Abstract
BACKGROUND
Several acute complications related to takotsubo cardiomyopathy (TTC) have been documented recently. However, the incidence and clinical significance of acute thromboembolic events in TTC is not well established.
METHODS
A detailed investigation of the clinical characteristics and in-hospital complications of 114 consecutive patients diagnosed with TTC between January 2003 and September 2015 was carried out. This study was initiated to reveal the predictors, clinical significance, and short-term and long-term outcomes of patients with TTC associated with acute thromboembolic events on index presentation.
RESULTS
The incidence of acute thromboembolic events related to TTC was around 12.2%, and these included ventricular thrombi, cerebrovascular events, retinal and brachial artery pathologies, renal, splenic, and aortic involvement. The most frequent complication on initial presentation was cardiogenic shock (20%) accompanied with pulmonary congestion (20%). Interestingly, patients experiencing thromboembolic events had higher C-reactive protein (CRP) levels as compared to the non-thromboembolic group (P = 0.02). Certain thromboembolic events were characterized by the presence of ST-segment elevation in electrocardiogram (P = 0.02). Chest pain was the primary symptom in these patients (P = 0.09). Furthermore, there was significant right ventricular involvement (as assessed by transthoracic echocardiography) in patients presenting with an acute thromboembolic event (P = 0.08). A Kaplan–Meier analysis indicated a significantly higher mortality rate over a mean follow-up of three years in the thromboembolic group than the non-thromboembolic group (log-rank, P = 0.02).
CONCLUSIONS
Our results confirmed the relative common occurrence of thromboembolic events in the setting of TTC. Inflammation might play an important role in the development of thromboembolic events, and a right ventricular involvement and ST-segment elevation could be positive predictors for this occurrence. In order to circumvent the risk of a negative outcome, it is recommended that an anticoagulation therapy be initiated in all high-risk patients.
Keywords: takotsubo cardiomyopathy, thromboembolic events, LV thrombus, stroke
Introduction
Takotsubo cardiomyopathy (TTC), a well-known reversible disease, was first described in 1991.1 It is characterized by the transient apical ballooning of the left ventricle (LV) with wall motion abnormalities of the middistal and apical regions and is generally associated with a decreased ejection fraction (EF). Right ventricular (RV) involvement has been described in up to 27% of patients,2,3 and a spontaneous recovery of the myocardial muscle is usually observed within a few days or weeks.
Patients present initially with symptoms, such as chest pain and dyspnea, which may mimic an acute coronary artery syndrome (ACS), and treatment is begun as per the ACS protocol by admitting to the hospital. Arrhythmias, cardiogenic shock, thromboembolic events, and in worst cases, in-hospital death are the well documented TTC-related complications. The in-hospital mortality rate of TTC is comparable with the mortality rate of ACS.4,22 A few thromboembolic events in patients with TTC have been described in some case reports5–7; however, there has been no documented systemic review available yet to address this acute scenario.
Materials and Methods
We retrospectively describe a collection of 114 consecutive patients diagnosed with TTC between January 2003 and September 2015 at our institution. The patients were diagnosed according to the Mayo Clinic Criteria,8 which outlines the clinical features associated with TTC.
The first criterion describes the transient wall motion abnormality in the left ventricular mid segments associated with or without apical involvement; regional wall motion abnormalities that extend beyond a single epicardial vascular distribution; and frequently in the event of a stressful trigger. The second criterion outlines the absence of obstructive coronary disease. The third criterion mentions the appearance of new ECG pathologies, which mimic ACS or modest elevations in cardiac troponin levels. The final criterion is the absence of pheochromocytoma and myocarditis in the patient.
The study population underwent a 12-lead ECG, echocardiography, coronary angiography, and in some cases, cardiac magnetic resonance tomography. Most patients were monitored in the intensive care unit for at least 24 hours.
The angiograms, echocardiograms, and ECGs were reviewed by two independent cardiologists who evaluated the diagnosis of TTC. This study was conducted in compliance with the Declaration of Helsinki with regard to investigations in human subjects, and the study protocol was approved by the Ethics Committee of University Medical Centre Mannheim.
In-hospital events, such as life-threatening arrhythmias (ventricular tachycardia, ventricular fibrillation, asystole, and AV-block II type mobitz and complete AV block), cardiac rupture, thromboembolic events, pulmonary congestion with the use of noninvasive positive-pressure ventilation, endotracheal intubation, use of a temporary pacemaker, use of inotropic agents, and in-hospital death, were assessed based on chart review.
