Abstract
IMPORTANCE
Alzheimer’s disease (AD) is the most common neurodegenerative disorder, and lacks effective disease modifying therapies. In 2001 we initiated a clinical trial of Nerve Growth Factor (NGF) gene therapy in AD, the first effort at gene delivery in an adult neurodegenerative disorder. This program aimed to determine whether a nervous system growth factor prevents or reduces cholinergic neuronal degeneration in AD patients. We present post-mortem findings in 10 subjects with survival times ranging from 1 to 10 years post-treatment.
OBJECTIVE
To determine whether degenerating neurons in AD retain an ability to respond to a nervous system growth factor delivered after disease onset.
DESIGN, SETTING, AND PARTICIPANTS
10 patients with early AD underwent NGF gene therapy using either ex vivo or in vivo gene transfer. The brains of all eight patients in the first Phase 1 ex vivo trial and two patients in a subsequent Phase 1 in vivo trial were examined.
MAIN OUTCOME MEASURES
Brains were immunolabeled to evaluate in vivo gene expression, cholinergic neuronal responses to NGF, and activation of NGF-related cell signaling. In two cases, NGF protein levels were measured by ELISA.
RESULTS
Degenerating neurons in the AD brain respond to NGF. All patients exhibited a trophic response to NGF, in the form of axonal sprouting toward the NGF source. Comparing treated and non-treated sides of the brain in three patients that underwent unilateral gene transfer, cholinergic neuronal hypertrophy occurred on the NGF-treated side (P>0.05). Activation of cellular signaling and functional markers were present in two patients that underwent AAV2-mediated NGF gene transfer. Neurons exhibiting tau pathology as well as neurons free of tau expressed NGF, indicating that degenerating cells can be infected with therapeutic genes with resulting activation of cell signaling. No adverse pathological effects related to NGF were observed.
CONCLUSIONS AND RELEVANCE
These findings indicate that neurons of the degenerating brain retain the ability to respond to growth factors, with axonal sprouting, cell hypertrophy and activation of functional markers. NGF-induced sprouting persists over ten years. Growth factor therapy appears safe over extended time periods and merits continued testing as a means of treating neurodegenerative disorders.
Trial Registration: NCT00087789 and NCT00017940
INTRODUCTION
Alzheimer’s disease (AD) is the most common neurodegenerative disorder, afflicting 50 million people worldwide. Therapies to slow the course of the disease do not exist, and constitute an objective of great medical importance. While amyloid modifying approaches have substantial mechanistic appeal for slowing disease progression, early clinical trial results with amyloid-depleting drugs have been disappointing, leading to the initiation of clinical trials in which amyloid-modifying treatment is initiated in pre-symptomatic or very early stage patients. There remains a great unmet need to identify therapies with the potential to slow disease progression and improve cognitive function in AD.
Nervous system growth factors prevent neuronal death in a variety of correlative animal models of AD, including amyloid overexpressing mice, aged rats and primates, and lesioned rats and primates 1–5. Of the approximately 50 identified nervous system growth factors, two are of particular relevance to AD: Nerve Growth Factor (NGF), and Brain-Derived Neurotrophic Factor (BDNF). NGF specifically prevents the death and stimulates the function of basal forebrain cholinergic neurons that undergo early and prominent degeneration in AD. NGF influences the nervous system in several species, including primates, and is present in the human brain 4, 6, 7. Indeed, NGF levels in the basal forebrain region decline in AD 8. BDNF prevents the death and stimulates the function of cortical neurons, representing a second candidate growth factor treatment for AD 1, 9. Work with NGF in models of AD was initiated approximately a decade earlier than BDNF, and it accordingly transitioned to human clinical trials in AD first 6, based on an extensive set of pre-clinical efficacy and safety studies.
In 2001 we initiated the first human clinical trial of gene delivery in an adult neurodegenerative disorder, administering the NGF gene to patients with early stage AD 6. We employed gene delivery of NGF for two reasons: first, NGF is a large and polar protein, thus it does not penetrate the blood brain barrier after peripheral administration and requires central administration to exert its effects on degenerating neurons. Second, if NGF broadly circulates throughout the brain, it elicits intolerable adverse affects, including pain and weight loss, by stimulating nociceptive and hypothalamic neurons, respectively 10, 11. Moreover, NGF induces Schwann cell migration into the brain 11. Ironically, these effects of broad NGF availability in the nervous system reflect its biological potency on mature neurons. Accordingly, growth factor testing in humans requires a long-term delivery method that achieves both central delivery and restricted distribution to only regions of degenerating basal forebrain cholinergic neurons. Gene therapy is one of the few means of achieving this goal.
We now report biological responses to NGF in the largest series of brain samples to date of humans that have undergone gene therapy: ten patients with AD. We find that NGF elicits classic “trophic” responses in the AD brain, including neuronal hypertrophy, axonal sprouting and activation of cell signaling. These findings provide evidence that growth factors consistently stimulate the functional state of degenerating neurons in chronic neurodegenerative disorders, constituting a rationale for the continued testing of growth factors as neuroprotective agents in human degenerative nervous system disorders.
