Abstract
Background
Idiopathic pulmonary fibrosis is a progressive lung disease with variable course. The Gender-Age-Physiology (GAP) Index and staging system uses clinical variables to stage mortality risk. It is unknown whether clinical staging predicts future decline in pulmonary function. We assessed whether the GAP stage predicts future pulmonary function decline and whether interval pulmonary function change predicts mortality after accounting for stage.
Methods
Patients with idiopathic pulmonary fibrosis (N = 657) were identified retrospectively at three tertiary referral centers, and baseline GAP stages were assessed. Mixed models were used to describe average trajectories of FVC and diffusing capacity of the lung for carbon monoxide (Dlco). Multivariable Cox proportional hazards models were used to assess whether declines in pulmonary function ≥ 10% in 6 months predict mortality after accounting for GAP stage.
Results
Over a 2-year period, GAP stage was not associated with differences in yearly lung function decline. After accounting for stage, a 10% decrease in FVC or Dlco over 6 months independently predicted death or transplantation (FVC hazard ratio, 1.37; Dlco hazard ratio, 1.30; both, P ≤ .03). Patients with GAP stage 2 with declining pulmonary function experienced a survival profile similar to patients with GAP stage 3, with 1-year event-free survival of 59.3% (95% CI, 49.4-67.8) vs 56.9% (95% CI, 42.2-69.1).
Conclusions
Baseline GAP stage predicted death or lung transplantation but not the rate of future pulmonary function decline. After accounting for GAP stage, a decline of ≥ 10% over 6 months independently predicted death or lung transplantation.
Key Words: idiopathic pulmonary fibrosis, interstitial lung disease, lung function
Abbreviations: Dlco, diffusing capacity of the lung for CO2; GAP, Gender-Age-Physiology; IPF, idiopathic pulmonary fibrosis; PFT, pulmonary function test
Idiopathic pulmonary fibrosis (IPF) is a fibrotic lung disease of unknown etiology with a prevalence estimated between 14 and 43 per 100,000 subjects.1 Median survival from the time of diagnosis is approximately 3 years, but disease course varies, with some subjects having a slow decline in lung function over time and others having a course marked by rapid deterioration and death.2 Many clinical, radiographic, physiologic, and pathologic predictors are associated with increased or decreased survival in IPF, some with more consistent association than others.3 Lower baseline FVC and diffusing capacity of the lung for carbon monoxide (Dlco), as well as declining FVC or Dlco during 6 or 12 months of follow-up, are predictive of worse survival.4, 5, 6, 7, 8, 9, 10
In addition to assessment of individual risk factors, several staging models have been developed to predict mortality in IPF,9, 11, 12 including the Gender-Age-Physiology (GAP) Index.13 The GAP index stratifies patients into three stages based on clinical (eg, sex, age) and physiologic (eg, FVC, Dlco) variables. It provides a 1-, 2-, and 3-year mortality estimate, with patients with GAP stage 3 experiencing the worst outcomes. A clinical staging system in IPF could prove useful for additional applications such as predicting change in pulmonary function over time and informing physicians regarding the best application of other key prognostic factors. The utility of existing staging models for these applications is currently unknown, and better knowledge in this area would be useful for several reasons. First, previous clinical trials evaluating pharmacologic therapy for IPF have used decline in pulmonary function as a primary end point.14, 15, 16, 17, 18, 19, 20 Our current inability to predict subsequent change in lung function has complicated study designs regarding power calculations and planning for adequate enrollment. Second, although staging can provide rough estimates of mortality for groups of patients, knowledge of how physiologic change over time in an individual interacts with baseline predictors could be used by physicians for making therapy decisions.
The present study evaluated whether the GAP stage provides information on the rate of future pulmonary function decline and on the predictive value of interval decline regarding mortality. We hypothesized that subjects with more advanced disease, as defined according to higher GAP index stage, would experience a more rapid decline in pulmonary function with greater yearly decrements in FVC and Dlco. We further hypothesized that a relative decline in FVC or Dlco ≥ 10% over 6 months would independently predict mortality after accounting for GAP stage.
Methods
Patients
We identified subjects with IPF (diagnosed according to results of lung biopsy or CT scan [as previously described])1, 21 through review of interstitial lung disease databases at the Royal Brompton and Harefield National Health Service Foundation Trust, National Jewish Health, and the University of Michigan Health System from 1981 to 2008. For each patient, age, sex, and each pulmonary function test (PFT) were captured, with PFTs performed as described.22, 23, 24, 25 Patients with missing baseline Dlco data were excluded because this variable is needed to calculate GAP stage,13 and we were not able to distinguish in our database patients with missing data due to respiratory limitation from those in whom the test was not ordered. This dataset was also used in a previously published study26 approved by research oversight committees at each participating institution (studies HUM00018279, 01-246, and HS-1603).
