM, NDM, and NG patients were enrolled for study participation sequentially, with no attempt to balance the numbers of subjects in each group. Demographics, medical history, and anthropometric measurements were recorded at enrollment. Sputum culture was performed at the National Institute for Research in Tuberculosis reference laboratory in Chennai, India. Complete blood counts, serum creatinine, lipid panel (total, low-density lipoprotein and high-density lipoprotein cholesterol, triglycerides), and 25-hydroxyvitamin D were measured in the laboratory of the M. V. Hospital for Diabetes in Chennai. Chest radiographs produced on enrollment were graded by two blinded readers at the University of Massachusetts Medical School, using a validated severity score based on the percent area of lung involved with TB disease and the presence or absence of cavities.8 Participants returned to the TUs three times weekly for directly observed therapy, short course (DOTS), and were evaluated monthly by the study team. Sputum culture was repeated monthly and HbA1c determination was repeated at month 3. Chest radiography was repeated on TB treatment completion.
Figure 1.
Summary of patients screened, enrolled, and monitored in the study. See the Materials and Methods section for details of subjects who were excluded or withdrawn. DOTS = directly observed therapy, short course; FPG = fasting plasma glucose; KDM = known diabetic; NDM = new diabetic; NG = normoglycemic; OGTT = oral glucose tolerance test; pre-DM = prediabetic. aSix patients withdrew after OGTT (two NDMs and four NG patients). bNegative enrollment culture. cWithdrawn from study. dTwenty-four enrolled patients were unavailable for month-3 analysis as detailed in the Materials and Methods section.
Six patients who consented for OGTT declined further participation. Of 159 patients enrolled between January 2014 and July 2015, six were withdrawn when it was determined that they did not have active TB disease based on negative sputum culture results at enrollment and normal chest radiographs. Four patients died during the study, two defaulted from treatment, seven were withdrawn on request, and five missed the month-3 visit. A total of 129 patients were included in the month-3 data analysis (Fig 1); six patients included in the baseline analysis had not reached that time point.
Statistical Analysis
Sociodemographic, lifestyle, and anthropometric characteristics are shown in Table 1. For categorical variables, percentages are reported. For continuous variables, median and interquartile ranges are reported since many were skewed. Between-group differences were calculated and compared by χ2 tests for categorical variables or by Kruskal-Wallis rank tests for continuous variables across multiple groups. All data were analyzed with Stata MP 13.0 (StataCorp). Statistical significance was set at .05.
Table 1.
Patient Demographic, Lifestyle, and Anthropometric Details at Enrollment
| Variable | KDM (n = 75) | NDM (n = 33) | NG (n = 45) | P Valuea |
|---|---|---|---|---|
| Age, y | 48.6 ± 9.3 | 46.7 ± 10.7 | 40.5 ± 10.6 | < .001 |
| Male | 72.0 | 87.5 | 88.9 | .041 |
| Education | ||||
| Educated | 88.0 | 60.6 | 80.9 | … |
| Uneducated | 12.0 | 39.4 | 19.1 | .005 |
| Occupation | ||||
| Unemployed | 13.3 | 22.6 | 11.4 | … |
| Unskilled worker | 37.3 | 35.5 | 59.1 | … |
| Skilled worker | 18.7 | 16.1 | 15.9 | .169 |
| Business/professional | 9.3 | 16.1 | 4.5 | … |
| Retired/housewife | 21.4 | 9.7 | 9.1 | … |
| Smoking | ||||
| Current | 16.0 | 27.3 | 28.9 | … |
| Former | 20.0 | 30.3 | 31.1 | .084 |
| Never | 64.0 | 42.2 | 40.0 | … |
| Current alcohol consumption | 26.7 | 24.2 | 48.9 | .022 |
| Family history of TB | 26.7 | 15.6 | 22.2 | .457 |
| Family history of DM | 50.7 | 30.3 | 17.8 | .001 |
| BMI, kg/m2 | 20.8 ± 3.8 | 18.3 ± 3.6 | 17.5 ± 2.5 | < .001 |
| Waist circumference, cm | 79.1 ± 9.7 | 77.2 ± 18.1 | 70.8 ± 10.6 | .002 |
Data are presented as percentages or as means ± SD. DM = diabetes mellitus; KDM = known diabetic; NDM = new diabetic; NG = normoglycemic.
