Abstract
Background
Chronic cough is a common clinical problem worldwide. Although many patients have underlying precipitating conditions such as asthma, gastroesophageal reflux, or rhinitis, many remain symptomatic despite treating these conditions. New approaches are needed for the treatment of this group of patients.
Methods
We conducted a randomized, double-blind, placebo-controlled trial to determine whether 250 mg of azithromycin three times a week for 8 weeks would affect the Leicester Cough Questionnaire (LCQ) score in 44 patients with treatment-resistant cough. Cough severity on a visual analog scale and bronchial exhaled nitric oxide were measured as secondary outcomes.
Results
There was a clinically important improvement in LCQ score with azithromycin (mean change, 2.4; 95% CI, 0.5 to 4.2) but not placebo (mean change, 0.7; 95% CI, −0.6 to 1.9), but the between-group difference was not statistically significant (P = .12). There were no significant between-group differences for any of the secondary outcome measures. Looking at subgroups of responders, there was a large and significant improvement in LCQ score in patients with chronic cough and a concurrent diagnosis of asthma who were treated with azithromycin (mean, 6.19; 95% CI, 4.06 to 8.32).
Conclusions
Treatment with low-dose azithromycin for 8 weeks did not significantly improve LCQ score compared with placebo. The use of macrolides for treatment-resistant cough cannot be recommended from this study, but they may have a place in the treatment of chronic cough associated with asthma; this is worthy of further investigation.
Trial Registry
WHO International Clinical Trials Registry; No.: ISRCTN75749391. URL: http://apps.who.int
Key Words: airway inflammation, antibiotic therapy, cough
Abbreviations: CSS, cough severity score; Feno, fraction of exhaled nitric oxide; LCQ, Leicester Cough Questionnaire; PD20, provocative dose causing a 20% drop in FEV1
Chronic cough (cough persisting for 8 weeks or more) is a common clinical problem worldwide and in Western settings accounts for at least 10% of respiratory referrals to secondary care.1 Chronic cough can be difficult to treat, and is associated with impairment of quality of life, which may be comparable to that seen in severe COPD, with a significant impact on physical, psychological, and social wellbeing.2
Several conditions, such as asthma, gastroesophageal reflux, and rhinitis, are associated with cough, and should be appropriately investigated and treated. In up to 20% of cases the cause of cough remains unclear despite extensive investigations. Patients who have no evidence of structural lung disease and do not have one of these related conditions are considered to have treatment-resistant cough. Current treatment options are limited to nonspecific antitussive therapy.
Patients with chronic cough have been found to have elevated levels of neutrophils in induced sputum, along with increased markers of neutrophilic inflammation such as tumor necrosis factor alpha and IL-8.3 Macrolides have been shown to have antineutrophil and antiinflammatory effects independent of their antimicrobial activity, which makes them a potential therapeutic option in this group.4 Based on a clinical observation of a dramatic improvement in cough symptoms for several patients treated with azithromycin, we set out to explore this potential effect in more detail.
We conducted a randomized, double-blind, placebo-controlled trial to determine whether treatment with low-dose azithromycin for 8 weeks in patients with treatment-resistant chronic cough would affect the Leicester Cough Questionnaire (LCQ) score, cough severity on a visual analog scale, and fraction of exhaled nitric oxide (Feno).
Methods
Design
We conducted a randomized, double-blind, placebo-controlled parallel group trial with an 8-week treatment period and follow-up visits at 4, 8. and 12 weeks.
Participants
Nonsmoking patients who were being investigated for chronic cough were identified from respiratory clinics at Nottingham University Hospitals National Health Service Trust in the United Kingdom between July 2009 and August 2012. Before the study all patients were seen in the respiratory research clinic, where they underwent investigation and treatment as outlined in Figure 1.
Figure 1.
Flowchart for investigation of chronic cough. ACE = angiotensin-converting enzyme; GORD = gastroesophageal reflux disease; MCT = macrolide therapy.
