Abstract
Background
OSA associates with insulin resistance (IR), hyperglycemia, and dyslipidemia consistently in adults, but inconsistently in children. We set out to quantify the impact of OSA treatment upon obesity and metabolic outcomes and thus assess causality.
Methods
Sixty-nine children with OSA; mean age, 5.9 years (range, 3-12.6); 55% boys; and 68% nonobese (NOB) underwent baseline overnight polysomnography, anthropometric and metabolic measurements, adenotonsillectomy (T&A), and follow-up testing a mean 7.9 months (range, 2-20) later.
Results
Fifty-three children (77% of study cohort; 91% of obese children) had residual OSA (apnea-hypopnea index > 1 event/h) post-T&A. Fasting plasma insulin (FPI, 14.4 ± 9.4 → 12.6 ± 9.7 μIU/mL, P = .008), homeostasis model assessment-IR (3.05 ± 2.13 → 2.62 ± 2.22, P = .005), and high-density lipoprotein (HDL) (51.0 ± 12.9 → 56.5 ± 14.4 mg/dL, P = .007) improved despite increased BMI z score (1.43 ± 0.78 → 1.52 ± 0.62, P = .001); changes did not differ significantly between sexes or NOB and obese participants; however, post-T&A BMI z score rather than apnea-hypopnea index was the main predictor of levels of follow-up FPI, HDL, and other metabolic parameters. Higher baseline FPI and BMI-z predicted likelihood of residual OSA; conversely, on regression analysis, follow-up IR, HDL, and triglycerides were predicted by BMI z score, not residual OSA.
Conclusions
T&A improved IR and HDL, and residual OSA is predicted by baseline FPI and BMI z score, indicating a causal relationship; however, following T&A, residual metabolic dysfunction related to underlying adiposity rather than remaining sleep-disordered breathing. Finally, T&A cured OSA in < 25% of all children and only 10% of obese children; post-T&A polysomnography is indicated to assess which children still require treatment.
Key Words: adenotonsillectomy, children, insulin resistance, lipids, OSA
Abbreviations: AHI, apnea-hypopnea index; CHAT, Randomized Controlled Study of Adenotonsillectomy for Childhood Sleep Apnea; CV, coefficient of variation; FPG, fasting plasma glucose; FPI, fasting plasma insulin; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; IR, insulin resistance; LDL, low-density lipoprotein; MetSyn, the metabolic syndrome; NOB, nonobese; PSG, polysomnography; T&A, adenotonsillectomy; T2DM, type 2 diabetes mellitus; TChol, total cholesterol; TG, triglyceride; TST, total sleep time
The prevalence of pediatric obesity1 and concomitant comorbidities, including type 2 diabetes mellitus (T2DM),2 dyslipidemia, and the metabolic syndrome (MetSyn),3 has increased in recent decades. Another common comorbidity of obesity is OSA, characterized by repetitive upper airway collapse during sleep, episodic oxyhemoglobin desaturations, and sleep fragmentation.4 OSA in adults is associated with increased risk of insulin resistance (IR),5, 6 MetSyn,7, 8 and T2DM6, 7 and poorer glycemic control in adults with existing T2DM.9, 10, 11 Similarly, OSA prevalence is greater among adults with T2DM12 vs community-based cohorts.13 Treating OSA in adults using CPAP improves IR, although the impact on glycemia is more unpredictable.14 In children, the association of OSA with IR and MetSyn has been inconsistent: some studies have found that OSA increases risk of IR15 and dyslipidemia,16 whereas others have found that IR and MetSyn are primarily determined by obesity.17, 18 Most studies have examined associations rather than causal relationships. However, the few interventional studies of metabolic outcome in children with OSA after adenotonsillectomy (T&A, the gold standard pediatric OSA treatment) have had similarly contradictory results.19, 20, 21, 22 Thus, we set out to prospectively study a cohort of children with OSA and examine the contribution of OSA to IR, glucose homeostasis, and dyslipidemia independently of body habitus by assessing changes in metabolic and anthropometric parameters following T&A.
Materials and Methods
This was an observational study of otherwise healthy, habitually snoring children, and was approved by the University of Chicago Institutional Review Board Committee (protocol #09-115-B-AM029). Habitually snoring children ages 3 to 16 years who were found to have clinically significant OSA after baseline polysomnography were prospectively recruited. Children with chronic medical conditions (eg, genetic syndromes, craniofacial anomalies, chronic illnesses excepting well-controlled asthma on no controller medications) were excluded. Children receiving medications potentially affecting sleep, lipids, insulin, or glucose homeostasis (eg, systemic glucocorticoids within a month of the study) were also excluded. Informed consent and age-appropriate assent were obtained. In our center, all children undergoing evaluation for suspected OSA undergo anthropometric measurements (height and weight), overnight polysomnography (PSG), and fasting blood draw for insulin, glucose, and lipoprotein profile at baseline. Children who were found to have OSA (apnea-hypopnea index [AHI] > 1.0 event/h) with adenotonsillar hypertrophy requiring T&A were approached for enrollment. Those who consented were recruited into the study, underwent clinical T&A, and returned for follow-up research PSG and anthropometric and metabolic evaluations.
Height was assessed via a wall-mounted stadiometer. Weight was assessed by electronic scale. BMI was calculated as BMI = kg/m2. Age- and sex-adjusted standard scores (z scores) for BMI were calculated using Centers for Disease Control and Prevention 2000 growth chart reference data.23 Participants with a BMI z score ≥ 1.64, corresponding to BMI ≥ 95th percentile for age and sex, were considered to be obese24; other children were classified as nonobese (NOB).
All participants underwent overnight PSG, conducted and scored as previously reported.25 Sleep architecture, respiratory events, and arousals were scored using standard pediatric criteria.26 Apneas were defined as ≥ 90% airflow decrement lasting at least two breaths, and hypopneas were defined as > 50% decrement in nasal airflow accompanied by a 3% desaturation and/or an EEG arousal. Arousals were defined per the revised American Academy of Sleep Medicine guidelines26; arousal indices (arousals/h of sleep) were calculated. Children with AHI (number of apneas and hypopneas per hour of sleep) < 1.0, ≥ 1, < 5, and ≥ 5 events/hour total sleep time (TST) were deemed to have normal breathing during sleep, mild OSA, and moderate-severe OSA, respectively.18 Only children with moderate-severe OSA were enrolled in this interventional study.
Following overnight PSG, participants underwent a fasting blood draw. Fasting plasma insulin levels were measured via solid-phase, two-site chemiluminescent immunometric assay (Immulite 2000; Siemens Healthcare Diagnostics), range 2 to 300 μIU/mL, intra-assay coefficient of variation (CV) ≤ 8.0%. Fasting plasma glucose (FPG) was measured via ultraviolet enzymatic method with hexokinase (Roche Cobas 8000 702 platform), range 10 to 1,200 mg/dL, CV ≤ 5%. High-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol (TChol), and triglycerides (TGs) were measured via homogenous enzymatic colorimetric assays (Roche Cobas 8000 502 platform). Analytical measuring range and assay CVs follow.
-
•
TChol: range, 3.86 to 800 mg/dL; CV ≤ 1.6%.
-
•
HDL cholesterol: range, 3 to 120 mg/dL; CV ≤ 1.5%.
-
•
LDL cholesterol: range, 3.86 to 548 mg/dL; range ≤ 2.7%.