The aim of this study was to better understand the predictors, clinical significance, and short-term and long-term outcomes, defined as all cause of death of patients with TTC complicated with an acute thromboembolic event on initial presentation.
Statistics
Data are presented as mean ± SD for continuous variables with a normal distribution, as median (inter-quartile range) for continuous variables with a non-normal distribution, and as frequency (%) for categorical variables. The Kolmogorov–Smirnov test was used to assess normal distribution. Student’s t-test and the Mann–Whitney U-test were used to compare continuous variables with normal and non-normal distributions, respectively. The chi-squared test or Fisher’s exact test was used to compare categorical variables. In all analyses, P < 0.05 (two-tailed) was taken to indicate statistical significance.
Results
Clinical features of patients with TTC
The mean age of patients with TTC was 67 ± 11 years with female predominance (83%) (Table 1). Seventy percent of the patients were admitted to the hospital with clinical symptoms of ACS, the most common clinical symptom being chest pain (50.8%) followed by dyspnea (37%). ST-segment elevation in ECG was observed in 34 patients (30%), and inverted T-waves were observed in 102 patients (89.5%). Patient histories revealed emotional and physical stresses in 29% and 56% of the cases, respectively. A mild elevation of cardiac enzymes, such as troponin I and creatine kinase, was confirmed in 95% of the cases for troponin I and in 43% of the cases for creatine kinase. The left ventricular function, measured by transthoracic echocardiography and laevocardiography, was significantly reduced in almost all patients during hospital admission (EF 38.3%). RV involvement was documented in 22.8% of the cases. An apical type TTC was observed in 71.9% (n = 82) of the patients, whereas midventricular type and reversed type were diagnosed in n = 30, 26.3% and n = 2, 1.7% respectively.
Table 1.
Baseline characteristics of 114 patients presenting with Takotsubo cardiomyopathy.
| VARIABLES | ALL PATIENTS (n = 114) | TTC WITHOUT TE EVENTS (n = 100) | TTC WITH TE EVENTS (n = 14) | P VALUE* |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean ± SD | 67 ± 11 | 67.1 ± 11 | 67 ± 13.1 | 0.956 |
| Female, n (%) | 95 (83) | 82 (82) | 13 (92.8) | 0.313 |
| Symptoms, n (%) | ||||
| Chest pain | 58 (50.8) | 54 (54) | 4 (28.5) | 0.077 |
| Dyspnea | 43 (37) | 37 (37) | 6 (42.8) | 0.677 |
| ECG Data, n (%) | ||||
| ST-segment elevation | 34 (30) | 26 (26) | 8 (57.1) | 0.018 |
| Inverted T-waves | 102 (89.4) | 89 (89) | 13 (92.8) | 0.459 |
| QTc (ms), mean ± SD | 474 ± 69 | 478.5 ± 55 | 483 ± 24 | 0.634 |
| Stress factor, n (%) | ||||
| Emotional sress | 30 (29) | 28 (28) | 2 (14.2) | 0.280 |
| Physical stress | 64 (56) | 53 (53) | 11 (78.5) | 0.073 |
| None | 25 (22) | 23 (23) | 2 (14.2) | 0.466 |
| Laboratory values, mean ± SD | ||||
| Troponin I (µg/L) | 3.3 ± 5 | 3.3 ± 5 | 4.6 ± 6.6 | 0.558 |
| Creatine phosphatkinase (U/L) | 674 ± 2773 | 680 ± 2786 | 330 ± 307 | 0.521 |
| C-Reactive protein (mg/l) | 38.5 ± 57.9 | 40 ± 58 | 118 ± 145 | 0.021 |
| Echocardiography data | ||||
| LV EF% ± SD | 38.3 ± 9.4 | 38 ± 9 | 38.6 ± 11.8 | 0.918 |
| Right ventricular involvement, n (%) | 26 (22.8) | 20 (20) | 6 (42.8) | 0.056 |
| Apical type | 82 (71.9) | 71 (71) | 11 (78.5) | 0.677 |
| Midventricular type | 30 (26.3) | 27 (27) | 3 (21.4) | 0.677 |
| Reversed type | 2 (1.7) | 2 (2) | 0 (0) | 1 |
| Medical history and risk factors, n (%) | ||||
| Smoking | 36 (31) | 33 (33) | 3 (21) | 0.388 |
| Atrial fibrillation | 21 (18.4) | 17 (17) | 4 (28.5) | 0.301 |
| Obesity (BMI >25 kg/m2) | 31 (27.2) | 27 (27) | 4 (28.5) | 1 |
| Arterial hypertension | 66 (58) | 58 (58) | 8 (57) | 0.956 |
| COPD | 22 (19.2) | 20 (20) | 2 (14.2) | 0.618 |
| Complications, n (%) | ||||
| Life-threatening arrhythmias | 13 (11.4) | 10 (10) | 3 (21) | 0.199 |
| Invasive respiratory support | 23 (20) | 20 (20) | 3 (21) | 0.794 |
| Recurrence | 6 (5) | 5 (5) | 1 (7) | 0.746 |
| Cardiogenic shock | 22 (19.2) | 18 (18) | 4(11) | 0.466 |
| In-hospital death | 9 (7.8) | 7 (7) | 2 (14) | 0.304 |
Note:
P values for the comparison between TE group and non-TE group.
