Abstract
Background
Relative pulmonary arterial enlargement, defined by a pulmonary artery to aorta (PA/A) ratio > 1 on CT scanning, predicts hospitalization for acute exacerbations of COPD (AECOPD). However, it is unclear how AECOPD affect the PA/A ratio. We hypothesized that the PA/A ratio would increase at the time of AECOPD and that a ratio > 1 would be associated with worse clinical outcomes.
Methods
Patients discharged with an International Classification of Diseases, Ninth Revision, diagnosis of AECOPD from a single center over a 5-year period were identified. Patients were included who had a CT scan performed during the stable period prior to the index AECOPD episode as well as a CT scan at the time of hospitalization. A subset of patients also underwent postexacerbation CT scans. The pulmonary arterial diameter, ascending aortic diameter, and the PA/A ratio were measured on CT scans. Demographic data, comorbidities, troponin level, and hospital outcome data were analyzed.
Results
A total of 134 patients were included in the study. They had a mean age of 65 ± 10 years, 47% were male, and 69% were white; overall, patients had a mean FEV1 of 47% ± 19%. The PA/A ratio increased from baseline at the time of exacerbation (0.97 ± 0.15 from 0.91 ± 0.17; P < .001). Younger age and known pulmonary hypertension were independently associated with an exacerbation PA/A ratio > 1. Patients with PA/A ratio > 1 had higher troponin values. Those with a PA/A ratio > 1 and troponin levels > 0.01 ng/mL had increased acute respiratory failure, ICU admission, or inpatient mortality compared with those without both factors (P = .0028). The PA/A ratio returned to baseline values following AECOPD.
Conclusions
The PA/A ratio increased at the time of severe AECOPD and a ratio > 1 predicted cardiac injury and a more severe hospital course.
Key Words: acute exacerbation of COPD, COPD, CT scan, enzymes (cardiology), pulmonary circulation
Abbreviations: AECOPD, acute exacerbations of COPD; ANOVA, analysis of variance; BNP, brain natriuretic peptide; CHF, congestive heart failure; PA/A, pulmonary artery to aorta; UAB, University of Alabama at Birmingham
COPD is a complex disease of airflow limitation accompanied by significant extrapulmonary effects that have major health implications.1 Acute exacerbations of COPD (AECOPD) are critical events in the natural history of COPD and are a significant cause of morbidity and mortality, accounting for more than $11 billion in direct costs annually.2, 3, 4 AECOPD are defined by an increase in dyspnea, sputum purulence, and sputum volume that warrant a change in therapy.1 Although these events are typically caused by viral or bacterial infections,5, 6 cardiovascular and pulmonary vascular causes have been implicated in precipitating up to 25% of AECOPD.7, 8, 9
Pulmonary vascular disease and cardiovascular disease often coexist with COPD and have major effects on its disease course.10 Pulmonary hypertension in the setting of COPD is associated with accelerated loss of lung function, increased risk for AECOPD, and death.11, 12, 13 Likewise, patients with COPD are at increased risk for hospitalization and mortality related to underlying acute myocardial infarction, congestive heart failure (CHF), or pulmonary thromboembolism.14, 15 Insight into the cardiopulmonary interactions that occur during acute exacerbations is therefore of major importance.16
We have previously identified the pulmonary artery to aorta (PA/A) ratio as a noninvasive marker of pulmonary vascular disease17 that predicts the development of AECOPD, including severe episodes requiring hospitalization.18 In fact, pulmonary arterial enlargement, defined as a PA/A ratio > 1, outperforms traditional risk factors for development of AECOPD, including prior exacerbations, FEV1, St George’s Respiratory Questionnaire score, and gastroesophageal reflux.19 Although these studies established the PA/A ratio as a clinically useful tool, little is known about the longitudinal stability of the metric, how it changes during AECOPD, and whether it could lend insight into the pathophysiology of these events. We hypothesized that the PA/A ratio increases from baseline at the time of severe AECOPD (defined by hospitalization) and that patients with pulmonary arterial enlargement would be at risk for greater cardiac injury and worse clinical outcomes.
