Abstract
Objective
To determine if differences exist in the pharmacokinetics (PK) of levonorgestrel-based emergency contraception (LNG-EC) in obese and normal body mass index (BMI) users and test whether doubling the dose of LNG-EC in obese women increases total and free (active) LNG serum concentrations.
Study design
Healthy, reproductive-age women with obese and normal BMIs received 1.5 mg LNG orally (ECx1) and then in a subsequent menstrual cycle, the obese group also received 3mg LNG (ECx2). Dosing occurred during the follicular phase. Total and free LNG PK parameters were obtained via serum samples through an indwelling catheter at 0, 0.5, 1, 1.5, 2, and 2.5 hours. The primary outcome was the difference in total and free LNG concentration maximum (Cmax) between ECx1 and ECx2 in the obese group.
Results
A total of 10 women enrolled and completed the study (normal BMI = 5, median 22.8 kg/m2, range 20.8–23.7; obese BMI = 5, 39.5 kg/m2, range 35.9–46.7). The total LNG Cmax for obese subjects following ECx1 (5.57±2.48 ng/mL) was significantly lower than the level observed in normal BMI women (10.30±2.47, p=0.027). Notably, ECx2 increased the Cmax significantly (10.52±2.76, p=0.002); approximating the level in normal BMI subjects receiving ECx1. Free LNG Cmax followed a similar pattern.
Conclusion
Obesity adversely impacts both the total and free Cmax levels of LNG EC and this likely explains its lack of efficacy in obese women. Doubling the dose appears to correct the obesity-related PK changes but additional research is needed to determine if this also improves EC effectiveness in obese women.
Implications
This study demonstrates that obesity interferes with the pharmacokinetics of LNG EC, and that doubling the dose may be an effective strategy to improve its efficacy in obese women.
Keywords: pharmacokinetics, obesity, body weight, emergency contraception
Introduction
Approximately 50% of all pregnancies in the United States are unintended [1]. The availability of emergency contraception (EC) provides women with an additional line of defense against unintended pregnancy following unprotected intercourse with the potential to decrease the risk of pregnancy by 81–90% [2]. The leading form of EC, known as Plan B One Step® or Next Choice®, is available over-the-counter in the U.S. to adults and following a recent court decision (2013), is available to adolescents as well. The use of EC among reproductive-age women has doubled from 2006 to 2008 [3] and is likely to continue increasing with this recent legislative change. Unfortunately, the levonorgestrel (LNG)-based method appears to be significantly less effective in obese women, failing 4 times as often as in non-obese women [4]. The mechanism for this phenomenon is unknown but likely due to differences in LNG pharmacokinetics (PK) and not patient adherence given that EC is a single-dose therapy.
As a single-dose therapy, EC is likely reliant on achieving a rapid peak level at a critical time point prior to the LH surge [5–7]. Drug levels were not done in the Glasier [4] study but we suspect that the changes in LNG PK caused by obesity likely resulted in lower peak levels or a delay in time to reach the therapeutic level. Obesity has been proven to adversely affect the PK of combined oral contraceptives containing LNG and ethinyl estradiol, in particular half-life and clearance; these in turn, cause a delay in achieving maximum concentration (Cmax) levels and steady state [8–12]. As the PK profile of the LNG-EC is similar to that of LNG-based OCs, only a magnitude higher due to differences in the dosage [13], we believe these changes could explain the failure seen in EC users. We hypothesize that obesity impacts LNG PK such that the critical peak level needed to prevent the LH surge and ovulation is not achieved. However, baseline differences between normal and obese BMI EC-users have not been studied.
LNG clearance is highly dependent on the availability of unbound drug [14]. LNG is a highly bound drug, mainly to SHBG, with only a small fraction unbound (2–3%) [15,16]. In theory, drug clearance is a function of blood flow to the organ, drug enzyme/transporter activity (i.e. intrinsic clearance) and plasma protein binding. For a low clearance drug like LNG, blood flow is less critical thus plasma protein binding and intrinsic clearance are highly influential. Compared to normal BMI women, levels of sex hormone binding globulin are lower in the obese [17]. Since LNG is bound to SHBG, free fraction of hormone could be elevated resulting in unpredictable effects on clearance. Furthermore, it is unclear whether SHBG associated increase in free fraction would also alter free concentrations, the pharmacologically active form of the drug.