Patients with evidence of bronchial hyperresponsiveness were eligible to be included in the study if they had normal spirometry (defined as FEV1 > 80% predicted and FEV1/FVC > 70%) and their cough did not improve with a 2-week trial of prednisolone. Patients with a history of reflux or rhinitis were included if they had failed to improve with appropriate trials of treatment. Patients with bronchiectasis (all patients underwent a high-resolution CT scan), or current infection (positive sputum culture within 4 weeks), patients who were pregnant or breastfeeding and those who had a previous intolerance of, or contraindication to, macrolide therapy were excluded. Prior to entry all patients underwent a 12-lead ECG to exclude QTc prolongation and phlebotomy to ensure normal liver function.
The study was approved by the UK Medicines and Healthcare Products Regulatory Authority and Nottingham Research Ethics Committee 2, and all patients provided written informed consent before any study-related procedures were performed.
Interventions
The active intervention was azithromycin capsules, 500 mg daily for 3 days followed by 250 mg three times a week for 8 weeks. The control intervention was lactose-containing placebo capsules (Bilcare GCS Europe, Ltd) taken according to the same dosing schedule.
Outcomes
The primary outcome measure was change from baseline in LCQ score at week 8. The LCQ is a validated, self-completed 19-item quality-of-life measure for chronic cough. It has been shown to be repeatable and responsive to change.5 The minimal important difference, which is the smallest change in LCQ score that can be considered clinically meaningful, has been demonstrated to be ±1.3 points.6
Specified secondary outcome measures were cough severity score (CSS) assessed using a 10-cm visual analog scale between 0 (no cough) and 9 (severe cough), and Feno (NIOX Flex Aerocrine AB).
Sample Size
Based on data using opiate therapy for chronic cough,7 we planned to recruit until 29 patients in each group completed the study to give 80% power to detect an effect on LCQ score equivalent to that of low-dose morphine (difference in LCQ score of 2, SD 2.7, α = 0.05).
Randomization and Blinding
Patients were randomly assigned using a computerized random code generator in permuted blocks by a clinical trials pharmacist to receive either azithromycin or matched placebo capsules for 8 weeks. Patients and clinicians were blinded to treatment allocation throughout.
Statistical Methods
The primary end point was change in LCQ score at 8 weeks, and secondary outcome measures were change in cough severity and Feno at 8 weeks. Statistical analysis was performed using Stata SE version 11.2 (Statacorp). Values for Feno were log transformed to assume normality prior to analysis. Within-group change was assessed using a paired samples t test, whereas between-group change from baseline was assessed using an independent samples t test. Analysis was performed on an intention-to-treat basis, with subjects who withdrew after randomization assigned posttreatment values using the baseline value carried forward.
Results
Participants
Fifty-four patients were approached to take part in the study. One patient declined to take part in the study, and 9 patients improved on empirical treatment for cough even though they had been previously investigated. Forty-four consented to take part in the study and were randomly assigned to receive either azithromycin or placebo (Fig 2, Table 1). Recruitment was stopped when 44 patients had been enrolled because of expiration of the investigational medicinal product; with 20 patients in each arm, the study had sufficient power to detect a difference in LCQ score of 2.4.
Figure 2.
Consort diagram for macrolides in chronic cough. IMP = investigational medicinal product; ITT = intention to treat.
Table 1.