Methods
Study Population and Design
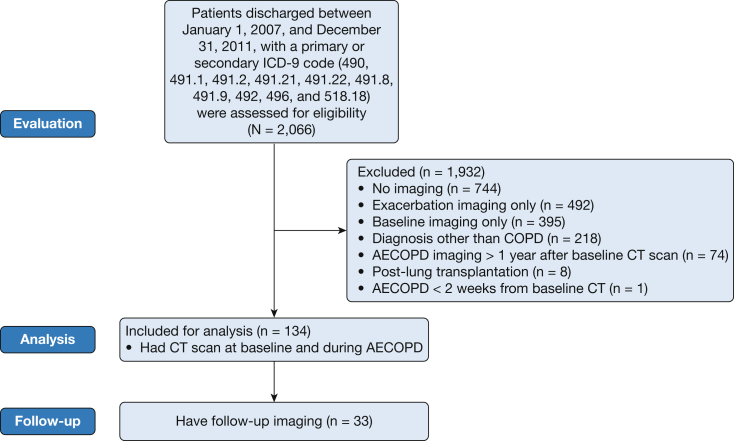
We identified 2,066 patients discharged from the University of Alabama at Birmingham (UAB) Hospital with AECOPD as indicated by using codes from the International Classification of Diseases, Ninth Revision (490-492 or 496 as the primary diagnosis code or as secondary with 518.18 [respiratory failure] as primary) between January 1, 2007 and December 31, 2011 (Fig 1). Patients were included in the study if they had a baseline CT scan (defined as a scan performed during the stable state preceding the index hospitalization) plus an exacerbation scan (defined as a CT scan obtained during the hospitalization for AECOPD) within 12 months of each other. Although not required for inclusion in the study, follow-up CT scans were analyzed and were defined as a CT scan obtained between 1 and 12 months following the index hospitalization in the subgroup that had these data. Patients were excluded from the analysis if they undergone a lung transplantation or if acute pulmonary embolism was present on the AECOPD scan. The latter were excluded to minimize confounder bias based on known associations between acute pulmonary embolism, PA size, and outcomes.20, 21, 22 This study was approved by the UAB Institutional Review Board (number X120730004).
Figure 1.
Patient flow diagram. AECOPD = acute exacerbations of COPD; ICD-9 = International Classification of Diseases, Ninth Revision.
Exacerbation Determination and Parameter Collection
Patients were identified by using International Classification of Diseases, Ninth Revision, coding, and detailed chart reviews were then performed via the electronic medical record system used by the UAB Hospital (Cerner IMPACT, Kansas City, Missouri). A pulmonologist (J. B. M.) reviewed all cases and included only patients who met the following criteria according to a standard abstraction tool: enhanced dyspnea with increased cough lasting > 48 h with either increased sputum volume or purulence prompting an ED visit. We recorded age at the time of admission, sex, race, BMI, smoking status, pack-year smoking history, and pulmonary function measurements (prebronchodilator FEV1, FVC, and FEV1/FVC ratio), as well as comorbidities (hypertension, coronary artery disease, CHF, obstructive sleep apnea, history of venous thromboembolic disease, gastroesophageal reflux, and known pulmonary hypertension). Any condition listed in the medical history from the admission history and physical examination was included as comorbidity. Outcome variables included length of hospital stay, need for ICU stay, acute respiratory failure defined by the need for noninvasive ventilation or invasive mechanical ventilation, in-hospital mortality, and serum troponin and brain natriuretic peptide (BNP) levels at admission.
Review of Imaging
Chest CT images were viewed by using the iSite digital image viewer (Philips Medical Systems). A single blinded reviewer measured the pulmonary arterial diameters in the tubular portion of the main pulmonary artery at the level of its bifurcation and the mean of two perpendicular measurements of the ascending aorta to represent the aortic diameter, as previously reported (Fig 2).17, 18, 23 The median time between baseline and AECOPD scans was 99 days (range, 7-352 days) and 103 days (range, 35-299 days) between AECOPD and follow-up imaging. Reviewers of the CT scans were blinded to all other clinical data.
Figure 2.
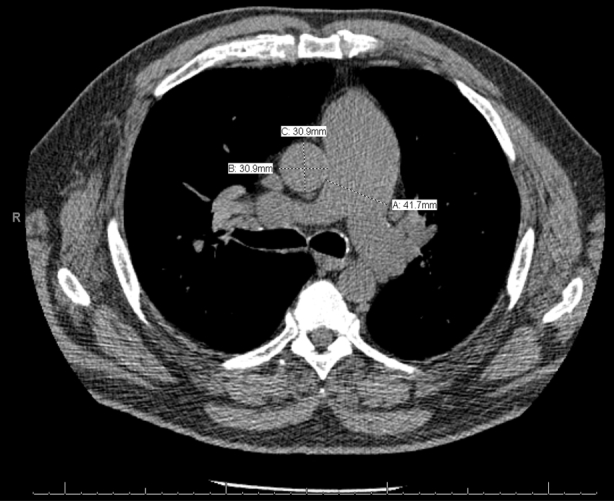
Pulmonary artery enlargement measured on CT scans. The pulmonary arterial diameter (A) and perpendicular measurements of the ascending aortic diameter (B and C) are measured on the same axial CT image to calculate the pulmonary artery to aorta ratio.
