Abstract
Matrix and cellular alignment are critical factors in the native function of many tissues, including muscle, nerve, and ligaments. Collagen is frequently a component of these aligned tissues, and collagen biomaterials are widely used in tissue engineering applications. However, the generation of aligned collagen scaffolds that maintain the native architecture of collagen fibrils has not been straightforward, with many methods requiring specialized equipment or technical procedures, extensive incubation times, or denaturing of the collagen. Herein, we present a simple, rapid method for fabrication of highly aligned collagen scaffolds. Collagen was assembled to form a fibrillar hydrogel in a cylindrical conduit with high aspect ratio and then frozen and lyophilized. The resulting collagen scaffolds demonstrated highly aligned topographical features along the scaffold surface. This presence of an initial fibrillar network and the high-aspect ratio vessel were both required to generate alignment. The diameter of fabricated scaffolds was found to vary significantly with both the collagen concentration of the hydrogel suspension and the diameter of conduits used for fabrication. Additionally, the size of individual aligned topographical features was significantly dependent on the conduit diameter and the freezing temperature. When cultured on aligned collagen scaffolds, both rat dermal fibroblasts and axons emerging from chick dorsal root ganglia explants demonstrated elongated, aligned morphology and growth on the aligned topographical features. Overall, this method presents a simple means for generating aligned collagen scaffolds that can be applied to a wide variety of tissue types, particularly those where such alignment is critical to native function.
Keywords: tissue engineering, regenerative medicine, collagen, scaffold, anisotropy
Graphical Abstract
INTRODUCTION
Structural anisotropy is a factor in the native function of a variety of biological tissues. Structural alignment imparts necessary mechanical strength to load bearing tissues such as skeletal muscle, cardiac muscle, the smooth muscle lining of blood vessels and intestines, ligaments, and tendons.1–8 Cell and matrix alignment also provide a guidance field for migrating cells or cell processes, which plays an important role in tissue morphogenesis, wound healing, and tissue regeneration.9,10 Matrix alignment can also guide tissue regeneration in neural tissues such as the spinal cord and peripheral nerves.11,12 Tissue engineering strategies that incorporate structural anisotropy are crucial in the development of next generation scaffolds fabricated from synthetic materials or natural polymers, such as collagen.13–17
Collagen is the most abundant protein in the human body and a key component of naturally occurring extracellular matrix (ECM).18 Type I collagen is easily extracted from animal sources and can be further processed to produce scaffolds that retain a fibrillar architecture characteristic of native ECM.19 Further, collagen is degraded and remodeled through natural enzymatic pathways, in contrast to many synthetic polymers that degrade hydrolytically into products that can be cytotoxic.19–22 Extracted type I collagen is biocompatible, FDA-approved for a number of in vivo uses, and supports cellular growth and infiltration.23,24 At physiological temperature and pH, a solution of collagen triple-helical monomers self-assembles into a network of D-banded, triple-helical fibers surrounding a hydrated solution.25 This fibrillar collagen network imparts mechanical strength to a gel or tissue, presents cell adhesion epitopes naturally within the matrix, and has the potential to guide cells within the network, similar to the topography of natural ECM.25,26
There are many approaches to align collagen for use as scaffolds, which have different advantages and drawbacks. Electrospinning is a relatively simple method that can easily generate highly aligned fiber scaffolds starting with collagen as a base solution. However, the volatile solvents, high shear, and harsh electrical fields have been shown to irreversibly alter collagen’s native structure, resulting in denatured, gelatin fibers.27 Alignment can also be induced by applying an external potential field during collagen self-assembly, such as a magnetic field or an electrochemical pH gradient; however, these methods require highly specialized and often costly apparatus and as a result are difficult to scale up.28,29 Several approaches utilize directional freezing and freeze-drying to form aligned scaffolds from natural polymers such as silk and