Baseline Characteristics of Trial Subjects
| Characteristic | Azithromycin (n = 22) | Placebo (n = 22) |
|---|---|---|
| Age,a years | 59.6 (11.0) | 56.9 (9.0) |
| Female, % | 73 | 64 |
| BMI,a kg/m2 | 28.3 (4.0) | 27.8 (5.2) |
| Smoking,b pack-years | 0 (0-0) | 0 (0-5) |
| Asthma diagnosis | 8/22 (36%) | 11/22 (50%) |
| Reflux symptoms | 6/22 (27%) | 9/22 (41%) |
| Rhinitis | 3/22 (14%) | 3/22 (14%) |
| PD20 methacholine,c μg | 660.7 (0.6) | 709.6 (0.4) |
| LCQ scorea | 10.2 (3.3) | 11.5 (3.1) |
| Cough severity scorea | 6.4 (1.9) | 6.0 (1.9) |
| Feno,c ppb | 21.1 (0.6) | 17.3 (0.7) |
| Blood neutrophils,b ×109/L | 3.75 (3.25-4.6) | 4.2 (3.6-5.1) |
Data are presented as No. (%) unless indicated otherwise. Feno = fraction of exhaled nitric oxide; LCQ = Leicester Cough Questionnaire; PD20 = provocative dose causing a 20% drop in FEV1. There were no significant differences between azithromycin and placebo groups.
Mean (SD).
Median (range).
Geometric mean (log SD).
Numbers Analyzed
Two subjects withdrew from the study before starting their allocated treatment, and two further patients withdrew during the study, one with gastrointestinal side effects (active) and one with perceived lack of efficacy (placebo). Twenty patients in each group contributed primary outcome data, completing all trial visits. All participants were asked to return unused medication, and none returned more than expected from their dosing schedule.
Outcomes and Estimation
There was a clinically important and statistically significant improvement in LCQ score in the azithromycin group from 10.2 to 12.6 (mean change, 2.4; 95% CI, 0.5 to 4.2; P = .01), not seen in the placebo group (mean change, 0.7; 95% CI, −0.6 to 1.9). There was also a significant reduction in Feno (fold change, 0.81; 95% CI, 0.69 to 0.95; P = .01) but not CSS (mean change, −1.0; 95% CI, −2.2 to 0.1) with azithromycin (Table 2).
Table 2.
Changes Within and Between Groups Between Baseline and Week 8
| Variable | Azithromycin (n = 21) |
Placebo (n = 21) |
Between Group P Value | ||||
|---|---|---|---|---|---|---|---|
| Randomization | Week 8 | P Value | Randomization | Week 8 | P Value | ||
| LCQ score | 10.2 (3.3) | 12.6 (4.9) | .01 | 11.5 (3.1) | 12.1 (4.2) | .275 | .12 |
| CSS | 6.4 (1.9) | 5.4 (2.9) | .07 | 6.0 (1.9) | 5.8 (2.3) | .626 | .21 |
| Feno,a ppb | 20.1 (0.06) | 16.3 (0.06) | .01 | 18.7 (0.07) | 16.9 (0.08) | .204 | .13 |
Date are presented as mean (SD) unless indicated otherwise. CSS = cough severity score. See Table 1 legend for expansion of other abbreviations.
Geometric mean (log SD).
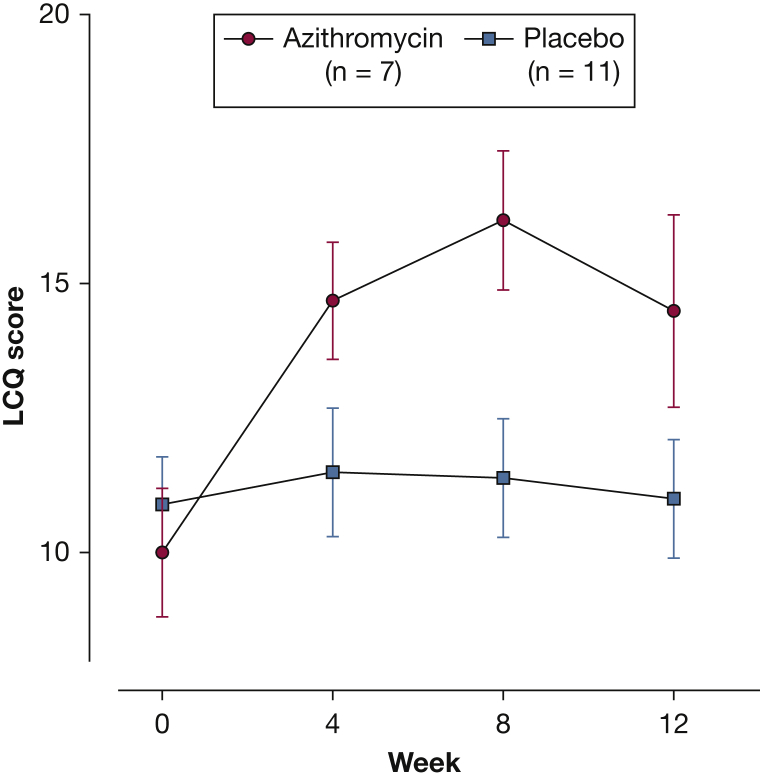
After adjusting for baseline values, the between-group difference in LCQ score was significant at week 4 (mean difference, 1.9; 95% CI, 0.1 to 3.8; P = .04), but this was not maintained across the rest of the trial (Fig 3). The differences in CSS and Feno between groups were also not significant.