Predictor and Outcome Variables
Predictor variables included the baseline GAP stage, calculated as previously described,13 and 10% relative decline in FVC or Dlco over 6 months of follow-up. Primary outcomes included average yearly absolute change and relative change from baseline for percent-predicted FVC and Dlco, as well as transplantation-free survival (defined as absence of death or lung transplantation during follow-up, measured from the date of the initial PFT). Vital status was confirmed through the Social Security Death Registry Index or the UK National Health Service censored by 3 months to account for reporting lag.
Statistical Methods
Demographic characteristics are displayed according to GAP stage (1, 2, or 3), with means ± SDs for continuous variables and percentages for categorical variables. Statistically significant differences between GAP stages for continuous and categorical variables were assessed by using an analysis of variance and Pearson’s χ2 methods, respectively. The Kaplan-Meier method illustrates survival according to GAP stage, with statistically significant differences in event rates assessed via the log-rank test.
Mixed models with linear spline components at yearly intervals describe average trajectories in pulmonary function over time as measured in absolute and relative change per year for FVC and Dlco; this method addresses issues with irregularly spaced measurement times and adjusts for dropout due to attrition or death. Additional inclusion of linear spline terms allowing trajectory changes at 6-month intervals were assessed but were not found to improve model fit. Lower Akaike information criteria were used to select the best variability terms in the mixed models. This resulted in the use of random intercept and time terms to account for variability in the data; in addition, random linear spline terms allowing variation in individual trajectories at yearly intervals were included when found to improve model fit via the lower Akaike information criteria. Notably, because the GAP Index stage incorporates baseline age, sex, FVC, and Dlco information, we did not initially adjust our model for these parameters. Adjustments were added later for baseline percent-predicted FVC, sex, and age. The adjusted outputs were not significantly different from unadjusted values and actually resulted in worse model fit; we therefore cite unadjusted model outputs in the main text. The average yearly absolute change was calculated, for example, as FVCbaseline – FVC12 months or FVC12 months – FVC24 months, and relative change from baseline as (FVCbaseline – FVC12 months)/FVCbaseline or (FVCbaseline – FVC24 months)/FVCbaseline, as estimated by using mixed model methods. The same methods were used for Dlco. Because of the differences in baseline pulmonary function when patients were grouped according to GAP stage, we felt that assessment of both absolute and relative change in lung function was appropriate, as the magnitude of relative change in pulmonary function is affected by baseline pulmonary function, whereas the magnitude of absolute change per year is not. The section on Statistical Methods in e-Appendix 1 presents greater details on the use of mixed model methods.
To assess whether decline in pulmonary function over 6 months predicts mortality when accounting for GAP stage, 6 months of follow-up data were used to estimate patient-specific FVC and Dlco trajectories and corresponding 6-month changes. Univariable and multivariable Cox proportional hazards models were used to assess whether relative decreases in either FVC or Dlco ≥ 10% in 6 months independently predicts death or lung transplantation after accounting for GAP stage. For each GAP stage, Kaplan-Meier plots were used to display survival differences according to whether patients had a decrease in FVC ≥ 10% irrespective of Dlco change, a decrease in Dlco ≥ 10% irrespective of FVC change, both types of decline, or neither. For this survival analysis, time zero was considered as the time of the follow-up PFT in the first 6 months. The baseline GAP stage was also calculated at the time of the follow-up PFT (new time zero) for this portion of the analysis.
Because a large number of P values are tabulated throughout this article, we offer a reminder of how to interpret statistical significance at the .05 level in the context of multiple comparisons. On average, if there are no associations of interest, spuriously one of every 20 P values is likely to be < .05; of 26 P values in the main text of this article, one to two of the 15 reported P values < .05 are likely spurious associations.
Statistical analyses were performed by using SAS version 9.4 (SAS Institute, Inc) and R version 3.1.0 (R Foundation for Statistical Computing) for plots.
Results
Patient Population and Survival Comparisons
From the interstitial lung disease databases, 734 patients with IPF were identified; 657 had FVC and Dlco data available at baseline and were included in the present analysis. Patient characteristics for the included group as a whole and according to GAP stage are shown in Table 1. Overall, 70% were men, and the mean age was 62.9 years at the time of the baseline PFT. The mean ± SD percent-predicted baseline FVC was 68.0 ± 17.0, and for Dlco it was 45.7 ± 16.0.
Table 1.