Due to the safety of progestins even at higher doses, many health care providers and expert panels have recommended that obese women take double the LNG EC dose (e.g. “take two”) to increase the effectiveness of the method. Although this strategy is one commonly used in pharmacotherapeutics [18], there is currently no evidence to support this approach for obese EC users.
The objectives of this study are to determine if differences exist in the PK of LNG-EC in obese and normal BMI-users, and to test whether dose escalation of LNG-EC in obese women increases total- and free-LNG levels. The overall goal of this research is to improve EC effectiveness and quality of clinical care for obese women seeking EC.
Materials and Methods
A prospective open-label study was conducted at Oregon Health & Science University (OHSU) in Portland, Oregon from March 2015 to August 2015. The OHSU Institutional Review Board approved the study protocol and all subjects underwent informed written consent.
Otherwise healthy, obese (BMI ≥30 kg/m2, n = 5) and normal (BMI <25 kg/m2, n = 5) reproductive-aged (18–35 years old) women with regular menstrual cycles (21–35 days) were recruited. Subjects were required to be either heterosexually abstinent or, if heterosexually active, to use a non-hormonal, non-IUD method of contraception. Major exclusion criteria included: metabolic disorders including uncontrolled thyroid dysfunction and Polycystic Ovarian Syndrome; impaired liver or renal function; actively seeking or involved in a weight loss program (must be weight stable); pregnancy, breastfeeding, or seeking pregnancy; recent (8 week) use of hormonal contraception; current use of drugs that interfere with metabolism of sex steroids; smokers.
All EC dosing occurred during the follicular phase of the menstrual cycle and ingestion occurred under direct observation. Both the normal and obese BMI groups received a standard oral dose of EC (ECx1, 1.5 mg LNG; Next Choice™, Actavis Pharma, Parsippany, NJ). In a subsequent cycle following at least a one-cycle washout, the obese group received a double dose of EC (ECx2, 3.0 mg LNG). PK parameters were obtained via serum samples through and indwelling catheter at 0, 0.5, 1, 1.5, 2, and 2.5 hours to evaluate the maximum serum LNG concentration (Cmax). Total and Free LNG serum concentrations were measured at all time points. Estradiol (E2), LH, progesterone (P4), albumin, and sex hormone binding globulin (SHBG) were obtained once at the beginning of each PK visit.
Assay characteristics
Serum samples were assayed at the Endocrine Technologies Support Core (ETSC) at the Oregon National Primate Research Center (ONPRC, Beaverton, Oregon http://www.ohsu.edu/xd/research/centers-institutes/onprc/research-services/research-support/endocrine-technology.cfm). The ultra-high performance liquid chromatography-tandem triple quadrupole mass spectrometry (LC-MS/MS) assays utilized for this study were developed following the FDA’s bioanalytical method validation including selectivity, accuracy, precision, recovery, calibration/standard curves, and stability. Total serum LNG levels were measured by (LC-MS/MS).One-hundred and fifty μl of serum were mixed with one hundred ul ultrapure water (Milli-Q, EMD Millipore, Billerica, MA) containing 0.3 ng/ml LNG-d6 isotopic standard (Toronto Research Chemicals, Toronto, ON, Canada) and added to a 400 μl SLE+ extraction plate (Biotage, Charlotte, NC). LNG was eluted with 2 × 900 μl dichloromethane (Sigma), dried with forced air and reconstituted in 25% (v:v) methanol in ultrapure water. Quality controls (QCs) were prepared by spiking LNG standard (Sigma) into normal human serum (Golden West Biologicals) at concentrations of 0.10 ng/ml and 1.00 ng/ml. QCs were subjected to the same SLE+ extraction procedure with four replicates in