-
•
TG: range, 8.85–885 mg/dL; CV ≤ 2 .0%.
TChol/HDL, LDL/HDL, and TG/HDL ratios were calculated, with higher values denoting greater cardiovascular risk.27
Calculated insulin sensitivity parameters included the following:
-
•
Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as: fasting plasma index [FPI] (μIU/mL) × FPG (mg/dL)/405.28
-
•
The McAuley index, a measure incorporating a weighted combination of FPI and TGs,29 was calculated as: exp[2.63 – 0.28 ln(FPI in μIU/mL) – 0.31 ln(TG [mmol/L]). Lower values indicate greater IR.
Statistical analyses were performed using IBM SPSS Statistics v22.0 (IBM SPSS). Normality of distribution of continuous variables was assessed; skewed variables were natural log-transformed. Paired t tests or related samples Wilcoxon signed-rank tests were used to assess changes pre- and post-T&A in normally distributed and skewed variables, respectively. Because susceptibility to OSA-related metabolic sequelae could vary by obesity status and/or sex, independent sample t tests/Mann Whitney U test were used to assess differences among NOB and obese participants and among boys and girls of sleep and metabolic variables pre- and post-T&A and of the pre-post delta values; χ2 analysis was used to compare categorical characteristics among residual OSA groups. Correlation coefficients were used to examine associations between residual AHI and pre-T&A metabolic measures of interest, including anthropometric and metabolic variables, to assess which would associate with residual OSA. Stepwise linear regression models were constructed to assess relationships between post-T&A AHI and metabolic measures. P value < .05 was used as the cutoff for statistical significance. Finally, subjects were divided into three groups of residual OSA severity (none, mild, and moderate-severe OSA), and χ2 (categorical variables) and ANOVA/Kruskal-Wallis tests (continuous variables) were performed to ascertain how many in each group had improvement, no change, or worsening of the metabolic outcomes of interest.
Results
One hundred fourteen subjects with moderate-severe baseline OSA consented to participate in the study; of these, 69 children completed all pre- and post-T&A testing. There were no significant sex, race, ethnicity, age, BMI z score, metabolic, or PSG differences between those who completed all study visits and those who did not. Thirty-eight (55%) of study participants were boys, 50 (72%) were NOB at baseline, and 47 (68%) were NOB at follow-up (net three participants became obese in the interim). Fifty (72%) were Caucasian, 16 (23%) were African American, and seven (10%) were Hispanic. Baseline age, BMI z score, and baseline PSG measures are shown in Table 1, column 1. Participants returned for follow-up PSG and evaluation a mean of 7.9 ± 3.1 months (range, 2.2-12.2 months) post-T&A. Age, ethnicity, and baseline PSG variables did not differ among boys and girls or between NOB vs obese participants (data not shown); baseline metabolic variables differed as anticipated between NOB and obese participants, with higher glucose and insulin levels, higher HOMA-IR and lower McAuley index (signifying greater IR), trend toward higher TGs, higher total and LDL cholesterol, and lower HDL cholesterol in participants with obesity (e-Table 1).
Table 1.
Age and Polysomnographic Measures Pre- and Post-T&A
| Variable | Pre-T&A Value | Post-T&A Value | P Value | Mean Delta (Post-Pre T&A) |
|---|---|---|---|---|
| No. | 69 | 69 | ||
| Age, y | 6.33 ± 2.04 (3.8-12.2) | 6.99 ± 2.05 (4.2-12.6) | < .0005 | 0.66 ± 0.26 (0.18-1.68) |
| AHI, events/h TST | 22.2 ± 15.6 (3.6-70.3)a | 5.9 ± 6.5 (0.2-34.6)a | < .0005a | −16.3 ± 16.4ǂ (−69.5 to 20.9) |
| Lowest SpO2, % | 82.2 ± 7.4 (50-93)a | 88.5 ± 6.9 (72-97) | < .0005a | 6.3 ± 10.0 (−15.0 to 43) |
| Spontaneous ArI | 9.9 ± 3.4 (1.6-24.4)a | 12.1 ± 4.9 (1.8-36.4)a | < .0005a | 2.2 ± 5.2 (−9.3 to 23.9)a |
| Respiratory ArI | 5.5 ± 4.3 (0.1-26.5)a | 1.4 ± 2.0 (0-13.0)a | < .0005a | −4.1 ± 4.7 (−25.9 to 7.3)a |
| Total ArI | 16.4 ± 5.7 (6.9-39.1)a | 12.0 ± 4.6 (2.8-37.4)a | < .0005a | −4.3 ± 7.0 (−28.2 to 12.9)a |
| Peak ETCO2 | 49.6 ± 5.5 (33.5-65.3) | 47.3 ± 4.9 (36.5-62.3) | .009 | −2.3 ± 7.0ǂ (−14.8 to 26.4) |
Data represent mean ± SD (range).
AHI = apnea-hypopnea index; ArI = arousal index; ETCO2 = end-tidal CO2; SpO2 = oxyhemoglobin saturation; T&A = adenotonsillectomy; TST = total sleep time.
Non-normal distribution by nonparametric testing.
Changes in OSA Measures Following T&A
Several PSG measures improved significantly following T&A (polysomnographic and age changes depicted in Table 1). Post-T&A PSG variables and pre-post changes in PSG variables were similar among boys and girls and NOB vs obese children (data not shown). Of note, although OSA improved significantly with surgery, the mean residual AHI post-T&A was still consistent with moderate OSA. Following T&A, 16 children (23% of study cohort; 14 NOB, two obese) had complete OSA resolution, whereas 53 (77% of study cohort) had residual OSA. Of these, 23 children (15 NOB, eight obese) had mild residual OSA and 30 (18 NOB, 12 obese) had residual moderate-severe OSA (AHI < 1, 1-5, and >5/h TST), although even in the moderate-severe OSA group (residual AHI > 5 events/h TST), mean AHI decline from 22.9 ± 15.6 to 5.9 ± 6.5/h TST. Proportions of NOB and obese participants did not differ among residual OSA subgroups (χ2 P = .21), nor between those with and without residual OSA (P = .12). Proportions of boys vs girls in the residual AHI groups did not differ (data not shown).
Body Habitus and Metabolic Alterations Following T&A
Changes in metabolic parameters after T&A are shown in Table 2. BMI z score increased significantly, whereas FPI and HOMA-IR were significantly lower and McAuley index trended toward being higher, indicating improved IR. FPG did not change significantly. The degree of change in metabolic parameters did not differ between boys and girls or between NOB and obese study participants (data not shown). HDL was significantly higher and TChol/HDL and LDL/HDL ratios significantly lower, indicating more favorable lipoprotein profiles.
Table 2.