Patient Characteristics
| Characteristic | All (N = 657) |
GAP Index Stage 1 (n = 306) |
GAP Index Stage 2 (n = 288) |
GAP Index Stage 3 (n = 63) |
P Value |
|---|---|---|---|---|---|
| Age, y | 62.9 ± 10.0 | 59.2 ± 9.5 | 65.2 ± 9.7 | 70.6 ± 5.0 | < .0001 |
| Male sex, % | 70 | 56.2 | 79.9 | 92.1 | < .0001 |
| FVC, % predicted | 68.0 ± 17.0 | 75.6 ± 16.0 | 63.3 ± 15.1 | 52.7 ± 11.1 | < .0001 |
| Dlco, % predicted | 45.7 ± 16.0 | 54.9 ± 14.9 | 39.5 ± 11.9 | 29.1 ± 8.2 | < .0001 |
Unless otherwise indicated, data are given as mean ± SD. Dlco = diffusing capacity of the lung for CO2; GAP = Gender-Age-Physiology.
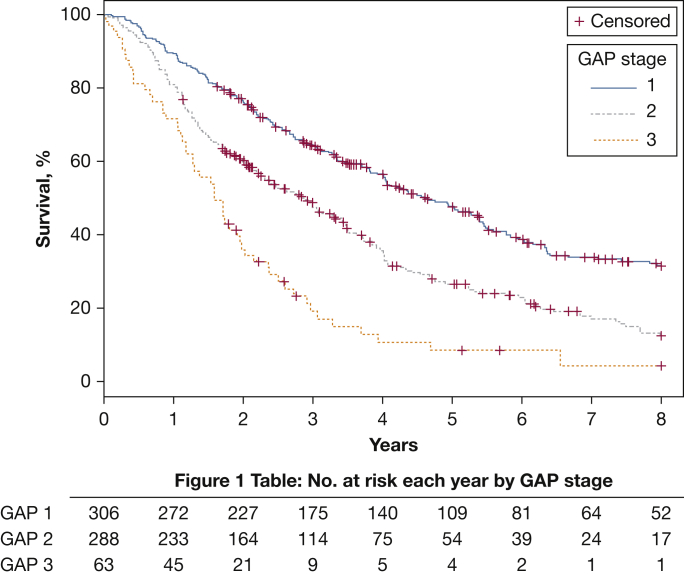
For the combined end point of time-to-lung transplantation or death, 11 patients achieved the end point via a transplant and 482 separate patients died. Figure 1 displays the Kaplan-Meier event-free survival rates according to GAP stage. Higher baseline GAP stages in our cohort were consistently associated with higher event rates: 35.9%, 51.7%, and 80.9% by 3 years and 68.5%, 87.5%, and 95.8% through 8 years for GAP stages 1, 2, and 3, respectively (P < .0001 for comparisons across either follow-up period).
Figure 1.
Kaplan-Meier transplant-free survival according to GAP stage. Transplant-free survival, defined as time from first pulmonary function test to death or lung transplantation, is displayed with patients grouped according to their GAP Index stage at the time of the baseline pulmonary function test. Patients with GAP stage 1 experienced the lowest event rates, and patients with GAP stage 3 had the highest event rates; GAP stage 2 was intermediate over 3 years of follow-up (P < .0001). Figure 1 Table shows the number at risk each year according to GAP stage. GAP = Gender-Age-Physiology.
Because a number of patients were excluded due to missing baseline Dlco or FVC data (n = 77 total; n = 2 were missing baseline FVC), baseline characteristics were evaluated in these patients and compared with included patients. For patients excluded, the 3-year transplant-free survival rate was 82.5% vs 53.3% for patients included (P < .0001), indicating that patients excluded were relatively healthier than patients included. The reason for this survival difference is not clear. Age, sex, and FVC were not significantly different between patients included and excluded (P > .16). e-Table 1 presents the complete demographic data for patients excluded because of missing baseline pulmonary function data.
