Abstract
Background
Randomized trials of percutaneous coronary intervention (PCI) versus coronary artery bypass graft (CABG) surgery have routinely excluded patients with chronic kidney disease (CKD).
Objectives
To evaluate the outcomes with PCI vs. CABG in patients with CKD.
Methods
Patients with CKD (eGFR <60mL/min/1.73m2) who underwent PCI using everolimus eluting stents (EES) were propensity score matched to patients who underwent isolated CABG surgery for multivessel coronary disease in New York State. The primary outcome was all-cause mortality. Secondary outcomes were myocardial infarction(MI), stroke and repeat revascularization.
Results
Among 11,305 patients with CKD, 5,920 patients (2,960 pairs) were propensity score matched. At short-term (within 30 days), PCI was associated with a lower risk of death [HR=0.55; 95% CI=0.35-0.87], stroke [HR=0.22; 95% CI=0.12-0.42] and repeat revascularization [HR=0.48; 95%CI=0.23-0.98] when compared with CABG. At longer-term (mean 2.9 years), PCI was associated with a similar risk of death [HR=1.07; 95% CI=0.92-1.24], higher risk of myocardial infarction [HR=1.76; 95% CI=1.40-2.23], a lower risk of stroke [HR=0.56; 95% CI=0.41-0.76] and higher risk of repeat revascularization [HR=2.42; 95% CI=2.05-2.85]. In the subgroup of patients who underwent who underwent complete revascularization with PCI, the increased risk of myocardial infarction was no longer statistically significant [HR=1.18, 95% CI=0.67-2.09]. In the 243 pairs of patients with end stage renal disease on hemodialysis, PCI was associated with a significant higher risk of death [HR=2.02; 95% CI=1.40-2.93] and repeat revascularization [HR=2.44; 95% CI=1.50-3.96] when compared with CABG.
Conclusions
In subjects with CKD, CABG is associated with higher short-term risk of death, stroke and repeat revascularization whereas PCI with EES is associated with higher long-term risk of repeat revascularization and perhaps MI (in those with incomplete revascularization), with no mortality difference between CABG and PCI long-term. However, in the subgroup on dialysis, the results favored CABG over PCI.
Keywords: coronary artery bypass graft surgery, chronic kidney disease, multivessel disease, percutaneous coronary intervention
Introduction
The chronic kidney disease (CKD) population has grown exponentially over the past decade and is projected to grow consistently in the next decade due to an increase in the incidence of obesity and diabetes and a decrease in mortality rates.(1) Cardiovascular disease is the leading cause of morbidity and mortality in patients with CKD.(1,2) However, there is a ‘treatment risk paradox’ in that these high risk patients have lower rates of medical therapy, referral for stress testing, cardiac catheterization and revascularization when compared with low risk patients.(2) Moreover, the majority of cardiovascular clinical trials routinely exclude this high risk group of patients.(3) The evidence based management of these patients is therefore based on extrapolating the results from clinical trials in non CKD cohorts and applying them to patients with CKD. However, it is not known if this extrapolation is accurate.
In patients with obstructive coronary artery disease (CAD), percutaneous coronary intervention (PCI) and coronary artery bypass graft surgery (CABG) are both treatment options. Although a number of clinical trials have been performed to evaluate the efficacy and safety of PCI vs. CABG, the trials have generally excluded patients with CKD. The 2014 European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Guidelines on myocardial revascularization recommends CABG over PCI (class IIa) in patients with moderate to severe CKD and multivessel disease when the surgical risk profile is acceptable and life expectancy is beyond one year.(4) The ACC/AHA guidelines also recommend CABG to improve survival in patients with ESRD and three vessel coronary artery disease or proximal left anterior descending artery plus one other major vessel. Most of these recommendations stem from observational studies of CABG with PCI using older generation stents. In the absence of randomized trials comparing CABG vs. PCI with second generation drug eluting stents (DES) in patients with CKD, we used data from the New York State registries to compare outcomes for CABG and PCI in subjects with CKD.
Methods
Study Population
Patients with CKD who underwent either PCI with EES or isolated CABG surgery for multivessel coronary artery disease between January 1, 2008 and December 31, 2011 in New York State were included in the study. The patients were identified using the New York State Percutaneous Coronary Intervention Reporting System (PCIRS) and the Cardiac Surgery Reporting System (CSRS) registries. The PCIRS and CSRS are mandatory reporting systems for all PCI and CABG procedures performed in non-federal hospitals in New York State. Data are entered by trained data coordinators at participating hospitals. Audits of samples of medical records are conducted regularly to ensure data quality.
Patient Inclusion and Exclusion Criteria
The inclusion criteria were the following: 1) Patients with CKD defined as those with an estimated glomerular filtration rate (eGFR) (calculated using the Modification of Diet in Renal Disease (MDRD) Study equation) of less than 60 mL/min/1.73m2; 2) Patients with multivessel disease defined as severe stenosis (≥70%) in at least 2 major epicardial coronary arteries; and 3) Patients undergoing PCI with implantation of EES or those undergoing isolated CABG.
The exclusion criteria were the following: 1) Prior cardiac surgery (CABG or valve surgery) as such patients are unlikely to undergo repeat surgery; 2) Myocardial infarction (MI) within 24 hours preceding the index procedure as these patients preferentially undergo PCI; 3) Severe left main coronary artery disease (degree of stenosis ≥50%) as these patients preferentially undergo CABG; 4) PCI with a stent other than EES or using a mixture of stents; 5) Revascularization within 1 year prior to the index procedure; and 6) Unstable hemodynamics or in cardiogenic shock.
Patient Follow-up
The PCIRS and CSRS registries collect data on in-hospital events and are linked across time and with each other to capture subsequent revascularization procedures. In addition, the registries were linked with the New York State Vital Statistics registry to obtain information on mortality. The registries were also linked with the Statewide Planning and Research Cooperative System (SPARCS) registry to obtain follow-up information for patients admitted with MI and stroke. The SPARCS registry collects comprehensive information on discharges from all nonfederal hospitals in New York State and contains information on patient diagnoses, procedures, admission and discharge dates, and discharge disposition for hospital discharges, ambulatory surgery, and emergency department admissions. Data are edited monthly to identify errors, audit reports are generated following monthly updates, and related data are verified with 2 data sources for consistency (5).