METHODS
Standard protocol approvals, registrations, and patient consents
All aspects of the study were approved by the institutional review boards for each of the participating sites. Consents for autopsy were obtained from all patients per approved HRRP protocols. The clinical trial identifiers for described in the article are NCT00087789 and NCT00017940
Patients that were subjects of this anatomico-pathological study were enrolled in clinical trials over a time period ranging from 2001 to 2012. Eight patients were enrolled in the first Phase 1 clinical trial, which used methods of ex vivo gene therapy to deliver NGF. Briefly, autologous fibroblasts were expanded from skin biopsies, transduced to express human NGF using Moloney leukemia viral (MLV) vectors, and implanted into the basal forebrain region to act as biological “minipumps” for NGF secretion 6. The basal forebrain region contains cholinergic cell bodies that send their projections throughout the cortex and hippocampus; these projections are necessary for maintenance of cognitive function, and undergo atrophy in early AD.12–14 The clinical findings of the Phase 1 ex vivo study have been reported,6 suggesting increases in cortical PET metabolism and possible reductions in cognitive decline over a two-year observation period compared to pre-treatment rates of cognitive decline. Ten patients were enrolled in a second Phase 1 clinical trial from 2005 – 2007, in which the method of gene delivery was changed to in vivo gene therapy: adeno-associated viral (AAV) vectors (serotype 2) were injected into the basal forebrain region, genetically modifying cells of the brain itself, rather than employing grafts of autologous cells in the Phase 1 ex vivo study. AAV2-NGF vectors represent a simpler and less costly method of gene delivery, and result in long-term gene expression in the non-human primate brain of at least seven years duration.15 Vectors were prepared by Ceregene, Inc. as indicated in public filing documents (Recombinant Advisory Commitee filings) using good manufacturing process (GMP) practices. Subjects 1–3 received a total vector dose of 1·2×1010 vector particles; subjects 4-6 received a total vector dose of 5·8×1010 vector particles; and patients 7–10 received a total vector dose of 1·2 ×1011 vector particles.16
Enrollment criteria have been reported 6, 16; in brief, patients had a clinical diagnosis of probable AD based on NINDS criteria, were between the ages of 50 and 80, and had baseline MMSE scores in the range of 20–30 for the ex vivo trial and 16-26 for the in vivo trial.
Details of the surgical methods for gene delivery have been summarized previously.6 Briefly, MRI scans were performed with the patient in an MRI-compatible stereotaxic head frame to identify brain targets for cell or viral vector injection. In the ex vivo gene delivery study, cells were stereotaxically implanted into five sites equally spaced over the rostral-to-caudal extent of the cholinergic Nucleus Basalis of Meynert, a total distance of approximately 12 mm (i.e., injections were spaced at ~2·5 mm intervals; this spacing reflected the distance over which NGF spreads from gene delivery sites in non-human primates).16 In the in vivo gene therapy trial, three vector injections were made targeting the caudal two-thirds of the Nucleus Basalis of Meynert (NBM) that constitute the majority cholinergic projection to the cortex, spaced at ~2·5 mm intervals.
Patient characteristics are summarized in Table 1. Five males and five females were enrolled in the trial. The mean age at diagnosis was 67.5 ± 2.2 years and the mean age at gene transfer was 69.3 ± 2.2 years. Patients survived a mean time period of 5.4 ± 1.0 years after gene transfer. Three control AD brains were included in the analyses and consisted of two males and 1 female with a mean age at death of 79.3 ± 0.6 years (79, 79, and 80 years old) and Braak stage of V, IV, and IV, respectively.
Table 1.
Subject Characteristics
| Subject # |
Age at Diagnosis |
Age at Gene Transfer |
Years to Death After Gene Transfer |
Gender | MMSE at entry |
Type of Gene Delivery |
Braak Stage |
|---|---|---|---|---|---|---|---|
| 1 | 59 | 60 | 5 | F | 13 | Ex Vivo | V/VI |
| 2 | 69 | 70 | 7 | F | 20 | Ex Vivo | V/VI |
| 3 | 70 | 72 | 10 | F | 27 | Ex Vivo | V/VI |
| 4 | 71 | 72 | 7 | F | 21 | Ex Vivo | V/VI |
| 5 | 71 | 72 | 3mo | M | 20 | Ex Vivo | V/VI |
| 6 | 69 | 73 | 9 | M | 25 | Ex Vivo | V/VI |
| 7 | 63 | 64 | 7 | F | 18 | Ex Vivo | V/VI |
| 8 | 75 | 76 | 5 | M | 25 | Ex Vivo | V/VI |
| 9 | 53 | 56 | 3 | F | 17 | In Vivo | V/VI |
| 10 | 75 | 78 | 11mo | M | 21 | In Vivo | V/VI |
Histology and Analyses
Consents for autopsy were obtained from all patients per approved HRRP protocols. Brains were harvested and placed in a solution of 4% paraformaldehyde for 72 hours, then switched to a 20% glycerin solution for two weeks. The brains were blocked into anterior, middle and posterior sections, and blocks containing the Nucleus Basalis of Meynert were sectioned on a freezing microtome set at 40 µm intervals. To evaluate effects of NGF delivery in the AD brain, we performed the following sets of tissue labels: 1) Nissl stain for general cellular morphology, including visualization of fibroblast grafts in ex vivo patients; 2) NGF immunolabeling (NGF antibody: 1:500 dilution for fluorescence, 1:1000 dilution for light-level labeling) 17; 3) p75 immunolabeling, which detects the low-affinity NGF receptor and is expressed by NGF-responsive cholinergic neurons of the basal forebrain 2–4, to assess NGF whether gene delivery altered the size or function of NGF-responsive cells; (1:1000 anti-human monoclonal antibody, clone NGFR5, produced by a hybridoma cell line, gift of Dr. M Bothwell); 4) c-fos, to assess activation of cell signlaing classically related to NGF signaling (1:100 dilution, goat anti-human, Santa Cruz Biotechnology); and 5) Phospho-tau immunolabeling to detect neurofibrillary degeneration (AT180; 1:50 dilution Mouse anti-PHF, Thermo). In addition, brain tissue from different regions (frontal cortex, superior temporal cortex occipital cortex, substantia nigra, basal ganglia, amygdala, and hippocampus/entorhinal cortex) were processed at the Human and Animal Tissue Technology Core at UCSD using an A-beta antibody (69D at 1:1500 from Dr. Edward Koo), Tau phosphorylation (PHF-1 at 1:300 from Dr. Peter Davies) and phospho-synuclein antibody (81A at 1:15000 from Dr. Virginia Lee); these sections were used to determine the Braak stage of the patients.