Pulmonary Function Trajectory
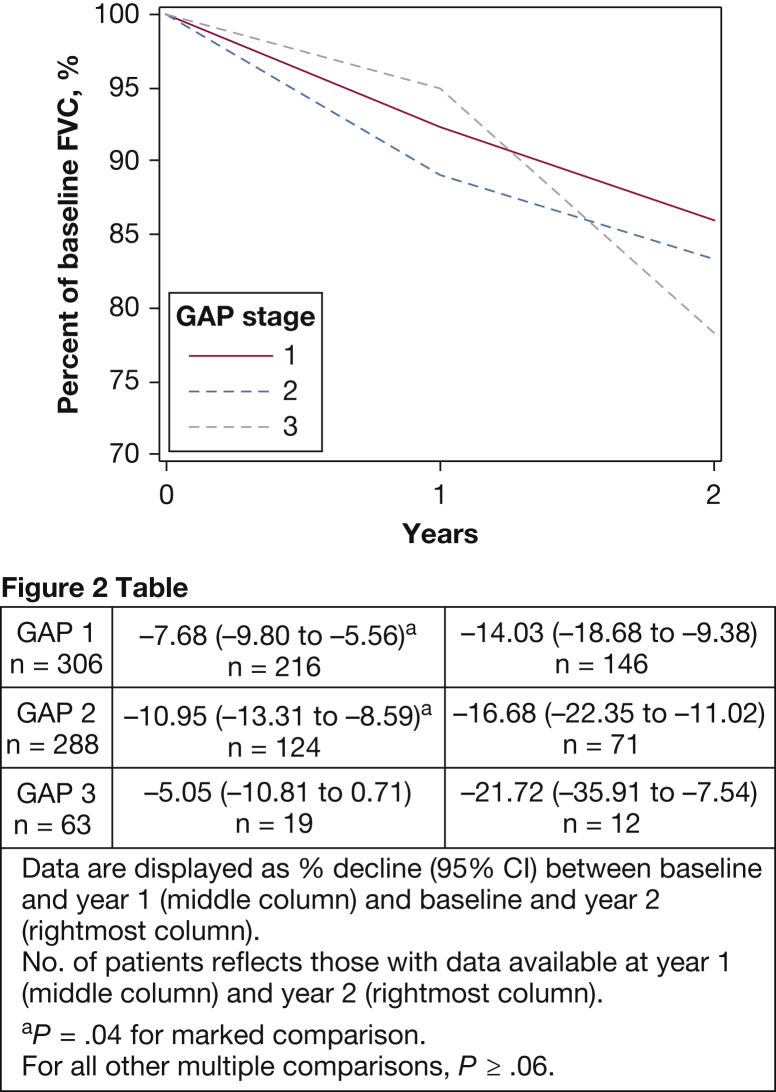
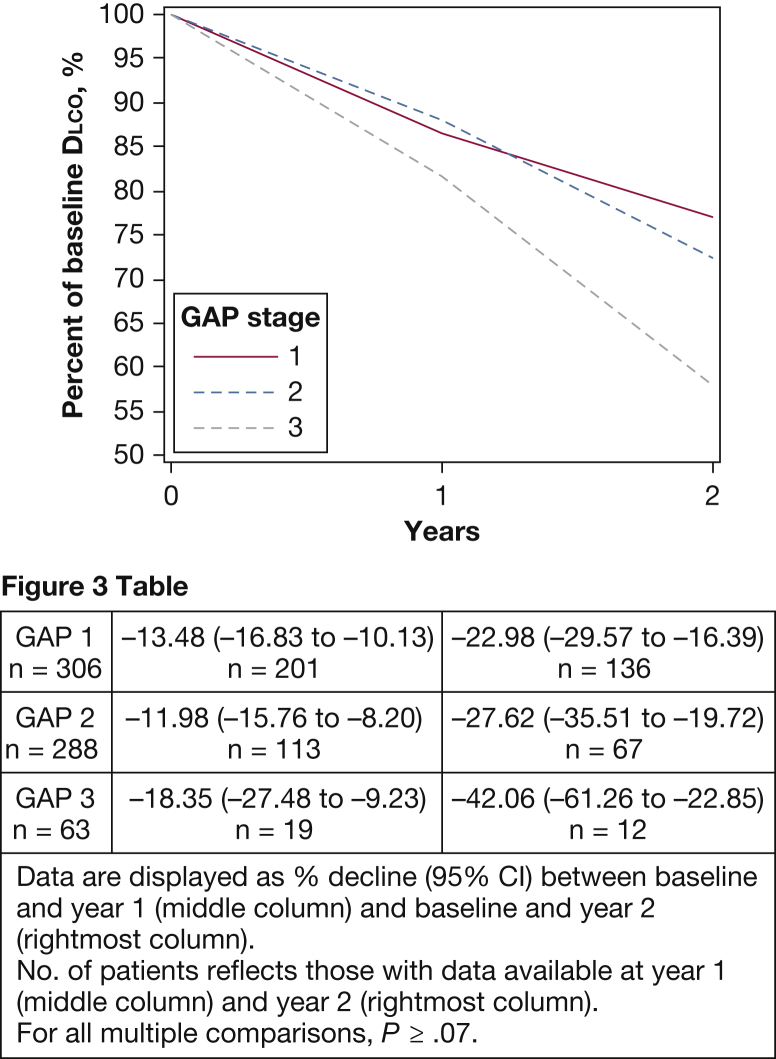
Although FVC and Dlco declined across GAP stages, the GAP stage was not associated with differences in yearly lung function decline over 2 years. As shown in Figure 2, there was no difference in relative decline from baseline for FVC at year 2 of follow-up (P ≥ .31). Patients with GAP stage 2 had the largest relative FVC decline between baseline and year 1 at –10.95% (95% CI, –13.31 to –8.59) vs GAP stage 1 at –7.68% (95% CI, –9.80 to –5.56) and GAP stage 3 at –5.05% (95% CI, –10.81 to 0.71) (P = .04 for GAP stage 1 vs GAP stage 2; other, P ≥ .06). There was no significant difference between groups for relative Dlco decline at the first year of follow-up or after 2 years (Fig 3). For simplicity, only relative change from baseline for percent-predicted FVC and Dlco are presented here. Side-by-side comparison of absolute yearly change and relative change from baseline are shown in e-Figure 1 and e-Figure 2. As noted previously, our models were evaluated by using both yearly and 6-month splines, and we assessed model fit with and without adjustment for baseline age, sex, and FVC. Yearly splines in unadjusted models attained the best model fit and are shown here; the outputs with 6-month splines (e-Table 2 and e-Table 3 for FVC and Dlco, respectively), and preadjustment and postadjustment outputs (e-Table 4 and e-Table 5 for FVC and Dlco) are shown in the online article.
Figure 2.
Relative FVC decline according to GAP stage. The percent-predicted FVC trajectory is assessed by using the mixed models method with splines at yearly intervals. The relative decline from baseline at years 1 and 2 is shown with patients stratified according to baseline GAP stage. The point estimates for relative decline from baseline at years 1 and 2 for each GAP stage group are shown in Figure 2 Table. Although patients with GAP stage 2 declined significantly more compared with patients with GAP stage 1 during year 1 (P = .04), no significant difference was seen between groups by year 2 (P ≥ .31). See Figure 1 legend for expansion of abbreviation.
Figure 3.
