Abstract
Objective
Younger persons with COPD report worse health-related quality of life (HRQL) than do older individuals. The factors explaining these differences remain unclear. The objective of this article was to explore factors associated with age-related differences in HRQL in COPD.
Methods
Cross-sectional analysis of participants with COPD, any Global Initiative for Chronic Obstructive Lung Disease grade of airflow limitation, and ≥ 50 years old in two cohorts: the Genetic Epidemiology of COPD (COPDGene) study and the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS). We compared St. George’s Respiratory Questionnaire (SGRQ) scores by age group: middle-aged (age, 50-64) vs older (age, 65-80) adults. We used multivariate linear modeling to test associations of age with HRQL, adjusting for demographic and clinical characteristics and comorbidities.
Results
Among 4,097 participants in the COPDGene study (2,170 middle-aged and 1,927 older adults) SGRQ total scores were higher (worse) among middle-aged (mean difference, −4.2 points; 95% CI, −5.7 to −2.6; P < .001) than older adults. Age had a statistically significant interaction with dyspnea (P < .001). Greater dyspnea severity (modified Medical Research Council ≥ 2, compared with 0-1) had a stronger association with SGRQ score among middle-aged (β, 24.6; 95% CI, 23.2-25.9) than older-adult (β, 21.0; 95% CI, 19.6-22.3) participants. In analyses using SGRQ as outcome in 1,522 participants in SPIROMICS (598 middle-aged and 924 older adults), we found similar associations, confirming that for the same severity of dyspnea there is a stronger association with HRQL among younger individuals.
Conclusions
Age-related differences in HRQL may be explained by a higher impact of dyspnea among younger subjects with COPD.
Trial Registry
ClinicalTrials.gov; No.: NCT00608764 and No.: NCT01969344; URL: www.clinicaltrials.gov.
Key Words: aging, COPD, dyspnea, geriatrics, health status, obstructive lung disease, quality of life
Abbreviations: COPDGene, Genetic Epidemiology of COPD; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HRQL, health-related quality of life; mMRC, modified Medical Research Council; SGRQ, St. George’s Respiratory Questionnaire; SPIROMICS, Subpopulations and Intermediate Outcome Measures in COPD Study
The experience of living with a chronic disease, such as COPD, not only affects physiological domains but also impacts and requires significant adjustments in other physical, mental, and emotional domains of life. For this reason, the overall impact of chronic diseases is best described using measures of health status or health-related quality of life (HRQL), which provide information beyond organ-centered physiological descriptors.1, 2 Studies of HRQL in COPD show that, independent of lung function, younger adults tend to report worse scores than do older adults, suggesting a pattern of more negative perception of health among younger subjects.3, 4 Little is known about the reasons for these differences and how they may impact the provision of care in COPD.
In this article, using data from two contemporary, well-characterized COPD cohorts with a wide spectrum of disease severities, we evaluated both the presence of age-related differences in HRQL and whether factors influencing this measure of disease impact differ across age groups. We hypothesized that age-related differences in HRQL may be explained by different levels of tolerance of respiratory symptoms. Specifically, we hypothesized that dyspnea has a stronger impact on HRQL among middle-aged than older adults with COPD.
Materials and Methods
Design and Study Participants
This study is a cross-sectional analysis of data from participants with COPD in two cohorts: the Genetic Epidemiology of COPD (COPDGene) study5 and the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS).6 The protocols for SPIROMICS and the COPDGene study were approved by the institutional review boards of all participating institutions. COPDGene data were used to examine the main hypothesis, and the robustness of the findings was tested in participants in SPIROMICS. For this analysis, we selected participants with COPD, both former and current smokers, who met Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria for COPD, were ≥ 50 years old, and had any GOLD grade of airflow limitation. For comparison, participants were further classified as middle-aged (age, 50-64 years) or older (age, 65-80 years) adults. An expanded version of the methods is available as a supplementary e-Appendix 1.
Data Collection
Demographic data, smoking history, symptoms, exacerbation history, comorbidities, and medical history were collected using self-administered questionnaires.5, 6 Dyspnea was assessed using the modified Medical Research Council (mMRC) scale,7 which was further categorized using the cut points suggested by GOLD recommendations8 (0-1 vs 2-4). Frequent exacerbation was defined as ≥ 2 per year.9, 10 Baseline St. George’s Respiratory Questionnaire (SGRQ) total score was the main outcome measure of this study.11, 12
Statistical Analysis
Respondents were analyzed according to their age at enrollment. We used descriptive statistics for demographic and clinical variables (means and proportions) for each cohort. Multivariate linear models were used to identify the effect of age group (predictor of interest) on SGRQ total score, while controlling for covariates relevant to theoretical models of HRQL (demographics, smoking and exacerbation history, lung function, respiratory symptoms, and comorbidities). We included interaction terms between age and dyspnea score on the basis of our a priori hypothesis; for significant interactions, we calculated the effect of each variable by age group. To test the robustness of our results, we replicated the initial analyses in the COPDGene cohort (discovery cohort) by using the SPIROMICS cohort (validation cohort) for the shared outcome (SGRQ total score). All analyses were performed using Stata version12 software (StataCorp).