Outcomes
The primary outcome of the study was long-term all-cause death. Secondary outcomes were MI, stroke and repeat revascularization tabulated separately. Short-term (within 30 days) and longer-term outcomes were evaluated. MI included both procedural MI (defined as new Q waves in both the PCIRS and the CSRS) and spontaneous MI (defined as an emergency admission with a principal diagnosis of MI or principal diagnosis of cardiogenic shock with a secondary diagnosis of MI). Similarly stroke was identified either as a complication at the time of index procedure or at readmission (principal diagnosis of stroke). Repeat revascularization was identified as any unstaged revascularization after the index procedure. Staged revascularization was defined as a non-target vessel revascularization within 90 days of the index procedure that was coded as intended to be staged in the index procedure and at the time of the staged procedure.
Statistical Analysis
Propensity Score Matching
In order to adjust for differences in measured baseline characteristics between the PCI and CABG groups and to assemble a cohort of patients with similar baseline characteristics, propensity score matching was used. The propensity score is a conditional probability of having a particular exposure (EES vs. CABG) given a set of baseline measured covariates.(6,7) A non-parsimonious multivariable logistic regression model(8) using EES use as the dependent variable and all the baseline characteristics outlined in Table 1 as covariates was used to estimate the propensity scores. Matching was performed using a 1:1 matching protocol without replacement (Greedy matching algorithm) using a caliper width equal to 0.2 of the standard deviation of the logit of the propensity score. Absolute standardized differences (ASD) were estimated for all the baseline covariates before and after matching to assess pre-match and post-match imbalance.(9) ASD<10% for a given covariate indicate a relatively small imbalance.(9) The risks of outcomes were analyzed in the matched cohort using a Cox proportional regression model after stratifying on the matched pairs.
Table 1.
Baseline characteristics before and after propensity score matching
| Pre-Matching | Post-Matching | |||||
|---|---|---|---|---|---|---|
| Variables | EES (n=5,058) | CABG (n=6,247) | ASD | EES (n=2,960) | CABG (n=2,960) | ASD |
| Age (%) | ||||||
| <59 | 17.16 | 18.20 | 2.70 | 18.00 | 17.80 | 0.60 |
| 60-69 | 26.59 | 28.37 | 4.00 | 26.40 | 26.80 | 1.00 |
| 70-79 | 35.77 | 37.14 | 2.90 | 36.70 | 36.30 | 0.80 |
| >=80 | 20.48 | 16.30 | 10.80 | 18.90 | 19.10 | 0.50 |
| Mean Age (yr) | 70.21±10.60 | 69.13±10.32 | 10.30 | 69.94±10.67 | 69.62±10.53 | 3.00 |
| Body Surface Area | 2.00±0.27 | 2.02±0.27 | 5.80 | 2.00±0.27 | 2.00±0.27 | 0.10 |
| Sex (%) | ||||||
| Male | 60.50 | 64.80 | 8.90 | 62.00 | 61.50 | 1.00 |
| Female | 39.50 | 35.20 | 8.90 | 38.00 | 38.50 | 1.00 |
| Hispanic ethnic background (%) | 13.15 | 8.90 | 13.60 | 12.00 | 11.20 | 2.50 |
| Race (%) | ||||||
| White | 78.39 | 85.51 | 18.60 | 81.30 | 81.50 | 0.40 |
| Black | 11.05 | 7.88 | 10.90 | 9.80 | 10.10 | 1.20 |
| Other | 10.56 | 6.61 | 14.10 | 8.90 | 8.40 | 1.90 |
| Smoking (%) | 26.69 | 26.12 | 1.30 | 26.40 | 26.80 | 1.10 |
| Diabetes Mellitus (%) | 47.57 | 48.97 | 2.80 | 48.90 | 48.30 | 1.20 |
| Hypertension (%) | 75.58 | 77.70 | 5.00 | 77.00 | 78.20 | 2.80 |
| Hyperlipidemia (%) | 63.54 | 60.30 | 6.70 | 62.40 | 63.30 | 1.90 |
| Prior PCI (%) | 35.03 | 17.72 | 40.10 | 26.30 | 25.60 | 1.70 |
| Unstable Angina (%) | 20.32 | 19.72 | 1.50 | 20.30 | 20.50 | 0.60 |
| Ejection Fraction (%) | ||||||
| <20% | 0.81 | 2.19 | 11.40 | 1.20 | 1.20 | 0.30 |
| 20-29% | 4.74 | 8.58 | 15.40 | 6.60 | 6.60 | 0.10 |
| 30-39% | 6.68 | 13.57 | 23.00 | 9.40 | 9.30 | 0.20 |
| 40-49% | 12.77 | 19.63 | 18.70 | 16.80 | 16.10 | 1.70 |
| >=50% | 68.70 | 55.50 | 27.50 | 65.30 | 65.70 | 0.90 |
| missing | 6.29 | 0.53 | 32.10 | 0.80 | 1.10 | 2.50 |
| Previous myocardial infarction (%) | ||||||
| Within 1-7 days | 16.01 | 19.51 | 9.20 | 17.20 | 17.40 | 0.40 |
| Within 8-14 days | 2.17 | 7.89 | 26.40 | 3.10 | 3.20 | 0.80 |
| Within 15-20 days | 0.55 | 1.38 | 8.40 | 0.90 | 0.80 | 0.70 |
| >20 days | 19.24 | 23.16 | 9.60 | 21.60 | 21.20 | 0.80 |
| No previous MI | 62.02 | 48.06 | 28.40 | 57.30 | 57.40 | 0.20 |
| Cerebrovascular disease (%) | 3.70 | 9.48 | 23.50 | 5.30 | 5.60 | 1.30 |
| COPD (%) | 7.26 | 13.53 | 20.70 | 9.20 | 9.60 | 1.20 |
| Peripheral arterial disease (%) | 13.66 | 17.00 | 9.30 | 14.80 | 15.60 | 2.40 |
| Congestive heart failure (%) | ||||||
| None | 87.52 | 74.68 | 33.30 | 83.20 | 83.40 | 0.60 |
| At current admission | 7.85 | 20.55 | 37.00 | 11.60 | 11.70 | 0.30 |
| Before current admission | 4.63 | 4.77 | 0.70 | 5.30 | 4.90 | 1.50 |
| Malignant ventricular arrhythmia (%) | 0.51 | 1.23 | 7.70 | 0.70 | 0.60 | 0.80 |
| Prior PCI (%) | 87.52 | 74.68 | 33.30 | 27.35 | 27.27 | 0.20 |
| eGFR Categories | ||||||
| 30≤eGFR<60 | 84.12 | 81.21 | 7.70 | 83.38 | 82.91 | 1.30 |
| 15≤eGFR<30 | 6.88 | 9.65 | 10.10 | 7.53 | 7.84 | 1.10 |
| eGFR<15 | 0.73 | 1.01 | 3.00 | 0.78 | 0.88 | 1.10 |
| dialysis | 8.26 | 8.13 | 0.50 | 8.31 | 8.38 | 0.20 |
| No. of diseased vessels (%) | ||||||
| 2, with proximal LAD artery | 18.25 | 17.03 | 3.20 | 23.90 | 23.80 | 0.30 |
| 2, without proximal LAD artery | 52.77 | 15.26 | 86.20 | 30.10 | 30.10 | 0.10 |
| 3, with proximal LAD artery | 10.16 | 35.92 | 64.30 | 16.80 | 17.40 | 1.40 |
| 3, without proximal LAD artery | 18.82 | 31.79 | 30.20 | 29.10 | 28.80 | 0.70 |
Plus-minus values are means±SD. CABG = coronary artery bypass graft surgery; COPD = chronic obstructive pulmonary disease; EES = everolimus eluting stent; eGFR= estimated glomerular filtration rate; LAD = left anterior descending artery. ASD reported are percentages. ASD less than 10% denotes relatively small imbalance.