For light-level immunolabeling, sections were rinsed in 0.1M tris-buffered saline (TBS), incubated in 0·1M Sodium Periodiate for 20 min, blocked in 3% donkey serum and 2% BSA in 0.05% Triton-X in TBS for 1 hr, and incubated in primary antibody (2.5 µg/ml for the 192 IgG) in 1% donkey serum + 1% BSA in 0.05% Triton-TBS for 72 hr at 4°C. Bound antibodies were detected by incubating sections in 1.5 µg/ml biotinylated IgG (Vector Labs, Burlingame, CA) for 2 hr and in an avidin-biotin peroxidase reagent (1:250 dilution ABC Elite, Vector Labs) for 90 min. Sections were then rinsed in imidazole-acetate buffer (pH = 7·4) and treated with a solution containing 0.05% diaminobenzidine tetrahydrachloride, 2.5% nickel ammonium sulfate and 0.15% hydrogen peroxide in imidazole-acetate solution. For NGF light level immunolabeling, sections first went through an antigen retrievel protocol and were post-fixed with 2% paraformaldehyde and 0.2% parabenzoquinone.
For fluorescent immunolabeling, sections were post-fixed in 2% paraformaldehyde and 0.2% parabenzoquinone. To block autofluorescence, sections were treated with 0.1M sodium borohydride for 30 min followed by 30 min in 0.5% Sudan black. Sections then underwent blocking with 3% donkey serum and 2% bovine serum albumin in TBS. Sections were incubated in primary antibodies for 72 hr at 4°C, and were detected using AlexaFluor secondary IgG antibodies and 1:1000 dapi for nuclear labeling; c-fos labeling was enhanced using a tyramide signal amplifcation (TSA) protocol.
Quantification of the size of p75 cell bodies were performed in three patients that received unilateral transplantation of the autologous fibroblasts expressing NGF. Using stereological microscope system (StereoInvestigator; Microbrightfield Bioscience), the size of the p75 cells were measured in the intermediate region of the NBM on the side of the graft and in the control hemisphere. A counting frame of 300×300 µm was used to with a sampling grid of either 600×600 (1:4 sample ratio) or 900×900 µm (1:9 sample ratio) to sample p75 neuron in the intermediate NBM. Cell body size was measured using a cross-sectional area of the soma at the level of the cell body based on the intersection of 6-ray extension from the nucleus.
NGF ELISA
NGF levels were measured by ELISA in one patient that received AAV2-NGF and survived for 11 months (patient 10 in Table 1), and another patient that underwent ex vivo NGF gene transfer and survived for 7 years (patient 7 in Table 1), using methods previously described 18. Briefly, brains were blocked into 1cm-thick coronal segments. The crossing of the anterior commissure across the midline demarcated the approximate first deposit of AAV2-NGF, while the presence of the mamillary bodies demarcated the approximate location of the third deposit. Brain punches were extracted from these regions, slightly dorsal to the known location of the cholinergic cell bodies, using 2mm diameter skin biopsy punches. These specimens were snap frozen to −80°C. When all specimens were collected, NGF ELISA was performed as reported 18.
Statistics
Within-subjects comparisons were made using paired t-test with a significance criterion of P<0.05. Results are reported as mean ± SEM.
RESULTS
Pathological Evaluation of Brains
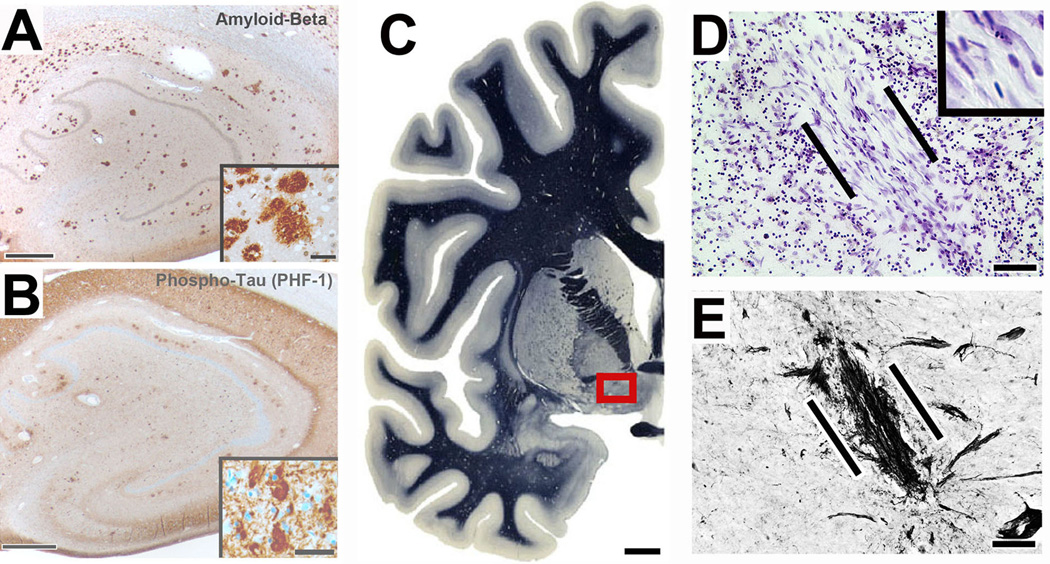
A pathological diagnosis of AD was confirmed in all study subjects (Fig. 1), with late-stage disease evident in each subject at the time of death (Braak stage V/VI, Table 1). Labeling for β-amyloid and tau showed classic signs of AD in all patients, including NGF-treated subjects and control AD brains (Fig. 1A–B). In patients treated with autologous fibroblasts genetically modified to secrete NGF (ex vivo gene therapy), surviving deposits of fibroblasts were observed within the region of the Nucleus Basalis of Meynert up to ten years post-gene delivery (Fig. 1C–E).
Figure 1. AD pathology and site of gene delivery.