Results
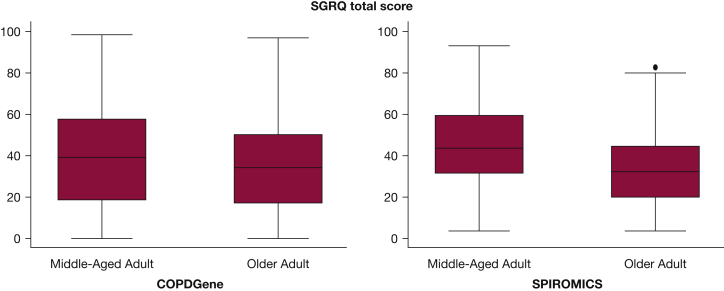
The two cohorts were similar in overall composition (Table 1), including a substantial proportion of women (range, 41.0%-45.0%), current smokers (range, 21.5%-57.3%), and individuals with chronic bronchitis (range, 18.5%-30.6%). Differences between the cohorts included a greater proportion of African Americans, higher cumulative smoking history, and more severe dyspnea in the COPDGene cohort. Almost all comorbidities were more frequent among older adult participants, with the exception of asthma. A similar pattern of demographic and clinical differences was found among participants in SPIROMICS (Table 1). Additionally, among participants in SPIROMICS, anxiety and depression were more common in the middle-aged adults (data on these conditions were not collected by the COPDGene study). Older adults in both cohorts reported better HRQL (lower SGRQ scores) (Fig 1).
Table 1.
General Characteristics of Participants With COPD in the COPDGene Study and SPIROMICS, Ages 50 to 80 Years
| Characteristics | COPDGene Study |
SPIROMICS |
||
|---|---|---|---|---|
| Middle-aged Adults (n = 2,170) |
Older Adults (n = 1,927) |
Middle-aged Adults (n = 598) |
Older Adults (n = 924) |
|
| Sociodemographic characteristics | ||||
| Age, mean (SD), y | 58.3 (4.2) | 71.0 (4.0) | 58.4 (4.2) | 70.8 (4.1) |
| Female, % | 45.0 | 42.8 | 44.3 | 41.0 |
| African American, % | 28.3 | 12.3 | 22.6 | 8.4 |
| Current smoker, % | 57.3 | 21.5 | 46.0 | 22.5 |
| Pack-y smoked, mean (SD) | 49.1 (24.5) | 57.2 (29.9) | 48.2 (20.5) | 56.5 (27.1) |
| BMI ≥ 30, % | 31.2 | 31.3 | 30.9 | 30.4 |
| BMI, mean (SD) | 27.9 (6.2) | 27.8 (5.8) | 27.1 (5.5) | 27.5 (5.1) |
| Time with COPD diagnosis, in y, mean (SD) | 7.6 (7.3) | 9.9 (9.0) | 7.4 (6.3) | 10.4 (8.6) |
| Respiratory status | ||||
| FEV1 % predicted, mean (SD) | 58.2 (22.9) | 54.9 (22.5) | 54.4 (22.7) | 62.3 (22.1) |
| mMRC score, mean (SD) | 1.9 (1.5) | 1.9 (1.4) | 1.5 (1.1) | 1.1 (1.0) |
| mMRC dyspnea score ≥ 2, % | 59.8 | 58.1 | 43.1 | 26.3 |
| GOLD III-IV, % | 37.8 | 44.2 | 44.0 | 31.6 |
| Chronic bronchitis, % | 29.1 | 21.9 | 30.6 | 18.5 |
| Six-minute walking distance, in m, mean (SD) | 385 (126) | 359 (122) | 399 (138) | 389 (123) |
| Exacerbation per y, mean (SD) | 0.7 (1.2) | 0.6 (1.1) | 0.8 (1.2) | 0.4 (0.8) |
| Severe exacerbations prior y, mean (SD) | 0.2 (0.4) | 0.2 (0.3) | 0.4 (0.8) | 0.1 (0.5) |
| Frequent exacerbations (≥ 2/y), % | 17.3 | 13.8 | 19.9 | 9.4 |
| Comorbidities, % | ||||
| Asthma | 24.3 | 19.3 | 30.4 | 20.1 |
| Coronary artery disease | 5.8 | 13.4 | 6.7 | 13.2 |
| Myocardial infarction | 6.9 | 9.5 | 5.7 | 8.7 |
| Peripheral vascular disease | 2.3 | 4.6 | 6.9 | 8.9 |
| Stroke | 3.2 | 4.0 | 4.5 | 4.4 |
| Congestive heart failure | 3.9 | 5.5 | 1.8 | 4.0 |
| Diabetes mellitus | 11.0 | 14.6 | 12.2 | 14.7 |
| Gastroesophageal reflux disease | 28.6 | 31.7 | 29.1 | 31.3 |
| Musculoskeletal disease | 33.1 | 44.9 | 58.0 | 65.7 |
| Anxiety or depression | … | … | 37.8 | 28.1 |
| HRQL, SGRQ score, mean (SD) | ||||
| Total | 38.9 (23.7) | 34.6 (21.3) | 44.7 (19.5) | 32.8 (17.4) |
| Symptoms | 45.8 (26.3) | 38.4 (24.2) | 60.2 (22.8) | 45.8 (22.8) |
| Activity | 52.5 (29.7) | 51.3 (28.7) | 58.1 (24.9) | 46.6 (23.4) |
| Impact | 29.0 (22.9) | 24.0 (19.8) | 31.7 (19.7) | 20.5 (16.3) |
Data are presented as mean (SD) or percentage. COPDGene = Genetic Epidemiology of COPD; GOLD = Global Initiative for Chronic Obstructive Lung Disease; HRQL = health-related quality of life; mMRC = modified Medical Research Council; SGRQ = St. George’s Respiratory Questionnaire; SPIROMICS = Subpopulations and Intermediate Outcome Measures in COPD Study.