Subgroup Analyses
Pre-specified subgroup analyses were performed based on anatomy: 1) 3-vessel disease vs. 2-vessel disease; and 2) based on complete vs. incomplete revascularization in the PCI cohort. For the subgroup analysis only the corresponding matched pairs in a subgroup were chosen in order to maintain the baseline balance between EES and CABG groups. In addition, pre-specified analysis was performed on the cohort of patients on dialysis. Subgroup analysis using corresponding match pairs of patients on dialysis yielded a small sample size. Therefore, separate propensity score matching was performed by first choosing patients on dialysis from the unmatched cohort of patients and matching them on their baseline measure covariates as described above. In addition, in the cohort not on dialysis (separate propensity score matching), subgroup analyses were performed to evaluate the outcomes based on eGFR categories of 45-60, 30-45, 15-30 and <15 ml/min. However, the number of patients in the eGFR <15 and eGFR 15-30 was small and this group was combined with the eGFR 30-45 category.
All reported P values are two-sided and are not adjusted for multiple testing. All analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC). The event rates presented are Kaplan-Meier estimates.
Results
We identified 11,305 patients with CKD and multivessel disease who fulfilled the entry criteria (eTable 1). Among the patients, 5,058 (45%) underwent PCI with EES and 6,247 (55%) patients underwent CABG. The cohort included 48% of patients with diabetes and 8.2% of patients on dialysis (Table 1). Propensity score matching matched 2,960 PCI patients with 2,960 CABG patients with similar propensity scores. After matching, the ASD was <10% for all variables, indicating an adequate match and no significant baseline difference between the two groups (Table 1). The C-statistics for the model was 0.81. All outcomes presented below are for the matched cohort.
Short-term (within 30 days) Outcomes
Among the 5,920 patients in the matched cohort, PCI was associated with a 45% lower risk of death [1.0% vs. 1.7%; HR=0.55; 95% CI 0.35-0.87; P=0.01], a 78% lower risk of stroke [0.4% vs. 1.7%; HR=0.22; 95% CI 0.12-0.42; P<0.0001], and a 52% lower risk of repeat revascularization [0.4% vs. 0.8%; HR=0.48; 95%CI 0.23-0.98; P=0.04] with no significant difference in myocardial infarction when compared with CABG (Table 2).
Table 2.
Short and long-term outcomes in the propensity score matched cohort
| Outcome | No. of Events | Event Rate (KM estimate) | Hazard Ratio (95% CI) | P-value |
|---|---|---|---|---|
| Short-term outcomes | ||||
| Death | ||||
| EES | 29 | 1.0 | 0.55(0.35,0.87) | 0.01 |
| CABG | 51 | 1.7 | Reference | |
| MI | ||||
| EES | 20 | 0.7 | 1.33(0.68,2.60) | 0.40 |
| CABG | 15 | 0.5 | Reference | |
| Stroke | ||||
| EES | 11 | 0.4 | 0.22(0.12,0.42) | <0.0001 |
| CABG | 50 | 0.5 | Reference | |
| Repeat Revascularization | ||||
| EES | 11 | 0.4 | 0.48(0.23,0.98) | 0.04 |
| CABG | 23 | 0.8 | Reference | |
| Long-term outcomes | ||||
| Death | ||||
| EES | 458 | 22.7 | 1.07(0.92,1.24) | 0.40 |
| CABG | 469 | 20.5 | Reference | |
| MI | ||||
| EES | 233 | 10.7 | 1.76(1.4,2.23) | <0.0001 |
| CABG | 153 | 7.0 | Reference | |
| Stroke | ||||
| EES | 90 | 4.5 | 0.56(0.41,0.76) | 0.0002 |
| CABG | 143 | 6.4 | Reference | |
| Repeat Revascularization | ||||
| EES | 582 | 26.1 | 2.42(2.05,2.85) | <0.0001 |
| CABG | 284 | 13.1 | Reference | |
CABG = coronary artery bypass graft surgery; EES = everolimus eluting stent; MI =myocardial infarction.
Longer-term Outcomes
Primary Outcome (Death)
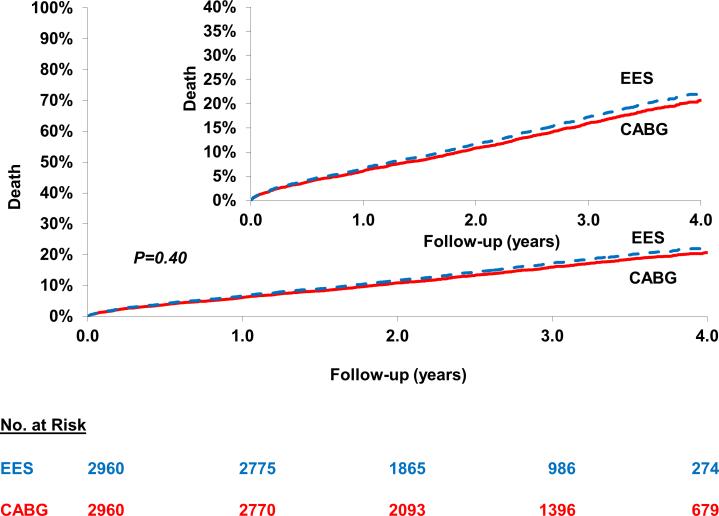
PCI was associated with a similar risk of death [22.7% vs. 20.5%; HR=1.07; 95% CI 0.92-1.24; P=0.40] when compared with CABG at a mean follow-up of 2.9 years (2.7 years for EES and 3.2 years for CABG) (Figure 1) (Table 2). This was consistent across anatomic subgroups based on number of diseased vessels and on completeness of revascularization (Pinteraction >0.05) (Table 3). In addition, in the cohort not on dialysis, subgroup analysis based on eGFR categories yielded largely similar results (Pinteraction >0.05) (Table 4).