Figure 3.
Mean (SEM) LCQ score from baseline to week 12. LCQ = Leicester Cough Questionnaire.
Ancillary Analyses
Because treatment-resistant cough is likely to be multifactorial, we performed a post hoc analysis comparing clinical responders (defined as those subjects demonstrating an increase in LCQ score of ≥ 1.3 at week 8) with nonresponders to determine whether there is a subgroup of patients who will potentially benefit from this treatment.
In total, 11 of 21 subjects in the azithromycin group (52%) had a clinically significant improvement in their LCQ score. Looking at their baseline characteristics, there was a significant association between a current or previous diagnosis of asthma and response to azithromycin (Table 3). In this group all (n = 7) of the subjects with coexisting asthma had a clinically important improvement in LCQ score. An eighth subject in the azithromycin group with a diagnosis of asthma dropped out of the study because of GI side effects before the week 4 study visit.
Table 3.
Characteristics of Clinical Responders vs Nonresponders in the Azithromycin Group
| Characteristic | Responders (n = 11) | Nonresponders (n = 10) | P Value |
|---|---|---|---|
| Female | 8 (73%) | 8 (80%) | .7 |
| Age,a years | 59.1 (12.6) | 60.5 (10.1) | .82 |
| BMI,a kg/m2 | 30.9 (3.1) | 26.3 (2.6) | < .01 |
| Asthma | 7 (64%) | 0 (0%) | < .01 |
| Reflux | 3 (27%) | 2 (20%) | .7 |
| Rhinitis | 1 (9%) | 1 (10%) | .94 |
| Baseline LCQa | 10.0 (3.0) | 10.0 (3.9) | .1 |
| Baseline CSSa | 6.4 (1.8) | 6.7 (2.2) | .72 |
| Baseline Fenob | 20.3 (0.58) | 20.3 (0.54) | .1 |
| Baseline sputum scorea | 3.7 (2.3) | 3.6 (2.1) | .1 |
The effect of asthma diagnosis on response to azithromycin was explored using two-way analysis of variance. There was a large and significant improvement in LCQ score with azithromycin (mean 6.19; 95% CI, 4.06 to 8.32; P = .01) but not placebo (mean, 0.42; 95% CI, −1.28 to 2.12) in the asthma cohort (n =18) (Table 4). This difference was apparent by week 4 and was maintained across both the treatment and follow-up periods (Fig 4). The between-group difference in the asthma cohort was statistically significant at week 8 (mean difference, 5.77; 95% CI, 2.75 to 8.79) (Fig 5). There was also a significant reduction in CSS with azithromycin (mean reduction, 3.14; 95% CI, 1.69 to 4.59; P = .01) but not with placebo in the asthma cohort (Table 5). Again, the between-group difference was statistically significant (mean difference, −2.82; 95% CI, −0.98 to −4.67). There were no significant changes in Feno level in either cohort (Table 6).
Table 4.