Figure 1.
SGRQ total score by age group in each cohort. All differences between middle-aged and older adult groups, in each cohort, are significant at P < .05. The box represents the interquartile range and the central line the median. COPDGene = Genetic Epidemiology of COPD; SGRQ = St. George’s Respiratory Questionnaire; SPIROMICS = Subpopulations and Intermediate Outcome Measures in COPD Study.
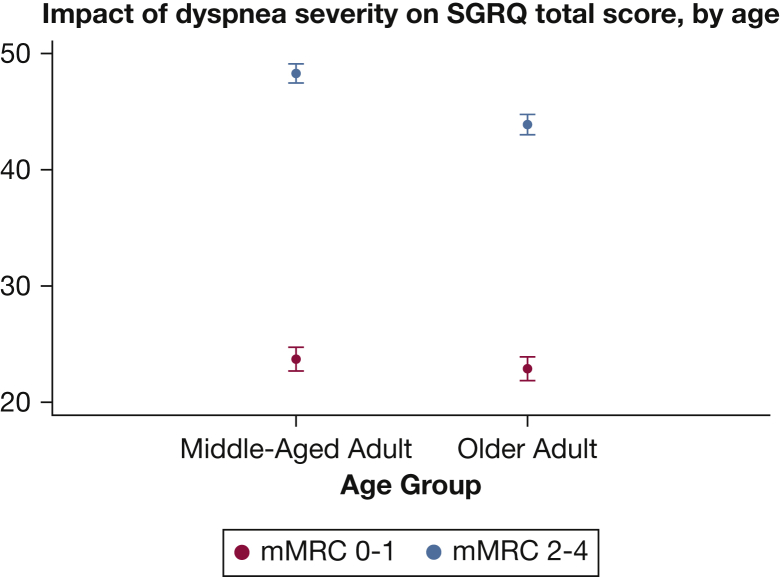
Linear models of HRQL demonstrated a significant association between age and SGRQ. Analyses of data from participants in the COPDGene study (Table 2) showed that being older (vs middle-aged) was associated with a lower SGRQ total score (better HRQL; β, −3.0; 95% CI, −3.9 to −2.0). As predicted by theoretical models,13 demographic (African American race), smoking status, and respiratory factors (moderate to severe dyspnea, exacerbation frequency, and presence of chronic bronchitis symptoms) were also associated with worse HRQL. We found an interaction between age and dyspnea severity (P < .001), which we included in the models. The linear models with age-by-dyspnea interaction also show that the impact of dyspnea was significant for all ages (for the whole population: β, 22.8; 95% CI, 21.8-23.9) but was more pronounced in the middle-aged adults. Thus, severe dyspnea was responsible for 24.6 points in the SGRQ total score in middle-aged adults, vs 21.0 points in older adults (Table 2), with a mean score difference of −4.4 points (95% CI, −5.5 to −3.2), which exceeds the minimum clinically important difference of 4 units on the SGRQ. Visual representation of the effect of dyspnea by age group (Fig 2) confirmed that HRQL differed by dyspnea severity at any age but also showed that the impact (difference in SGRQ total score) was more severe among the middle-aged adults.
Table 2.
Linear Models of SGRQ Total Score Among Participants With COPD in the COPDGene Study, Ages 50 to 80 Years (n = 4,097)
| Variable | β (95% CI) |
|---|---|
| Age,a y | |
| < 65 | Reference |
| ≥ 65 | −3.0 (−3.9 to −2.0) |
| Interaction age × dyspneab | |
| Effect of severe dyspnea if age < 65 y | 24.6 (23.2-25.9) |
| Effect of severe dyspnea if age ≥ 65 y | 21.0 (19.6-22.3) |
| Mean score difference by age when dyspnea is severe | −4.4 (−5.5 to −3.2) |
| Demographic factors | |
| Sex | |
| Male | Reference |
| Female | −0.3 (−1.2 to 0.6) |
| Race | |
| Non-Hispanic white | Reference |
| African American | 3.0 (1.9-4.1) |
| Smoking status | |
| Past smoker | Reference |
| Current smoker | 2.3 (1.3-3.2) |
| BMI | |
| < 30 | Reference |
| ≥ 30 | 2.2 (1.2-3.2) |
| Respiratory factors | |
| mMRC dyspnea scorec | |
| < 2 | Reference |
| ≥ 2 | 22.8 (21.8-23.9) |
| Chronic bronchitis | |
| No | Reference |
| Yes | 9.6 (8.5-10.6) |
| Frequent exacerbations | |
| No (< 2/y) | Reference |
| Yes (≥ 2/y) | 6.8 (5.6-8.0) |
Models additionally were adjusted for FEV1 % predicted, time with a COPD diagnosis, and comorbidities. COPDGene = Genetic Epidemiology of COPD; mMRC = modified Medical Research Council; SGRQ = St. George’s Respiratory Questionnaire.