Changes in BMI z Score and Metabolic Variables Following T&A
| Variable | Pre T&A Value | Post-T&A Value | P Value | Mean Delta (Post-Pre) |
|---|---|---|---|---|
| No. | 69 | 69 | ||
| BMI z score | 1.43 ± 0.78 (−1.29 to 4.45) |
1.52 ± 0.62ǂ (−0.74 to 4.17) |
.001a | 0.09 ± 0.88 |
| Fasting plasma glucose, mg/dL | 84.6 ± 9.0 (67-112) |
82.3 ± 9.3 (57-103) |
.21 | −2.3 ± 11.9 (−32 to 30) |
| Fasting plasma insulin, μIU/mL |
14.4 ± 9.4 (2.0-49.0)a |
12.6 ± 9.7 (2.0-45.0) |
.008a | −1.8 ± 10.5a (−27 to 32) |
| HOMA-IR |
3.05 ± 2.13 (0.39-11.25)a |
2.62 ± 2.22 (0.28-11.11)a |
.005a | −0.43 ± 2.33a (−5.41 to 8.03) |
| McAuley Index | 7.56 ± 1.73 (3.94-12.46) |
8.06 ± 1.98 (4.36-13.17) |
.053 | 0.50 ± 2.05 (−4.26 to 7.22) |
| Total cholesterol (mg/dL) | 157.6 ± 31.2 (105-249) |
155.5 ± 28.7a (118-269) |
.60a | −2.1 ± 33.3 (−75 to 65) |
| Triglycerides, mg/dL | 75.8 ± 23.4a (43-207) |
77.6 ± 22.8a (45-179) |
.54a | 1.8 ± 30.0a (−121 to 103) |
| HDL cholesterol, mg/dL |
51.0 ± 12.9 (25-87) |
56.5 ± 14.4 (25-91) |
.007 | 5.5 ± 16.1 (−29 to 54) |
| LDL cholesterol, mg/dL | 108.8 ± 34.1 (39.9-216.3) |
103.5 ± 31.1 (47.4-205.0) |
.24 | −5.2 ± 35.7 (−107.9-94) |
| TChol/HDL ratio |
3.31 ± 1.02 (1.57-5.79) |
2.92 ± 0.96 (1.48-5.67) |
.001 | −0.38 ± 0.95 (−3.32 to 1.10) |
| LDL/HDL ratio |
2.30 ± 0.99 (0.60-5.03) |
1.98 ± 0.91a (0.59-5.69) |
.012a | −0.31 ± 1.01 (−3.45 to 2.69) |
| TG/HDL ratio | 1.60 ± 0.78a (0.72-5.60) |
1.51 ± 0.78a (0.56-4.97) |
.093a | −0.09 ± 0.88ǂ (−3.21 to 3.16) |
Data represent mean ± SD (range). Numbers in bold are significantly different between the pre- and post-T&A conditions.
HDL = high-density lipoprotein; HOMA-IR = homeostasis model assessment of insulin resistance; LDL = low-density lipoprotein; TChol = total cholesterol; TG = triglycerides. See Table 1 legend for expansion of other abbreviations.
Non-normal distribution assessed by nonparametric test.
Predictors of Residual OSA
We examined associations between baseline age, BMI z score and metabolic parameters and post-T&A AHI to assess whether any baseline variable predicted posttreatment OSA (Table 3). FPI and HOMA-IR were strongly positively associated with post-T&A AHI; trends were seen toward associations between follow-up AHI and BMI z score and McAuley index. Correlation analyses between post-T&A AHI and anthropometric variables showed a trend toward association of post-T&A AHI with BMI z score (r = 0.202, P = .096), but no relationships with age, insulin sensitivity, glucose levels, or lipid levels (data not shown). On stepwise linear regression models, FPI significantly predicted residual AHI, whereas BMI z score did not: model adjusted R2 = 0.086, FPI β-coefficient = 0.315 (P = .008), and BMI z score β-coefficient = 0.17 (P = .19).
Table 3.
Associations Between Baseline Parameters and Residual AHI
| Variable | AHI |
|---|---|
| Age, y | 0.093 |
| BMI z score | 0.227 (P = .061) |
| Glucose, mg/dL | 0.024 |
| Insulin, μIU/mL | 0.263 (P = .029) |
| HOMA-IR | 0.258 (P = .033) |
| McAuley Index | −0.204 (P = .092) |
| TG, mg/dL | −0.111 |
| TChol, mg/dL | −0.023 |
| HDL, mg/dL | −0.159 |
| LDL, mg/dL | 0.029 |
| TChol:HDL ratio | 0.142 |
| LDL:HDL ratio | 0.144 |
| TG:HDL ratio | −0.023 |
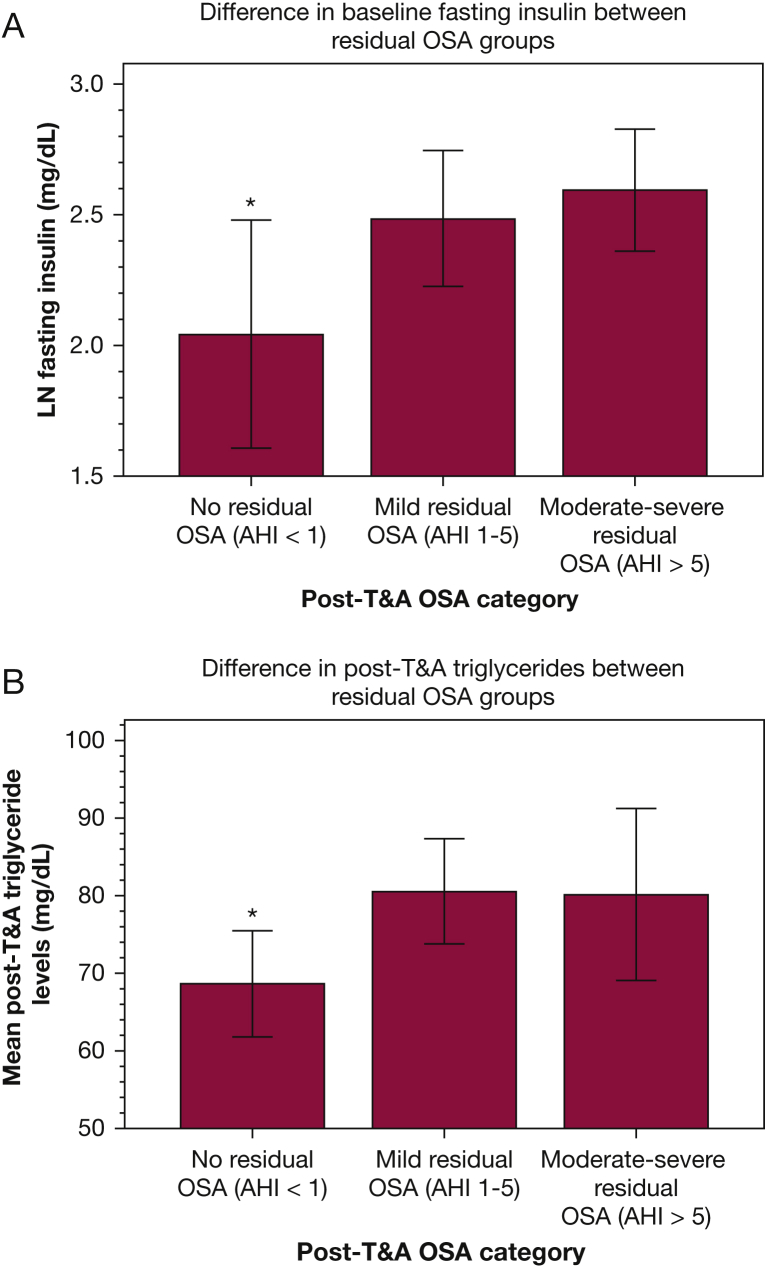
We also examined demographic, metabolic, and sleep parameters at baseline and at follow-up across the groups of subjects who had no OSA, mild OSA, and moderate-severe OSA on follow-up PSG (Table 4, Table 5) to explore whether subtle baseline differences or postoperative metabolic markers might predict likelihood of residual OSA. We found a trend toward lower BMI z score pre-T&A in participants who exhibited complete OSA resolution (Kruskal-Wallis P = .053). Of baseline metabolic variables, only FPI differed, with lowest values in those who would go on to have OSA resolution and highest in those who would go on to have the most severe persistent OSA (Table 4, Fig 1A); a parallel trend was seen with McAuley index values, with highest values (denoting greatest insulin sensitivity) in those who would have no OSA on follow-up. No baseline PSG variables differed significantly among the post-T&A ad hoc groups. Post-T&A PSG variables differed significantly as anticipated: lowest AHI (the group defining criterion) and respiratory arousal index levels and highest oxyhemoglobin saturation nadir levels were seen in those without OSA. Post-T&A, TGs differed significantly among post-T&A OSA groups, with lowest levels seen in those with no OSA at follow-up, but with no differences among those with mild vs moderate-severe OSA (Table 4, Fig 1B); other post-T&A metabolic parameters, including FPI, did not differ among the groups. On regression analyses, follow-up BMI z score was found to be the primary predictor of post-T&A fasting insulin, HOMA-IR, McAuley index, TGs, and HDL; neither age nor post-T&A AHI was a significant predictor of any of these metabolic variables (data not shown).