All subjects in this study exhibited Alzheimer’s pathology; a case seven years post-gene transfer is shown. (A) Hippocampus shows numerous plaques. Inset shows diffuse and dense-core plaques. (B) Tangles and dense neurofibrillary tau pathology are present, evident also in inset. (C) Overview of left half of normal human brain; boxed region indicates region containing neurons of nucleus basalis of Meynert that are targeted by NGF gene delivery. Myelin stain reproduced by permission from Mai et al., Atlas of the Human Brain, 2003, Elsevier 36. (D) Nissl stain of graft of autologous fibroblasts, genetically modified to secrete human NGF and injected into the nucleus basalis. Cells were injected seven years previously; graft is indicated between lines. Cells survive and exhibit typical fibroblast morphology (inset). (E) In the adjacent section of Panel D, p75 neurotrophin receptor immunolabeling shows basal forebrain cholinergic axons penetrating into the graft in a linear fashion and this concentration of p75 fibers are only present at the sites of grafts (confirmed by Nissl staining). Scale bar A, 300µm (Inset 50µm); B, 300µm (Inset 50µm); C, 6mm; D, 30µm; E, 25µm.
NGF Expression After NGF Gene Transfer
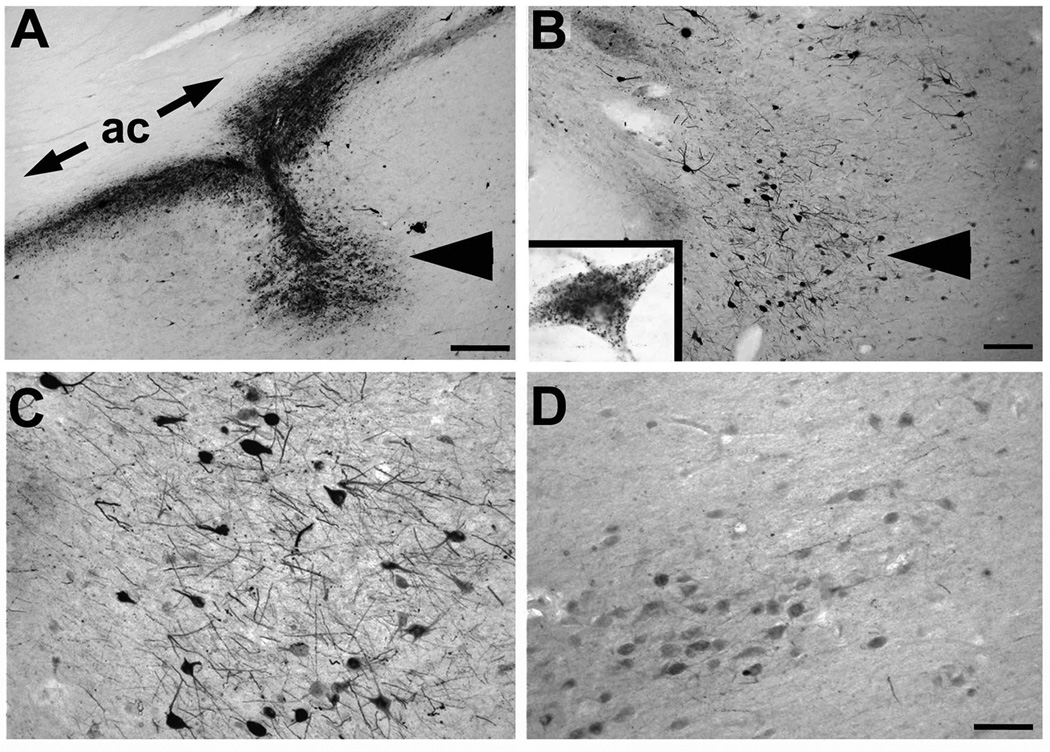
The two patients that underwent in vivo AAV2-NGF mediated gene therapy exhibited targeting of the vector to the region of the Nucleus Basalis of Meynert, revealed by NGF immunolabeling, one and three years after gene transfer, respectively (Fig. 2A–D). Neuronal NGF accumulation was distinctly more intense in neurons adjacent to sites of NGF delivery compared to neurons more distantly located (>3mm; Fig. 2C,D). The presence of NGF labeling indicated that gene expression persisted throughout this time period. NGF protein levels by ELISA were measured in one AAV2-NGF treated patient who underwent gene delivery one year earlier and detected 1,230 pg NGF/gm tissue in the Nucleus Basalis of Meynert; an adjoining control region of the Nucleus Basalis located 5mm from the site of NGF delivery in the same patient contained only 22 pg NGF/gm tissue. Thus, AAV2-NGF gene delivery increased NGF protein by more than 50-fold one year after gene delivery in this subject. In a patient that underwent ex vivo gene transfer using grafts of autologous fibroblasts who died 7 years after treatment, NGF ELISA from the same region detected 50 pg NGF/gm tissue; this two-fold increase compared to control regions is within the variance of the assay.
Figure 2. AAV2-NGF gene expression.
(A) NGF labeling shows site of NGF gene delivery in nucleus basalis of Meynert (arrowhead), under anterior commissure (ac). Patient injected three years previously. (B) Another site from same brain showing NGF uptake in neurons in region of nucleus basalis (arrowhead). Inset shows single neuron with granular intraneuronal labeling. (C) Higher magnification of NGF-expressing neurons, compared to (D) less intense labeling in nucleus basalis neurons located 3mm from injection site. Scale bar A 325µm; B 250µm; C–D, 100µm.