Effect of age unweighted average over both levels of dyspnea.
P < .001.
Effect of severe dyspnea unweighted average over both groups of age.
Figure 2.
Average effect of different degrees of dyspnea on SGRQ total score by age group among participants in the Genetic Epidemiology of COPD study. mMRC = modified Medical Research Council. See Figure 1 legend for expansion of other abbreviation.
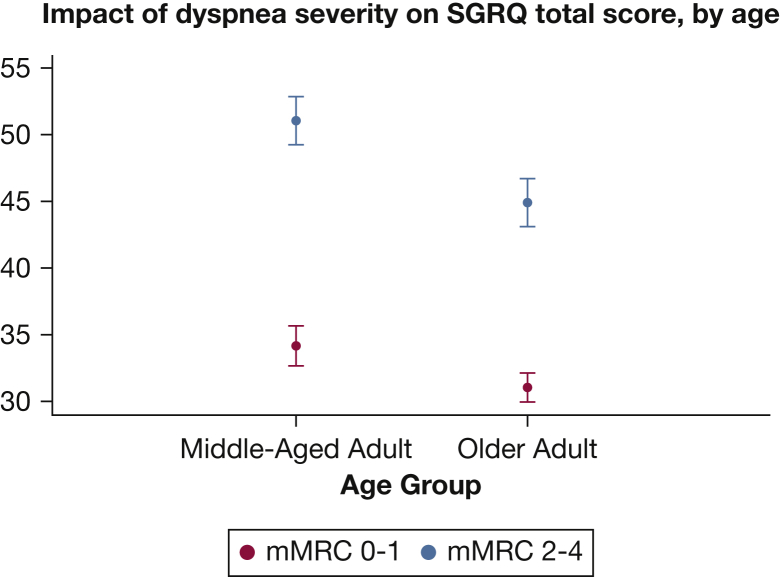
These findings were supported by similar associations in the SPIROMICS cohort. Linear models of HRQL testing the age group relations with SGRQ total score (Table 3) confirmed that being older was independently associated with better HRQL (lower SGRQ total score; difference of −4.2 points; 95% CI, −5.7 to −2.6). There was also an age-by-dyspnea interaction (P = .04), with the impact of dyspnea being higher in the middle-aged (16.9 points in SGRQ total score) than in the older (13.9 points) adults (Table 3, Fig 3).
Table 3.
Linear Models of SGRQ Total Score Among Participants With COPD in SPIROMICS, Ages 50 to 80 Years (n = 1,522)
| Variable | β (95% CI) |
|---|---|
| Age,a y | |
| < 65 | Reference |
| ≥ 65 | −4.2 (−5.7 to −2.6) |
| Interaction age × dyspneab | |
| Effect of severe dyspnea if age < 65 y | 16.9 (14.6-19.2) |
| Effect of severe dyspnea if age ≥ 65 y | 13.9 (11.7-16.0) |
| Mean score difference by age when dyspnea is severe | −6.2 (−8.7 to −3.7) |
| Demographic factors | |
| Sex | |
| Male | Reference |
| Female | 1.5 (0.0-3.0) |
| Race | |
| Non-Hispanic white | Reference |
| African American | 1.7 (−0.5 to 3.8) |
| Smoking status | |
| Past smoker | Reference |
| Current smoker | 3.4 (1.8-5.0) |
| BMI | |
| < 30 | Reference |
| ≥ 30 | 3.5 (1.9-5.0) |
| Respiratory factors | |
| mMRC dyspnea scorec | |
| < 2 | Reference |
| ≥ 2 | 15.3 (13.6-16.9) |
| Chronic bronchitis | |
| No | Reference |
| Yes | 6.1 (4.4-7.9) |
| Frequent exacerbations | |
| No (< 2/y) | Reference |
| Yes (≥ 2/y) | 6.3 (4.2-8.4) |
Models additionally were adjusted for FEV1 % predicted and comorbidities. mMRC = modified Medical Research Council; SGRQ = St. George’s Respiratory Questionnaire; SPIROMICS = Subpopulations and Intermediate Outcome Measures in COPD Study.
Effect of age unweighted average over both levels of dyspnea.
P = .04.
Effect of severe dyspnea unweighted average over both groups of age.
Figure 3.
Average effect of different degrees of dyspnea on SGRQ total score by age group among participants in the Subpopulations and Intermediate Outcome Measures in COPD Study. See Figure 1 and 2 legends for expansion of abbreviations.