each assay. For standard curves, normal charcoal-stripped human serum (Golden West Biologicals) was spiked with LNG standard (Sigma) in methanol and serial diluted to final concentrations between 0.009 and 20 ng/ml. The spiked standards were then subjected to the SLE+ extraction procedure. LNG was eluted with a Raptor 2.7 μm Biphenyl 50 mm X 2.1 mm column. Mobile phase consisted of 0.2 mM ammonium fluoride (Sigma) in water (A) and methanol (B) with a flow rate of 0.2 ml/min. Gradient elution started at 70% B and linearly increased to 73% B over 1 minute followed by an isocratic hold for 1 minute followed by a linear increase to 76% over 1 minute and then linearly to 100% over 1 minute. After chromatography, 3.75 minutes were spent re-equilibrating the column back to 70% B for a total of 7.75 minutes/sample. LNG was detected in positive ion mode with multiple reaction monitoring (Shimadzu LCMS-8050 tandem triple-quadrupole with heated electrospray ionization (ESI)): LNG, 313.10→109.20 (quant), 313.10→245.25(qual), m/z; LNG-d6, 319.10→251.35(quant), 319.10→113.15 (qual), m/z. The dynamic range for the LNG standard curve was 0.009 to 20 ng/ml; intra-assay variation was 6% and inter-assay variation was 8% (n=3). The sensitivity of the LNG assay was 0.009 ng/mL as determined by the lowest integrated point on the calibration curve. Intra-assay and inter-assay variations were calculated using LNG values determined in a QC sample with 0.100 ng/mL or 1.500 ng/mL standard spiked into normal human serum. Variation was consistent using QC samples at both LNG concentrations.
For free serum LNG determination, 500ul of serum were centrifuged through Ultracel PL Regenerated Cellulose Centrifugal Filters (EMD Millipore). Filtrate volume was measured and then subjected to same analytical procedure as described for total serum LNG.
Serum E2, P, LH, and SHBG were analyzed by a Roche Modular E170 chemiluminescence-based automatic clinical platform (Roche Diagnostics, Indianapolis, IN). The sensitivities of the E2, P, LH, FSH and SHBG assays for the Roche E170 is 5 pg/ml, 30 pg/ml, 0.1 mIU/ml, 0.1 mIU/ml, and 0.35 nmol/L, respectively. The intra- and inter-assay variation with the Roche E170 is consistently less than 7% for all assays. Quality control samples and validations were repeated prior to each assay run. Serum albumin was measured by ELISA following the manufacturer’s instructions (Sigma, St. Louis, MO). The limit of sensitivity for this assay was 49.15 pg/ml, and the intra-assay variation was 8.5%. No inter-assay variation was calculated as all samples were analyzed in a single assay.
PK parameter analysis
LNG PK data were analyzed separately by non-compartmental method using Microsoft Excel 2010 (Richmond, WA). Cmax were observed values. Area under the curve (AUC0-2.5h) was calculated from time zero to last time point (2.5h) of measurable levels using the linear trapezoidal rule. Fraction unbound was computed as a ratio of free and total LNG concentrations and reported as a percentage.
Following a single dose administration of LNG 1.5 mg under fasting conditions, maximum plasma concentrations of levonorgestrel of 19.1 ng/mL were reached at 1.7 hours [19]. To determine sample size, we based our effect size on our prior results with a combined oral contraceptive (ethinyl estradiol + LNG) that demonstrated that the peak level LNG in obese women was approximately 67% that of normal BMI women (Edelman 2009). Our sample size of 5 per group had 85% power to detect a difference in Cmax of 19 ng/mL in normal BMI and 13 ng/mL in obese BMI with a pooled standard deviation of 2.8. Paired t-tests were performed to assess PK parameters between ECx1 versus ECx2 in the obese group. A two sample t-test was used to compare PK parameters of ECx1 (baseline) between obese and normal BMI women.