Abbreviations: TE, Thromboembolic; LV, Left ventricular; EF, Ejection fraction.
The most frequent complications associated with TTC were life-threatening arrhythmias (11.4%), pulmonary congestion with need for invasive respiratory support (20%), thromboembolic events (12.2%), cardiogenic shock (19.2%), and in-hospital death (7.8%).
Clinical findings of patients with thromboembolism
The thromboembolic events occurred exclusively in the arterial system and patients suffering from this complication were predominantly (92.8%) postmenopausal females with a mean age of 67 ± 13.1 years. Also, initial ECGs revealed ST-segment elevation in 57.1% of the patients.
A physical trigger was more than often responsible for the chain of events as compared to an emotional cause (78.5% versus 14.2%). The apical variant of TTC was identified in 11 (78.5%) patients, whereas the midventricular variant of TTC was identified in 3 patients (21.4%). RV involvement was diagnosed in six patients (42.8%). Due to worsening circulatory status, catecholamine therapy was initiated in four cases, whereas invasive respiratory support was required in five patients due to impending cardiogenic shock. One patient received extracorporeal membrane oxygenation therapy.
The database documented a single female patient experiencing a recurrent TTC. Interestingly, this patient showed a biventricular form of TTC the second time around compared to a left ventricular TTC in the past. The in-hospital death of patients associated with TTC complicated by a thromboembolic event was calculated to be 14%. The cause of death was attributed to an acute cardiac failure (respiratory distress triggered by cardiogenic shock and pulseless electrical activity).
The distribution of the thromboemboli and its presenting location are indicated in Table 2. Only one patient with previously diagnosed atrial fibrillation presented with a thrombus in the LV. Newly diagnosed atrial fibrillation at index event of TTC was similar in both groups (14.2% versus 4%; P > 0.1).
Table 2.
Clinical findings of patients with acute TE events.
| CHARACTERISTIC | CASE 1 | CASE 2 | CASE 3 | CASE 4 | CASE 5 | CASE 6 | CASE 7 | CASE 8 | CASE 9 | CASE 10 | CASE 11 | CASE 12 | CASE 13 | CASE 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Cerebrovascular | Cerebrovascular, spleen | Thoracic aorta | Biventricular | LV | LV | Retinal artery | LV | Ramus marginalis sinister | Pulmonary artery | Left kidney, LV | Cerebrovascular | LV | Brachial artery |
| Age | 79 | 81 | 81 | 70 | 68 | 53 | 64 | 69 | 76 | 53 | 68 | 83 | 57 | 55 |
| Sex | Female | Female | Female | Female | Female | Female | Female | Female | Female | Female | Female | Female | Male | Female |
| Chest pain | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | + | + | 0 | 0 | + | 0 |
| Dyspnea | 0 | + | 0 | + | 0 | + | 0 | + | 0 | + | + | 0 | + | 0 |
| Stress | Physical | Physical | Emotional | Physical | Emotional | Physical | Physical | Physical | Physical | Emotional | Physical | Physical | Physical | |
| CK (U/L) | 760 | 117 | 143 | 423 | 48 | 389 | 182 | 114 | 939 | 856 | 60 | 323 | 118 | 89 |
| TNI (µg/L) | 24 | 1.25 | 1.5 | 0.9 | 0.13 | 7.9 | 3.1 | 1.84 | 0.73 | 12 | 0.3 | 4.3 | 0.05 | 0.124 |
| ST elevation | + | 0 | + | 0 | + | + | 0 | 0 | + | + | 0 | + | + | 0 |
| Inverted T waves | + | + | 0 | + | 0 | 0 | + | + | 0 | 0 | + | + | 0 | + |