Change in LCQ Score Comparing Subjects With an Asthma Diagnosis to Those Without for Both Azithromycin and Placebo
| Subjects | Treatment Group | Change in LCQ Score | P Value |
|---|---|---|---|
| Asthma | Azithromycin (n = 7) | 6.19 (4.06 to 8.32) | < .01 |
| Placebo (n = 11) | 0.42 (−1.28 to 2.12) | .63 | |
| No Asthma | Azithromycin (n = 14) | 0.44 (−1.07 to 1.94) | .57 |
| Placebo (n = 10) | 0.92 (−0.86 to 2.71) | .31 |
Data are presented as marginal means plus 95% CI. See Table 1 legend for expansion of abbreviation.
Figure 4.
Mean (SEM) LCQ score in subjects with a diagnosis of asthma. See Figure 3 legend for expansion of abbreviation.
Figure 5.
Mean (SEM) change in LCQ score for azithromycin and placebo groups ∗∗∗P < .001. See Figure 3 legend for expansion of abbreviation.
Table 5.
Change in Cough Severity Score, Comparing Subjects With an Asthma Diagnosis to Those Without for Both Azithromycin and Placebo
| Subjects | Treatment Group | Change in Cough Severity Score | P Value |
|---|---|---|---|
| Asthma | Azithromycin (n = 7) | −3.14 (−4.59 to −1.69) | < .01 |
| Placebo (n = 11) | −0.32 (−1.48 to 0.84) | .59 | |
| No Asthma | Azithromycin (n = 14) | 0.00 (−1.03 to 1.03) | 1.00 |
| Placebo (n = 10) | −0.05 (−1.26 to 1.16) | .94 |
Data are presented as marginal means plus 95% CI.
Table 6.
Change in Feno, Comparing Subjects With a Diagnosis of Asthma to Those Without for Both Azithromycin and Placebo
| Subjects | Treatment group | Change in Feno (ppb) | P Value |
|---|---|---|---|
| Asthma | Azithromycin (n = 7) | −4.24 (−9.94 to 1.45) | .14 |
| Placebo (n = 11) | −0.98 (−5.52 to 3.57) | .67 | |
| No Asthma | Azithromycin (n = 14) | −3.78 (−8.33 to 0.76) | .10 |
| Placebo (n = 10) | 1.49 (−4.2 to 7.19) | .61 |
Data are presented as marginal means plus 95% CI. See Table 1 legend for expansion of abbreviation.
BMI was also significantly different between responders and nonresponders (Table 3), but it did not have a significant association with change in LCQ score when analyzed for the whole trial population using analysis of covariance, and did not significantly change the adjusted means when incorporated into the analysis of variance model. Although anecdotally patients who complain of a chronic productive cough seem to do well with macrolides, there was no association between baseline sputum score (one of the domains of the LCQ questionnaire) and response to treatment.
Adverse Effects
The azithromycin was well tolerated, with only one dropout in the azithromycin group as a result of gastrointestinal side effects (Fig 2, Table 7). No patients exhibited a clinically significant elevation of liver function tests at any point. There was no reported hearing loss, although this was not specifically asked about.
Table 7.
Adverse Events During Trial Period
| System | Detail | Azithromycin | Placebo | P Value |
|---|---|---|---|---|
| Gastrointestinal | Diarrhea | 4 | 2 | … |
| Heartburn | 1 | 1 | … | |
| Abdominal pain | 2 | 1 | … | |
| Nausea | 1 | 1 | … | |
| Total | 8 | 5 | .29 | |
| Respiratory | Worsening cough | 1 | 5 | .08 |
| CNS | Headache | 1 | 0 | .31 |
| Musculoskeletal | Back pain | 0 | 1 | … |
| Rib pain | 0 | 1 | … | |
| Fall | 0 | 1 | … | |
| Total | 0 | 3 | .07 |
Discussion
There was a clinically important and significant improvement in LCQ score within the first 4 weeks in the azithromycin group but because of differences in baseline and a small placebo effect, the difference between groups was not significant. A type 2 error cannot, therefore, be excluded, although looking at the subgroup analysis described subsequently, any beneficial effect would seem to be restricted to a specific patient phenotype rather than all patients with refractory cough. We believe a longer treatment period would be unlikely to affect our results because when effects were seen, they seemed to be present at 4 weeks.