χ2 test for categorical variables. Kruskal-Wallis rank tests of between-group equivalence for continuous variables.
Results
Of 578 patients reported as suspected of having TB, 209 were eligible for participation. The remaining 369 were excluded on the basis of age ≤ 25 or > 60 years (27.0%), TB treatment started > 7 days before screening (24.9%), fluoroquinolone treatment (30.5%), and HIV seropositivity (1.1%). Sixty-one patients (16.5%) declined participation. Of the 209 eligible patients, 113 (54.1%) had DM and 44 (21.0%) had prediabetes (pre-DM) while 52 (24.9%) were classified as NG (Fig 1). Among 113 patients with diabetes, 76 (66.3%) were classified as KDM and 38 (32.7%) as NDM. Candidates with pre-DM were excluded. Enrolled patients received DOTS administered by TU staff.9 The analysis included 129 subjects (93 with DM, 36 NG) who completed the first 3 months of TB treatment, at which point HbA1c determination was repeated.
Sociodemographic, behavioral, and anthropometric characteristics of the cohort are shown in Table 1. Patients with diabetes were older and more likely to have a family history of DM than were NG patients. Alcohol consumption was more common among NG patients than among patients with diabetes. There was no difference between groups in family history of TB. Waist circumference and body mass index was higher in NDM and KDM than in NG patients, although mean body mass index fell below the WHO cutoff for undernutrition (18.5 kg/m2) in NDM and NG patients.10
Baseline laboratory data are shown in Table 2. The median HbA1c level was significantly different between all patient groups. The hemoglobin level did not vary by glycemic status, and regression adjustment for hemoglobin did not change the between-group differences in HbA1c; we conclude that anemia did not confound comparison of HbA1c levels. Total and low-density lipoprotein cholesterol were higher in KDM compared with NDM and NG patients, while NDMs had lower high-density lipoprotein cholesterol than the other two groups. Serum triglycerides were higher in KDM and NDM than in NG patients. All three groups were vitamin D insufficient (≤ 20 ng/mL), with lower values in patients with diabetes vs those who were nondiabetic.11
Table 2.
Laboratory Data at Enrollment
| Variable | KDM (n = 75) | NDM (n = 33) | NG (n = 45) | P Valuea |
|---|---|---|---|---|
| Hemoglobin, g/dL | 13.0 (11.6-14.5) | 12.2 (11.3-13.8) | 12.6 (11.4-14.0) | .334 |
| Hemoglobin A1c, % | 10.4 (9.4-11.8) | 6.8 (6.2-9.3) | 5.7 (5.4-5.9) | < .001 |
| Creatinine, mg/dL | 0.80 (0.80-0.90) | 0.80 (0.70-0.90) | 0.90 (0.80-0.95) | .190 |
| Total cholesterol, mg/dL | 179 (137-202) | 159 (129-182) | 155 (128-172) | .011 |
| LDL cholesterol, mg/dL | 97 (74-117) | 87 (70-101) | 83 (70-93) | .004 |
| HDL cholesterol, mg/dL | 38 (33-46) | 33 (27-38) | 37 (30-44) | .013 |
| Triglycerides, mg/dL | 111 (90-137) | 100 (77-128) | 73 (64-91) | < .001 |
| 25-Hydroxyvitamin D, ng/mL | 13 (8-20) | 15 (9-21) | 17 (13-28) | .026 |
Data are presented as medians (interquartile range). HDL = high-density lipoprotein; LDL = low-density lipoprotein. See Table 1 legend for expansion of other abbreviations.
Kruskal-Wallis rank test of equivalence between groups.
The KDMs did not undergo OGTT; their classification was based on history and HbA1c level. NDMs and NG patients were classified on the basis of OGTT and FPG test results, in accordance with WHO guidelines.7 There was only moderate agreement between OGTT and HbA1c for DM diagnosis (κ 0.6411) (Table 3). Of 77 patients who underwent both tests, 32 (41.6%) were diabetic by OGTT result, with 2-h glucose ≥ 200 mg/dL, while 25 (32.5%) were diabetic as indicated by HbA1c ≥ 6.5%. The lower sensitivity of HbA1c for DM diagnosis in our cohort matches results from a study conducted in a similar population12 and a trend identified in most studies comparing these methods for DM diagnosis.13
Table 3.