| Pattern | Apical | Apical | Apical | Apical | Apical | Apical | Apical | MV | Apical | Apical | MV | Apical | MV | Apical |
| EF | 30% | 60% | 40% | 30% | 38% | 43% | 40% | 26% | 40% | 60% | 30% | 47% | 25% | 55% |
| RV-involvement | + | + | + | + | 0 | 0 | 0 | + | 0 | + | 0 | 0 | 0 | 0 |
| Hypertension | + | + | + | 0 | + | 0 | + | 0 | + | 0 | 0 | + | + | 0 |
| Diabetes mellitus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 |
| COPD | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | + | 0 | 0 | + | 0 |
| Arrhythmias | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | VT | 0 | 0 | 0 | Asystole | VF |
| Respiratory support | 0 | + | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 |
| Shock | + | + | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | + | |
| In-hospital death | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 |
| ECMO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 |
| Recurrence | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tumor | 0 | Colon | 0 | 0 | 0 | 0 | 0 | Rectum | 0 | 0 | 0 | 0 | 0 | Colon |
Notes: + for positive, (0) for negative,
Abbreviations: TE, Thromboembolic; TNI, Troponin I; CK, Creatine Kinase; EF, Ejection fraction; LV, Left ventricular; RV, Right ventricular; MV, Midventricular; VT, Ventricular tachycardia; VF, Ventricular fibrillation; ECMO, Extracorporeal membrane oxygenation; COPD, Chronic obstructive pulmonary disease.
In this study, 11 patients with proven thromboembolic events were started on an anticoagulant therapy with either warfarin or heparin. Three patients (stroke and occlusion of retinal artery) were treated with aspirin. In one patient, a thromboendarterectomy was performed.
Patients with TTC presenting with thromboembolic events were compared with patients with TTC who were not affected by such complications, and this revealed a similar predominance of females suffering from physical stress (78.5% versus 53%; P = 0.08). Also, no significant difference between the two groups could be elucidated when clinical symptoms, cardiac enzyme levels, creatinine levels, and hemoglobin levels were compared. However, patients who experienced thromboembolic events did show higher CRP levels (118 ± 145 mg/dL versus 40 ± 58 mg/L; P = 0.02). Furthermore, ST-segment elevation was more frequent in patient with thromboembolic events (57.1% versus 26%; P = 0.02). The frequency of cardiovascular risk factors, such as obesity, arterial hypertension, diabetes mellitus, and history of smoking, was similar in both groups. The EF and the takotsubo variant were also similar in both groups.
Pertaining to RV involvement, this was documented more often in patients who had experienced a thromboembolic event (42.8% versus 19%; P = 0.05). Additionally, the incidence of other TTC-related complications, such as cardiogenic shock, respiratory distress, in-hospital death, and life-threatening arrhythmias (ventricular tachycardia, ventricular fibrillation, asystole, and AV-block II type mobitz and complete AV block), were similar in both groups.
Long-term prognosis
Kaplan–Meier analysis indicated that short-term mortality rate (30 days) was similar in both groups. But nevertheless, long-term mortality rate was significantly higher in the thromboembolic group (Fig. 1).
Figure 1.
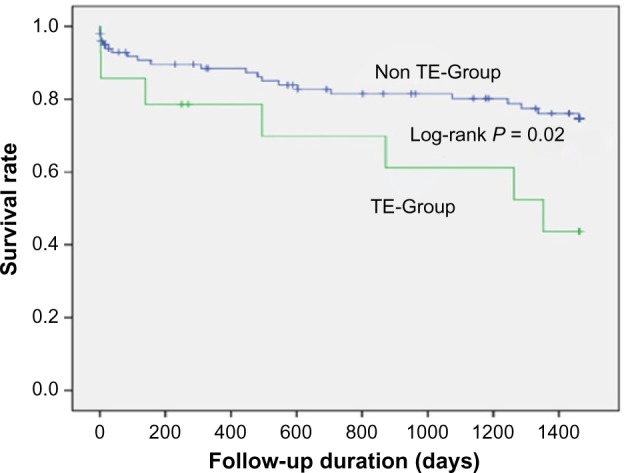
Survival rate in TTC patients with and without thromboembolic events.