Statistical Analysis
Baseline data were expressed as means ± SDs for normally distributed values or median with interquartile ranges for nonnormally distributed values; continuous variables were compared by using two-sided Student t tests or Mann-Whitney U tests as appropriate. Categorical variables were examined by using the Fisher exact test. Univariate and multivariate backward logistic regression analyses were used to determine associations between demographic characteristics and comorbidity variables and a PA/A ratio > 1 at the time of hospital admission. Student t tests and the Fisher exact test were used to measure differences between outcome variables in patients with a PA/A ratio > 1 at admission and those with a PA/A ratio < 1. An analysis of variance (ANOVA) with Tukey’s multiple comparison testing and posttests for linear trends were used to evaluate the differences in clinical outcomes between the group of patients with a PA/A ratio > 1 plus a troponin level > 0.01 ng/mL at admission compared with the group with only one positive variable versus those with neither. Paired t tests or repeated measures ANOVA were used to evaluate the differences in pulmonary arterial diameter, ascending aortic diameter, and the PA/A ratio over time.
SPSS for Windows version 22 (IBM SPSS Statistics, IBM Corporation) was used for all analyses, and figures were designed in Prism Version 5 (GraphPad Software, Inc). Statistical tests were two-sided, and significance was assigned to tests with P values < .05.
Results
Baseline Characteristics
A total of 134 patients were included in the analysis. Patients had a mean age of 65 ± 10 years, 47% were male, 69% were white, and 40% were current smokers; all had airflow obstruction, with a mean FEV1 of 47% ± 19% predicted (Table 1). Comorbid conditions included hypertension (70%), coronary artery disease (31%), CHF (29%), sleep apnea (19%), thromboembolic disease (19%), gastroesophageal reflux disease (37%), and known pulmonary hypertension (12%). The pulmonary arterial diameter was 2.88 ± 0.52 cm, the ascending aortic diameter was 3.21 ± 0.44 cm, and the PA/A ratio was 0.91 ± 0.17, corresponding to a baseline PA/A ratio > 1 in 35 (26%) patients of the cohort (Table 2).
Table 1.
Baseline Characteristics at the Time of Index Admission for AECOPD
| Characteristic | All (N = 134) | PA/A < 1 (n = 77) | PA/A > 1 (n = 57) | P Value |
|---|---|---|---|---|
| Age, ya | 65 ± 10 | 67 ± 10 | 63 ± 10 | .025 |
| White race | 93 (69) | 57 (74) | 36 (63) | .181 |
| Male sex | 63 (47) | 39 (51) | 24 (42) | .383 |
| BMI, kg/m2 | 26.8 ± 8.0 | 25.7 ± 6.4 | 28.2 ± 9.7 | .203 |
| Hypertension | 94 (70) | 51 (66) | 43 (75) | .340 |
| CAD | 42 (31) | 20 (26) | 22 (39) | .135 |
| CHF | 39 (29) | 17 (22) | 22 (39) | .054 |
| OSA | 26 (19) | 11 (14) | 15 (26) | .121 |
| VTE | 25 (19) | 13 (17) | 12 (21) | .655 |
| GERD | 50 (37) | 28 (36) | 22 (39) | .857 |
| Known PHb | 16 (12) | 4 (5) | 12 (21) | .007 |
| Current smoker | 53 (40) | 30 (39) | 23 (40) | .999 |
| Pack-year tobacco | 52 ± 32 | 51 ± 32 | 53 ± 32 | .684 |
| Supplemental oxygen use | 76 (57) | 38 (49) | 38 (67) | .054 |
| FEV1, percent predicted | 47 ± 19 | 45 ± 17 | 48 ± 20 | .363 |
| FEV1/FVC ratio | 52 ± 15 | 50 ± 13 | 55 ± 16 | .100 |
Data are represented as mean ± SD or No. (%). AECOPD = acute exacerbation of COPD; CAD = coronary artery disease; CHF = congestive heart failure; GERD = gastroesophageal reflux disease; OSA = obstructive sleep apnea; PH = pulmonary hypertension; VTE = venous thromboembolism.
P < .05 by two-sided Student t test.
P < .01 by the Fisher exact test.
Table 2.
Changes in PA Size in the Setting of AECOPD
| Variable | Baseline (n = 134) | Exacerbation (n = 134) | Follow-up (n = 33) |
|---|---|---|---|
| PA, cm | 2.88 ± 0.52 | 3.07 ± 0.49a | 2.85 ± 0.56b |
| A, cm | 3.21 ± 0.44 | 3.12 ± 0.40 | 3.15 ± 0.42 |
| PA/A | 0.91 ± 0.17 | 0.97 ± 0.15a | 0.91 ± 0.15b |
Data are presented as mean ± SD. Paired t tests were used to detect differences between baseline and exacerbation-related values. A = aorta; PA = pulmonary artery; PA/A = pulmonary artery to aorta ratio. See Table 1 for expansion of other abbreviation.
P < .001 between baseline and exacerbation values.
P < .001 between exacerbation and follow-up values according to paired Student t tests.