Figure 1.
Everolimus eluting stent (EES) versus Coronary Artery Bypass Graft surgery (CABG) for the risk of longer-term death. In the propensity score matched cohort of patients who underwent either percutaneous coronary intervention with EES or CABG there was no statistically significant difference in death between the two cohorts.
Table 3.
Risk of primary and secondary outcomes in anatomic subgroups
| Variables | No. of Patients | No. of Events | Event Rate (%) (KM Estimate) | Hazard Ratio (95% CI) | P-value | P-value for interaction |
|---|---|---|---|---|---|---|
| Outcome: Death | ||||||
| 3 Diseased Vessels | 0.23 | |||||
| EES | 541 | 95 | 24.7 | 1.15[0.81,1.61] | 0.44 | |
| CABG | 541 | 93 | 24.0 | Reference | ||
| 2 Diseased Vessels | ||||||
| EES | 779 | 88 | 17.7 | 0.86[0.61,1.19] | 0.35 | |
| CABG | 779 | 108 | 18.1 | Reference | ||
| Outcome: Myocardial Infarction | ||||||
| 3 Diseased Vessels | 0.04 | |||||
| EES | 541 | 51 | 13.8 | 2.75[1.55,4.87] | 0.0005 | |
| CABG | 541 | 24 | 6.1 | Reference | ||
| 2 Diseased Vessels | ||||||
| EES | 779 | 45 | 8.0 | 1.26[0.76,2.09] | 0.37 | |
| CABG | 779 | 36 | 6.3 | Reference | ||
| Outcome: Repeat Revascularization | ||||||
| 3 Diseased Vessels | 0.05 | |||||
| EES | 541 | 125 | 32.0 | 3.75[2.47,5.69] | <0.0001 | |
| CABG | 541 | 44 | 10.4 | Reference | ||
| 2 Diseased Vessels | ||||||
| EES | 779 | 134 | 22.2 | 2.18[1.56,3.03] | <0.0001 | |
| CABG | 779 | 68 | 12.5 | Reference | ||
| Outcome: Death | ||||||
| Complete Revascularization | 0.89 | |||||
| EES | 541 | 72 | 16.9 | 1.09[0.76,1.58] | 0.64 | |
| CABG | 541 | 74 | 18.4 | Reference | ||
| Incomplete revascularization* | ||||||
| EES | 2419 | 386 | 23.9 | 1.06[0.90,1.25] | 0.48 | |
| CABG | 2419 | 395 | 21,0 | Reference | ||
| Outcome: Myocardial Infarction | ||||||
| Complete Revascularization | 0.13 | |||||
| EES | 541 | 33 | 8.3 | 1.18[0.67,2.09] | 0.56 | |
| CABG | 541 | 33 | 8.56 | Reference | ||
| Incomplete Revascularization* | ||||||
| EES | 2419 | 200 | 11.2 | 1.91[1.48,2.47] | <0.0001 | |
| CABG | 2419 | 120 | 6.5 | Reference | ||
| Outcome: Repeat Revascularization | ||||||
| Complete Revascularization | 0.05 | |||||
| EES | 541 | 79 | 19.8 | 1.68[1.13,2.48] | 0.01 | |
| CABG | 541 | 61 | 15.9 | Reference | ||
| Incomplete Revascularization* | ||||||
| EES | 2419 | 503 | 27.5 | 2.60[2.17,3.13] | <0.0001 | |
| CABG | 2419 | 223 | 12.4 | Reference | ||
Based on incomplete revascularization in the PCI group.
CABG = coronary artery bypass graft surgery; EES = everolimus eluting stent.
Table 4.
Subgroup analyses based on eGFR categories in subjects not on dialysis
| Variables | No. of Patients | No. of Events | Event Rates* | Hazard Ratio (95% CI) | P-value | Test of Interaction |
|---|---|---|---|---|---|---|
| Outcome: Death | 0.15 | |||||
| eGFR <45 | ||||||
| EES | 395 | 61 | 21.74 | 0.71[0.49,1.03] | 0.07 | |
| CABG | 395 | 83 | 25.95 | Reference | ||
| eGFR 45-60 | ||||||
| EES | 1073 | 99 | 14.4 | 1.01[0.74,1.38] | 0.94 | |
| CABG | 1073 | 109 | 13.0 | Reference | ||
| Outcomes: Myocardial Infarction | 0.96 | |||||
| GFR <45 | ||||||
| EES | 395 | 26 | 8.64 | 1.75[0.86,3.56] | 0.12 | |
| CABG | 395 | 14 | 4.73 | Reference | ||
| GFR 45-60 | ||||||
| EES | 1073 | 60 | 7.56 | 1.79[1.12,2.84] | 0.01 | |
| CABG | 1073 | 37 | 4.5 | Reference | ||
| Outcome: Stroke | 0.20 | |||||
| GFR <45 | ||||||
| EES | 395 | 14 | 4.95 | 0.71[0.34,1.48] | 0.36 | |
| CABG | 395 | 20 | 6.31 | Reference | ||
| GFR 45-60 | ||||||
| EES | 1073 | 18 | 2.4 | 0.37[0.20,0.70] | 0.002 | |
| CABG | 1073 | 43 | 4.9 | Reference | ||
| Outcome: Repeat Revascularization | 0.58 | |||||
| GFR <45 | ||||||
| EES | 395 | 68 | 22.32 | 1.69[1.04,2.75] | 0.03 | |
| CABG | 395 | 32 | 11.15 | Reference | ||
| GFR 45-60 | ||||||
| EES | 1073 | 200 | 24.9 | 1.98[1.51,2.58] | <0.0001 | |
| CABG | 1073 | 114 | 14.2 | Reference | ||
Kaplan-Meier Estimate.
CABG = coronary artery bypass graft surgery; EES = everolimus eluting stent.