Table 4.
Demographic, Metabolic, and Sleep Results Across Residual AHI Groups
| Variable | AHI 0-1/h TST: No OSA (n = 16) | AHI 1-5/h TST: Mild OSA (n = 23) | AHI >5/h TST: Moderate-Severe OSA (n = 30) |
|---|---|---|---|
| Baseline demographic, metabolic variables | |||
| Baseline age, y | 6.34 ± 2.03 (4.0-11.7) | 6.14 ± 2.60 (3.8-16.2) | 6.47 ± 2.34 (3.8-14.1) |
| Baseline BMI z scorea | 1.05 ± 0.72 (−1.01 to 1.94) | 1.53 ± 0.96 (−1.29 to 4.45) | 1.56 ± 0.52 (0.89-2.87)b |
| Baseline FPG, mg/dL | 84.8 ± 9.5 (69-100) | 85.8 ± 8.5 (68-101) | 83.9 ± 9.1 (67-112) |
| Baseline FPI, μIU/mLa | 10.3 ± 7.6 (2.0-27.0) | 14.1 ± 8.1 (4.0-35.0) | 16.2 ± 10.7 (4.0-49.0) |
| Baseline LN FPI | 2.04 ± 0.82 (0.69-3.30) | 2.49 ± 0.60 (1.39-3.56) | 3.59 ± 0.63 (1.39-3.89)c |
| Baseline HOMA-IRa | 2.20 ± 1.76 (0.39-6.53) | 2.94 ± 1.65 (0.94-6.91) | 3.46 ± 2.50 (0.78-11.25) |
| Baseline McAuley Index | 8.47 ± 2.36 (5.66-12.46) | 7.56 ± 1.69 (3.94-10.67) | 7.28 ± 1.37 (5.13-10.26)b |
| Baseline TG, mg/dLa | 77.9 ± 17.9 (43-116) | 77.0 ± 35.5 (48-207) | 73.2 ± 11.8 (55-105) |
| Baseline TChol, mg/dL | 160.0 ± 34.2 (105-212) | 153.5 ± 23.7 (110-198) | 159.7 ± 31.0 (117-249) |
| Baseline HDL cholesterol, mg/dL | 54.9 ± 13.1 (37-81) | 50.7 ± 13.4 (34-87) | 49.5 ± 12.4 (25-76) |
| Baseline LDL cholesterol, mg/dL | 108.4 ± 38.7 (39.9-176.4) | 103.9 ± 25.8 (58.8-167.0) | 111.8 ± 37.3 (50.4-216.3) |
| Baseline TChol/HDL ratio | 3.05 ± 0.93 (1.57-4.82) | 3.30 ± 1.0 (1.64-5.11) | 3.43 ± 1.09 (1.63-5.79) |
| Baseline LDL/HDL ratio | 2.06 ± 0.96 (0.60-4.01) | 2.25 ± 0.91 (0.68-4.28) | 2.42 ± 1.07 (0.66-5.03) |
| Baseline TG/HDL ratioa | 1.50 ± 0.59 (0.98-3.14) | 1.66 ± 1.11 (0.82-5.96) | 1.59 ± 0.54 (0.72-2.96) |
| Baseline sleep variables | |||
| Baseline AHI, events/h TSTa | 20.3 ± 16.5 (6.2-70.3) | 22.6 ± 18.0 (7.4-66.1) | 22.9 ± 15.6 (3.6-45.8) |
| Baseline lowest SpO2 (%)a | 82.0 ± 7.0 (68-92) | 81.7 ± 8.9 (50-93) | 82.7 ± 6.6 (61-92) |
| Baseline spontaneous ArI, events/h TSTa | 9.7 ± 2.6 (5.3-13.3) | 11.5 ± 4.1 (5.9-24.4) | 8.9 ± 2.9 (1.6-14.3)b |
| Baseline respiratory ArI, events/h TSTa | 5.8 ± 5.7 (2.1-26.5) | 5.9 ± 5.1 (0.1-25.3) | 5.2 ± 2.4 (1.8-14.7) |
| Baseline total ArI, events/h TSTa | 16.3 ± 6.2 (10.6-37.6) | 18.3 ± 7.2 (9.0-39.1) | 15.0 ± 5.7 (6.9-23.0) |
| Post T&A demographic, metabolic variables | |||
| Follow-up age, ya | 6.98 ± 2.03 (4.8-12.5) | 6.71 ± 2.62 (4.4-16.6) | 7.2 ± 2.35 (4.2-14.8) |
| Follow-up BMI z scorea | 1.17 ± 0.62 (−0.74 to 1.96) | 1.69 ± 0.69 (0.71-4.17) | 1.57 ± 0.48 (0.93-2.82)b |
| Follow-up FPG, mg/dL | 80.9 ± 7.5 (68-98) | 84.7 ± 8.5 (65-100) | 81.2 ± 10.6 (57-103) |
| Follow-up FPI, μIU/mLa | 9.9 ± 7.1 (3.0-27.0) | 14.1 ± 11.1 (2.0-45.0) | 13.0 ± 9.9 (2.0-42.0) |
| Follow-up LN FPI | 2.07 ± 0.67 (1.10-3.30) | 2.40 ± 0.82 (0.69-3.81) | 2.28 ± 0.80 (0.69-3.74) |
| Follow-up HOMA-IRa | 1.99 ± 1.47 (0.64-5.80) | 3.13 ± 2.66 (0.39-11.11) | 2.61 ± 2.21 (0.28-10.37) |
| Follow-up McAuley Index | 8.65 ± 1.91 (5.85-11.61) | 7.78 ± 2.04 (4.94-11.69) | 7.93 ± 1.99 (4.36-13.17) |
| Follow-up TG, mg/dLa | 68.6 ± 12.9 (45-94) | 80.6 ± 15.4 (45-111) | 80.2 ± 29.7 (46-179)c |
| Follow-up TChol, mg/dLa | 148.2 ± 22.5 (122-206) | 154.7 ± 22.1 (120-204) | 159.9 ± 35.2 (118-269) |
| Follow-up HDL cholesterol, mg/dL | 58.8 ± 12.8 (36-81) | 55.5 ± 15.7 (25-85) | 56.1 ± 14.7 (35-91) |
| Follow-up LDL cholesterol, mg/dL | 98.2 ± 26.6 (64-160) | 102.7 ± 31.5 (59-205) | 105.7 ± 33.4 (47-178) |
| Follow-up TChol/HDL ratio | 2.67 ± 0.82 (1.61-4.92) | 2.02 ± 1.04 (1.76-5.67) | 3.04 ± 1.00 (1.48-5.50) |