Changes to the PA/A Ratio at the Time of AECOPD
At the time of hospitalization, the pulmonary arterial diameter increased to 3.07 ± 0.49 cm (P < .001 compared with baseline), and the PA/A ratio increased to 0.97 ± 0.15 (P < .001 compared with baseline), with no change in the ascending aortic diameter (Table 2). At the time of exacerbation, 57 patients (43%) had a PA/A ratio > 1 (Table 1). Patients with a PA/A ratio > 1 at admission were younger (63 ± 10 years vs 67 ± 10 years; P = .025) and had higher rates of known pulmonary hypertension (21% vs 5%; P = .007) than those with an exacerbation PA/A ratio < 1. Clinical factors associated with a PA/A ratio > 1 at the time of AECOPD according to univariate logistic regression analysis included the following: age (OR, 0.96; 95% CI, 0.93-0.99; P = .027), CHF (OR, 2.18; 95% CI, 1.02-4.66; P = .044), home use of supplemental oxygen (OR, 2.05; 95% CI, 1.01-4.17; P = .047), and known pulmonary hypertension (OR, 4.87; 95% CI, 1.48-16.0; P = .009). Of these factors, age (OR, 0.95; 95% CI, 0.91-0.99; P = .031) and known pulmonary hypertension (OR, 5.77; 95% CI, 1.44-21.2; P = .013) were independently associated with a PA/A ratio > 1 at exacerbation on multivariate logistic regression analysis; this analysis adjusted for age, race, sex, FEV1 percent predicted, CHF, supplemental oxygen use, and known pulmonary hypertension.
Associations Between the PA/A Ratio and Markers of Cardiovascular Injury and Exacerbation Severity
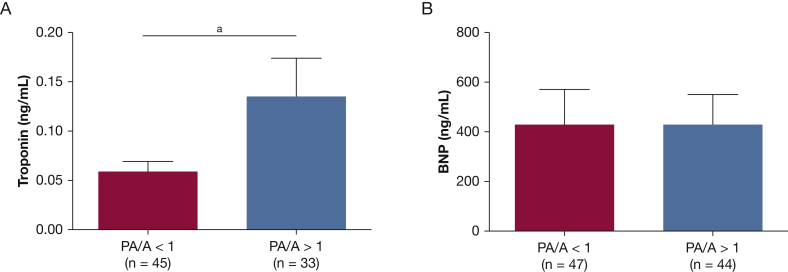
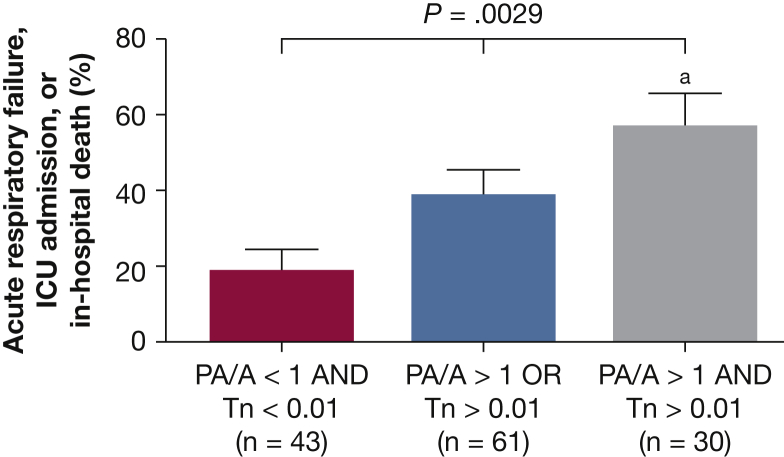
As seen in Figure 3, patients with a PA/A ratio > 1 at the time of hospitalization had increased serum troponin levels (0.13 ± 0.04 ng/mL vs 0.06 ± 0.01 ng/mL; P = .043) with no difference in BNP (433 ± 123 pg/mL vs 434 ± 140 pg/mL; P = .407). We evaluated the contribution of a PA > 1 and a troponin level > 0.01 ng/mL to the risk of ICU admission, acute respiratory failure, or in-hospital mortality. Patients who had both a PA/A ratio > 1 and a troponin level > 0.01 ng/mL were more likely to have one of these outcomes vs patients with a PA/A ratio < 1 and troponin levels < 0.01 ng/mL (57% vs 19%; P = .001 according to the Fisher exact test). Thirty-nine percent of patients with either a PA/A ratio > 1 or a troponin level > 0.01 ng/mL at admission developed acute respiratory failure, required ICU admission, or died in the hospital (P = .0029 by one-way ANOVA, P = .0008 for linear trend between groups) (Fig 4). There was no difference in hospital duration (8.6 ± 1.5 days vs 7.0 ± 1.0 days; P = .36) for patients with a PA/A ratio > 1 compared with those with a PA/A ratio < 1. There was no association between the change in PA/A ratio from baseline to exacerbation and either serum troponin levels or clinical outcomes.
Figure 3.