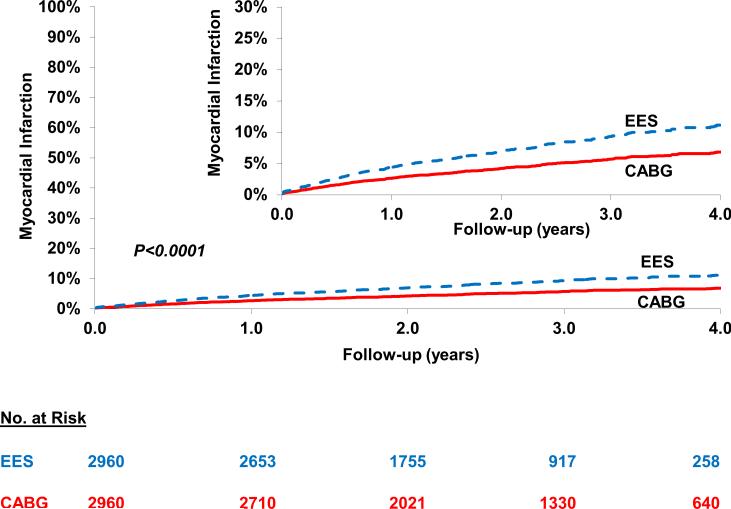
Myocardial Infarction
PCI was associated with a higher risk of myocardial infarction [10.7% vs. 7.0%; HR=1.76; 95% CI 1.40-2.23; P<0.0001] when compared with CABG (Figure 2) (Table 2). This higher risk of myocardial infarction with PCI was driven by patients with three diseased vessels (HR=2.75; 95% CI 1.55-4.87; P=0.0005) but not in those with two diseased vessels (HR=1.26, 95% CI 0.76-2.09; P=0.37) (Pinteraction =0.04) and by patients who underwent incomplete revascularization(HR=1.91;95%CI: 1.48-2.47; P<0.0001) but not in those who underwent complete revascularization (HR=1.18, 95% CI 0.67-2.09; P=0.56) although the test for interaction for the latter was not significant (Pinteraction =0.13) (Table 3). In addition, in the cohort not on dialysis, subgroup analysis based on eGFR categories yielded largely similar results (Pinteraction =0.94) (Table 4).
Figure 2.
Everolimus eluting stent (EES) versus Coronary Artery Bypass Graft surgery (CABG) for the risk of longer-term myocardial infarction (MI). In the propensity score matched cohort of patients there was a higher risk of MI in the cohort who underwent percutaneous coronary intervention with EES when compared with those who underwent CABG. In the subgroup of patients who underwent who underwent complete revascularization with PCI, the increased risk of MI was no longer statistically significant [HR=1.18, 95% CI=0.67-2.09].
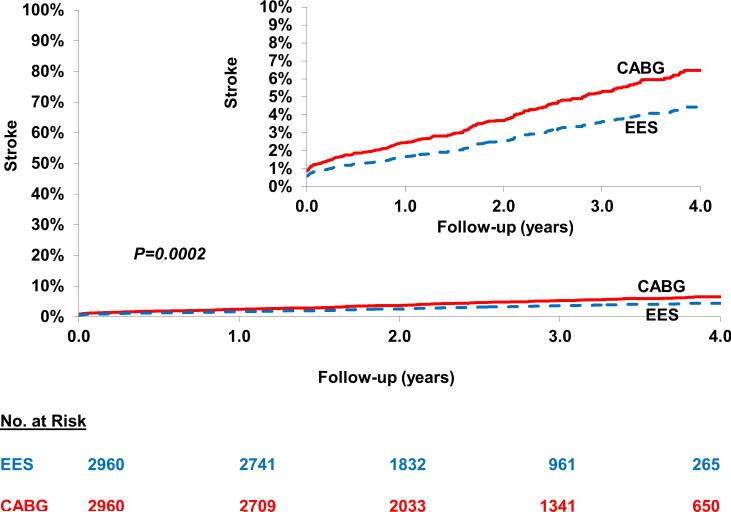
Stroke
PCI was associated with a lower risk of stroke [4.5% vs. 6.4%; HR=0.56; 95% CI 0.41-0.76; P=0.0002] when compared with CABG (Figure 3) (Table 2). In addition, in the cohort not on dialysis, subgroup analysis based on eGFR categories yielded largely similar results (Table 4).
Figure 3.
Everolimus eluting stent (EES) versus Coronary Artery Bypass Graft surgery (CABG) for the risk of longer-term stroke. In the propensity score matched cohort of patients there was a lower risk of stroke in the cohort who underwent percutaneous coronary intervention with EES when compared with those who underwent CABG.
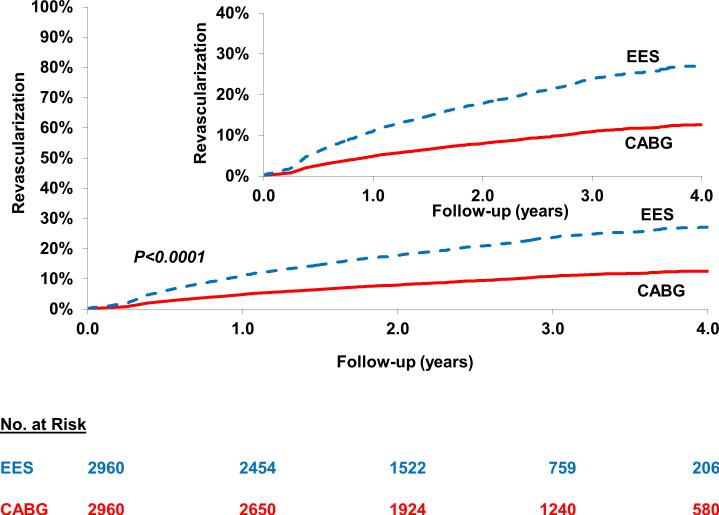
Repeat Revascularization
PCI was associated with a higher risk of repeat revascularization [26.1% vs. 13.1%; HR=2.42; 95% CI 2.05-2.85; P<0.0001] when compared with CABG (Figure 4) (Table 2). The test for interaction was borderline significant (Pinteraction =0.05) both for the number of vessels diseased and completeness of revascularization for the magnitude of effect size rather than the direction such that the risk of repeat revascularization with PCI (vs. CABG) was significantly higher in those with 3-vessel disease (vs. 2-vessel disease) and in those with incomplete revascularization (vs. complete revascularization) (Table 3). In addition, in the cohort not on dialysis, subgroup analysis based on eGFR categories yielded largely similar results (Table 4).
Figure 4.
Everolimus eluting stent (EES) versus Coronary Artery Bypass Graft surgery (CABG) for the risk of longer-term repeat revascularization. In the propensity score matched cohort of patients there was a higher risk of repeat reavscularization in the cohort who underwent percutaneous coronary intervention with EES when compared with those who underwent CABG.
Patients on Dialysis
A separate propensity score matching was performed in patients on dialysis who would otherwise satisfy all of the inclusion and exclusion criteria. Propensity score matching identified 486 patients on dialysis with similar baseline characteristics. At long-term, in the matched cohort of patients on dialysis, PCI was associated with a significantly higher risk of death (54.3% vs. 39.1%; HR=2.02; 95% CI 1.40-2.93; P=0.0002), a numerically higher risk of myocardial infarction (31.9% vs. 16.7%; HR=1.68; 95% CI 0.99-2.85; P=0.05), significantly higher risk of repeat revascularization (48.3% vs. 25.0%; HR=2.44; 95% CI 1.50-3.96; P=0.0003) with no difference in stroke, when compared with CABG (Table 5).