In an exploratory subgroup analysis, there was a large treatment benefit for subjects with treatment-resistant cough and a background of asthma. The improvement in this cohort accounted for nearly all of the treatment effect seen in the azithromycin group, with a mean (SD) improvement in LCQ of 6.19 (3.09) compared with 0.44 (2.95) in those without asthma. These subjects had been treated with the combination of inhaled corticosteroids and long-acting β-agonists either currently or in the past 3 months, they all had normal spirometry, and 12 of 19 had a positive methacholine challenge (geometric mean provocative dose causing a 20% drop in FEV1 [PD20], 346.7 μg) compared with 1 of 25 patients without asthma (geometric mean PD20, 1104.3 μg). All subjects had failed to improve with an increase in regular asthma medication and a trial of prednisolone, and most (14 of 19) had received empirical short courses of nonmacrolide antibiotics before the trial without benefit. The study was not designed to detect subgroup effects, so this may be a chance finding rather than a true treatment effect. There have, however, been several studies reporting an alteration in the bacterial flora of the respiratory tract in patients with asthma who are using inhaled steroids, with an increase in colonization by organisms such as Streptococcus pneumoniae,8 Haemophilus influenzae,9 and nontuberculous Mycobacteria.10 A recent Cochrane review demonstrated increased relative risk of pneumonia (OR, 1.55; 95% CI, 1.20 to 2.01) and upper respiratory tract infection (OR, 1.32; 95% CI, 1.12 to 1.55) with high-dose inhaled steroids in COPD, and similar effects have been seen in asthma.11 It is therefore possible that treatment-resistant cough in this group of patients is a consequence of asthma or inhaled steroid treatment modifying the airway flora, and macrolide antibiotics may attenuate this change. This is worthy of further investigation, as it is not clear whether the benefit in this cohort is related to the antibiotic or immunomodulatory properties of macrolides. Macrolides have been shown to improve markers of inflammation12 and reduce the frequency of exacerbations13 in patients with asthma and predominantly neutrophilic inflammation.
To date there has been one other randomized, controlled trial of macrolide antibiotics in chronic cough. In a study comparing erythromycin 250 mg once daily for 12 weeks with placebo, Yousaf et al14 recruited 30 patients with chronic cough. All patients had normal spirometry, a provocative concentration of methacholine of greater than 8 mg/mL, normal induced sputum eosinophil count, and a normal high-resolution CT chest scan. In addition, all patients had failed to improve with empirical trials of treatment for reflux and postnasal drip. The primary end point was change in 24-h cough frequency, measured using the Leicester Cough Monitor. Although at 12 weeks there was a significant between-treatment difference in change in sputum neutrophil count (−10.2% vs +6.6% in the placebo group), there was no between-group difference in 24-h cough frequency, or other secondary outcome measures such as LCQ score or CSS.
Although many studies of patients with treatment-resistant cough exclude patients with other comorbidities such as asthma, rhinitis, or reflux, we elected to include this group of patients because it more accurately reflects the patient group that we see in clinical practice. Patients with evidence of bronchiectasis were, however, excluded, as there is already evidence that macrolides improve symptoms and reduce exacerbation frequency in this group.
We elected not to use cough reflex sensitivity in this study in addition to subjective measurements of cough severity. This is partly because there is no consensus on methodology for this test, and the range of normal cough reflex sensitivity is very wide. In addition, in a real-life clinical setting, patients are unlikely to continue with a treatment long-term if they do not experience a subjective improvement in symptoms.
As a result of the lack of between-group differences, the results from our study do not support the routine use of low-dose macrolides in patients with treatment-resistant chronic cough, but chronic cough in association with asthma is worthy of further investigation.