Results
Ten subjects signed informed consent (normal BMI n = 5; obese BMI, n= 5) and completed study procedures. The majority of the participants characterized themselves as Caucasian/not hispanic (9/10, 90%). With the exception of BMI [medians: normal BMI 22.8 kg/m2 (range 20.8–23.7), obese 39.5 kg/m2 (range 35.9–46.7)], there were no notable differences in the baseline demographic characteristics, ovarian hormones, albumin, or LH levels between the two BMI groups or between the obese cohorts two treatment cycles. No serious adverse events occurred but one normal BMI participant experienced emesis within 30 minutes of EC dosing. The evaluation was cancelled and she repeated her study visit in a subsequent menstrual cycle without mishap.
Pharmacokinetics
Compared to normal BMI subjects (10.3 ng/mL), total LNG Cmax for obese subjects following ECx1 was significantly lower (5.57 ng/mL, p = 0.027) (Table 1). However, when obese subjects received ECx2, a significant increase in LNG Cmax was observed (10.5 ng/mL, p=0.002) that approximated the Cmax of a normal BMI ECx1 user. The calculated AUC0-2.5h total showed a similar pattern.
Table 1.
PK parameters of exposure in obese and normal BMI women.
| Obese ECx1 | Normal ECx1 | Obese ECx2 | |
|---|---|---|---|
| Cmax, total (ng/mL) | 5.57±2.48a | 10.30±2.47 (p=0.027b) | 10.52±2.76 (p=0.002c) |
| Cmax, free (ng/mL) | 0.065±0.038 | 0.089±0.033 (p=0.37) | 0.126±0.025 (p=0.013) |
| AUCo-2.5h, total (h*ng/mL) | 9.05±4.95 | 17.62±5.89 (p=0.056) | 16.90±5.07 (p=0.044) |
| AUCo-2.5h, free (h*ng/mL) | 0.096±0.056 | 0.145±0.054 (p=0.250) | 0.196±0.044 (p=0.021) |
data represents mean±S.D of n=5 women.
compared to Obese ECx1 using 2-tailed student t-test.
compared to Obese ECx1 using paired t-test.
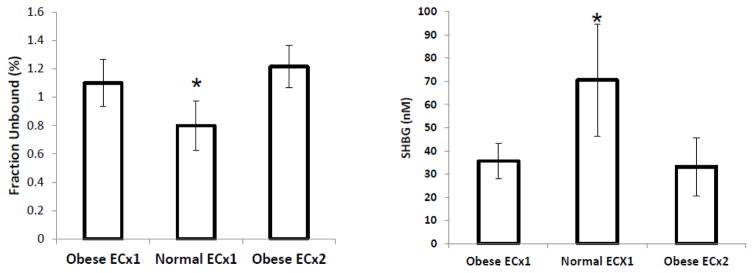
Levels of free LNG were approximately 1% of the total levels (see Table 1, Figure 1). Although there was no significant difference in the free Cmax between obese and normal BMI women that received ECx1 (0.065 ng/mL vs 0.089, p = 0.37), the absolute proportion of free drug was somewhat higher in the obese group (1.2% versus 0.8%, Figure 1). A higher free Cmax (0.126 ng/mL) was also seen in the obese ECx2 group as compared to both the obese ECx1 (0.065 ng/mL, p = 0.013) and the normal ECx1 (0.089 ng/mL, p = 0.081). Again, the findings with free AUC were comparable.
Figure 1.
Fraction of LNG unbound (free-fraction) and SHBG levels. [* denotes significantly (p<0.05) different from Obese ECx1]. Each bar represents mean±S.D of n=5 women.