Agreement Between HbA1c and OGTT Results for Diabetes Mellitus Classificationa
| DM Status | Total | OGTT |
HbA1c |
||
|---|---|---|---|---|---|
| < 200 mg/dL | ≥ 200 mg/dL | < 6.5% | ≥ 6.5% | ||
| NDM | 33 | 1 | 32 | 10 | 23 |
| NG | 44 | 44 | 0 | 42 | 2 |
| Total | 77 | 45 | 32 | 52 | 25 |
HbA1c = glycohemoglobin; OGTT = oral glucose tolerance test (plasma glucose 2 h post-challenge). See Table 1 legend for expansion of other abbreviations.
Cohen’s κ coefficient = 0.6411, P < .001.
Enrolled patients reported to TUs 3 d/wk for DOTS, coinciding once per month with the study team visit. Compliance ranged from 92.5% to 100% on a monthly basis. The present analysis was conducted when 129 participants completed ≥ 3 months of treatment. Sputum was collected at enrollment and monthly during TB treatment. By month 1, 62.1% of KDMs and 59.3% of NDMs had negative sputum culture results vs 78.8% of NG patients (Fig 2; e-Tables 1, 2). Month-2 culture conversion was 92.3% in both KDM and NDM vs 96.7% in NG patients. Chest radiographs were obtained from all patients at enrollment and again at month 6 from 43 patients who completed TB treatment by July 2015. Images were read by blinded reviewers using a validated radiographic TB severity score.8 Median scores at enrollment were not significantly different between patients with diabetes and patients who were nondiabetic (Fig 3, e-Table 3). Scores improved significantly at month 6 for all three patient groups, with greater improvement in NG patients (73.3% reduction) than in KDMs and NDMs (42.9% and 33.3% reduction, respectively).
Figure 2.
Sputum culture conversion by diabetic status. The curves for KDMs and NDMs virtually overlapped. Trends for differences between groups did not reach statistical significance. See Figure 1 legend for expansion of abbreviations.
Figure 3.
Radiographic TB severity score by diabetic status and time point. The top and bottom of each box represent the first and third quartiles, respectively; the line within each box indicates the median, and the ends of the whiskers represent the maximum and minimum range. The differences between scores at enrollment (0) and the completion of TB treatment (month 6) within each group were statistically significant (P ≤ .006). At month 0 there were 73 KDMs, 32 patients newly diagnosed with DM (New DM), and 44 normoglycemic (NG) patients. At month 6 there were 19 KDMs, 13 patients newly diagnosed with DM, and 11 NG patients. The trends for differences between groups did not reach statistical significance. See Figure 1 legend for expansion of abbreviations.
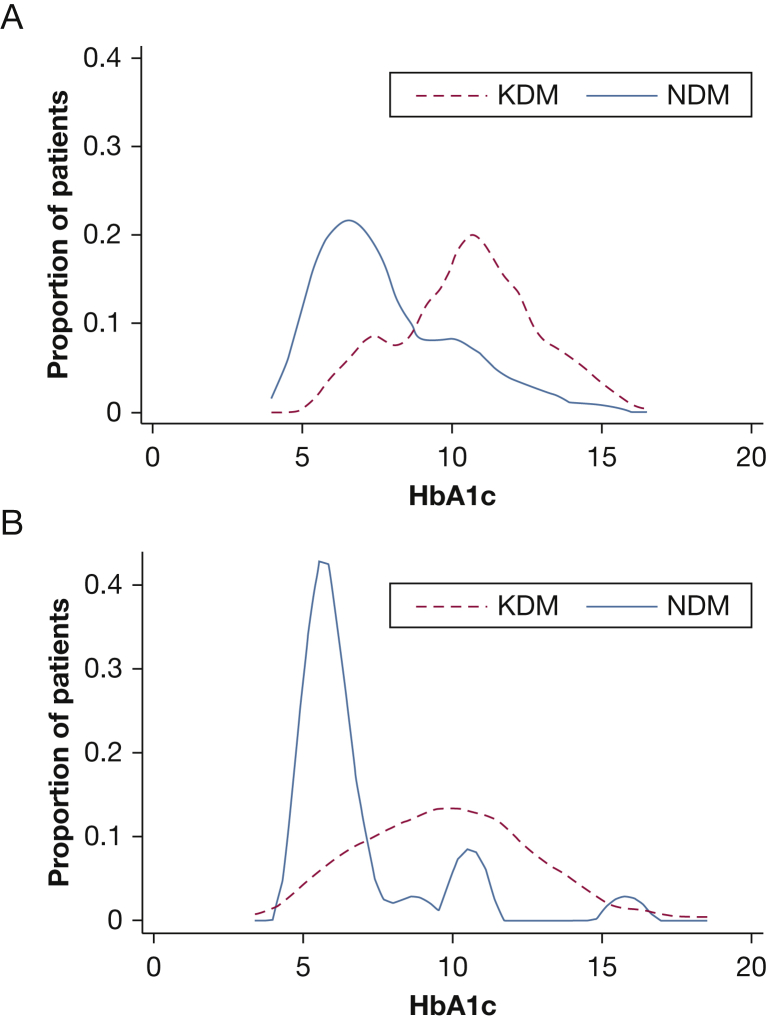
Eighty-eight percent of KDMs took antidiabetic medication at enrollment. Metformin, which was reported to improve TB outcomes,14 was used by 40 (53.3%) of these patients. No NDMs used antidiabetic medication at enrollment. Following EDOTS screening, they were referred to community providers for DM treatment. Antidiabetic treatment was given to 75.9% of NDMs by month 3, and metformin was included in the regimen for 53.6% of them. The median HbA1c level in NDMs fell by a statistically significant 1.0% from enrollment to month 3 (Fig 4, e-Fig 1, e-Table 4). There was a significant 0.4% drop in HbA1c for NG patients and a 0.6% reduction in KDMs, with borderline statistical significance.