Acknowledgments
Author contributions: T. H. is the guarantor of the content of the manuscript, including the data and analysis. he had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. T. H., D. H., J. A., C. R., J. O., G. M., H. B., D. S., and K. M. contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor and funder did not input into the development of the research or manuscript.
Footnotes
FUNDING/SUPPORT: This paper summarizes research completed as part of a National Institute for Health Research (NIHR) Biomedical Research Fellowship (BRF-2011-010). The views expressed are those of the author(s) and not necessarily those of the National Health Service, the NIHR, or the Department of Health.
References
- 1.Morice A.H., McGarvey L., Pavord I., British Thoracic Society Cough Guideline Group Recommendations for the management of cough in adults. Thorax. 2006;61(suppl 1):i1–i24. doi: 10.1136/thx.2006.065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.French C.L., Irwin R.S., Curley F.J., Krikorian C.J. Impact of chronic cough on quality of life. Arch Intern Med. 1998;158(15):1657–1661. doi: 10.1001/archinte.158.15.1657. [DOI] [PubMed] [Google Scholar]
- 3.Jatakanon A., Lalloo U.G., Lim S., Chung K.F., Barnes P.J. Increased neutrophils and cytokines, TNF-alpha and IL-8, in induced sputum of non-asthmatic patients with chronic dry cough. Thorax. 1999;54(3):234–237. doi: 10.1136/thx.54.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz M.J. Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and cystic fibrosis. J Antimicrob Chemother. 2004;54(1):21–28. doi: 10.1093/jac/dkh309. [DOI] [PubMed] [Google Scholar]
- 5.Birring S.S., Prudon B., Carr A.J., Singh S.J., Morgan M.D., Pavord I.D. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58(4):339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raj A.A., Pavord D.I., Birring S.S. Clinical cough IV: What is the minimal important difference for the Leicester Cough Questionnaire? Handb Exp Pharmacol. 2009;(187):311–320. doi: 10.1007/978-3-540-79842-2_16. [DOI] [PubMed] [Google Scholar]
- 7.Morice A.H., Menon M.S., Mulrennan S.A. Opiate therapy in chronic cough. Am J Respir Crit Care Med. 2007;175(4):312–315. doi: 10.1164/rccm.200607-892OC. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L., Prietsch S.O., Mendes A.P. Inhaled corticosteroids increase the risk of oropharyngeal colonization by Streptococcus pneumoniae in children with asthma. Respirology. 2013;18(2):272–277. doi: 10.1111/j.1440-1843.2012.02280.x. [DOI] [PubMed] [Google Scholar]
- 9.Hilty M., Burke C., Pedro H. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andréjak C., Nielsen R., Thomsen V.O., Duhaut P., Sørensen H.T., Thomsen R.W. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68(3):256–262. doi: 10.1136/thoraxjnl-2012-201772. [DOI] [PubMed] [Google Scholar]
- 11.O’Byrne P.M., Pedersen S., Carlsson L.G. Risks of pneumonia in patients with asthma taking inhaled corticosteroids. Am J Respir Crit Care Med. 2011;183(5):589–595. doi: 10.1164/rccm.201005-0694OC. [DOI] [PubMed] [Google Scholar]
- 12.Simpson J.L., Powell H., Boyle M.J., Scott R.J., Gibson P.G. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008;177(2):148–155. doi: 10.1164/rccm.200707-1134OC. [DOI] [PubMed] [Google Scholar]
- 13.Brusselle G.G., Vanderstichele C., Jordens P. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68(4):322–329. doi: 10.1136/thoraxjnl-2012-202698. [DOI] [PubMed] [Google Scholar]
- 14.Yousaf N., Monteiro W., Parker D., Matos S., Birring S., Pavord I.D. Long-term low-dose erythromycin in patients with unexplained chronic cough: a double-blind placebo controlled trial. Thorax. 2010;65(12):1107–1110. doi: 10.1136/thx.2010.142711. [DOI] [PubMed] [Google Scholar]