Table 5.
Primary and Secondary outcomes in subjects on dialysis
| Outcomes | No. of Patients | No. of Events | Event Rate (%)* | Hazard Ratio (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Death | ||||||
| EES | 243 | 98 | 54.3 | 2.02[1.40,2.93] | 0.0002 | |
| CABG | 243 | 69 | 39.1 | Ref | ||
| Myocardial Infarction | ||||||
| EES | 243 | 52 | 31.9 | 1.68[0.99,2.85] | 0.05 | |
| CABG | 243 | 30 | 16.7 | Ref | ||
| Stroke | ||||||
| EES | 243 | 12 | 10.7 | 1.17[0.39,3.47] | 0.78 | |
| CABG | 243 | 7 | 4.1 | Ref | ||
| Repeat Revascularization | ||||||
| EES | 243 | 79 | 48.3 | 2.44[1.50,3.96] | 0.0003 | |
| CABG | 243 | 39 | 25.0 | Ref | ||
Kaplan-Meier Estimate.
CABG = coronary artery bypass graft surgery; EES = everolimus eluting stent.
Discussion
The results from this study of 5920 subjects with CKD and multivessel disease who underwent either PCI using the latest generation stents (EES) or CABG, showed that PCI was associated with lower short-term risk (death, stroke and repeat revascularization), similar long-term risk of death, higher risk of MI (in those with incomplete revascularization), and a lower risk of stroke but higher risk of repeat revascularization when compared with CABG. However, in the subgroup on dialysis, the results favored CABG over PCI with an increase in death and repeat revascularization with PCI.
Revascularization in Patients with CKD
Patients with CKD are at high risk for cardiovascular disease and have increased risk of death from CAD. Although the major concern in patients with CAD and CKD is to prevent/avoid acute kidney injury (during PCI or CABG), data shows that CKD patients are 5-10 times more likely to die (mainly from cardiovascular causes) than to develop ESRD requiring dialysis.(10,11) In a longitudinal follow up of a large managed care organization, Keith et al reported that the rate of ESRD was only 1.3% whereas patients were more likely to die, with a mortality rate of 24.3%.(11)
CAD in CKD patients tends to present with unique challenges, including earlier onset, more rapid progression and stronger association with calcification and vascular stiffness. As such, revascularization with PCI or CABG poses challenges. When compared with patients without CKD, patients with CKD have increased rates of repeat revascularization following PCI with bare metal stents (BMS),(12-15) although these rates have improved considerably compared with balloon angioplasty alone.(16) With the advent of drug eluting stents, the incidence of restenosis after PCI has further decreased when compared with BMS in the non-dialysis CKD population,(17) as well as for patients on dialysis,(18-22) although most of the evidence is from non-randomized studies (23). In addition, both PCI and CABG are associated with increased risk of acute kidney injury with some studies showing a 2- to 3-fold higher risk with CABG in the short-term than PCI. (24)
The question of PCI vs. CABG in patients with multivessel disease and CKD is not readily answered from randomized trials as the majority of randomized trials of PCI vs. CABG have either excluded patients with CKD or included only a small subgroup of such patients. In the SYNTAX trial of CABG versus PCI using paclitaxel eluting stents,(25) only 264 patients with renal insufficiency (eGFR <60) were included. In this small subgroup of patients, CABG was superior to PCI for reduction of cardiovascular events (15.6% vs. 27.4%; P<0.05) driven by a reduction in repeat revascularization (5.0% vs. 14.9%; P<0.05) with no difference in death (9.9% vs. 11.8%). (26) When compared with the first generation DES used in SYNTAX, the second generation DES (such as EES) has thinner struts and thinner and more biocompatible polymer-properties which reduce inflammation, promote faster vessel healing and reduce the risk of restenosis and stent thrombosis. As such data from an all-comers population from randomized controlled trials,(27) observational registries(28) and meta-analyses of randomized trials(27,29) indicate reduction in death, MI and stent thrombosis with newer generation stents when compared with older generation stents. Data in the CKD cohort suggests similar low rates of repeat revascularization with EES even when compared with the cohort with no CKD.(30) In the BEST trial (31) PCI with EES was associated with increased risk of MI and repeat revascularization without any mortality difference when compared with CABG, largely similar to our publication on the overall cohort from the New York State registries. (32) However, the trial did not report on the CKD subgroup.
The results of this study with data from 5920 propensity score matched patients therefore offers important insights into the outcomes of patients who underwent PCI with the latest generation DES (EES) or CABG. For the overall CKD cohort, PCI using EES was associated with lower short-term risk (death, stroke and repeat revascularization) when compared with CABG, consistent with the results from previous studies. In addition, the primary outcome of longer-term death was also not statistically different between PCI vs. CABG. PCI was associated with a higher risk of MI (in those with incomplete revascularization), and repeat revascularization when compared with CABG. The higher risk of MI in those with incomplete revascularization with PCI is an important outcome as patients on dialysis who present with an MI have high mortality from cardiac cause and poor long-term survival (33). In a recent analysis of 1786 propensity-matched patients with CKD from Ontario, CABG was associated with improved survival over PCI using DES.(34) However, the study did not report outcomes with second generation DES. Our study has 3.3 times the sample size of the Ontario analysis and restricts the comparison to 2nd generation DES (EES). Consequently, it offers important additional insights as well as being the largest series comparing CABG with 2nd generation DES. Although there was excess stroke with CABG when compared with PCI in the current study, consistent with prior data, more recent trials have shown that the risk of stroke to be similar between CABG and PCI (31), likely due to increased use of off-pump surgery and avoidance of aortic cross clamp.
In our study, in the subgroup on dialysis, the results favored CABG over PCI with increase in death, repeat revascularization and numerically higher MI with PCI. The results in the dialysis cohort is consistent with data from the CREDO-Kyoto PCI/CABG registry analysis of 388 patients on dialysis where CABG was associated with lower risk of cardiac death, MI and repeat revascularization, compared with PCI using BMS or 1st generation DES, although there was no difference in all-cause death between the two groups.(35) However, other studies comparing CABG with PCI using 1st generation DES (N=104 patients) have shown a lower risk of both all-cause death and cardiac death with CABG in patients on hemodialysis (36). The results are also consistent with the analysis by Shroff et al., where short term mortality was higher after CABG but long-term survival was superior, but the Shroff study did not directly compare CABG vs. PCI and PCI was performed mainly using 1st generation DES.(37) Patients with CKD and especially patients on dialysis have increased prevalence of medial calcification,(38) which impairs the response to PCI with increase in prevalence of under expanded stents, reduced efficacy of antiproliferative drug which is eluted from the stent and impaired endothelialization resulting in propensity for restenosis and stent thrombosis. The newer generation DES and especially the EES have thinner strut as well as thinner and more biocompatible polymer which is thromboresistant.(39) Although stenting results in “spot” treatment- the risk of restenosis and stent thrombosis in this stented segment contributes to a proportion of long-term events with PCI. EES, with all the favorable properties described above likely reduces the ‘stent associated’ future events and can potential bridge the outcomes gap between PCI and CABG.