We attempted to identify alternate explanations for the differences in HRQL and the disparate impact of dyspnea by age in a post hoc analysis by using interaction analyses and evaluation of potential pathways. For example, several somatic and emotional factors have been associated with dyspnea, including age, respiratory symptoms, exacerbation history, anxiety, and depression.10, 14, 15, 16, 17 We tested the following interactions in both cohorts: age-by-chronic bronchitis, age-by-exacerbation frequency, and dyspnea-by-asthma. In the COPDGene study, we also tested an age-by-time interaction with a COPD diagnosis. For this purpose, we used the answer to the question about participants’ time with COPD diagnosis (categorized as < 10 years vs ≥ 10 years, with those with no prior diagnosis or without a valid response considered as having had COPD diagnosed for < 10 years). We also tested interactions among age, dyspnea, and self-reported physician-diagnosed anxiety or depression among participants in SPIROMICS (these variables were not collected at entry to the COPDGene study). With the exception of an interaction between age and history of frequent exacerbations among participants in SPIROMICS, but not in the COPDGene study, none of these analyses (e-Table 1) indicated alternative explanations.
We tested whether the findings regarding the SGRQ total score were maintained for SGRQ subscales. For all SGRQ subscales in both cohorts, there was a consistent association between age and subscale score. The age-dyspnea interactions showing a greater impact of dyspnea in middle-aged participants were also present for all subscales in the COPDGene study and for SGRQ impact in both cohorts (e-Table 2). Additional analysis restricted to those with a GOLD grade of airflow limitation of 1 to 2 confirmed that the age-related differences in the impact of dyspnea are present, in both cohorts, even with mild to moderate obstruction (e-Table 3). Moreover, these results were maintained when we used the full spectrum of mMRC values (e-Fig 1) and also when an alternative cut point of dyspnea severity (mMRC, 0-2 vs 3-4) was tested in participants in the COPDGene study (e-Table 4, e-Fig 2).
Lastly, we tested the specific contribution of dyspnea to explain the differences in SGRQ total score by age group. We used a strategy based on examining the change in odds ratio of the exposure variable (age group) after the inclusion of other variables or sets of variables. A change in the odds toward the null hypothesis suggests a mediating effect of the variable being tested.18, 19 We examined not just dyspnea, but all the variables tested as interaction terms in the main analyses for their contribution (potential mediation effect) to the HRQL-age association, in adjusted logistic models using as outcome the higher tertile of SGRQ total score (worse HRQL). In participants in the COPDGene study, a base model without dyspnea showed reduced odds of reporting low HRQL as age increases (odds ratio, 0.58), and its value changed in the direction of the null hypothesis after inclusion of dyspnea (with a new odds ratio of 0.62, representing a 9.5% contribution of dyspnea to reducing the differences between age groups). In similar models using SPIROMICS data, dyspnea changed the odds of reporting poor HRQL by 11.3% (base model without dyspnea odds ratio of 0.47 vs 0.53 with inclusion of dyspnea). No other variable tested, in either cohort, had a contribution of this magnitude. A detailed description of the contribution of each variable to changing the older age odds of reporting poor HRQL is presented in e-Table 5.
Discussion
Our investigation of middle-aged vs older Americans with COPD (1) confirms that younger age is independently associated with worse HRQL, (2) provides evidence that the same level of dyspnea has greater impact on HRQL among middle-aged adults, and (3) indicates that this age-related disparity in impact of dyspnea is the main contributor to the differences in HRQL identified between the two age-defined groups. The greater impact of dyspnea in middle-aged subjects was not explained by other factors usually linked to breathlessness (sex, presence of asthma, anxiety, depression), by differences in the distribution of comorbidities or disease phenotypes (chronic bronchitis, frequent exacerbations), or by differences in walk distance. This pattern of differences was robust and was maintained when similar analyses were applied to participants in a large independent cohort.
Our analyses indicated that younger adults with COPD consistently score worse in different HRQL instruments. This finding is in agreement with findings from studies of the general population and persons with other chronic diseases, including fibromyalgia,20 gastroesophageal reflux,21 HIV infection,22 and breast cancer.23 Using data from patients with chronic lung diseases (advanced COPD and interstitial lung diseases), Berry et al.3 recently found that older adults had lower odds of reporting health-related limitations. Our investigation, restricting the population to COPD, confirmed a similar pattern of differences. Importantly, these results were maintained in analyses additionally adjusted for the presence of other factors associated with HRQL, including comorbidities, exacerbation history, and chronic bronchitis symptoms. The apparent dissociation between the level of dyspnea and the patient’s perception of disease severity was reported by investigators from the Confronting COPD International Survey24; we extended their findings with an evaluation of age-related differences in the impact of dyspnea on HRQL.
Although describing the presence of age-related differences in HRQL is interesting, it is even more important to find an explanation for the differences, as they could have implications for the design of rehabilitation programs and the selection of age-specific outcomes for COPD interventions. The reasons for worse perceived HRQL among younger participants are still subject to debate and speculation and have been subject to limited testing. We tested potential explanations of this phenomenon.