Relative Dlco decline according to GAP stage. The percent-predicted Dlco trajectory was assessed by using the mixed models method with splines at yearly intervals. The relative decline from baseline at years 1 and 2 with patients stratified according to baseline GAP stage is shown. The point estimates for relative decline from baseline at years 1 and 2 for each GAP stage group are shown in Figure 3 Table. There is no accelerated decline seen for any GAP stage, with no significant difference in the relative decline from baseline at year 1 or 2 of follow-up (P ≥ .07). Dlco = diffusing capacity of the lung for CO2. See Figure 1 legend for expansion of other abbreviation.
Predicting Survival: Changes in Pulmonary Physiology
Using univariable and multivariable Cox proportional hazards models incorporating each GAP stage, as well as a 10% decline in FVC or 10% decline in Dlco (with GAP stage 1 as reference), the multivariable hazard ratio for a 10% decline in FVC was 1.37 (P = .01); for a 10% decline in Dlco, it was 1.30 (P = .03) (Table 2). We tested for interactions to determine if these associations varied according to GAP stage, and the results were nonsignificant (P > .4). Of the 657 patients in our cohort with available baseline PFT data, 412 had 6-month follow-up data and participated in this analysis. e-Figure 3 shows a CONSORT diagram detailing reasons for exclusion. To ensure that the results were not influenced by missing Dlco data (ie, a patient with available follow-up FVC measurement excluded from multivariable analysis due to inability to perform Dlco), the results of a multivariable model incorporating GAP stages 1 through 3 along with FVC and Dlco declines separately is shown in e-Table 6.
Table 2.
Univariable and Multivariable Cox Proportional Hazards for the Combined End Point of Death or Lung Transplantation
| Predictor | Univariable |
Multivariable |
||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | No. | HR | 95% CI | P Value | No. | |
| GAP stage 1 | Ref | 170 | Ref | 170 | ||||
| GAP stage 2 | 1.93 | 1.51-2.46 | < .0001 | 191 | 1.83 | 1.43-2.34 | < .0001 | 191 |
| GAP stage 3 | 3.16 | 2.23-4.49 | < .0001 | 51 | 2.49 | 1.72-3.61 | < .0001 | 51 |
| 10% FVC decrease | 1.69 | 1.35-2.13 | < .0001 | 143 | 1.37 | 1.07-1.74 | .01 | 137 |
| 10% Dlco decrease | 1.59 | 1.27-1.99 | < .0001 | 189 | 1.30 | 1.03-1.65 | .03 | 189 |
HR = hazard ratio. See Table 1 legend for expansion of other abbreviations.