| Follow-up LDL/HDL ratioa | 1.82 ± 0.82 (0.79-3.34) | 2.06 ± 1.08 (0.83-5.69) | 2.04 ± 0.87 (0.59-4.11) |
| Follow-up TG/HDL ratioa | 1.26 ± 0.47 (0.56-2.05) | 1.63 ± 0.80 (0.87-4.08) | 1.57 ± 0.88 (0.65-4.97) |
| Post-T&A sleep variables | |||
| Follow-up AHI, events/h TSTa | 0.50 ± 0.22 (0.2-0.8) | 2.52 ± 1.23 (1.0-4.9) | 5.9 ± 6.5 (5.7-34.6)d |
| Follow-up lowest SpO2, % | 95.9 ± 1.0 (94-97) | 90.6 ± 4.9 (77-97) | 82.9 ± 5.2 (72-91)d |
| Follow-up spontaneous ArI, events/h TSTa | 13.5 ± 2.9 (6.6-16.6) | 12.3 ± 6.8 (3.7-36.4) | 10.9 ± 4.0 (1.8-17.2)b |
| Follow-up respiratory ArI, events/h TSTa | 0.2 ± 0.3 (0-1) | 1.6 ± 1.6 (0-5) | 2.0 ± 2.5 (0-13)d |
| Follow-up total ArI, events/h TSTa | 12.1 ± 2.3 (6.7-14.7) | 12.5 ± 6.5 (4.7-37.4) | 11.3 ± 3.7 (2.9-21.9) |
| Change (delta) in age and metabolic variables, post-pre | |||
| Delta age, y | 0.64 ± 0.15 (0.4-0.88) | 0.57 ± 0.25 (0.18-1.20) | 0.72 ± 0.29 (0.20-1.68)b ƒ |
| Delta BMI z scorea | 0.12 ± 0.16 (−0.07 to 0.54) | 0.16 ± 0.45 (−0.40 to 2.0) | 0.01 ± 0.17 (−0.45 to 0.41) |
| Delta FPG, mg/dL | −3.9 ± 14.8 (−30 to 29) | −1.0 ± 10.7 (−24 to 30) | −2.4 ± 11.3 (−32 to 21) |
| Delta FPI, μIU/mLa | −0.4 ± 4.3 (−9 to 6) | −1.0 ± 13.4 (−25 to 31) | −3.2 ± 10.8 (−27 to 32) |
| Delta LN FPI | 0.03 ± 0.53 (−0.75 to 0.92) | −0.21 ± 0.99 (−2.6 to 1.79) | −0.32 ± 0.78 (−2.67 to 1.61) |
| Delta HOMA-IRa | −0.21 ± 1.19 (−2.5 to 2.02) | −0.06 ± 3.15 (−5.41 to 8.03) | −0.80 ± 2.17 (−5.16 to 5.93) |
| Delta McAuley Index | 0.17+1.89 (−3.81 to 3.68) | 0.54 ± 2.34 (−4.26 to 6.09) | 0.66 ± 1.98 (3.90-7.22) |
| Delta TG, mg/dLa | −9.2 ± 24.6 (−62 to 31) | 2.8 ± 34.5 (−121 to 52) | 6.9 ± 28.4 (−31 to 103)b |
| Delta TChol, mg/dL | −11.5 ± 36.0 (−69.4 to 44.0) | 1.5 ± 31.5 (−73.0 to 67.0) | 0.2 ± 33.5 (−75.0 to 61.9) |
| Delta HDL cholesterol, mg/dL | 3.8 ± 17.5 (−20 to 39) | 5.2 ± 16.1 (−29 to 32) | 6.6 ± 15.7 (−27 to 54) |
| Delta LDL cholesterol, mg/dL | −8.0 ± 30.1 (−58.6 to 36.7) | −2.0 ± 39.5 (−107.9 to 94) | −6.1 ± 36.4 (−82 to 70.8) |
| Delta TChol/HDL ratio | −0.38 ± 0.84 (−2.48 to 0.75) | −0.37 ± 0.96 (−3.32 to 1.10) | −0.39 ± 1.02 (−3.15 to 1.04) |
| Delta LDL/HDL ratio | −0.17 ± 0.67 (−1.65 to 0.85) | −0.28 ± 1.15 (−3.45 to 2.69) | −0.41 ± 1.06 (−3.23 to 1.54) |
| Delta TG/HDL ratioa | −0.25 ± 0.76 (−2.42 to 0.70) | −0.07 ± 0.96 (−3.21 to 2.04) | −0.03 ± 0.91 (−1.48 to 3.16) |
| Change in sleep variables | |||
| Delta AHI, events/h TST | −19.8 ± 16.4 (−69.4 to 5.9) | −20.0 ± 18.1 (−63.7 to 3.7) | −11.5 ± 14.2 (−34.6 to 20.9)d |
| Delta lowest SpO2, % | 13.9 ± 6.8 (5.3-28.1) | 8.9 ± 9.9 (−7.1 to 43.0) | 0.2 ± 7.8 (−15.4 to 20.8)d |
| Delta spontaneous ArI, events/h TSTa | 4.1 ± 3.2 (−1.4 to 10.0) | 1.2 ± 7.4 (−9.3 to 23.9) | 2.0 ± 3.7 (−6.4 to 11.4)c |
| Delta respiratory ArI, events/h TSTa | −5.6 ± 5.7 (−25.9 to 1.5) | −4.3 ± 5.4 (−22.6 to 2.0) | −3.2 ± 3.3 (−25.9 to 7.3) |
| Delta total ArI, events/h TSTa | −3.9 ± 6.8 (−27.7 to 0) | −5.4 ± 9.6 (−28.2 to 12.9) | −3.8 ± 4.6 (−12.4 to 4.7) |
Data represent mean ± SD. Numbers in bold are significantly different between study participants across residual OSA groups.
AI = apnea index; FPG = fasting plasma glucose; FPI = fasting plasma insulin; OAI = obstructive apnea index. See Table 1 and 2 legends for expansion of abbreviations.
Non-normal distribution using nonparametric testing (Kruskal-Wallis).
P between .05 and .1,
P < .05, and
P < .01 for ANOVA/Kruskal-Wallis comparisons across OSA groups.
Table 5.