Discussion
TTC occurs predominantly in postmenopausal women.9–11,13 and the disease is usually provoked by emotional or physical stress.2–6 An enhanced sympathetic activity with an elevation of catecholamine levels has been documented in these patients.14 Furthermore, coronary vasospasm and widespread coronary microvascular dysfunction10,15 might be a contributing factor in the pathophysiologic mechanism of TTC. Nevertheless, a defining explanation to its underlying pathogenesis remains unresolved.
In this study, we first investigate the risk of acute thromboembolism in 114 patients diagnosed with TTC. To date, the incidence and clinical significance of acute (in-hospital) thromboembolic events in TCC have not been well established. Our literature search revealed single case report or cohort of maximum 21 patients.16–19,23 A 1%–4.2% in-hospital mortality rate of patients with TTC has also been reported.4,20,21 Interestingly, further TTC-related complications, such as congestive heart failure, cardiogenic shock, respiratory distress, and lethal arrhythmias, have been well presented.5,22 In this work, the frequency of acute thromboembolic events in patients with TTC was 12.2%, and this was predominantly documented in postmenopausal women. This might be due to the higher incidence of TTC in females and is in accordance with the further investigations of the TTC cohort of 21 patients mentioned earlier.19
In 42.8% of patients with thromboembolic events, there was a thrombus diagnosed in the left and/or right ventricle. A meta-analysis of Gregorio et al reported thrombi in the LV in 2.5% of patients with TTC.5 Our work demonstrates a risk of approximately 5.2%. The development of thrombus or emboli in the right ventricle in patients with TTC has not yet been reported. However, in our study, a single patient was diagnosed with thrombi in both ventricles. This patient was then classified as presenting with a biventricular form of TTC.
Ventricular thrombus can occur in the setting of ventricular dysfunction, especially in the acute stage postmyocardial infarction.24,25 In patients with noncompaction cardiomyopathy and dilated cardiomyopathy, the coincidental development of thrombi has been also reported.26,27 Left ventricular thrombus may be associated with antiphospholipid antibody syndrome, hypereosinophilic syndrome, and other autoimmune disorders, such as Adamantiadis-Behcet’s disease and lupus erythematosus.28–31 In TTC, an acute ventricular embolism is presumably explained due to the low blood flow in the ventricle. In addition, it has been hypothesized that the coagulation cascade plays a role influencing the underlying mechanism of thromboembolic development.32
In this study, we also demonstrate that acute cerebrovascular events occurred with a frequency of approximately 2.6%. However, data from existing literature revealed a higher rate of acute cerebrovascular events in TTC of up to 9%.19 In contrast, previous randomized controlled trials revealed that only 4.6% of patients with ACS experienced stroke.33
In this study, the incidence of thromboembolism in more than one organ was observed in 14.2% of the patients. Interestingly, the occlusion of retinal artery due to embolism in patients with TTC has not been reported yet.
In our thromboembolism cohort, anticoagulation treatment in most cases was initiated with heparin or warfarin (78%). Although anticoagulation is generally recommended, the type and duration of anticoagulant treatment remain unresolved.
Our report also revealed that patients with TTC who experience thromboembolic events have higher CRP levels. This indicates a potential pathogenic role of inflammation in the development of thromboembolic events. Similar findings have been reported in patients with thromboembolic events in patients with ACS.34
Furthermore, we demonstrated that RV involvement assessed by transthoracic echocardiography correlates with the thromboembolic events in TTC. Although the mildly reduced left EF was similar in both groups, patients with thromboembolic events presented a significant ST-segment elevation. It has been reported that a thromboembolic event, such as pulmonary artery embolism, is associated with ST-segment elevation.
Conclusions
Our results indicate that the prevalence of acute thromboembolic events is higher than expected in TTC and is associated with a high long-term mortality rate. This complication can occur in every organ, irrespective to the presence or absence of a ventricular thrombus. Anticoagulation therapy might be the sole therapy for all high-risk TTC patients and should be prescribed during the in-hospital phase to prevent such events. We recommend further studies to evaluate the occurrence and prevalence of out of hospital thromboembolic events.
Acknowledgments
We acknowledge the financial support of the Deutsche Forschungsgemeinschaft and Ruprecht-Karls-Universität Heidel-berg within the funding programme Open Access Publishing.
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
PEER REVIEW: Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 685 words, excluding any confidential comments to the academic editor.