Although the present study compared PCI and CABG, it is not known if revascularization is superior to optimal medical therapy alone in patients with CKD, as patients with CKD were excluded or formed a small proportion of enrolled patients in contemporary trials of revascularization vs. optimal medical therapy. The ISCHEMIA CKD trial (NCT01985360) will address the question of invasive versus conservative management in patients with advanced CKD and will provide valuable insights into this question.
Study Limitations
In our analysis, although propensity score matching adjusts for baseline imbalances, the analysis does not control for unmeasured confounders. We did not have data on medication use between the two cohorts. The analysis compared CABG with PCI using EES and hence the results may not be generalizable to other second generation DES. Moreover, the cohort of patients on dialysis was a small subgroup of patients and is likely underpowered. Although for the overall CKD cohort there was no difference in mortality between PCI and CABG, the mortality difference may appear at longer term follow-up. The registries do not collect data on the method used to measure serum creatinine nor whether the method was standardized to IDMS. However, this is unlikely to differentially affect the PCI and CABG outcomes. Similarly, although the MDRD formula was used to calculate the eGFR, the choice of formula to calculate eGFR is unlikely to differentially affect the PCI and CABG outcomes. The follow-up outcomes were based on registries in New York State and it is likely that patients who had outcomes outside of New York State could have been missed. However, a prior study evaluated the percent of deaths missed because of the above limitation and found that this was small (40) and is unlikely to differentially affect the PCI and CABG outcomes. We excluded patients with left main disease. In the timeframe of this study, PCI for left main was a Class III indication by the ACC/AHA guideline committee. As such only 159 patients with left main disease underwent PCI, whereas the majority (2715 patients) underwent CABG. Given the small numbers and likely selection bias in those who underwent PCI in deference to the Class III recommendations (likely patients with extensive comorbidities who were poor surgical candidates), we did not perform additional analyses on this cohort. In addition, the follow-up duration between the EES and CABG groups were different due to slower uptake of EES when the stent was first approved. However, we used time-to-event analysis and therefore incorporates the follow-up duration.
Conclusions
In this largest series of comparison of PCI with a 2nd generation DES vs. CABG in patients with CKD, CABG was associated with a higher short-term risk of death, stroke and repeat revascularization, whereas PCI with EES was associated with a higher long-term risk of repeat revascularization and perhaps MI (in patients who underwent incomplete revascularization). However, in patients on dialysis, the results favored CABG over PCI. These associations should be considered while choosing between PCI and CABG in patients with CKD and should be tested in future clinical trials.
Supplementary Material
Perspectives.
Competency in Patient Care
Data provide evidence for CABG versus PCI using latest generation stents in subjects with CKD. CABG was associated with a higher 30-day risk of death, stroke and repeat revascularization, whereas PCI with EES was associated with a higher long-term risk of repeat revascularization and perhaps MI (in patients who underwent incomplete revascularization).
Translational Outlook
Further clinical trials are needed to evaluate outcomes with CABG and PCI in subjects with CKD.
Acknowledgments
Funding Source:
The study was funded by Abbott-Vascular.
Disclosures:
Dr. Sripal Bangalore: Ad hoc consultant/speaker: Abbott Vascular. Research Grant: Abbott Vascular., NHLBI
Dr. Saul Blecker: Supported by Agency for Healthcare Research and Quality (AHRQ) grant K08 HS23683
Rest of the authors: None
Acronyms
- CABG
coronary artery bypass graft surgery
- CAD
coronary artery disease
- CKD
chronic kidney disease
- EES
everolimus eluting stent
- ESRD
end stage renal disease
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
Footnotes
Role of the funding source
Study design, data analysis and interpretation, as well as preparation, review, and approval of the manuscript, were done independently by academic authors who were not governed by the funding sponsors. The funding source had no role in the design; conduct of this analysis; interpretation of the data; or preparation or approval of this manuscript.
References
- 1.Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl. 2003:S24–31. doi: 10.1046/j.1523-1755.64.s87.5.x. [DOI] [PubMed] [Google Scholar]
- 2.Reddan DN, Szczech LA, Tuttle RH, et al. Chronic kidney disease, mortality, and treatment strategies among patients with clinically significant coronary artery disease. J Am Soc Nephrol. 2003;14:2373–80. doi: 10.1097/01.asn.0000083900.92829.f5. [DOI] [PubMed] [Google Scholar]
- 3.Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int. 2006;70:2021–30. doi: 10.1038/sj.ki.5001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention. 2015;10:1024–94. doi: 10.4244/EIJY14M09_01. [DOI] [PubMed] [Google Scholar]
- 5.Informatics BoH, Safety OoQaP, NYS Department of Health SPARCS Operations Guide. 2014 [Google Scholar]
- 6.Rosenbaum P, Rubin D. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 7.Rubin D. Using propensity score to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 8.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–9. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–98. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 10.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 11.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Archives of internal medicine. 2004;164:659–63. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 12.Azar RR, Prpic R, Ho KK, et al. Impact of end-stage renal disease on clinical and angiographic outcomes after coronary stenting. Am J Cardiol. 2000;86:485–9. doi: 10.1016/s0002-9149(00)00998-x. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed WH, Shubrooks SJ, Gibson CM, Baim DS, Bittl JA. Complications and long-term outcome after percutaneous coronary angioplasty in chronic hemodialysis patients. Am Heart J. 1994;128:252–5. doi: 10.1016/0002-8703(94)90476-6. [DOI] [PubMed] [Google Scholar]
- 14.Best PJ, Lennon R, Ting HH, et al. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2002;39:1113–9. doi: 10.1016/s0735-1097(02)01745-x. [DOI] [PubMed] [Google Scholar]