One proposed explanation is that older adults feel less impact of dyspnea as a result of tolerance and adaptation after a longer time with the disease.25 To test that possibility, we examined models and interactions including time with a diagnosis of COPD (in clinical terms always unknown) and frequency of chronic bronchitis and exacerbations. To evaluate whether the differences resulted from compensatory attempts by the elderly to avoid triggering dyspnea via lower activity levels,26, 27 we compared distance walked, an imperfect but useful surrogate of physical activity. Additional models including total lung capacity did not change the associations. To test whether less impact of dyspnea results from the competing effect of comorbidities more prevalent in the elderly,28 we included cardiovascular and musculoskeletal diseases in our models. Conversely, to test whether higher impact of dyspnea among middle-aged participants could be due to greater frequency of emotional factors, we also included in the models and tested interactions with anxiety and depression.15, 29 None of those pathways changed the differential impact of dyspnea, and the results were maintained when analyses were restricted to early disease (spirometry grade, 1-2). However, two explanations could not be tested in our participants. First, differences in perception of dyspnea and ventilatory loads, associated in some but not all studies with increased age,30, 31 could explain a lower impact of the same level of dyspnea in the elderly. Second, it is possible that personality changes, changing expectations, and adaptations with aging result in a more optimistic overall view about HRQL.32
Some strengths of our analysis include the use of detailed clinical information from participants in two large cohorts and our analytic approach, testing not just for differences but also for factors contributing to them. In fact, using two different cohorts in separate analyses allowed us to find a similar profile of associations, even when the absolute differences in HRQL scores by age group were not the same. Nevertheless, this is a cross-sectional analysis, a study design with limited capacity to evaluate causal pathways. Other potential limitations include the use of self-reported dyspnea and the inability to develop other physiological evaluations of ventilatory load; however, this is an accepted standard in the evaluation of HRQL, which is a description of a unique individual experience with disease.33
Our categorization of dyspnea severity was selected to match GOLD recommendations on how to judge symptom burden in COPD (mMRC, 0-1 vs 2-4); however, we also used COPDGene data to test the impact of the full spectrum of mMRC values (e-Fig 1), as well as a higher cut point of dyspnea severity (mMRC, 0-2 vs 3-4), and the results were maintained in those new analyses (e-Table 4, e-Fig 2). Our definition of age groups can be seen as a potential limitation, as it is in some way arbitrary, and other authors could group participants in different ways.34, 35 We selected as cut point age ≥ 65 years because it is close to retirement age and could have future public health implications; the lower boundary of 50 years of age was selected to facilitate clinical interpretation.
Socioeconomic disparities are also a factor associated with HRQL in chronic lung disease, particularly among minorities.36 In agreement with that possibility, we tested the impact of race and showed that being of African American race was associated with worse HRQL. It is still possible that there is an additional impact of income and other markers of socioeconomic disadvantages not tested in our models. It is also possible that better HRQL among older participants is a survivor effect, which cannot be tested in a cross-sectional study. We attempted to control in our models for time with diagnosis, an imperfect surrogate of disease duration. Finally, a response shift is also possible but needs to be tested in further longitudinal analyses.37 Discovering factors related to HRQL is of greater than theoretical interest because it can help to identify new targets for clinical interventions, a crucial goal in chronic and progressive conditions such as COPD. Our results, that even with the described limitations we consider robust, suggest that strategies to counteract dyspnea, relevant at any age, could be especially important for younger persons with COPD.
In conclusion, we demonstrated that older subjects with COPD report better HRQL than do their younger counterparts and that this age-related difference involves less impact of dyspnea with increased age. Although dyspnea is important at any age, for the same level of dyspnea, the decrement in HRQL is more profound among younger persons with COPD. Strategies to improve HRQL should consider the diverse impact of different factors across age groups.
Acknowledgments
Author contributions: C. H. M. had full access to all of the data in the study and takes responsibility for the integrity of the data, and the accuracy of the data analysis, and is the guarantor of this study. C. H. M. contributed to conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. A. A. D. contributed to conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. A. D. P. contributed to conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. S. I. R. contributed to designing the study, collecting the data, conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. R. E. K. contributed to designing the study, collecting the data, conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. N. N. H. contributed to designing the study, collecting the data, conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. D. C. contributed to designing the study, collecting the data, conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. K. E. H. contributed to conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. K. F. H. contributed to conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. J. L. C. contributed to designing the study, collecting the data, conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. F. J. M. contributed to designing the study, collecting the data, conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. N. A. H. contributed to designing the study, collecting the data, conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. E. A. R. contributed to designing the study, collecting the data, conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. R. P. contributed to designing the study, collecting the data, conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. C. T. C. contributed to conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript. M. K. H. contributed to designing the study, collecting the data, conceiving and writing the manuscript, data analysis and clinical interpretation of the data, and approving the final draft of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. I. R. has served as a consultant, participated in advisory boards, and received an honorarium for speaking or grant support from American Board of Internal Medicine, Advantage Healthcare, Almirall, American Thoracic Society, AstraZeneca, Baxter, Boehringer Ingelheim, Chiesi, ClearView Healthcare, Cleveland Clinic, Complete Medical Group, CSL, Daiichi Sankyo, Decision Resources Group, Forest, Gerson Lehrman Group, Grifols, groupH, Guidepoint Global, Haymarket, Huron Consulting Group, inThought, Johnson and Johnson, Methodist Health System Dallas, NCI Consulting, Novartis, Pearl, Penn Technology, Pfizer, PlanningShop, PSL FirstWord, Qessential Medical Market Research, Takeda, Theron, and WebMD. J. L. C. has received grant funding from MedImmune. F. J. M. has served as a consultant, participated in advisory boards, and received an honorarium for speaking or grant support from Nycomed/Takeda, Amgen, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Ikaria, Janssen, Johnson and Johnson, Roche, Veracyte, Bayer, Forest, Gilead Sciences, Promedior, and Novartis. N.A.H. has participated as a member of advisory boards or as a consultant for Boehringer Ingelheim, GlaxoSmithKline, Sunovion, Mylan, AstraZeneca, and Novartis and has received research funding (to her institution) from GlaxoSmithKline, Boehringer Ingelheim, Mylan, Sunovian, Chiesi, Genentech, Novartis, and Pfizer. M. K. H. participated in advisory boards for Boehringer Ingelheim and GlaxoSmithKline; participated in speaker’s bureaus for Boehringer Ingelheim, GlaxoSmithKline, Grifols, and Forest; has consulted for United BioSource Corporation; and has received royalties from UpToDate. None declared (C. H. M., A. A. D., A. D. P., R. E. K., N. N. H., D. C., K. E. H., K. F. H., E. A. R., R. P., and C. T. C.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: This work was performed at the University of Michigan Health System.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Cigolle and Han contributed equally to this manuscript.