Figure 4.
Glycohemoglobin (hemoglobin A1c, or HbA1c) levels in patients with diabetes (DM), stratified by time of DM diagnosis. The proportionate distribution of HbA1c at enrollment (A) and 3 months after enrollment and TB treatment initiation (B) is shown for KDMs and NDMs. See Figure 1 legend for expansion of abbreviations.
Discussion
The World Health Assembly goal of a 90% reduction in TB incidence by 2035 will not be achieved at the current rate of decline, and DM is contributing to that failure.5 Diabetes prevalence is rising at the highest rate in Asian countries where TB is already prevalent.3, 15 The TB-DM interaction will assume escalating clinical significance in the coming decade. Developing an evidence-based approach to combat this dual burden requires a foundation of high-quality clinical data and a better understanding of the susceptibility mechanisms and consequences of TB in patients with diabetes. Most clinical evidence on TB risk and outcomes in DM comes from retrospective studies, many conducted in countries less affected by the dual burden than India. The EDOTS study is intended to address this gap in knowledge. Findings from screening, enrollment, and partial follow-up with roughly half of the planned cohort merit consideration.
Of 209 screened patients with TB, only 24.9% were euglycemic while 75.1% had DM or pre-DM according to WHO criteria. This high prevalence of DM in patients with TB (54.1%) might be unique to South India, but given the population and TB prevalence in that region, any new approaches for DM-TB treatment will need to be highly scalable. Another novel finding concerned a dichotomy among patients with TB and DM. There was a bimodal distribution of diabetic severity within the cohort at enrollment, reflected by HbA1c level and the duration of DM (Fig 4A). Two-thirds of patients were KDMs, and their median HbA1c level was higher than that of NDMs. Evidence supports a correlation between poor glycemic control and adverse TB outcomes.1 Pending outcome data from the full cohort, it remains unknown whether TB severity differs between KDMs and NDMs. This heterogeneity within populations of patients with TB and DM must be considered in the design and interpretation of future clinical management and implementation research.
Diabetes increases TB disease risk approximately threefold.6 Since 54% of the patients in EDOTS were diabetic, the inferred community prevalence of DM would be about 22%. This exceeds the 10.4% DM prevalence reported from a 2011 population survey conducted in Tamil Nadu.16 The age range of our cohort might partially explain that difference since DM prevalence is low in younger people, who were excluded from the study, but we suspect that some NDMs were prediabetic before developing active TB and progressed to DM with the stress of infection. The high prevalence of pre-DM and NDM in patients with new smear-positive test results, indicating TB, raises important questions about the interaction of pre-DM and TB.