Enlargement in pulmonary arterial diameter is associated with increased cardiac injury during AECOPD. A, Serum troponin levels are increased at hospital admission in patients with a PA/A ratio > 1. B, There are no differences between admission serum BNP levels in patients with and without enlargement in pulmonary arterial diameter. aP < .05 according to Student t test.BNP = brain natriuretic peptide; PA/A = pulmonary artery to aorta ratio. See Figure 1 legend for expansion of other abbreviation.
Figure 4.
Association between enlargement of pulmonary arterial diameter and severity outcomes during AECOPD. Patients with a PA/A ratio > 1 and a Tn level > 0.01 ng/mL at the time of AECOPD had a higher rate of respiratory failure, ICU admission, or in-hospital mortality (59%) vs those with either a PA/A ratio > 1 or Tn level > 0.01 ng/mL (39%) and vs those with a PA/A ratio < 1 and a Tn level > 0.01 ng/mL (19%; P = .0008 for linear trend between groups, P = .0029 by one-way analysis of variance). aP = .001 between the PA/A ratio < 1 and Tn level < 0.01 ng/mL group and the PA/A ratio > 1 and Tn level > 0.01 ng/mL group by one-way analysis of variance with a Tukey post hoc analysis. Tn = troponin. See Figure 1, Figure 3 legends for other abbreviations.
Changes in the PA/A Ratio Following Index Hospitalization for AECOPD
Thirty-three patients had follow-up imaging (Table 2). The mean pulmonary arterial diameter and the PA/A ratio significantly decreased to 2.85 ± 0.56 cm and 0.91 ± 0.15, respectively, compared with AECOPD values (P < .001 for both). The follow-up pulmonary arterial diameter and PA/A values were not statistically different from baseline values. The ascending aortic diameter was not statistically different during exacerbation or follow-up compared with baseline.
Discussion
This study shows, for the first time, that the PA/A ratio increases during AECOPD, that enlargement of the pulmonary artery at the time of hospitalization is associated with markers of cardiac injury, and that the acute increase in pulmonary arterial size recovers to baseline following the event. The increase in the PA/A ratio was driven by changes in the diameter of the pulmonary artery, as the aortic diameter remained relatively constant at all three time points. These findings build on our prior observations relating pulmonary arterial enlargement to the risk of severe AECOPD by demonstrating that changes to size occur during hospitalization, are associated with cardiac injury, and may portend a worse prognosis.
Although we showed that both the pulmonary arterial diameter and PA/A increase during AECOPD, the mechanism for this change remains uncertain. As Boerrigter et al24 reported in patients with pulmonary arterial hypertension, progressive enlargement in the pulmonary arterial diameter measured by using cardiac MRI occurs independent of changes in hemodynamics. A possible explanation for this phenomenon is that underlying pulmonary vascular disease decreases pulmonary arterial wall distensibility during stability,25, 26 but the reduced vascular distensibility is overcome during AECOPD by factors such as dynamic hyperinflation, altered gas exchange, hypoxic vasoconstriction, increased circulating volume (pulmonary edema or diastolic dysfunction), increased cardiac output, and inflammation.27, 28, 29 Following exacerbations, the vessel returns to its normal size due to its impaired distensibility. In addition, these acute changes could be exaggerated by centralization of blood flow, similar to what we have observed in the stable state of COPD.23, 30 We also observed that younger age was associated with pulmonary arterial enlargement at the time of admission. The mechanism for this phenomenon is unknown and requires further study, although it could be due to impaired vascular distensibility in older patients.27, 31, 32
No patient in our cohort received a clinical discharge diagnosis of acute myocardial infarction, although a number did have elevated troponin levels. Instead, troponin elevations were more common in those with an increased PA/A ratio at admission. Troponin elevations, often occurring with chest pain and ECG changes, have been reported as frequently as one in 12 patients hospitalized for AECOPD.33 Previous factors associated with troponin elevations in AECOPD include increased neutrophil count, decreased hemoglobin concentrations, increased creatinine levels, and cardiac injury scores.34 Increased troponin levels with AECOPD independently predict in-hospital mortality,35, 36, 37 and Brekke et al38 have also shown that elevated troponin levels in the setting of AECOPD predicted all-cause mortality over a 1.9-year follow-up period. Elevated troponin levels in the setting of severe AECOPD are not exclusively related to acute myocardial infarctions,33 and our findings may establish a link between acutely decompensated COPD, right ventricular dysfunction, and pulmonary hypertension. In fact, emerging data suggest that elevated troponin levels are independently associated with an increased risk of rehospitalization in patients with chronic right-sided heart failure due to pulmonary hypertension.39 Although we observed no relationship between BNP and pulmonary arterial size, BNP levels were elevated in our cohort as a whole, suggesting there was increased ventricular stretch during these episodes of severe AECOPD. As others have shown, BNP is commonly elevated during COPD-related hospitalizations, and these elevations are related to early mortality.40
Our observations of pulmonary arterial enlargement and worsened clinical outcomes are important in understanding the prognosis of these acute events. Previously identified prognostic factors for patients hospitalized with AECOPD include age, organ failure, comorbidities, BMI, and the need for mechanical ventilation, but these factors do not completely explain the variability in risk of poor outcomes,41, 42, 43 and additional biomarkers are needed. Blood-based biomarkers, including procalcitonin, C-reactive protein, and copeptin, have been investigated at the time of AECOPD hospitalization; only copeptin exhibited associations with prognosis,44 and procalcitonin may have a role in guiding antibiotic therapy.45 Although our group and others have used noninvasive imaging modalities as biomarkers for COPD prognosis, none have evaluated imaging at the time of hospital admission.18, 46 The present study evaluating pulmonary arterial enlargement is the first radiologic metric evaluated in this context, and incorporating the PA/A ratio into routinely acquired CT reports in the setting of AECOPD may be useful for identifying those at greatest risk.