FUNDING/SUPPORT: Dr C. H. Martinez was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [NHLBI; Grant 3R01HL122438-02S1]. Dr Diaz was supported by the NHLBI [Grant K01HL118714] and the Brigham and Women’s Hospital Minority Faculty Career Development Award. Dr Hoth was supported by the NHLBI [Grant K23 HL095658]. Dr Curtis was supported by Clinical Science Research and Development, Department of Veterans Affairs [Merit Review Award I01 CX000911]. Dr Cigolle was supported by the National Institute on Aging [Grant 5K08AG031837] and by the Claude D. Pepper Older Americans Independence Center at the University of Michigan. Dr Han was supported by the NHLBI [Grant R01HL122438-01]. SPIROMICS was funded by the NHLBI [HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN2682009000019C, and HHSN268200900020C]. COPDGene was supported by the NHLBI [Grants R01HL089897 and R01HL089856].
Supplementary Data
References
- 1.Curtis J.R., Deyo R.A., Hudson L.D. Pulmonary rehabilitation in chronic respiratory insufficiency. 7. Health-related quality of life among patients with chronic obstructive pulmonary disease. Thorax. 1994;49(2):162–170. doi: 10.1136/thx.49.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srivastava K., Thakur D., Sharma S., Punekar Y.S. Systematic review of humanistic and economic burden of symptomatic chronic obstructive pulmonary disease. Pharmacoeconomics. 2015;33(5):467–488. doi: 10.1007/s40273-015-0252-4. [DOI] [PubMed] [Google Scholar]
- 3.Berry C.E., Han M.K., Thompson B. Older adults with chronic lung disease report less limitation compared with younger adults with similar lung function impairment. Ann Am Thoracic Soc. 2015;12(1):21–26. doi: 10.1513/AnnalsATS.201407-312OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holm K.E., Plaufcan M.R., Ford D.W. The impact of age on outcomes in chronic obstructive pulmonary disease differs by relationship status. J Behav Med. 2014;37(4):654–663. doi: 10.1007/s10865-013-9516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regan E.A., Hokanson J.E., Murphy J.R. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couper D., LaVange L.M., Han M. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69(5):491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bestall J.C., Paul E.A., Garrod R., Garnham R., Jones P.W., Wedzicha J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vestbo J., Hurd S.S., Agusti A.G. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 9.Wedzicha J.A., Brill S.E., Allinson J.P., Donaldson G.C. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181. doi: 10.1186/1741-7015-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurst J.R., Vestbo J., Anzueto A., Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 11.Jones P.W., Quirk F.H., Baveystock C.M. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- 12.Jones P.W. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 13.Costa D.S., King M.T. Conceptual, classification or causal: models of health status and health-related quality of life. Expert Rev Pharmacoecon Outcomes Res. 2013;13(5):631–640. doi: 10.1586/14737167.2013.838024. [DOI] [PubMed] [Google Scholar]
- 14.Diaz A.A., Morales A., Diaz J.C. CT and physiologic determinants of dyspnea and exercise capacity during the six-minute walk test in mild COPD. Respir Med. 2013;107(4):570–579. doi: 10.1016/j.rmed.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Borges-Santos E., Wada J.T., da Silva C.M. Anxiety and depression are related to dyspnea and clinical control but not with thoracoabdominal mechanics in patients with COPD. Respir Physiol Neurobiol. 2015;210:1–6. doi: 10.1016/j.resp.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Kim V., Han M.K., Vance G.B. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140(3):626–633. doi: 10.1378/chest.10-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardin M., Silverman E.K., Barr R.G. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee R., Brancati F.L., Shafi T. Non-traditional risk factors are important contributors to the racial disparity in diabetes risk: the atherosclerosis risk in communities study. J Gen Intern Med. 2014;29(2):290–297. doi: 10.1007/s11606-013-2569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birkmeyer J.D., Stukel T.A., Siewers A.E., Goodney P.P., Wennberg D.E., Lucas F.L. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 20.Jiao J., Vincent A., Cha S.S., Luedtke C.A., Oh T.H. Relation of age with symptom severity and quality of life in patients with fibromyalgia. Mayo Clin Proc. 2014;89(2):199–206. doi: 10.1016/j.mayocp.