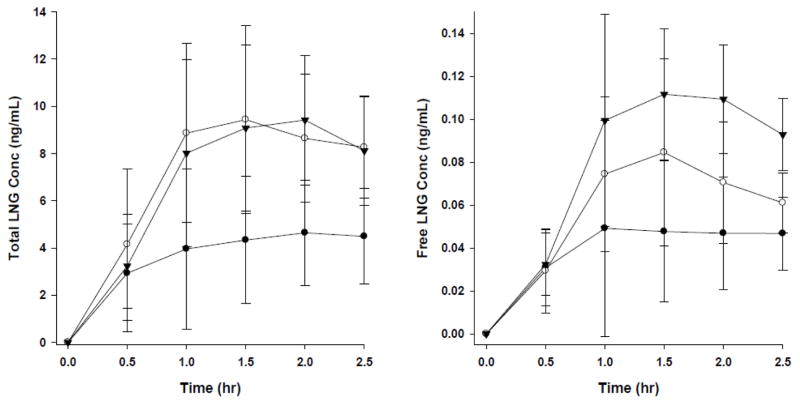
Concentration time curves are represented in Figure 2. Notably, the total concentration time curve for obese ECx2 mirrors the normal BMI ECx1 serum concentration time curve correcting the abnormality observed with ECx1 dosing. In terms of free concentration levels, the patterns are similar but the free LNG concentration level with ECx2 dosing exceeds the normal BMI ECx1 level. The higher fraction of LNG unbound in obese women likely explains this finding (Figure 1).
Figure 2.
Concentration time curves for total (left panel) and free (right panel) LNG serum concentrations (closed circle: obese ECx1, open circle: normal BMI ECx1, closed triangle: obese ECx2). Each data point represents mean±S.D of n=5 women.
The fraction of LNG unbound or free fraction percentage was inverse to SHBG levels (Figure 1). Serum SHBG levels were significantly lower in obese compared to normal BMI women (70.4 nmol/L±21.6; p=0.015). SHBG levels were similar in obese BMI women between the two dosing regimens (ECx1 35.6 nmol/L±6.8; ECx2 33.2 nmol/L±11.2; p=0.42). Compared to normal BMI women, the increase in fraction unbound was approximately 35% in obese women at both doses (Figure 1).
Discussion
Obesity adversely impacted both the Cmax and AUC of LNG EC. In fact, total LNG Cmax levels of obese ECx1 users are approximately 50% less than normal BMI users. Although free LNG Cmax levels of obese ECx1 were also lower, the difference between BMI groups was not significant likely reflecting the higher fraction of unbound drug due to lower SHBG levels in obese women. Although we do not have pharmacodynamic data to determine the impact of the observed PK changes, the direction of the changes correlates with the observed reduction in effectiveness seen in clinical trials [4].
Our data also supports that the simple intervention of doubling the dose in obese users may be an effective strategy to correct both the free and total LNG obesity-related PK changes. However, caution is recommended as additional research is needed to determine if this actually results in EC effectiveness for obese women. However, doubling the dose was well-tolerated by study participants and should be a very low risk intervention.
Although our study is limited in its ability to assess adverse outcomes due to its small sample size, we had sufficient power to detect the expected 30% difference in Cmax. As noted, a major limitation is the lack of pharmacodynamic data to evaluate ovulation or the main outcome of interest, pregnancy. Additionally, this is an abridged PK study. We did not perform extended PK sampling which limits the PK parameters we can calculate which may yield clues regarding the biochemical mechanism for these changes. For example, an extensive PK study design should yield a more accurate estimate of AUC, volume of distribution, half-life and clearance.
We utilized liquid chromatography-mass spectrometry (LC/MS), a highly sensitive and specific technique, for both the total and free LNG levels. The use of LC/MS, as opposed to radio-immunoassay, yielded accurate measurements of drug levels which are approximately 30–40% lower than the published values [19]. Our work as well as others has showed that radioimmunoassay overestimates LNG concentrations by ~20% [20,21]. Additionally, the matrix differences (serum in this study vs. plasma in the literature) likely contribute to the remainder of the difference [22,23]. More importantly, the free level analysis is a novel approach that we have developed in order to determine if the pharmacologically active form of the drug, i.e. “free” drug concentration, is different between normal and obese women. The relationship between free and total levels is different between BMI groups due to differences in protein binding. Our direct calculation of free-fraction LNG correlated with serum levels of SHBG at the time the drug was administered. While the exact free level of LNG needed to prevent ovulation is not known, it is reassuring that we achieved a level of active drug for obese women at or above the levels seen in normal BMI women with ECx2 dosing.