Residual OSA Group Comparisons: Percentage Improved, Unchanged, and Worsened
| Variable | Delta (Δ) Indicating Significant Change Pre- vs Post-T&A | Residual AHI 0-1/h TST; No OSA (n = 16) |
Residual AHI 1-5/h TST; Mild OSA (n = 23) |
Residual AHI >5/h TST; Moderate-Severe OSA (n = 30) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Better | % Worse | % No change | % Better | % Worse | % No change | % Better | % Worse | % No change | ||
| AHI | ≥ 1.5 events/h TST | 100 | 0 | 0 | 100 | 0 | 0 | 86.70 | 6.70 | 6.70 |
| BMI z score | > 0.05 | 6.25 | 56.25 | 37.50 | 17.40 | 60.90 | 21.70 | 33.30 | 20 | 46.70 |
| FPG, mg/dL | ≥ 5 mg/dL | 37.50 | 25 | 37.50 | 30.40 | 17.40 | 52.20 | 41.40 | 27.60 | 31.00 |
| FPI, μIU/mLa | > 5 μIU/mL | 6.25 | 18.75 | 75 | 38.10 | 14.30 | 47.60 | 36.70 | 16.70 | 46.70 |
| HOMA-IRa | ≥ 0.3 | 50 | 31.25 | 18.7% | 57.10 | 19 | 23.80 | 65.50 | 20.70 | 13.80 |
| McAuley Index | > 0.25 | 50 | 31.25% | 18.75 | 42.10 | 31.60 | 26.30 | 60 | 26.70 | 13.30 |
| Total cholesterol | > 10 mg/dL | 37.50 | 31.25 | 31.25 | 31.80 | 36.40 | 31.80 | 43.30 | 23.30 | 33.30 |
| HDL cholesterol | > 10 mg/dL | 37.50 | 25 | 37.50 | 36.40 | 22.70 | 40.90 | 40 | 13.30 | 46.70 |
| LDL cholesterol | > 10 mg/dL | 53.30 | 33.30 | 13.30 | 33.30 | 33.30 | 33.30 | 43.30 | 26.70 | 30 |
| TG | > 10 mg/dL | 56.25 | 18.75 | 25 | 13.60 | 50 | 36.40 | 20 | 26.70 | 53.30 |
| TChol/HDL ratio | > 0.4 | 37.50 | 6.25 | 56.25 | 42.90 | 23.80 | 33.30 | 50 | 23.30 | 26.70 |
| TG/HDL ratioa | > 0.44 | 18.75 | 12.50 | 68.75 | 18.20 | 13.60 | 68.10 | 26.70 | 20 | 53.30 |
| LDL/HDL ratio | > 0.3 | 33 | 20 | 46.70 | 47.60 | 23.80 | 28.60 | 58.60 | 27.90 | 13.80 |
Figure 1.
(A) Difference in baseline fasting insulin between residual OSA groups. (B) Difference in post-adenotonsillectomy (T&A) triglycerides between residual OSA groups. AHI = apnea-hypopnea index; LN = natural log. ∗P < .05 vs the other two groups.
Discussion
In this study, we found that T&A improved insulin sensitivity and HDL levels, but not fasting glucose or other lipoprotein levels despite a parallel increase in BMI z scores, suggesting that OSA is causally involved in creating an adverse metabolic state independent from obesity because the metabolic changes did not differ significantly between NOB and obese children or between boys and girls. Fasting insulin was most strongly associated with post-T&A AHI, such that more children with IR were more likely to have residual OSA. No baseline PSG variable predicted who would be at higher risk of having residual OSA at follow-up. Finally, although T&A ameliorated the severity of OSA, it was not highly efficacious at resolving OSA in a substantial proportion: only 23% of children studied (10% of obese and 30% of NOB) experienced full OSA resolution following T&A, and 43% (38% of NOB, 54% of obese) still had moderate-severe OSA.
OSA, Body Habitus, and Impact of T&A
Similar to previous studies,30 more overweight children were at greater risk of residual OSA post-T&A. However, children with more significant residual OSA were less likely to gain weight after surgery compared with children with mild or no OSA. OSA can increase energy expenditure,31 which improves following OSA treatment32; in children, OSA can even cause failure to thrive, and weight gain can improve following T&A.32 Thus, it is unsurprising that excess weight gain can be exacerbated by effective OSA treatment.
Metabolic Changes Following T&A
Remarkably, despite the weight gain in our study cohort at follow-up, IR measures and HDL improved significantly post-T&A (these positive changes were seen even in the group with residual moderate-severe OSA, although it should be noted that OSA still improved considerably even in this group following T&A). These changes suggest that OSA per se directly affects metabolic homeostasis, although following T&A, residual IR and dyslipidemia were primarily determined by adiposity rather than AHI. Thus, the current study adds further to the list of contradictory pediatric studies focused on OSA and metabolic function, whereby some have reported significant associations between OSA and IR independently of obesity, whereas others have found no independent associations.18, 33 Studies examining the impact of OSA treatment upon metabolic derangements in children have been similarly contradictory; we previously reported improvements in TChol, HDL, and LDL in all children, but with TG and IR improvements only in obese children,19 whereas other studies have found no improvements in IR.22 The Randomized Controlled Study of Adenotonsillectomy for Childhood Sleep Apnea (CHAT) study, the largest pediatric study to date examining the effect of T&A on OSA in children, found no improvement in lipoproteins or other cardiometabolic markers in children 5 to 9 years of age with OSA undergoing T&A.34 However, the CHAT study differed from ours in several ways: the age cutoff of the CHAT study was younger (up to 9 years in the CHAT study vs up to 12.6 years in ours), included a larger proportion of African American vs Caucasian children (53% African American and 35% Caucasian vs 23% African American and 72% Caucasian in our group.) The differences in age distribution between our study and the CHAT study may account for some of the discrepancies: younger children may be less likely to exhibit significant metabolic changes after T&A than older children. The difference in racial makeup of study participants may also be important: African American children are more likely to be IR than Caucasian children35 and may be at higher risk for residual OSA36 than Caucasians. Finally, the younger age group of the CHAT study means that the majority of participants were likely prepubertal or at most in early puberty; our study included adolescents of pubertal age. The distinction between prepubertal and pubertal status is potentially critical because studies examining associations in adolescents as opposed to prepubertal children have consistently found associations between OSA and IR independently of obesity.18, 33, 37 The endocrine milieu and body composition change considerably with puberty, accompanied by considerably increased IR38 and increased TGs and lower HDL and LDL levels39; thus, relationships with risk factors such as obesity or OSA could certainly differ in prepubertal vs pubertal cohorts. The prevalent OSA phenotype also changes with puberty, with a decline in the proportion of OSA attributable to lymphadenoidal hypertrophy and a rise in the proportion of OSA phenotype more related to obesity.40 Few studies of OSA’s metabolic sequelae have separated prepubertal and pubertal children and formally assessed pubertal staging,37 and no study of which we are aware has examined the impact of T&A on metabolic sequelae of OSA exclusively in adolescents.
Potential Predictors of Residual OSA
Of the factors examined, higher baseline insulin was most predictive of likelihood of residual OSA at follow-up, more than baseline BMI z score. In other studies examining metabolic changes in children with OSA following T&A, obesity,30, 41 AHI,41 and older age30, 41 at diagnosis were associated with greater likelihood of persistent OSA30; this is the first study to our knowledge in which baseline insulin was identified as being predictive of likelihood of residual OSA. Classically, OSA is thought to alter insulin sensitivity and glucose homeostasis via sleep fragmentation42 and intermittent hypoxia43 that induce sympathetic activation,44 catecholamine elevation, oxidative stress and inflammation,45 increased glucocorticoid levels,46 and adipose tissue hypoxia.47 Our findings suggest that there may also be a reciprocal relationship, whereby IR helps maintain OSA independently of obesity. In adults, exercise can reduce the frequency of apneas independently of obesity, age or sex,48 and women with polycystic ovary syndrome, a disorder of androgen excess in which IR is a key underlying anomaly, are more likely to have OSA than BMI-matched women without polycystic ovary syndrome,49 supporting the possibility that IR per se could exacerbate OSA, although the degree to which this is additive to the effect of BMI following T&A is unclear. Future studies should explore whether IR predicts likelihood of OSA persistence in children following treatment, especially in a pubertal cohort.