Our study has several limitations. First, it was a single-center retrospective analysis and is thus subject to the usual shortcomings of these types of studies; our observations, however, are mechanistically and physiologically sound and compatible with our earlier report as well as others linking the PA/A ratio and COPD-related pathophysiology.17, 18, 47 In addition, our sample size was relatively small, limiting our ability to detect statistical differences in some of the observed trends in clinical outcomes. Another limitation is that we did not evaluate the impact of pharmacologic therapies on clinical outcomes. However, > 90% of patients were treated with antibiotics and systemic corticosteroids, limiting any potential for confounding. Finally, < 10% of the discharged patients from our center had CT scans performed at the time of hospitalization for AECOPD, resulting in a selection bias; our findings should thus be prospectively validated.
Conclusions
To the best of our knowledge, this study is the first to longitudinally examine the PA/A ratio and evaluate it in the setting of severe acute exacerbations. We found that pulmonary arterial enlargement is acutely increased during hospitalizations for AECOPD and is associated with enhanced markers of cardiac injury and trends toward worsened clinical outcomes. These findings highlight the mechanistic plausibility for the PA/A ratio to be used as a biomarker for both acute and stable disease.
Acknowledgments
Author contributions: J. M. W. takes responsibility for the content of the manuscript, including the data and analysis. J. M. W. and M. T. D. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. J. M. W., J. B. M., S. P. B., H. N., and M. T. D. contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. M. W. has received grants from the National Institutes of Health/National Heart, Lung, and Blood Institute; had contracts with GlaxoSmithKline and Forest; and acted as a consultant for AstraZeneca. S. P. B. received a grant from the American Heart Association. M. T. D. received grants from the National Heart, Lung, and Blood Institute, the American Heart Association, Forest, and GlaxoSmithKline; had contracts with Aeris, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Pearl, PulmonX, PneumRx, Otsuka, Boston Scientific, and Pfizer; and acted as a consultant for Boehringer Ingelheim, Ikaria, and GlaxoSmithKline. None declared (J. B. M., H. N.).
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank the UAB informatics team for their assistance with patient identification and Tomasz Szul, PhD, for his assistance with figure formatting.
Footnotes
Drs Nath and Dransfield contributed equally to this work.
FUNDING/SUPPORT: The funding for this study came from the National Institutes of Health/National Heart, Lung, and Blood Institute (K08HL123940) and the Walter B. Frommeyer Jr. Fellowship in Investigational Medicine from the University of Alabama at Birmingham (to Dr Wells).