Obesity can affect any aspect of drug PK including absorption, distribution (including drug binding), metabolism, and excretion [15]. We are unable to determine a clear mechanism for obesity’s significant impact on LNG due to our limited PK sampling except that it does appear that binding proteins play a key role. Further studies that evaluate free-fraction through direct methodology may help elucidate the therapeutic drug levels needed for contraceptive effects at various sites (e.g. cervix, hypothalamus).
No prior studies exist comparing LNG serum levels between obese and normal BMI EC LNG users. Consistent with prior studies, we have demonstrated that obesity adversely impacts oral contraceptive steroid hormones [8–11]. As this is a one-time therapy and these PK studies were performed following direct observation of EC ingestion, we feel confident in the results. We believe that given the large differences in Cmax between obese and normal BMI women that this is the main reason for lack of LNG EC effectiveness in obese women [4]. Confirmation of the effect of PK normalization on ovulation inhibition by a double dose of EC will require appropriate pharmacodynamic studies and the endpoint of effectiveness will require a clinical trial. We look forward to future studies focusing on these end-points. However, given the great safety of LNG, until these studies are completed clinicians may wish to recommend a double dose of LNG EC for obese women when alternative regimens are not available.
Acknowledgments
Financial Support: Support for this research was from Medical Research Foundation of Oregon (Grant #1501) as well as grant support from National Institutes of Health the OHSU Oregon Clinical & Translational Research Institute (NIH NCRR 1 UL1 RR024120).
The authors would like to thank the Women’s Health Research Unit, the Department of Obstetrics & Gynecology, and the Oregon Clinical & Translational Research Institute at Oregon Health & Science University and the Endocrine Technologies Support Core at the Oregon National Research Primate Center.
Footnotes
Clinical trials#: NCT02408692.
Authors Disclosures: A. Edelman: consultant for World Health Organization, Gynuity Health Projects, Genzyme, and Agile Therapuetics. Nexplanon trainer for Merck. Author for UptoDate (Royalties received). J. Jensen has received payments for consulting from Agile Pharmaceuticals, Abbvie Pharmaceuticals, Bayer Healthcare, ContraMed, Evofem Inc, HRA Pharma, Merck Pharmaceuticals, Microchips, Teva Pharmaceuticals and the Population Council. He has also received research funding from Abbvie, Bayer, Merck, FHI360, Medicines 360, the Population Council, and the Bill & Melinda Gates Foundation. These companies and organizations may have a commercial or financial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU. G. Cherala, S Blue, and D Erikson have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health 2006. 2006;38:90–96. doi: 10.1363/psrh.38.090.06. [DOI] [PubMed] [Google Scholar]
- 2.Grimes D, Von Hertzen H, Piaggio G, Van Look DFA. Randomized controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428–433. [PubMed] [Google Scholar]
- 3.Kavanaugh ML, Williams S, Schwarz EB. Emergenecy contraception use and counseling after changes in United States prescription status. Fertil Steril. 2001;95:2578–2581. doi: 10.1016/j.fertnstert.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Glasier A, Cameron ST, Blithe D, Scherrer B, Mathe H, Levy D, Gainer E, Ulmann A. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84:363–367. doi: 10.1016/j.contraception.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Piaggio G, Von Hertzen H, Grimes DA, Van Look PFA. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353:721. doi: 10.1016/s0140-6736(98)05718-3. [DOI] [PubMed] [Google Scholar]