We propose two nonexclusive hypotheses: that TB disease stimulates progression from pre-DM to DM and/or that pre-DM impairs protective immunity, increasing TB susceptibility. Supporting the first hypothesis, HbAc1 declined in NG patients and some NDMs treated for TB in the absence of antidiabetic drugs (Fig 4B). In support of the second hypothesis, an inferred near-equal prevalence of DM and pre-DM in our cohort prior to incident TB suggests that pre-DM raises TB risk comparably to DM. Furthermore, reports of cytokine dysregulation in prediabetic patients with TB that mirrors an established pattern in TB-DM17, 18 imply an adverse impact of pre-DM on host defense. Metabolic stress from dyslipidemia or insulin resistance in pre-DM could impair immunity to TB, analogous to its impact on vascular complications.19, 20 An association between dyslipidemia and TB risk has not been established in humans, but we reported that hypercholesterolemia impairs TB defense in mice.21, 22 This could be clinically relevant since statins may offer adjunctive benefit in TB treatment, based on their antiinflammatory properties.23
Diabetes has been associated with delayed sputum conversion and radiographic severity in TB.4, 24, 25, 26 Delayed month-2 sputum conversion correlates with increased risk of TB relapse.27 Interim EDOTS data show a trend for delayed month-2 sputum conversion that did not reach statistical significance but might do so once a full cohort is analyzed. Diabetes has been linked to greater radiographic severity for TB in some but not all studies.1 We found that patients with diabetes have greater residual lung TB pathology after treatment completion than do patients who are not diabetic (Fig 3). Our study was not funded to perform pulmonary function tests, but the data suggest that DM might increase the risk for pulmonary impairment after TB, with its attendant negative impact on quality of life and capacity to work.28
Our study has several weaknesses. Only 36.3% of notified cases were eligible for screening. Patient selection was designed to limit confounding variables affecting immunity and TB treatment response. The sample size for NG patients was relatively small, but the data supporting our conclusions are robust. Because the observed differences were large, between-group comparisons were statistically significant for many outcome variables despite the sample size. We expect that the trends in DM and pre-DM prevalence will be confirmed when the planned accrual is achieved. The varied prevalence of other TB comorbidities (smoking, alcohol consumption, undernutrition, and vitamin D insufficiency) and metformin treatment may also have influenced TB severity and treatment response. This will be comprehensively analyzed when full cohort data are available.
In conclusion, interim EDOTS study results demonstrate a remarkably high prevalence of DM and pre-DM in adult patients with pulmonary TB in South India, emphasizing the need for urgent action to better understand and address the dual burden of TB and DM. The data indicate that OGTT results and HbA1c levels, as well as patient history, define subpopulations of patients with diabetes for whom the susceptibility mechanisms and natural history of TB may differ. Finally, our data suggest that the population of people at risk for TB because of glucose metabolic disorders is larger than reflected by DM prevalence alone. If pre-DM hampers immunity to TB to a degree approaching that of DM, then the estimation of the population-attributable fraction of TB cases among people with disorders of glucose metabolism would need to be revised upward.
Acknowledgments
Author contributions: H. K. and V. V. conceived and organized the study, drafted the manuscript, and take responsibility for the integrity of the work as a whole, from inception to published article. K. W. was responsible for data cleaning and data entry. K. K. was responsible for programming related to database construction, data extraction, and analysis. S. K. was responsible for clinical laboratory assay performance. R. R. Z. and C. M.-B. scored chest radiographs. W. L. was responsible for statistical analysis and contributed to study design and manuscript preparation.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank Vigneswari Aravindalochanan, BMSM, MSc, and Saigopal Sathyamurthy, MBBS, MPH, and Kavitha Selvaraj, MBBS, for assistance in data collection and management.
Additional information: The e-Figure and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was jointly sponsored by the Indian Department of Biotechnology; the Indian Council of Medical Research; and the National Institute for Allergy and Infectious Diseases, National Institutes of Health; and administered by CRDF Global [grant USB1-31149-XX-13].