References
- 1.Vestbo J., Hurd S.S., Agusti A.G. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Perera P.N., Armstrong E.P., Sherrill D.L., Skrepnek G.H. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. COPD. 2012;9(2):131–141. doi: 10.3109/15412555.2011.650239. [DOI] [PubMed] [Google Scholar]
- 3.Khakban A., Sin D.D., FitzGerald J.M. Ten-year trends in direct costs of COPD: a population-based study. Chest. 2015;148(3):640–646. doi: 10.1378/chest.15-0721. [DOI] [PubMed] [Google Scholar]
- 4.McGhan R., Radcliff T., Fish R., Sutherland E.R., Welsh C., Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748–1755. doi: 10.1378/chest.06-3018. [DOI] [PubMed] [Google Scholar]
- 5.Anzueto A., Sethi S., Martinez F.J. Exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4(7):554–564. doi: 10.1513/pats.200701-003FM. [DOI] [PubMed] [Google Scholar]
- 6.Sethi S., Evans N., Grant B.J., Murphy T.F. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 7.Zvezdin B., Milutinov S., Kojicic M. A postmortem analysis of major causes of early death in patients hospitalized with COPD exacerbation. Chest. 2009;136(2):376–380. doi: 10.1378/chest.08-2918. [DOI] [PubMed] [Google Scholar]
- 8.Smeeth L., Thomas S.L., Hall A.J., Hubbard R., Farrington P., Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 9.Rizkallah J., Man S.F., Sin D.D. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009;135(3):786–793. doi: 10.1378/chest.08-1516. [DOI] [PubMed] [Google Scholar]
- 10.Divo M., Cote C., de Torres J.P. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi: 10.1164/rccm.201201-0034OC. [DOI] [PubMed] [Google Scholar]
- 11.Weitzenblum E., Hirth C., Ducolone A., Mirhom R., Rasaholinjanahary J., Ehrhart M. Prognostic value of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax. 1981;36(10):752–758. doi: 10.1136/thx.36.10.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurdman J., Condliffe R., Elliot C.A. ASPIRE registry: assessing the spectrum of pulmonary hypertension identified at a referral centre. Eur Respir J. 2012;39(4):945–955. doi: 10.1183/09031936.00078411. [DOI] [PubMed] [Google Scholar]
- 13.Kessler R., Faller M., Weitzenblum E. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(2):219–224. doi: 10.1164/ajrccm.164.2.2006129. [DOI] [PubMed] [Google Scholar]
- 14.Patel A.R., Kowlessar B.S., Donaldson G.C. Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188(9):1091–1099. doi: 10.1164/rccm.201306-1170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curkendall S.M., DeLuise C., Jones J.K. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16(1):63–70. doi: 10.1016/j.annepidem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt S.P., Wells J.M., Dransfield M.T. Cardiovascular disease in COPD: a call for action. Lancet Respir Med. 2014;2(10):783–785. doi: 10.1016/S2213-2600(14)70197-3. [DOI] [PubMed] [Google Scholar]
- 17.Iyer A.S., Wells J.M., Vishin S., Bhatt S.P., Wille K.M., Dransfield M.T. CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest. 2014;145(4):824–832. doi: 10.1378/chest.13-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells J.M., Washko G.R., Han M.K. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurst J.R., Vestbo J., Anzueto A. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 20.George E., Kumamaru K.K., Ghosh N. Computed tomography and echocardiography in patients with acute pulmonary embolism: part 2: prognostic value. J Thorac Imaging. 2014;29(1):W7–W12. doi: 10.1097/RTI.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 21.Zhao D.J., Ma D.Q., He W., Wang J.J., Xu Y., Guan C.S. Cardiovascular parameters to assess the severity of acute pulmonary embolism with computed tomography. Acta Radiol. 2010;51(4):413–419. doi: 10.3109/02841851003649266. [DOI] [PubMed] [Google Scholar]
- 22.Sanal S., Aronow W.S., Ravipati G., Maguire G.P., Belkin R.N., Lehrman S.G. Prediction of moderate or severe pulmonary hypertension by main pulmonary artery diameter and main pulmonary artery diameter/ascending aorta diameter in pulmonary embolism. Cardiol Rev. 2006;14(5):213–214. doi: 10.1097/01.crd.0000181619.87084.8b. [DOI] [PubMed] [Google Scholar]
- 23.Wells J.M., Iyer A.S., Rahaghi F.N. Pulmonary artery enlargement is associated with right ventricular dysfunction and loss of blood volume in small pulmonary vessels in chronic obstructive pulmonary disease. Circ Cardiovasc Imaging. 2015;8(4) doi: 10.1161/CIRCIMAGING.114.002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boerrigter B., Mauritz G.J., Marcus J.T. Progressive dilatation of the main pulmonary artery is a characteristic of pulmonary arterial hypertension and is not related to changes in pressure. Chest. 2010;138(6):1395–1401. doi: 10.1378/chest.10-0363. [DOI] [PubMed] [Google Scholar]
- 25.Kang K.W., Chang H.J., Kim Y.J. Cardiac magnetic resonance imaging-derived pulmonary artery distensibility index correlates with pulmonary artery stiffness and predicts functional capacity in patients with pulmonary arterial hypertension. Circ J. 2011;75(9):2244–2251. doi: 10.1253/circj.cj-10-1310. [DOI] [PubMed] [Google Scholar]