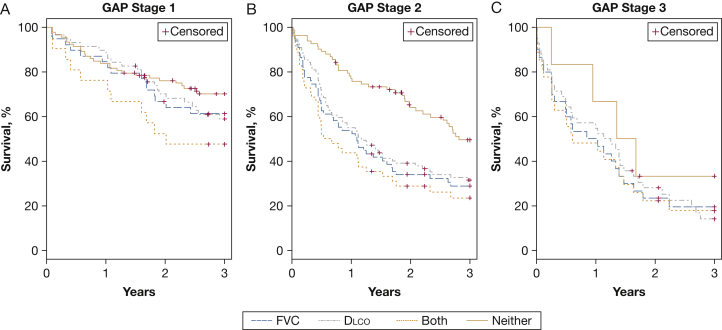
For GAP stages 1, 2, and 3 (Figs 4A, 4B, and 4C), Kaplan-Meier transplant-free survival was highest for those not experiencing a relative decrease ≥ 10% in either FVC or Dlco over 6 months and is lowest for those previously experiencing both types of relative decline in pulmonary function in patients with GAP stage 1 (log-rank test, P = .03) and GAP stage 2 (log-rank test, P < .0001). In GAP stage 3, all but 6 patients experienced a 6-month FVC or Dlco decline, and the power was low to differentiate between groups with neither decline and those with both types of decline (P = .37). Notably, for patients with GAP stage 2, those with any decline in FVC or Dlco experienced a survival profile similar to patients with GAP stage 3 (regardless of decline). Specifically, after 1 year, the event-free survival for patients with GAP stage 2 with any decline was 59.3% (95% CI, 49.4 to 67.8) vs 56.9% (95% CI, 42.2 to 69.1) for patients with GAP stage 3.
Figure 4.
A-C, Kaplan-Meier transplant-free survival according to GAP stage in those experiencing a 10% decline in FVC, Dlco, both, or neither. Kaplan-Meier transplant-free survival, defined as time from the follow-up pulmonary function test (PFT) to death or lung transplantation, is shown for patients with (A) GAP stage 1, (B) GAP stage 2, and (C) GAP stage 3. Patients were grouped based on whether they experienced a ≥ 10% decline in FVC, Dlco, both FVC and Dlco, or neither during the first 6 months of follow-up. Patients with GAP stage 1 and 2 with a decline in both FVC and Dlco experienced higher event rates compared with those experiencing no change in either parameter. In GAP stage 3, only 6 patients experienced no decline in pulmonary function over 6 months, and the power was low to find differences between groups. See Figure 1 and 3 legends for expansion of abbreviations.
Discussion
Pulmonary function trajectories were modeled in a large retrospective cohort of patients with IPF seen at 3 referral centers to determine if the GAP Index stage, a validated estimate of mortality risk, was also predictive of the future rate of decline in pulmonary function. Our findings are most notable for the lack of a consistently larger absolute or relative decline in FVC or Dlco for a particular GAP stage over 2 years of follow-up. Several modestly significant P values were reported in comparing year-to-year differences in relative or absolute change (see e-Figure 1 and e-Figure 2 for absolute yearly change); these could be due to a type I error given the overall large number of comparisons made in this study. If a Bonferroni correction is applied to the P values tabulated from the mixed models yearly PFT change outputs, statistical significance is lost throughout. Because of the inherent differences in baseline pulmonary function when a cohort is stratified according to GAP stage, we assessed absolute yearly change and relative change from baseline, both of which are predictive of mortality in IPF.27 We propose that assessment of relative change is a more relevant marker when assessing differences in the rate of change in our study because it takes into account the baseline pulmonary function.
Although many studies have assessed for predictors of mortality in IPF, few have assessed for predictors of future decline in pulmonary function. Our study is the only one, to the best of our knowledge, that evaluated the baseline GAP stage as a predictor of future rate of pulmonary function change. Schmidt et al26 previously examined whether future decline in pulmonary function can be predicted from earlier trends in this variable. They found that a relative 10% FVC or 15% Dlco decline, while predictive of greater mortality, was not predictive of future declines in pulmonary function. Richeldi et al27 assessed baseline pulmonary function as a predictor of future declines in this variable. They found no significant relationship between baseline FVC and the prevalence of a ≥ 10% absolute or relative decline in FVC over 12 months. These findings are compatible with our results when considering the significant differences in baseline pulmonary function in our comparison groups. Taken together, our results and the previously published data support the idea that although pulmonary function variables are predictors of subsequent mortality, they do not significantly predict subsequent pulmonary function trajectory. Further study is needed to identify factors predictive of rapid vs slow decline in lung function. These factors are likely to be biologic in nature rather than related to demographic characteristics, imaging, or pulmonary physiology.