Supplementary Data
References
- 1.Martinez N., Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol. 2014;44(3):617–626. doi: 10.1002/eji.201344301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L., Magliano D.J., Zimmet P.Z. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2012;8(4):228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO Press; Geneva: 2014. Global Tuberculosis Report 2014. [Google Scholar]
- 4.Baker M.A., Harries A.D., Jeon C.Y. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odone A., Houben R.M., White R.G. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. Lancet Diabetes Endocrinol. 2014;2(9):754–764. doi: 10.1016/S2213-8587(14)70164-0. [DOI] [PubMed] [Google Scholar]
- 6.Jeon C.Y., Murray M.B. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . WHO Press; Geneva: 2006. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia. [Google Scholar]
- 8.Ralph A.P., Ardian M., Wiguna A. A simple, valid, numerical score for grading chest x-ray severity in adult smear-positive pulmonary tuberculosis. Thorax. 2010;65(10):863–869. doi: 10.1136/thx.2010.136242. [DOI] [PubMed] [Google Scholar]
- 9.Granich R., Chauhan L.S. The Revised National Tuberculosis Control Programme (RNTCP) In: Sharma S.K., Mohan A., editors. Tuberculosis. 2nd ed. Jaypee Brothers Medical Publishers; New Delhi: 2009. pp. 894–917. [Google Scholar]
- 10.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [published correction appears in Lancet. 2004;363(9412):902] [DOI] [PubMed] [Google Scholar]
- 11.Thacher T.D., Clarke B.L. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumpatla S., Aravindalochanan V., Rajan R. Evaluation of performance of A1c and FPG tests for screening newly diagnosed diabetes defined by an OGTT among tuberculosis patients—a study from India. Diabetes Res Clin Pract. 2013;102(1):60–64. doi: 10.1016/j.diabres.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Inzucchi S.E. Clinical practice: diagnosis of diabetes. N Engl J Med. 2012;367(6):542–550. doi: 10.1056/NEJMcp1103643. [DOI] [PubMed] [Google Scholar]
- 14.Singhal A., Jie L., Kumar P. Metformin as adjunct antituberculosis therapy. Sci Transl Med. 2014;6(263):263ra159. doi: 10.1126/scitranslmed.3009885. [DOI] [PubMed] [Google Scholar]
- 15.Rhee E.J. Diabetes in Asians. Endocrinol Metab (Seoul) 2015;30(3):263–269. doi: 10.3803/EnM.2015.30.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anjana R.M., Pradeepa R., Deepa M. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54(12):3022–3027. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 17.Kumar N.P., Banurekha V.V., Nair D. Coincident pre-diabetes is associated with dysregulated cytokine responses in pulmonary tuberculosis. PLoS One. 2014;9(11):e112108. doi: 10.1371/journal.pone.0112108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar N.P., Sridhar R., Banurekha V.V. Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17 and other pro-inflammatory cytokines. Ann Am Thorac Soc. 2013;10(5):441–449. doi: 10.1513/AnnalsATS.201305-112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otani H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxid Redox Signal. 2011;15(7):1911–1926. doi: 10.1089/ars.2010.3739. [DOI] [PubMed] [Google Scholar]
- 21.Martens G.W., Arikan M.C., Lee J. Hypercholesterolemia impairs immunity to tuberculosis. Infect Immun. 2008;76(8):3464–3472. doi: 10.1128/IAI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martens G.W., Vallerskog T., Kornfeld H. Hypercholesterolemic LDL receptor-deficient mice mount a neutrophilic response to tuberculosis despite the timely expression of protective immunity. J Leukoc Biol. 2012;91(6):849–857. doi: 10.1189/jlb.0311164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skerry C., Pinn M.L., Bruiners N. Simvastatin increases the in vivo activity of the first-line tuberculosis regimen. J Antimicrob Chemother. 2014;69(9):2453–2457. doi: 10.1093/jac/dku166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jimenez-Corona M.E., Cruz-Hervert L.P., Garcia-Garcia L. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax. 2013;68(3):214–220. doi: 10.1136/thoraxjnl-2012-201756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viswanathan V., Vigneswari A., Selvan K. Effect of diabetes on treatment outcome of smear-positive pulmonary tuberculosis—a report from South India. J Diabetes Complications. 2014;28(2):162–165. doi: 10.1016/j.jdiacomp.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Chiang C.Y., Lee J.J., Chien S.T. Glycemic control and radiographic manifestations of tuberculosis in diabetic patients. PLoS One. 2014;9(4):e93397. doi: 10.1371/journal.pone.0093397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallis R.S., Wang C., Meyer D. Month 2 culture status and treatment duration as predictors of tuberculosis relapse risk in a meta-regression model. PLoS One. 2013;8(8):e71116. doi: 10.1371/journal.pone.0071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasipanodya J.G., McNabb S.J., Hilsenrath P. Pulmonary impairment after tuberculosis and its contribution to TB burden. BMC Public Health. 2010;10:259. doi: 10.1186/1471-2458-10-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.