FUNDING: We acknowledge the financial support of the Deutsche Forschungsgemeinschaft and Ruprecht-Karls-Universität Heidelberg within the funding program Open Access Publishing. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: IEl-B, IA, M Borggrefe, JK, D Haghi, FH. Analyzed the data: SL, D Hillenbrand, CF, TB. Wrote the first draft of the manuscript: IEl-B, IA, M Borggrefe. Contributed to the writing of the manuscript: IEl-B, IA, M Behnes, TP, SL, JK. Agree with manuscript results and conclusions: IEl-B, M Behnes, D Hillenbrand, D Haghi, UH, TP, SL, CF, TB, SB, FH, JK, M Borggrefe, IA. Jointly developed the structure and arguments for the paper: IEl-B, IA, M Behnes. Made critical revisions and approved final version: M Borggrefe, UH, IEl-B, IA. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases. J Cardiol. 1991;21(2):203–14. [PubMed] [Google Scholar]
- 2.Elesber A, Lerman A, Bybee KA, et al. Myocardial perfusion in apical ballooning syndrome correlate of myocardial injury. Am Heart J. 2006;152(3):469, e9–13. doi: 10.1016/j.ahj.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Haghi D, Papavassiliu T, Fluchter S, et al. Variant form of the acute apical ballooning syndrome (takotsubo cardiomyopathy): observations on a novel entity. Heart. 2006;92(3):392–4. doi: 10.1136/hrt.2005.061044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinjikji W, El-Sayed AM, Salka S. In-hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J. 2012;164(2):215–21. doi: 10.1016/j.ahj.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 5.de Gregorio C, Cento D, Di Bella G, Coglitore S. Minor stroke in a takotsubo-like syndrome: a rare clinical presentation due to transient left ventricular thrombus. Int J Cardiol. 2008;130(2):e78–80. doi: 10.1016/j.ijcard.2007.11.104. [DOI] [PubMed] [Google Scholar]
- 6.Gnanenthiran SR, Amos D, Kamaladasa K, Lowe HC. Occlusive radial artery thrombosis following severe radial artery spasm during coronary angiography in takotsubo cardiomyopathy: should this access route be avoided in this condition? Int J Cardiol. 2015;179:38–9. doi: 10.1016/j.ijcard.2014.10.086. [DOI] [PubMed] [Google Scholar]
- 7.Hrymak C, Liu S, Koulack J, Funk DJ, Schaffer SA, Tam JW. Embolus from probable takotsubo cardiomyopathy: a bedside diagnosis. Can J Cardiol. 2014;30(12):1732.e9–11. doi: 10.1016/j.cjca.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Movahed MR, Donohue D. Review: transient left ventricular apical ballooning, broken heart syndrome, ampulla cardiomyopathy, atypical apical ballooning, or takotsubo cardiomyopathy. Cardiovasc Revasc Med. 2007;8(4):289–92. doi: 10.1016/j.carrev.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Ito K, Sugihara H, Kawasaki T, et al. Assessment of ampulla (takotsubo) cardiomyopathy with coronary angiography, two-dimensional echocardiography and 99 mTc-tetrofosmin myocardial single photon emission computed tomography. Ann Nucl Med. 2001;15(4):351–5. doi: 10.1007/BF02988242. [DOI] [PubMed] [Google Scholar]
- 10.Kurisu S, Inoue I, Kawagoe T. Conditions associated with left ventricular apical ballooning. Clin Cardiol. 2010;33(6):E123–4. doi: 10.1002/clc.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (takotsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408–17. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118(25):2754–62. doi: 10.1161/CIRCULATIONAHA.108.767012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94(3):343–6. doi: 10.1016/j.amjcard.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539–48. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001;38(1):11–8. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 16.Korosoglou G, Haars A, Kuecherer H, Giannitsis E, Katus HA. Prompt resolution of an apical left ventricular thrombus in a patient with takotsubo cardiomyopathy. Int J Cardiol. 2007;116(3):e88–91. doi: 10.1016/j.ijcard.2006.08.089. [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka K, Nakayama S, Okubo S, Fujii E, Uchida F, Nakano T. Transient cerebral ischemic attack induced by transient left ventricular apical ballooning. Eur J Intern Med. 2004;15(6):393–5. doi: 10.1016/j.ejim.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Mitsuma W, Kodama M, Ito M, et al. Thromboembolism in takotsubo cardiomyopathy. Int J Cardiol. 2010;139(1):98–100. doi: 10.1016/j.ijcard.2008.06.089. [DOI] [PubMed] [Google Scholar]