- 15.Gruberg L, Weissman NJ, Waksman R, et al. Comparison of outcomes after percutaneous coronary revascularization with stents in patients with and without mild chronic renal insufficiency. Am J Cardiol. 2002;89:54–7. doi: 10.1016/s0002-9149(01)02163-4. [DOI] [PubMed] [Google Scholar]
- 16.Rubenstein MH, Harrell LC, Sheynberg BV, Schunkert H, Bazari H, Palacios IF. Are patients with renal failure good candidates for percutaneous coronary revascularization in the new device era? Circulation. 2000;102:2966–72. doi: 10.1161/01.cir.102.24.2966. [DOI] [PubMed] [Google Scholar]
- 17.Shenoy C, Boura J, Orshaw P, Harjai KJ. Drug-eluting stents in patients with chronic kidney disease: a prospective registry study. PLoS One. 2010;5:e15070. doi: 10.1371/journal.pone.0015070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das P, Moliterno DJ, Charnigo R, et al. Impact of drug-eluting stents on outcomes of patients with end-stage renal disease undergoing percutaneous coronary revascularization. J Invasive Cardiol. 2006;18:405–8. [PubMed] [Google Scholar]
- 19.Halkin A, Selzer F, Marroquin O, Laskey W, Detre K, Cohen H. Clinical outcomes following percutaneous coronary intervention with drug-eluting vs. bare-metal stents in dialysis patients. J Invasive Cardiol. 2006;18:577–83. [PubMed] [Google Scholar]
- 20.Yachi S, Tanabe K, Tanimoto S, et al. Clinical and angiographic outcomes following percutaneous coronary intervention with sirolimus-eluting stents versus bare-metal stents in hemodialysis patients. Am J Kidney Dis. 2009;54:299–306. doi: 10.1053/j.ajkd.2009.01.263. [DOI] [PubMed] [Google Scholar]
- 21.Ishio N, Kobayashi Y, Takebayashi H, et al. Impact of drug-eluting stents on clinical and angiographic outcomes in dialysis patients. Circ J. 2007;71:1525–9. doi: 10.1253/circj.71.1525. [DOI] [PubMed] [Google Scholar]
- 22.Athappan G, Ponniah T. Clinical outcomes of dialysis patients after implantation of DES: meta analysis and systematic review of literature. Minerva Cardioangiol. 2009;57:291–7. [PubMed] [Google Scholar]
- 23.Bangalore S, Vlachos HA, Selzer F, et al. Percutaneous coronary intervention of moderate to severe calcified coronary lesions: insights from the National Heart, Lung, and Blood Institute Dynamic Registry. Catheter Cardiovasc Interv. 2011;77:22–8. doi: 10.1002/ccd.22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang TI, Leong TK, Boothroyd DB, Hlatky MA, Go AS. Acute kidney injury after CABG versus PCI: an observational study using 2 cohorts. J Am Coll Cardiol. 2014;64:985–94. doi: 10.1016/j.jacc.2014.04.077. [DOI] [PubMed] [Google Scholar]
- 25.Kappetein AP, Feldman TE, Mack MJ, et al. Comparison of coronary bypass surgery with drug-eluting stenting for the treatment of left main and/or three-vessel disease: 3-year follow-up of the SYNTAX trial. Eur Heart J. 2011;32:2125–34. doi: 10.1093/eurheartj/ehr213. [DOI] [PubMed] [Google Scholar]
- 26.Holmes J,DR, Mohr FW, Serruys PW, et al. The impact of renal insufficienty on 2-year outcomes following PCI or CABG in the SYNTAX trial. JACC. 2010;55:A184, E1724. [Google Scholar]
- 27.Dangas GD, Serruys PW, Kereiakes DJ, et al. Meta-analysis of everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease: final 3-year results of the SPIRIT clinical trials program (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients With De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 2013;6:914–22. doi: 10.1016/j.jcin.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Räber L, Magro M, Stefanini G, et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. 2012;125:1110–21. doi: 10.1161/CIRCULATIONAHA.111.058560. [DOI] [PubMed] [Google Scholar]
- 29.Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ. 2013;347:f6625. doi: 10.1136/bmj.f6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruberg L, Jeremias A, Rutledge DR, et al. Clinical outcomes in real-world patients receiving the Xience V Everolimus-eluting stent presenting with chronic kidney disease: One-year results fromt eh Xience V USA study. JACC. 2011;57:E1896. [Google Scholar]
- 31.Park SJ, Ahn JM, Kim YH, et al. Trial of Everolimus-Eluting Stents or Bypass Surgery for Coronary Disease. N Engl J Med. 2015;372:1204–12. doi: 10.1056/NEJMoa1415447. [DOI] [PubMed] [Google Scholar]
- 32.Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL. Everolimus-eluting stents or bypass surgery for multivessel coronary disease. N Engl J Med. 2015;372:1213–22. doi: 10.1056/NEJMoa1412168. [DOI] [PubMed] [Google Scholar]
- 33.Herzog CA, Ma JZ, Collins AJ. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med. 1998;339:799–805. doi: 10.1056/NEJM199809173391203. [DOI] [PubMed] [Google Scholar]
- 34.Chan W, Ivanov J, Ko D, et al. Clinical outcomes of treatment by percutaneous coronary intervention versus coronary artery bypass graft surgery in patients with chronic kidney disease undergoing index revascularization in Ontario. Circ Cardiovasc Interv. 2015:8. doi: 10.1161/CIRCINTERVENTIONS.114.001973. [DOI] [PubMed] [Google Scholar]
- 35.Marui A, Kimura T, Nishiwaki N, et al. Percutaneous Coronary Intervention Versus Coronary Artery Bypass Grafting in Patients With End-Stage Renal Disease Requiring Dialysis (5-Year Outcomes of the CREDO-Kyoto PCI/CABG Registry Cohort-2). Am J Cardiol. 2014;114:555–61. doi: 10.1016/j.amjcard.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 36.Sunagawa G, Komiya T, Tamura N, Sakaguchi G, Kobayashi T, Murashita T. Coronary artery bypass surgery is superior to percutaneous coronary intervention with drug-eluting stents for patients with chronic renal failure on hemodialysis. Ann Thorac Surg. 2010;89:1896–900. doi: 10.1016/j.athoracsur.2010.02.080. discussion 1900. [DOI] [PubMed] [Google Scholar]
- 37.Shroff GR, Solid CA, Herzog CA. Long-term survival and repeat coronary revascularization in dialysis patients after surgical and percutaneous coronary revascularization with drug-eluting and bare metal stents in the United States. Circulation. 2013;127:1861–9. doi: 10.1161/CIRCULATIONAHA.112.001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Genereux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63:1703–14. doi: 10.1016/j.jacc.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 39.Kolandaivelu K, Swaminathan R, Gibson WJ, et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400–9. doi: 10.1161/CIRCULATIONAHA.110.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hannan EL, Racz MJ, McCallister BD, et al. A comparison of three-year survival after coronary artery bypass graft surgery and percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1999;33:63–72. doi: 10.1016/s0735-1097(98)00540-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.