Trophic Responses to NGF in the Alzheimer’s Disease Brain
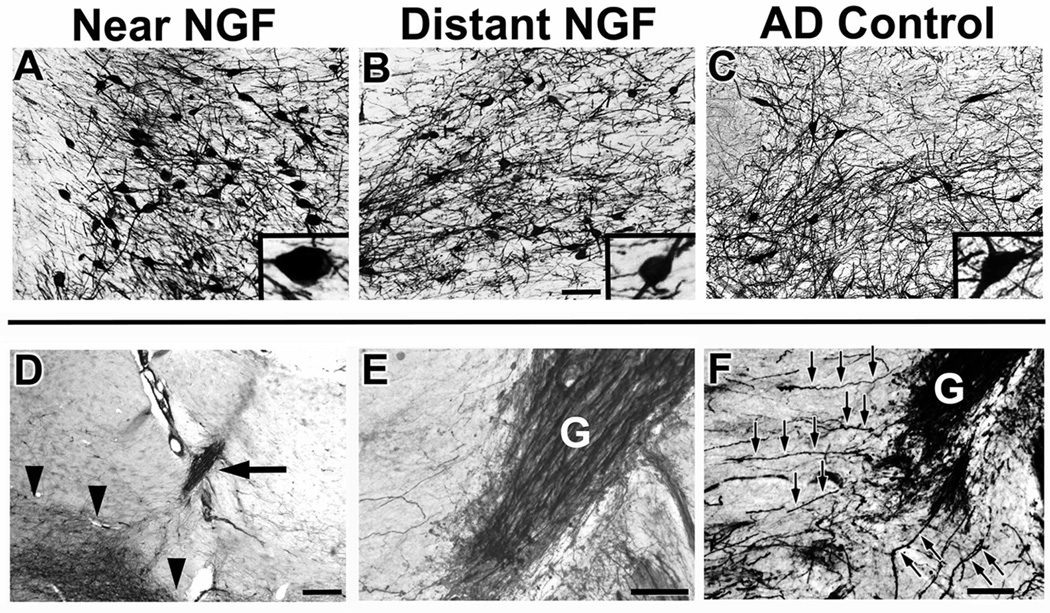
Each graft in patients in the ex vivo gene therapy trial showed penetration by cholinergic axons labeled for p75, the low affinity neurotrophin receptor that is exclusively expressed by cholinergic neurons and their axons in the basal forebrain region (Figs. 1E and 3). The growth of cholinergic axons toward and within an ectopic location reflects sprouting of these axons toward the growth factor produced by gene delivery, a classic “trophic” effect 4 has been reported in numerous animal studies 4, 19, 20. Cholinergic axonal sprouting into sites of NGF gene delivery was evident in all subject brains, including the longest surviving ex vivo gene transfer patient, ten years after treatment, that showed p75 axons (Fig. 3D–E).
Figure 3. Cholinergic neuronal hypertrophy and sprouting.
Labeling for p75, a neurotrophin receptor expressed on cholinergic neurons of the nucleus basalis of Meynert. A-C, images three years after AAV2-NGF delivery; D-F, images seven years after ex vivo gene transfer. (A) Cholinergic neurons labeled for p75 within the zone of NGF transduction, (B) 3mm from the zone of transduction, and (C) in control AD brain of the same Braak stage. Cells near to NGF transduction region appear larger. Inset, higher magnification views of typical neuron from each region. (D) Graft of fibroblasts transduced to secrete NGF (arrow), adjacent to nucleus basalis of Meynert (indicated by arrowheads). (E) Graft (g) at higher magnification is densely penetrated by p75-labeled axons arising from nucleus basalis of Meynert. These axons sprouting toward graft, a classic trophic response. (F) F) p75-labeled axons from nucleus basalis of Meynert sprouting toward graft. Individual axons coursing toward graft are evident (arrows). Scale bars A–C 125µm; D 500µm; E–F 100µm.
Effects of NGF on Cell Size and Functional Markers
p75-labeled cholinergic neurons located in regions of NGF delivery appeared to exhibit hypertrophy compared to neurons more remotely located (>3mm) and compared to neurons in AD control brains (Fig. 3A–C). To quantify this effect, cholinergic cell size in p75 labeled sections was measured in three subjects (Patients 1, 2, 5; Table 1) who received unilateral delivery of NGF to the Nucleus Basalis; mean somal area was measured on the treated vs. the untreated side of the brain. Significant hypertrophy was present on the treated side: mean cholinergic cell soma area was 948 ± 44 µm2 on the treated side and 890 ± 28 µm2 on the untreated side of the brain (Paired t-test: t(2)=3.62, p<0.05), a difference of 7%.
AAV2-NGF vectors infected neurons with nearly complete specificity: double labeling for NGF and NeuN, GFAP and Olig-2 demonstrated that >99% of cells expressing a cell-specific marker were neurons (data not shown), consistent with findings in studies of AAV2 gene transfer in the non-human primate brain 21.
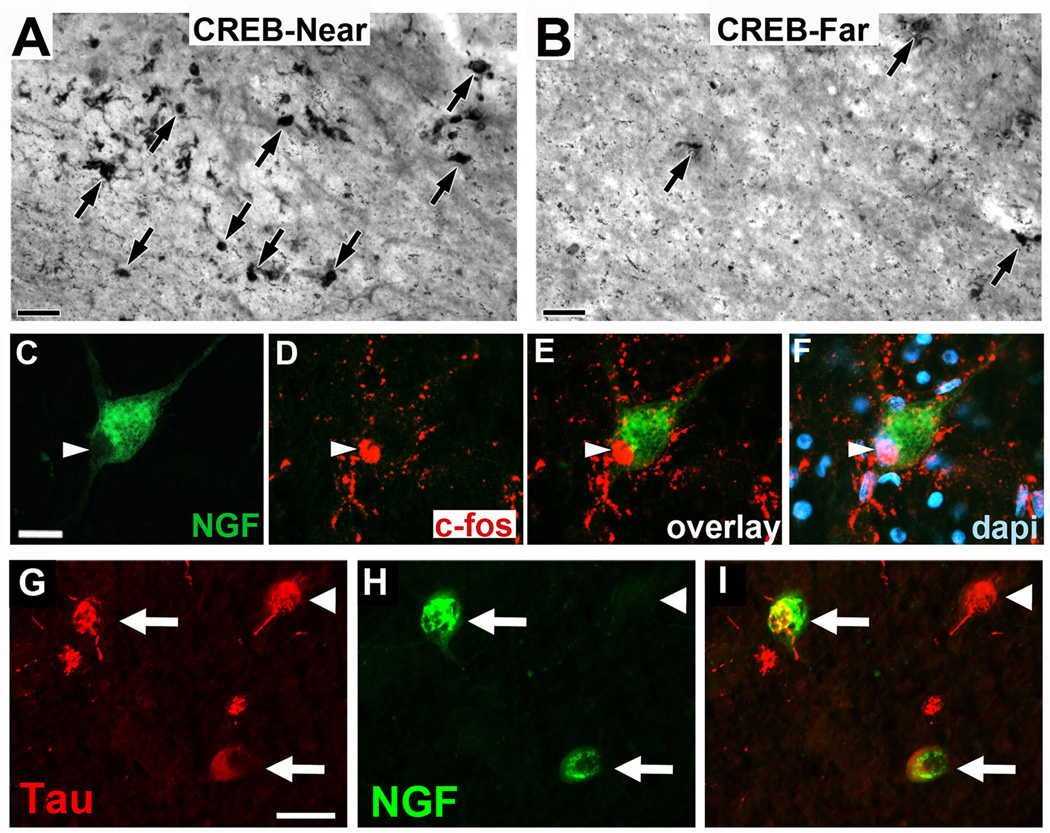
NGF gene transfer is predicted to stimulate the functional state of the neuron, based on canonical activation of Creb and c-fos signaling after trophic stimulation22. Phospho-Creb and c-fos expression were examined in the two patients that underwent AAV2-NGF gene transfer in the Nucleus Basalis of Meynert; these subjects were chosen because NGF expression was unequivocally detected by immunolabeling in both subjects (Fig 2 and ELISA data, above). Compared to regions outside the zone of NGF delivery, both Creb and fos expression were elevated (Fig. 4A–F). Moreover, neurons actively expressing tau pathology were infected by the AAV2-NGF vector, shown by co-labeling for NGF and Tau (Fig. 4G–I), indicating that actively degenerating neurons retain an ability to incorporate a therapeutic vector.