An additional important finding in our study is that a relative decline ≥ 10% in either FVC or Dlco over 6 months predicted mortality after adjusting for GAP stage. Our effect size for the 6-month FVC decline in multivariable analysis adjusting for GAP stage was similar to that seen in a recent analysis by Ley et al28 for FVC decline > 10% in 24 weeks after adjusting for GAP stage. Notably, our analysis reached statistical significance whereas the previous analysis exhibited only marginal significance. Various degrees of decline in FVC and Dlco are well known for predicting greater mortality in patients with IPF.4, 5, 6, 7, 8, 9, 10
Our study highlights that interval changes in pulmonary function may add clinically useful prognostic information to the GAP Index stage. For example, a physician evaluating a patient with IPF classified as being GAP stage 3 might consider referring the patient for lung transplantation evaluation based on a predicted 1-year mortality approaching 40%.13 In our analysis, we found that patients with GAP stage 2 (predicted 1-year mortality of 16%) previously experiencing a ≥ 10% decline in FVC or Dlco had a 1-year predicted event rate similar to patients with GAP stage 3 (approximately 41%). Based on the GAP stage alone, a physician might not refer this patient with GAP stage 2 for lung transplantation. However, given the additional hazard conveyed by a recent PFT decline, consideration may be given to a more expeditious transplantation evaluation in an appropriate patient with GAP stage 2 with declining FVC or Dlco.
Our results are limited primarily by the retrospective study design. In the originally derived GAP Index staging system, GAP stage can be calculated in patients unable to perform Dlco maneuver due to respiratory limitation. Unfortunately as our data was deidentified, we could not distinguish between patients missing a Dlco value due to respiratory limitation vs those in whom the test was not ordered for an unknown reason, therefore patients missing baseline Dlco measurement were excluded. As noted in the Results section, those subjects excluded due to missing Dlco data had significantly better 3-year survival than included patients (82.5% vs 53.3%). Because the predicted combined event rate seen in our population is similar to the predicted mortality in the original GAP cohort,13 we believe this difference in approach introduced minimal bias. There may have been referral bias in including only patients seen at a referral center; it has been previously reported that patients with IPF experience a steep decline in pulmonary function around the time of evaluation at a referral center.29 Given that this effect should be evenly distributed across comparison groups, it is unlikely to have influenced our ability to detect a difference in the rate of pulmonary function decline.
Conclusions
Our findings showed that pulmonary function trajectory does not vary based on disease severity as defined according to the GAP Index. We also found that 6-month declines in pulmonary function may add prognostic value to the baseline GAP stage. Further validation of our findings in additional cohorts is warranted, as well as a search for novel markers of future disease progression not tied to pulmonary function variables.
Acknowledgments
Author contributions: M. L. S. and K. R. F conceived and designed the study, had full access to all of the data, and take responsibility for the integrity of the data and the accuracy of the data analysis. M. L. S., M. X., and Y. Z. analyzed the data with supervision and assistance from S. M. and K. R.; F. N. T., K. K. B., A. U. W., S. L. S., and F. J. M. contributed data; and M. L. S. and K. R. F. prepared the manuscript. All other authors revised the manuscript and approved the final draft.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. M. reports receiving grants from the National Institutes of Health during the conduct of the study. A. U. W. reports personal fees from Actelion, Boehringer Ingelheim, InterMune, Roche, Bayer, Gilead, Takeda, MedImmune, Genentech, and Chiesi, outside the submitted work. F. J. M. reports grants from the National Institutes of Health; nonfinancial support from Bayer, Centocor, Gilead, and Promedior; and personal fees from Ikaria, Genentech, Nycomed/Takeda, Pfizer, Vertex, the American Thoracic Society, Inova Health System, MedScape, Spectrum Health System, University of Texas Southwestern, Stromedix/Biogen, Axon Communications, Johnson & Johnson, Genzyme, the National Association for Continuing Education, Boehringer Ingelheim, Veracyte, during the conduct of the study; personal fees from Forest, Janssen, GSK, Nycomed/Takeda, Actelion, Amgen, AstraZeneca, CSA Medical, Ikaria/Bellerophon, Genentech, Janssen, Merck, Pearl, Nycomed/Takeda, Pfizer, Roche, Sudler & Hennessey, the American College of Chest Physicians, CME Incite, Center for Health Care Education, Inova Health System, MedScape, Miller Medical, the National Association for Continuing Education, Paradigm, Peer Voice, Projects in Knowledge, St. John's Hospital, St. Mary's Hospital, University of Illinois Chicago, UpToDate, Wayne State University, Boehringer Ingelheim, Ikaria, Bayer, Grey Healthcare, Merion, Informa, Annenberg, and Forest, outside the submitted work. K.R.F. reports grants from the National Institutes of Health during the conduct of the study; personal fees from Boehringer Ingelheim, Fibrogen, Genentech, Gilead, Ikaria, ImmuneWorks, MedImmune, Novartis, Takeda, Vertex, Veracyte, Roche, and the Pulmonary Fibrosis Foundation; grants from ImmuneWorks and Bristol-Myers Squibb; and grants and personal fees from InterMune, outside the submitted work. None declared (M. L. S., M. X., N. T., K. K. B., S. L. S.).