- 6.Piaggio G, Kapp N, Von Hertzen H. Effect on pregnancy rates of the delay in the administration of levonorgestrel for emergency contraception: a combined analysis of four WHO trials. Contraception. 2011;84:35–39. doi: 10.1016/j.contraception.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Johansson E, Brache V, Alvarez F, Faundes A, Cochon L, Ranta S, Lovern M, Kuman N. Pharmacokinetic study of different dosing regimens of levornorgestrel for emergency contraception in healthy women. Hum Reprod. 2002;17:1472–6. doi: 10.1093/humrep/17.6.1472. [DOI] [PubMed] [Google Scholar]
- 8.Edelman AB, Carlson NE, Cherala G, Munar MY, Stouffer RL, Cameron JL, Stanczyk FZ, Jensen JT. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80:119–127. doi: 10.1016/j.contraception.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelman A, Cherala G, Munar MY, Dubois B, McInnis M, Stanczyk FZ, Jensen JT. Prolonged monitoring of ethinyl estradiol and levonorgestrel levels confirms an alterered pharmacokinetic profile in obese oral contraceptive users. Contraception. 2013;87:220–226. doi: 10.1016/j.contraception.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelman A, Cherala G, Munar MY, McInnis M, Stanczyk FZ, Jensen JT. Correcting oral contraceptive pharmacokinetic alterations due to obesity. A randomized controlled trial. Contraception. 2014;90:550–556. doi: 10.1016/j.contraception.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westhoff CL, Torgal AH, Mayeda ER, Pike MC, Stanczyk FZ. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474–480. doi: 10.1016/j.contraception.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croxatto HB, Diaz S, Pavez M, Cardenas H, Larsson M, Johansson EDB. Clearance of levonorgestrel from the circulation following removal of NORPLANT subdermal implants. Contraception. 1988;38:509–523. doi: 10.1016/0010-7824(88)90155-2. [DOI] [PubMed] [Google Scholar]
- 13.Johannsson E, Brache V, Alvarez F, Faundes A, Cochon L, Ranta S, Lovern M, Kumar N. Pharmacokinetic study of different dosing regimens of levnorgestrel for emergency contraception in healthy women. Hum Reprod. 2002;17:1472–6. doi: 10.1093/humrep/17.6.1472. [DOI] [PubMed] [Google Scholar]
- 14.Rowland M. Protein binding and drug clearance. Clin Pharmacokinet Suppl. 1994;1:10–7. doi: 10.2165/00003088-198400091-00002. [DOI] [PubMed] [Google Scholar]
- 15.Edelman A, Cherala G, Stanczyk F. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314–232. doi: 10.1016/j.contraception.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Stanczyk F. All progestins are not created equal. Steriods. 2003;68:879–890. doi: 10.1016/j.steroids.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Lindstedt G, Lundberg P, Lapidus L, Lundgren H, Bengtsson C, Bjorntorp P. Low sex-hormone-binding globulin concentration as independent risk factor for development of NIDDM. Diab. 1991;40:123–128. doi: 10.2337/diab.40.1.123. [DOI] [PubMed] [Google Scholar]
- 18.Edwards DJ. Beneficial pharmacokinetic drug interactions. Adv Pharmacoepidem Drug Safety. 2012:S1–002. [Google Scholar]
- 19. [Accessed November 2, 2015];FDA prescribing information, Plan B one-step. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021998lbl.pdf.
- 20.Cherala G, Edelman A, Thornburg K. Traditional assay methodology, drug species, and perinatal growth: a perfect storm for oral contraceptive failure among obese women. J Women’s Health. 2014;23:855. [Google Scholar]
- 21.Callahan R, et al. Measuring total plasma levonorgestrel (LNG) levels among users of contraceptive implants: a comparison of radioimmunoassay and mass spectrometry methods. Meeting of the Fertility Control Club; 2015; Barcelona, Spain. [Google Scholar]
- 22.Ohshima T, Hasegawa T, Johno I, Kitazawa S. Variations in protein binding of drugs in plasma and serum. Clin Chem. 1989;35:1722–5. [PubMed] [Google Scholar]
- 23.El-Migdadi F, Qaw F. Serum and plasma levels of total and free testosterone and of sex hormone binding globulins in rats growing in the below sea level environment of the Jordan Valley. The Internet Journal of Endocrinology. 2009 [Google Scholar]