- 19.Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27(13):1523–9. doi: 10.1093/eurheartj/ehl032. [DOI] [PubMed] [Google Scholar]
- 20.Pilgrim TM, Wyss TR. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: a systematic review. Int J Cardiol. 2008;124(3):283–92. doi: 10.1016/j.ijcard.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Stiermaier T, Eitel C, Denef S, et al. Prevalence and clinical significance of life-threatening arrhythmias in takotsubo cardiomyopathy. J Am Coll Cardiol. 2015;65(19):2148–50. doi: 10.1016/j.jacc.2015.02.062. [DOI] [PubMed] [Google Scholar]
- 22.Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929–38. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 23.Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol. 1989;14(4):903–11. doi: 10.1016/0735-1097(89)90463-4. [DOI] [PubMed] [Google Scholar]
- 24.Roifman I, Connelly KA, Wright GA, Wijeysundera HC. Echocardiography vs. cardiac magnetic resonance imaging for the diagnosis of left ventricular thrombus: a systematic review. Can J Cardiol. 2015;31(6):785–91. doi: 10.1016/j.cjca.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Mahmood MM, Mahmood S. Unusual echocardiographic appearance of left ventricular thrombi in a patient with dilated cardiomyopathy. BMJ Case Rep. 2014;2014:bcr2014204416. doi: 10.1136/bcr-2014-204416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aryal MR, Badal M, Giri S, Pradhan R. Left ventricular non-compaction presenting with heart failure and intramural thrombus. BMJ Case Rep. 2013;2013:bcr2013009757. doi: 10.1136/bcr-2013-009757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguilar JA, Summerson C. Intracardiac thrombus in antiphospholipid antibody syndrome. J Am Soc Echocardiogr. 2000;13(9):873–5. doi: 10.1067/mje.2000.106825. [DOI] [PubMed] [Google Scholar]
- 28.Barjatiya MK, Shah NK, Kothari SS, Shah PP, Trivedi HL. Spontaneous left ventricle cavity thrombus in a patient of systemic lupus erythematosus. J Assoc Physicians India. 1992;40(3):195–6. [PubMed] [Google Scholar]
- 29.Ejima J, Ohmura I, Kaji Y, Tsuda Y, Kanaya S, Fujino T. Diffuse endocardial thrombus in left ventricle associated with a case of hypereosinophilic syndrome. Jpn Heart J. 1991;32(2):267–72. doi: 10.1536/ihj.32.267. [DOI] [PubMed] [Google Scholar]
- 30.Vanhaleweyk G, el-Ramahi KM, Hazmi M, Sieck JO, Zaman L, Fawzy M. Right atrial, right ventricular and left ventricular thrombi in (incomplete) Behcet’s disease. Eur Heart J. 1990;11(10):957–9. doi: 10.1093/oxfordjournals.eurheartj.a059619. [DOI] [PubMed] [Google Scholar]
- 31.Parkkonen O, Mustonen P, Puurunen M, Valkonen K, Nieminen M, Sinisalo J. Coagulation changes in takotsubo cardiomyopathy support acute phase reaction and catecholamine excess, but not thrombus production. Int J Cardiol. 2014;177(3):1063–5. doi: 10.1016/j.ijcard.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Loh E, Sutton MS, Wun CC, et al. Ventricular dysfunction and the risk of stroke after myocardial infarction. N Engl J Med. 1997;336(4):251–7. doi: 10.1056/NEJM199701233360403. [DOI] [PubMed] [Google Scholar]
- 33.Seo Y, Maeda H, Ishizu T, et al. Peak C-reactive protein concentration correlates with left ventricular thrombus formation diagnosed by contrast echocardiographic left ventricular opacification in patients with a first anterior acute myocardial infarction. Circ J. 2006;70(10):1290–6. doi: 10.1253/circj.70.1290. [DOI] [PubMed] [Google Scholar]
- 34.Bakalli A, Georgievska-Ismail L, Kocinaj D, Musliu N, Krasniqi A, Pllana E. Prevalence of left chamber cardiac thrombi in patients with dilated left ventricle at sinus rhythm: the role of transesophageal echocardiography. J Clin Ultrasound. 2013;41(1):38–45. doi: 10.1002/jcu.21953. [DOI] [PubMed] [Google Scholar]