Role of sponsor: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Tables, e-Figures, and e-Appendix can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by the National Institutes of Health [Grant K24 HL111316 to Dr Flaherty and Grant T32 HL007749-22].
Supplementary Data
References
- 1.Raghu G., Collard H.R., Egan J.J. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collard H.R., Moore B.B., Flaherty K.R. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176(7):636–643. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley B., Collard H.R., King T.E., Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 4.Collard H.R., King T.E., Jr., Bartelson B.B., Vourlekis J.S., Schwarz M.I., Brown K.K. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168(5):538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty K.R., Andrei A.C., Murray S. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med. 2006;174(7):803–809. doi: 10.1164/rccm.200604-488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flaherty K.R., Mumford J.A., Murray S. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168(5):543–548. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 7.Hanson D., Winterbauer R.H., Kirtland S.H., Wu R. Changes in pulmonary function test results after 1 year of therapy as predictors of survival in patients with idiopathic pulmonary fibrosis. Chest. 1995;108(2):305–310. doi: 10.1378/chest.108.2.305. [DOI] [PubMed] [Google Scholar]
- 8.Jegal Y., Kim D.S., Shim T.S. Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. Am J Respir Crit Care Med. 2005;171(6):639–644. doi: 10.1164/rccm.200403-331OC. [DOI] [PubMed] [Google Scholar]
- 9.King T.E., Jr., Tooze J.A., Schwarz M.I., Brown K.R., Cherniack R.M. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164(7):1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 10.Latsi P.I., du Bois R.M., Nicholson A.G. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med. 2003;168(5):531–537. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 11.du Bois R.M., Weycker D., Albera C. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(4):459–466. doi: 10.1164/rccm.201011-1790OC. [DOI] [PubMed] [Google Scholar]
- 12.Wells A.U., Desai S.R., Rubens M.B. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med. 2003;167(7):962–969. doi: 10.1164/rccm.2111053. [DOI] [PubMed] [Google Scholar]
- 13.Ley B., Ryerson C.J., Vittinghoff E. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Daniels C.E., Lasky J.A., Limper A.H. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181(6):604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 15.Demedts M., Behr J., Buhl R. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 16.King T.E., Jr., Behr J., Brown K.K. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(1):75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 17.King T.E., Jr., Bradford W.Z., Castro-Bernardini S. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 18.Noble P.W., Albera C., Bradford W.Z. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 19.Raghu G., Brown K.K., Bradford W.Z. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350(2):125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- 20.Raghu G., Brown K.K., Costabel U. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008;178(9):948–955. doi: 10.1164/rccm.200709-1446OC. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 22.Crapo R.O., Morris A.H. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis. 1981;123(2):185–189. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- 23.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 24.Macintyre N., Crapo R.O., Viegi G. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 25.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt S.L., Tayob N., Han M.K. Predicting pulmonary fibrosis disease course from past trends in pulmonary function. Chest. 2014;145(3):579–585. doi: 10.1378/chest.13-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richeldi L., Ryerson C.J., Lee J.S. Relative versus absolute change in forced vital capacity in idiopathic pulmonary fibrosis. Thorax. 2012;67(5):407–411. doi: 10.1136/thoraxjnl-2011-201184. [DOI] [PubMed] [Google Scholar]
- 28.Ley B., Bradford W.Z., Weycker D., Vittinghoff E., du Bois R.M., Collard H.R. Unified baseline and longitudinal mortality prediction in idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1374–1381. doi: 10.1183/09031936.00146314. [DOI] [PubMed] [Google Scholar]
- 29.Strand M.J., Sprunger D., Cosgrove G.P. Pulmonary function and survival in idiopathic vs secondary usual interstitial pneumonia. Chest. 2014;146(3):775–785. doi: 10.1378/chest.13-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.