- 26.Stevens G.R., Garcia-Alvarez A., Sahni S., Garcia M.J., Fuster V., Sanz J. RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovasc Imaging. 2012;5(4):378–387. doi: 10.1016/j.jcmg.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Reeves J.T., Linehan J.H., Stenmark K.R. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol. 2005;288(3):L419–L425. doi: 10.1152/ajplung.00162.2004. [DOI] [PubMed] [Google Scholar]
- 28.de Matthaeis A., Greco A., Dagostino M.P. Effects of hypercapnia on peripheral vascular reactivity in elderly patients with acute exacerbation of chronic obstructive pulmonary disease. Clin Interv Aging. 2014;9:871–878. doi: 10.2147/CIA.S57548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarenbach C.F., Senn O., Sievi N.A. Determinants of endothelial function in patients with COPD. Eur Respir J. 2013;42(5):1194–1204. doi: 10.1183/09031936.00144612. [DOI] [PubMed] [Google Scholar]
- 30.Estepar R.S., Kinney G.L., Black-Shinn J.L. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188(2):231–239. doi: 10.1164/rccm.201301-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Challah M., Nadaud S., Philippe M. Circulating and cellular markers of endothelial dysfunction with aging in rats. Am J Physiol. 1997;273(4 pt 2):H1941–1948. doi: 10.1152/ajpheart.1997.273.4.H1941. [DOI] [PubMed] [Google Scholar]
- 32.Warnock M.L., Kunzmann A. Changes with age in muscular pulmonary arteries. Arch Pathol Lab Med. 1977;101(4):175–179. [PubMed] [Google Scholar]
- 33.McAllister D.A., Maclay J.D., Mills N.L. Diagnosis of myocardial infarction following hospitalisation for exacerbation of COPD. Eur Respir J. 2012;39(5):1097–1103. doi: 10.1183/09031936.00124811. [DOI] [PubMed] [Google Scholar]
- 34.Brekke P.H., Omland T., Holmedal S.H., Smith P., Soyseth V. Determinants of cardiac troponin T elevation in COPD exacerbation—a cross-sectional study. BMC Pulm Med. 2009;9:35. doi: 10.1186/1471-2466-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoiseth A.D., Neukamm A., Karlsson B.D., Omland T., Brekke P.H., Soyseth V. Elevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2011;66(9):775–781. doi: 10.1136/thx.2010.153122. [DOI] [PubMed] [Google Scholar]
- 36.Baillard C., Boussarsar M., Fosse J.P. Cardiac troponin I in patients with severe exacerbation of chronic obstructive pulmonary disease. Intensive Care Med. 2003;29(4):584–589. doi: 10.1007/s00134-003-1635-0. [DOI] [PubMed] [Google Scholar]
- 37.Singanayagam A., Schembri S., Chalmers J.D. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(2):81–89. doi: 10.1513/AnnalsATS.201208-043OC. [DOI] [PubMed] [Google Scholar]
- 38.Brekke P.H., Omland T., Holmedal S.H., Smith P., Soyseth V. Troponin T elevation and long-term mortality after chronic obstructive pulmonary disease exacerbation. Eur Respir J. 2008;31(3):563–570. doi: 10.1183/09031936.00015807. [DOI] [PubMed] [Google Scholar]
- 39.Roy A.K., McCullagh B.N., Segurado R. Detection of high-sensitivity troponin in outpatients with stable pulmonary hypertension identifies a subgroup at higher risk of adverse outcomes. J Card Fail. 2014;20(1):31–37. doi: 10.1016/j.cardfail.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Chang C.L., Robinson S.C., Mills G.D. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax. 2011;66(9):764–768. doi: 10.1136/thx.2010.155333. [DOI] [PubMed] [Google Scholar]
- 41.Tsimogianni A.M., Papiris S.A., Stathopoulos G.T., Manali E.D., Roussos C., Kotanidou A. Predictors of outcome after exacerbation of chronic obstructive pulmonary disease. J Gen Intern Med. 2009;24(9):1043–1048. doi: 10.1007/s11606-009-1061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weichenthal S., Dufresne A., Infante-Rivard C., Joseph L. Indoor ultrafine particle exposures and home heating systems: a cross-sectional survey of Canadian homes during the winter months. J Expo Sci Environ Epidemiol. 2007;17(3):288–297. doi: 10.1038/sj.jes.7500534. [DOI] [PubMed] [Google Scholar]
- 43.Almagro P., Soriano J.B., Cabrera F.J. Short- and medium-term prognosis in patients hospitalized for COPD exacerbation: the CODEX index. Chest. 2014;145(5):972–980. doi: 10.1378/chest.13-1328. [DOI] [PubMed] [Google Scholar]
- 44.Stolz D., Christ-Crain M., Morgenthaler N.G. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest. 2007;131(4):1058–1067. doi: 10.1378/chest.06-2336. [DOI] [PubMed] [Google Scholar]
- 45.Stolz D., Christ-Crain M., Bingisser R. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131(1):9–19. doi: 10.1378/chest.06-1500. [DOI] [PubMed] [Google Scholar]
- 46.Stone A.C., Machan J.T., Mazer J., Casserly B., Klinger J.R. Echocardiographic evidence of pulmonary hypertension is associated with increased 1-year mortality in patients admitted with chronic obstructive pulmonary disease. Lung. 2011;189(3):207–212. doi: 10.1007/s00408-011-9293-4. [DOI] [PubMed] [Google Scholar]
- 47.Shin S., King C.S., Brown A.W. Pulmonary artery size as a predictor of pulmonary hypertension and outcomes in patients with chronic obstructive pulmonary disease. Respir Med. 2014;108(11):1626–1632. doi: 10.1016/j.rmed.2014.08.009. [DOI] [PubMed] [Google Scholar]