Figure 4. Cell activation after NGF gene delivery.
(A, B) In regions of NGF expression (CREB-Near), there is induction of phospho-CREB expression, a canonical mediator of downstream neurotrophin signaling and cell activation 22, compared to regions of the NBM 3mm distant from site of NGF injection (CREB-Far). Patient received AAV2-NGF three years earlier. Similar response is evident in patient who received AAV2-NGF and survived one year (not shown). (C–F) An individual neuron expressing NGF (green) also expresses c-fos, another canonical marker of neurotrophin-mediated activation of cell signaling 22. c-fos labeling is nuclear (arrowheads), as expected. This image is also from the brain of the patient that underwent AAV2-NGF gene transfer three years earlier. (G–I) NGF expression in degenerating neurons expressing Tau Pathology. (G) Neurons in nucleus basalis of Meynert exhibit characteristic Tau pathology, illustrated by tau phosphorylation (AT180 antibody); (H) One of the neurons from panel A is also expressing NGF, shown in the merge panel; (I) These findings indicate that actively degenerating neurons can be infected by AAV2 vectors to express a potentially therapeutic gene product. Scale bars A–B 50µm, C–F 35µm, G–I 25 µm.
Safety: Absence of Off-Target Effects of NGF
In rat and non-human primate studies, NGF distribution throughout the neuraxis after intraventricular or intrathecal infusions results in Schwann cell migration and hypertrophy in the subpial space of the brainstem and spinal cord 11. This is a potential adverse effect that is not observed after gene delivery to focal parenchymal targets in animal studies 4, 23, 24. In all 10 human brains examined in this study, sectioning of the medulla failed to show evidence of Schwann cell hyperplasia (eFig. 1A–B). No tumor formation or evidence of neural toxicity, other than histological changes associated with AD, were detected.
DISCUSSION
Evidence from ten human AD patients that underwent NGF gene transfer over a period ranging from one month to ten years indicates that growth factors consistently promote classic “trophic” responses in neurons of the degenerating human brain. Axons sprouted toward the local source of NGF in every brain examined, following concentration gradients of NGF4. Sprouting is a classic neuronal response to trophic factors applied to the brain 4, 25–29. Cell hypertrophy, another classic response to a trophic stimulus 4, 30, 31, was also observed in the brains of three patients that underwent unilateral ex vivo NGF gene transfer; we measured cholinergic cell size in these subjects because the contralateral, untreated side of the brain provided a within-subject control. Further evidence for activation of growth factor-induced canonical trophic signaling 22 was observed in two patients that underwent AAV2-NGF in vivo gene transfer, with increased neuronal labeling for Creb in regions of gene delivery. Collectively, these anatomical findings support the rationale for clinical trials to test the hypothesis that sustained growth factor delivery over time can reduce cell degeneration and stimulate cell function in chronic neurodegenerative disorders, thereby slowing functional decline. A Phase 2 multicenter, randomized, sham surgery-controlled trial of AAV2-NGF gene delivery is underway that is measuring cognitive outcomes in AD patients.
The present study presents anatomical findings from the largest cohort to date of patients in any gene transfer clinical trial. Previous case reports suggested that growth factors could influence trophic responsiveness in AD 6 and Parkinsons disease 32, but the consistency and extent of these responses across patient cohorts have not been examined previously. The present findings demonstrate that every subject in an extended cohort of 10 patients exhibits trophic responses, reflecting the consistency and potency of the trophic response despite the presence of an ongoing and severe neurodegenerative process. We have demonstrated that cells exhibiting tau pathology can also express NGF, suggesting that individual neurons affected by neurodegeneration retain the ability to become infected with viral vectors and express a potentially therapeutic gene.
We also present the first actual measure of growth factor protein levels after gene transfer in the human brain by ELISA, and find that levels of the growth factor exceed physiological levels by approximately 50-fold in one patient that underwent AAV2-NGF gene transfer one year earlier. The AAV2 vector expressed NGF from the relatively potent CAG promoter, which has sustained in vivo gene expression for at least 6 years in the non-human primate brain 33. ELISA was performed in one other subject, a patient that underwent ex vivo NGF gene transfer 7 years earlier; in this patient, levels of NGF in the basal forebrain showed a small increase of 2-fold compared to a control region of the same brain, but this small increase could be within the variance of the assay. The lower level of NGF at this extended 7-year time points suggests, as expected, that gene expression after ex vivo gene transfer declines over time. In patients that underwent ex vivo gene transfer, NGF was expressed by the MLV promoter, which is generally less potent than the CAG promoter.