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.W., Chang C.M., Chang C.S., Kao A.W., Chou M.C. Comparison of presentation and impact on quality of life of gastroesophageal reflux disease between young and old adults in a Chinese population. World J Gastroenterol. 2011;17(41):4614–4618. doi: 10.3748/wjg.v17.i41.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skevington S.M. Is quality of life poorer for older adults with HIV/AIDS? International evidence using the WHOQOL-HIV. AIDS Care. 2012;24(10):1219–1225. doi: 10.1080/09540121.2012.661838. [DOI] [PubMed] [Google Scholar]
- 23.Champion V.L., Wagner L.I., Monahan P.O. Comparison of younger and older breast cancer survivors and age-matched controls on specific and overall quality of life domains. Cancer. 2014;120(15):2237–2246. doi: 10.1002/cncr.28737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rennard S., Decramer M., Calverley P.M. Impact of COPD in North America and Europe in 2000: subjects’ perspective of Confronting COPD International Survey. Eur Respir J. 2002;20(4):799–805. doi: 10.1183/09031936.02.03242002. [DOI] [PubMed] [Google Scholar]
- 25.Fischer M., Scharloo M., Abbink J. The dynamics of illness perceptions: testing assumptions of Leventhal’s common-sense model in a pulmonary rehabilitation setting. Br J Health Psychol. 2010;15(Pt 4):887–903. doi: 10.1348/135910710X492693. [DOI] [PubMed] [Google Scholar]
- 26.Spruit M.A., Watkins M.L., Edwards L.D. Determinants of poor 6-min walking distance in patients with COPD: the ECLIPSE cohort. Respir Med. 2010;104(6):849–857. doi: 10.1016/j.rmed.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Parada A., Klaassen J., Lisboa C., Saldias F., Mendoza L., Diaz O. Reduction of physical activity in patients with chronic obstructive pulmonary disease. Rev Med Chile. 2011;139(12):1562–1572. [in Spanish] [PubMed] [Google Scholar]
- 28.Frei A., Muggensturm P., Putcha N. Five comorbidities reflected the health status in patients with chronic obstructive pulmonary disease: the newly developed COMCOLD index. J Clin Epidemiol. 2014;67(8):904–911. doi: 10.1016/j.jclinepi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Hanania N.A., Mullerova H., Locantore N.W. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183(5):604–611. doi: 10.1164/rccm.201003-0472OC. [DOI] [PubMed] [Google Scholar]
- 30.Tack M., Altose M.D., Cherniack N.S. Effect of aging on the perception of resistive ventilatory loads. Am Rev Respir Dis. 1982;126(3):463–467. doi: 10.1164/arrd.1982.126.3.463. [DOI] [PubMed] [Google Scholar]
- 31.Ward M.E., Stubbing D.G. Effect of chronic lung disease on the perception of added inspiratory loads. Am Rev Respir Dis. 1985;132(3):652–656. doi: 10.1164/arrd.1985.132.3.652. [DOI] [PubMed] [Google Scholar]
- 32.Blachnio A., Bulinski L. Prejudices and elderly patients’ personality: the problem of quality of care and quality of life in geriatric medicine. Med Sci Monit. 2013;19:674–680. doi: 10.12659/MSM.889501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones P.W. Health status and the spiral of decline. COPD. 2009;6(1):59–63. doi: 10.1080/15412550802587943. [DOI] [PubMed] [Google Scholar]
- 34.Zizza C.A., Ellison K.J., Wernette C.M. Total water intakes of community-living middle-old and oldest-old adults. J Gerontol A Biol Sci Med Sci. 2009;64(4):481–486. doi: 10.1093/gerona/gln045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forman D.E., Berman A.D., McCabe C.H., Baim D.S., Wei J.Y. PTCA in the elderly: the “young-old” versus the “old-old”. J Am Geriatr Soc. 1992;40(1):19–22. doi: 10.1111/j.1532-5415.1992.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 36.Martinez C.H., Mannino D.M., Curtis J.L., Han M.K., Diaz A.A. Socioeconomic characteristics are major contributors to ethnic differences in health status in obstructive lung disease: an analysis of the National Health and Nutrition Examination Survey 2007-2010. Chest. 2015;148(1):151–158. doi: 10.1378/chest.14-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz C.E., Andresen E.M., Nosek M.A., Krahn G.L. RRTC Expert Panel on Health Status Measurement. Response shift theory: important implications for measuring quality of life in people with disability. Arch Phys Med Rehabil. 2007;88(4):529–536. doi: 10.1016/j.apmr.2006.12.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.