Impact of T&A on OSA
Finally, we found that 77% of all children studied and 90% of those with obesity still had OSA following T&A. Our findings add to a growing body of literature reporting that, following T&A, many children still have residual OSA.30, 50 Although T&A remains the gold standard first line of treatment for pediatric OSA and indeed ameliorates OSA severity, it has now been repeatedly demonstrated that T&A is not curative for a substantial majority; postoperative PSG is therefore necessary to assess who still has OSA.30, 50
Strengths and Limitations
Our study had a number of strengths. First, we examined potential causality by assessing metabolic changes after intervention aiming to improve or cure OSA and thus to assess the contribution of OSA to IR, hyperglycemia, and dyslipidemia independently of obesity. OSA diagnosis was based on the gold standard in-laboratory overnight PSG. We also studied several NOB children who are at lower a priori risk of IR and MetSyn and were able to evaluate the contribution of OSA to IR without the confounder of obesity. Our study also had several limitations. First, of the 114 children who initially consented to participate, only 69 returned for follow-up evaluation (approximately 60%); however, those who did not return for follow-up visits did not differ demographically, anthropometrically, metabolically, or in OSA severity from those who did return, so attrition is unlikely to have significantly biased our results. Also, the wide age distribution of our cohort could have affected interpretation of IR, hyperglycemia, and dyslipidemia, given the mixture of prepubertal and pubertal children. We also did not examine the participants’ diet and physical activity pre- and post-T&A, and thus cannot exclude the possibility that some participants significantly changed their food intake patterns and overall activity lifestyle following T&A. Lymphadenoidal hypertrophy also has been found to decline in adolescence,51 although adolescents with OSA vs without OSA are more likely to have adenotonsillar hypertrophy52; this variability may have affected how the underlying primary contributors to upper airway dysfunction impinge upon metabolic function. Furthermore, glycosylated hemoglobin was not examined, so the impact of OSA treatment on longstanding glycemia could not be assessed. Children who were found to have clinically significant OSA on follow-up PSG were offered CPAP as per clinical routine by their sleep medicine physicians; however, this was not part of our study and thus we do not have data regarding the impact of CPAP on metabolic outcomes in children with residual OSA. The metabolic impact of CPAP in children should be examined in future studies. Finally, only 22 subjects were obese, limiting our power to assess for separate relationships between OSA and metabolic sequelae in this population.
In conclusion, we found that T&A significantly improved IR and HDL, and that baseline FPI predicted residual OSA independently from BMI z score, suggesting that IR may contribute to worsening of untreated OSA. Future studies are clearly needed to better delineate putative associations between OSA and IR, especially in the context of puberty onset and progression and its related changes. Finally, we found that OSA persisted following T&A in the majority of children, especially those with obesity; the high prevalence of remaining OSA post-T&A not only demonstrates that T&A is not fully efficacious at resolving OSA, but suggests that objective follow-up assessment of sleep-breathing patterns is indicated after adenotonsillectomy.
Acknowledgments
Author contributions: L. K.-G. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis and serves as guarantor for the manuscript. D. K., D. G., and L. K.-G. contributed to study design, data analysis, and writing the manuscript. M. F. P. and R. B. contributed to data acquisition and to revising the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We acknowledge Ms Richa Kulkarni for coordinating this study, the sleep technologists who conducted the overnight sleep studies, the laboratory technicians who drew the laboratory fluids and performed the laboratory assays, and the children and their families for taking part in this study.
Additional information: The e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
Dr Bhattacharjee is currently at University of California at San Diego (San Diego, CA).
Dr Philby is currently at University Medical City (Riyadh, Kingdom of Saudi Arabia).
FUNDING/SUPPORT: This work was supported by the National Institutes of Health (grant HL65270) and the Herbert T. Abelson Chair in Pediatrics.
Supplementary Data
References
- 1.Ogden C.L., Carroll M.D., Kit B.K., Flegal K.M. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goran M.I., Ball G.D.C., Cruz M.L. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab. 2003;88(4):1417–1427. doi: 10.1210/jc.2002-021442. [DOI] [PubMed] [Google Scholar]
- 3.Weiss R., Dziura J., Burgert T.S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350(23):2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 4.Arens R., Muzumdar H. Childhood obesity and obstructive sleep apnea syndrome. J Appl Physiol. 2010;108(2):436–444. doi: 10.1152/japplphysiol.00689.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pamidi S., Wroblewski K., Broussard J. Obstructive sleep apnea in young lean men: impact on insulin sensitivity and secretion. Diabetes Care. 2012;35(11):2384–2389. doi: 10.2337/dc12-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ip M.S., Lam B., Ng M.M., Lam W.K., Tsang K.W., Lam K.S. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165(5):670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 7.Punjabi N.M., Shahar E., Redline S., Gottlieb D.J., Givelber R., Resnick H.E. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 8.Parish J.M., Adam T., Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3(5):467–472. [PMC free article] [PubMed] [Google Scholar]
- 9.Babu A.R., Herdegen J., Fogelfeld L., Shott S., Mazzone T. TYpe 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165(4):447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 10.Grimaldi D., Beccuti G., Touma C., Van Cauter E., Mokhlesi B. Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care. 2014;37(2):355–363. doi: 10.2337/dc13-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronsohn R.S., Whitmore H., Van Cauter E., Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181(5):507–513. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster G.D., Sanders M.H., Millman R. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32(6):1017–1019. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peppard P.E., Young T., Barnet J.H., Palta M., Hagen E.W., Hla K.M. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guest J.F., Panca M., Sladkevicius E., Taheri S., Stradling J. Clinical outcomes and cost-effectiveness of continuous positive airway pressure to manage obstructive sleep apnea in patients with type 2 diabetes in the U.K. Diabetes Care. 2014;37(5):1263–1271. doi: 10.2337/dc13-2539. [DOI] [PubMed] [Google Scholar]
- 15.de la Eva R.C., Baur L.A., Donaghue K.C., Waters K.A. Metabolic correlates with obstructive sleep apnea in obese subjects. J Pediatr. 2002;140(6):654–659. doi: 10.1067/mpd.2002.123765. [DOI] [PubMed] [Google Scholar]