Moreover, we demonstrate safety of growth factor gene transfer over the longest time periods yet examined: 7 years. Over this protracted time period, there remains persistent evidence of trophic responses to NGF. This raises the possibility that once-in-life treatment with growth factor gene transfer could enable protection for very extended periods of time without re-treatment.
Finally, we provide the greatest amount of long-term safety to date in a pathological study after gene transfer in the nervous system: growth factor gene expression in vivo did not lead to a detectable pathological change that has previously been reported after broad NGF distribution through the cerebrospinal fluid: the migration and proliferation of Schwann cells in the subpial space of the medulla 11. Moreover, at sites of NGF gene transfer in the Nucleus Basalis, there was no evidence of localized cell toxicity resulting from gene transfer, of broader cell toxicity, or of tumor formation.
These findings support the rationale for the continued development of growth factor therapy to determine whether this potent class of biologically active molecules will slow neurodegeneration and activate the functional state of neurons in human disease. A phase 2 multicenter trial of NGF gene therapy in Alzheimer’s disease, and a phase 2 trial of GDNF gene therapy in Parkinson’s disease (clinicaltrials.gov), are addressing this possibility. Preceding studies indicate that the types of trophic responses elicited by NGF in the present study have been associated with amelioration of cognitive deficits in animal models of neurodegeneration 20, 34, 35. Moreover, a number of new neurological gene therapy indications are undergoing clinical translation, including trials in spinal muscular atrophy, retinitis pigmentosa, Leber’s Congenital Ameurosis, Parkinson’s disease and other disorders
CONCLUSIONS
NGF gene therapy administered to patients with established Alzheimer’s disease results in classic trophic responses, characterized by axonal sprouting in all patients examined. Responses to NGF persist up to ten years after gene transfer. Subsets of patients examined for neuronal hypertrophy and Creb activation provide additional evidence for trophic activity. No adverse pathological effects are observed over this time period, supporting the safety and rationale for expanded clinical programs underway in Alzheimer’s disease, Parkinson’s disease and other neurological indications.
Supplementary Material
Acknowledgments
We thank R. Torres, M. Mateling, H. Zhang, N. Du, A. Hoang, and A. Blesch for technical assistance. Funding: This work was supported by the NIH (AG1035, AG043416), the Veterans Administration and the Alzheimer’s Association. Phase 1 ex vivo gene therapy clinical trials costs were generously supported by the Donald and Darlene Shiley Family Trust. Phase 1 in vivo gene therapy clinical trials costs were supported by Ceregene, Inc., but did not fund the present post-mortem analysis of specimens.
Footnotes
List Disclosures for all Authors: M.H.T. was the scientific founder of Ceregene, Inc., but has no present financial interest in the company. The other authors have no disclosures.
Author Contribution: M.H.T., S.R., E.M., and A.H.N contributed to the design of the study; M.H.T., D.B., R.B, and M.M.P contributed to performing the clinical trials; P.K., and S.R. performed pathological assessment and relevant histology of the brains; J.H.Y. and A.H.N participated in histology, quantification, and analyses of the brains; J.M.C. performed the NGF ELISA, M.H.T., J.H.Y., and A.H.N assisted in the statistical analyses, M.H.T., S.R., E.M., J.H.Y., and A.H.N. contributed to the interpretation of the data and writing the manuscript.
REFERENCES
- 1.Nagahara AH, Merrill DA, Coppola G, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kordower JH, Winn SR, Liu YT, et al. The aged monkey basal forebrain: rescue and sprouting of axotomized basal forebrain neurons after grafts of encapsulated cells secreting human nerve growth factor. Proc Nat Acad Sci. 1994;91:10898–10902. doi: 10.1073/pnas.91.23.10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuszynski MH, U HS, Amaral DG, Gage FH. Nerve growth factor infusion in the primate brain reduces lesion-induced cholinergic neuronal degeneration. J Neurosci. 1990;10:3604–3614. doi: 10.1523/JNEUROSCI.10-11-03604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conner JM, Darracq MA, Roberts J, Tuszynski MH. Nontropic actions of neurotrophins: subcortical nerve growth factor gene delivery reverses age-related degeneration of primate cortical cholinergic innervation. Proc Nat Acad Sci. 2001;98:1941–1946. doi: 10.1073/pnas.98.4.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagahara AH, Mateling M, Kovacs I, et al. Early BDNF Treatment Ameliorates Cell Loss in the Entorhinal Cortex of APP Transgenic Mice. J Neurosci. 2013;33:15596–15602. doi: 10.1523/JNEUROSCI.5195-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuszynski MH, Thal L, Pay M, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 7.Goedert M, Fine A, Dawbarn D, Wilcock GK, Chao MV. Nerve growth factor receptor mRNA distribution in human brain: normal levels in basal forebrain in Alzheimer's disease. Mol Brain Res. 1989;5:1–7. doi: 10.1016/0169-328x(89)90011-9. [DOI] [PubMed] [Google Scholar]
- 8.Scott SA, Mufson EJ, Weingartner JA, Skau KA, Crutcher KA. Nerve growth factor in Alzheimer's disease: Increased levels throughout the brain coupled with declines in nucleus basalis. J.Neurosci. 1995;15:6213–6221. doi: 10.1523/JNEUROSCI.15-09-06213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blurton-Jones M, Kitazawa M, Martinez-Coria H, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Nat Acad Sci. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams LR. Hypophagia is induced by intracerebroventricular administration of nerve growth factor. Exp.Neurol. 1991;113:31–37. doi: 10.1016/0014-4886(91)90143-z. [DOI] [PubMed] [Google Scholar]