- 16.Adedayo A.M., Olafiranye O., Smith D. Obstructive sleep apnea and dyslipidemia: evidence and underlying mechanism. Sleep Breath. 2014;18(1):13–18. doi: 10.1007/s11325-012-0760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koren D., Chirinos J.A., Katz L.E. Interrelationships between obesity, obstructive sleep apnea syndrome and cardiovascular risk in obese adolescents. Int J Obes (Lond) 2015;39(7):1086–1093. doi: 10.1038/ijo.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tauman R., O’Brien L.M., Ivanenko A., Gozal D. Obesity rather than severity of sleep-disordered breathing as the major determinant of insulin resistance and altered lipidemia in snoring children. Pediatrics. 2005;116(1):e66–e73. doi: 10.1542/peds.2004-2527. [DOI] [PubMed] [Google Scholar]
- 19.Gozal D., Capdevila O.S., Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177(10):1142–1149. doi: 10.1164/rccm.200711-1670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waters K.A., Sitha S., O'Brien L.M. Follow-up on metabolic markers in children treated for obstructive sleep apnea. Am J Respir Crit Care Med. 2006;174(4):455–460. doi: 10.1164/rccm.200401-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng D.K., Wong J.C., Chan C.H., Leung L.C., Leung S.Y. Ambulatory blood pressure before and after adenotonsillectomy in children with obstructive sleep apnea. Sleep Med. 2010;11(7):721–725. doi: 10.1016/j.sleep.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Apostolidou M.T., Alexopoulos E.I., Damani E. Absence of blood pressure, metabolic, and inflammatory marker changes after adenotonsillectomy for sleep apnea in Greek children. Pediatr Pulmonol. 2008;43(6):550–560. doi: 10.1002/ppul.20808. [DOI] [PubMed] [Google Scholar]
- 23.Ogden C.L., Kuczmarski R.J., Flegal K.M. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 24.Barlow S.E., Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharjee R., Alotaibi W.H., Kheirandish-Gozal L., Capdevila O.S., Gozal D. Endothelial dysfunction in obese non-hypertensive children without evidence of sleep disordered breathing. BMC Pediatrics. 2010;10:8. doi: 10.1186/1471-2431-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Academy of Sleep Medicine . American Academy of Sleep Medicine; Westchester, IL: 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [PMC free article] [PubMed] [Google Scholar]
- 27.Di Bonito P., Valerio G., Grugni G. Comparison of non-HDL-cholesterol versus triglycerides-to-HDL-cholesterol ratio in relation to cardiometabolic risk factors and preclinical organ damage in overweight/obese children: the CARITALY study. Nutr Metab Cardiovasc Dis. 2015;25(5):489–494. doi: 10.1016/j.numecd.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 29.Ascaso J.F., Pardo S., Real J.T., Lorente R.I., Priego A., Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26(12):3320–3325. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharjee R., Kheirandish-Gozal L., Spruyt K. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children. Am J Respir Crit Care Med. 2010;182(5):676–683. doi: 10.1164/rccm.200912-1930OC. [DOI] [PubMed] [Google Scholar]
- 31.Stenlof K., Grunstein R., Hedner J., Sjostrom L. Energy expenditure in obstructive sleep apnea: effects of treatment with continuous positive airway pressure. Am J Physiol. 1996;271(6 Pt 1):E1036–E1043. doi: 10.1152/ajpendo.1996.271.6.E1036. [DOI] [PubMed] [Google Scholar]
- 32.Marcus C.L., Carroll J.L., Koerner C.B., Hamer A., Lutz J., Loughlin G.M. Determinants of growth in children with the obstructive sleep apnea syndrome. J Pediatr. 1994;125(4):556–562. doi: 10.1016/s0022-3476(94)70007-9. [DOI] [PubMed] [Google Scholar]
- 33.Hannon T.S., Lee S., Chakravorty S., Lin Y.A.N., Arslanian S.A. Sleep-disordered breathing in obese adolescents is associated with visceral adiposity and markers of insulin resistance. Int J Pediatr Obes. 2011;6(2):157–160. doi: 10.3109/17477166.2010.482156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quante M., Wang R., Weng J. The effect of adenotonsillectomy for childhood sleep apnea on cardiometabolic measures. Sleep. 2015;38(9):1395–1403. doi: 10.5665/sleep.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goran M.I., Bergman R.N., Cruz M.L., Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care. 2002;25(12):2184–2190. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 36.Tan H.-L., Gozal D., Kheirandish-Gozal L. Obstructive sleep apnea in children: a critical update. Nat Sci Sleep. 2013;5:109–123. doi: 10.2147/NSS.S51907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly A., Dougherty S., Cucchiara A., Marcus C.L., Brooks L.J. Catecholamines, adiponectin, and insulin resistance as measured by HOMA in children with obstructive sleep apnea. Sleep. 2010;33(9):1185–1191. doi: 10.1093/sleep/33.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goran M.I., Gower B.A. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444–2450. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 39.Pinhas-Hamiel O., Lerner-Geva L., Copperman N.M., Jacobson M.S. Lipid and insulin levels in obese children: changes with age and puberty. Obesity. 2007;15(11):2825–2831. doi: 10.1038/oby.2007.335. [DOI] [PubMed] [Google Scholar]
- 40.Dayyat E., Kheirandish-Gozal L., Gozal D. Childhood obstructive sleep apnea: One or two distinct disease entities? Sleep Med Clin. 2007;2(3):433–444. doi: 10.1016/j.jsmc.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tauman R., Gulliver T.E., Krishna J. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149(6):803–808. doi: 10.1016/j.jpeds.2006.08.067. [DOI] [PubMed] [Google Scholar]
- 42.Stamatakis K.A., Punjabi N.M. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137(1):95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Punjabi N.M., Beamer B.A. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179(3):235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tasali E., Mokhlesi B., Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133(2):496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 45.Tauman R., O'Brien L.M., Gozal D. Hypoxemia and obesity modulate plasma C-reactive protein and interleukin-6 levels in sleep-disordered breathing. Sleep Breathing. 2007;11(2):77–84. doi: 10.1007/s11325-006-0085-7. [DOI] [PubMed] [Google Scholar]
- 46.Martins R.C., Andersen M.L., Tufik S. The reciprocal interaction between sleep and type 2 diabetes mellitus: facts and perspectives. Braz J Med Biol Res. 2008;41(3):180–187. doi: 10.1590/s0100-879x2006005000194. [DOI] [PubMed] [Google Scholar]
- 47.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 2009;33(1):54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vgontzas A.N., Bixler E.O., Tan T.L., Kantner D., Martin L.F., Kales A. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med. 1998;158(12):1333–1337. doi: 10.1001/archinte.158.12.1333. [DOI] [PubMed] [Google Scholar]
- 49.Vgontzas A.N., Legro R.S., Bixler E.O., Grayev A., Kales A., Chrousos G.P. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrino Metab. 2001;86(2):517–520. doi: 10.1210/jcem.86.2.7185. [DOI] [PubMed] [Google Scholar]
- 50.Kheirandish-Gozal L., Gileles-Hillel A., Alonso-Alvarez M.L. Effects of adenotonsillectomy on plasma inflammatory biomarkers in obese children with obstructive sleep apnea: a community-based study. Int J Obes. 2015;39(7):1094–1100. doi: 10.1038/ijo.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahne A., El-Haddad G., Alavi A. Assessment of age-related morphological and functional changes of selected structures of the head and neck by computed tomography, magnetic resonance imaging, and positron emission tomography. Semin Nucl Med. 2007;37(2):88–102. doi: 10.1053/j.semnuclmed.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Schwab R.J., Kim C., Bagchi S. Understanding the anatomic basis for obstructive sleep apnea syndrome in adolescents. Am J Respir Crit Care Med. 2015;191(11):1295–1309. doi: 10.1164/rccm.201501-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.