- 11.Winkler J, Ramirez GA, Kuhn HG, et al. Reversible Schwann cell hyperplasia and sprouting of sensory and sympathetic neurites after intraventricular administration of nerve growth factor. Ann Neurol. 1997;41:82–93. doi: 10.1002/ana.410410114. [DOI] [PubMed] [Google Scholar]
- 12.Wenk GL. The nucleus basalis magnocellularis cholinergic system: one hundred years of progress. Neurobiol Learn Mem. 1997;67:85–95. doi: 10.1006/nlme.1996.3757. [DOI] [PubMed] [Google Scholar]
- 13.Mufson EJ, Ma SY, Dills J, et al. Loss of basal forebrain P75(NTR) immunoreactivity in subjects with mild cognitive impairment and Alzheimer's disease. J Comp Neurol. 2002;443:136–153. doi: 10.1002/cne.10122. [DOI] [PubMed] [Google Scholar]
- 14.Counts SE, Nadeem M, Wuu J, Ginsberg SD, Saragovi HU, Mufson EJ. Reduction of cortical TrkA but not p75(NTR) protein in early-stage Alzheimer's disease. Ann Neurol. 2004;56:520–531. doi: 10.1002/ana.20233. [DOI] [PubMed] [Google Scholar]
- 15.Hadaczek P, Eberling JL, Pivirotto P, Bringas J, Forsayeth J, Bankiewicz KS. Eight years of clinical improvement in MPTP-lesioned primates after gene therapy with AAV2-hAADC. Mol Ther. 2010;18:1458–1461. doi: 10.1038/mt.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rafii MS, Baumann TL, Bakay RA, et al. A phase1 study of stereotactic gene delivery of AAV2-NGF for Alzheimer's disease. Alzheimers Dement. 2014;10:571–581. doi: 10.1016/j.jalz.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Conner JM, Muir D, Varon S, Hagg T, Manthorpe M. The localization of nerve growth factor-like immunoreactivity in the adult rat basal forebrain and hippocampal formation. J Comp Neurol. 1992;319:454–462. doi: 10.1002/cne.903190310. [DOI] [PubMed] [Google Scholar]
- 18.Conner JM, Varon S. Characterization of antibodies to nerve growth factor: assay-dependent variability in the cross-reactivity with other neurotrophins. J Neurosci Methods. 1996;65:93–99. doi: 10.1016/0165-0270(95)00151-4. [DOI] [PubMed] [Google Scholar]
- 19.Chen KS, Gage FH. Somatic gene transfer of NGF to the aged brain: behavioral and morphological amelioration. J Neurosci. 1995;15:2819–2825. doi: 10.1523/JNEUROSCI.15-04-02819.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuszynski MH, Gage FH. Bridging grafts and transient NGF infusions promote long-term CNS neuronal rescue and partial functional recovery. Proc. Nat. Acad. Sci. 1995;92:4621–4625. doi: 10.1073/pnas.92.10.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kordower JH, Herzog CD, Dass B, et al. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann Neurol. 2006;60:706–715. doi: 10.1002/ana.21032. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 23.Tuszynski MH, Roberts J, Senut MC, U HS, Gage FH. Gene therapy in the adult primate brain: intraparenchymal grafts of cells genetically modified to produce nerve growth factor prevent cholinergic neuronal degeneration. Gene Ther. 1996;3:305–314. [PubMed] [Google Scholar]
- 24.Mandel RJ, Gage FH, Clevenger DG, Spratt SK, Snyder RO, Leff SE. Nerve growth factor expressed in the medial septum following in vivo gene delivery using a recombinant adeno-associated viral vector protects cholinergic neurons from fimbria-fornix lesion-induced degeneration. Exp. 1999 Jan;155:59–64. doi: 10.1006/exnr.1998.6961. [DOI] [PubMed] [Google Scholar]
- 25.Levi-Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951;116:321–361. doi: 10.1002/jez.1401160206. [DOI] [PubMed] [Google Scholar]
- 26.Wong AW, J KPY, Payne SC, Keast JR, Osborne PB. Neurite outgrowth in normal and injured primary sensory neurons reveals different regulation by nerve growth factor (NGF) and artemin. Molec Cell Neurosci. 2015 Mar;65:125–134. doi: 10.1016/j.mcn.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Smithson LJ, Krol KM, Kawaja MD. Neuronal degeneration associated with sympathosensory plexuses in the trigeminal ganglia of aged mice that overexpress nerve growth factor. Neurobiol Aging. 2014;35:2812–2821. doi: 10.1016/j.neurobiolaging.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Killock D. Experimental arthritis: NGF promotes synovial nerve sprouting. Nat Rev Rheum. 2012;8:124. doi: 10.1038/nrrheum.2012.6. [DOI] [PubMed] [Google Scholar]
- 29.Lin CL, Heron P, Hamann SR, Smith GM. Functional distinction between NGF-mediated plasticity and regeneration of nociceptive axons within the spinal cord. Neurosci. 2014;272:76–87. doi: 10.1016/j.neuroscience.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holtzman DM, Li Y, Chen K, Gage FH, Epstein CJ, Mobley WC. Nerve growth factor reverses neuronal atrophy in a Down syndrome model of age-related neurodegeneration. Neurology. 1993;43:2668–2673. doi: 10.1212/wnl.43.12.2668. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Serrano A, Fischer W, Bjorklund A. Reversal of age-dependent cognitive impairments and cholinergic neuron atrophy by NGF-secreting neural progenitors grafted to the basal forebrain. Neuron. 1995 Ug;15:473–484. doi: 10.1016/0896-6273(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 32.Marks WJ, Jr, Bartus RT, Siffert J, et al. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- 33.Bankiewicz KS, Forsayeth J, Eberling JL, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Fischer W, Wictorin K, Bjorklund A, Williams LR, Varon S, Gage FH. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature. 1987;329:65–68. doi: 10.1038/329065a0. [DOI] [PubMed] [Google Scholar]
- 35.Markowska AL, Koliatsos VE, Breckler SJ, Price DL, Olton DS. Human nerve growth factor improves spatial memory in aged but not in young rats. J.Neurosci. 1994;14:4815–4824. doi: 10.1523/JNEUROSCI.14-08-04815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. Elsevier, Inc.; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.