collagen.30–36 Wray et al. used gradient freezing of a silk solution in a mold of arrayed wires to fabricate silk-based scaffolds with tunable porosity, channel architecture, mechanical properties, and degradation rates.30 Stoppel et al. then demonstrated that these aligned silk-based scaffolds could be combined with cardiac tissue-derived ECM to greatly enhance cellular infiltration and vascularization of the scaffold constructs when implanted subcutaneously in rats.31 Other work has utilized similar freezing and lyophilization methods to produce collagen based scaffolds with aligned pores. Yannas et al. have prepared collagen and glycosaminoglycan sponges with axially aligned pores through unidirectional freezing and subsequent lyophilization of a collagen-chondroitin sulfate suspension aimed at scaffolds for nerve regeneration.32–35 Unlike the methods described herein, this approach does not start with a continuous, entangled network of self-assembled, fibrillar collagen, but rather a mixture of soluble collagen and insoluble collagen fragments. More recent work from the same laboratory has demonstrated that collagen-only scaffolds with axially aligned pores can be fabricated through gradient freezing of an acidified collagen suspension. The setup for fabrication of these scaffolds required a custom built polyethylene mold with copper plugs and precise orientation of the molds to allow gradient freezing.36
Mechanical forces can also be used to generate alignment. Anisotropic strain will orient the collagen fibers in the direction of the maximum strain, and the strength of the alignment will be dependent on the degree of anisotropy.37 This alignment is local to the axis of maximum strain, and sections of the bulk gel that are not strained will maintain randomly oriented fibrils. One approach to introduce anisotropic strain is via cell-mediated compaction. Cells are included during the preparation of the collagen gel and allowed to compact the gel by generating traction, constraining the deformation of the gel in some directions but allowing deformation in others. The anisotropic strain develops, which leads to fiber alignment. This alignment is dependent on the cell type, number, and incubation time.38 Anisotropic strain can also be generated from extrinsically applied mechanical forces. In general, to apply this strain uniformly, repeatedly, and consistently, specialized equipment and apparatus are required, limiting the potential of this method for large scale fabrication of aligned collagen scaffolds.
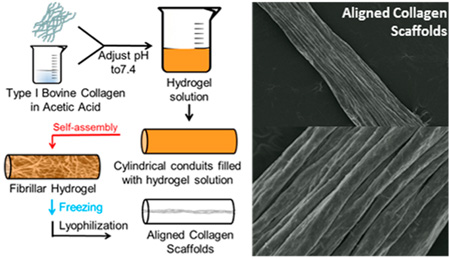
Herein, we present a simple, alternate method that allows for the rapid fabrication of aligned collagen scaffolds. We hypothesized that, by constraining the shape of a collagen hydrogel to a high aspect ratio vessel during freezing and lyophilization, we could control the direction of gel contraction. The combined effect of the high aspect ratio vessel and the high water fraction of the hydrogel that is removed during lyophilization causes the radial shrinkage to be much greater than the axial shrinkage, which would generate scaffold alignment. This method is different from previous approaches with respect to its simplicity, the mechanism by which alignment is generated, and the presence of a continuous, entangled fibrillar collagen network as a starting material.
MATERIALS AND METHODS
Scaffold Fabrication
All reagents were purchased from Sigma unless otherwise indicated. Type-I bovine collagen (Elastin Products Company) was reconstituted in 0.02N acetic acid at 3 mg/mL. Buffered hydrogel solutions were prepared using the following protocol for 1 mL: 20 µL of HEPES, 130 µL of 0.15N NaOH, 100 µL of 10 × MEM, 53 µL of Medium 199 (Life Technologies), 10 µL of l-glutamine, 10 µL of penicillin/streptomycin, and 677 µL of 3.0 mg/mL collagen solution. The final concentration of collagen in the buffered hydrogel suspension was 2.0 mg/mL. Cylindrical silicone conduits (SmallParts.com) of various diameter (2380 µm, 3175 µm, 4762 µm, and 6350 µm) but the same length (6 cm) were used to ensure a high aspect ratio (minimum aspect ratio, length/diameter, was 9.44). These conduits were filled with hydrogel solution, and the filled conduits were then incubated at 37 °C for 1 h to allow collagen self-assembly. Conduits were then frozen as described below and lyophilized (VirTis Benchtop K) overnight.
Parameter Variation in Scaffold Fabrication
The effects of several parameters on scaffold features were investigated. To study the effects of the freezing temperature and rate of freezing, fibrillar collagen within silicone conduits was placed in either (1) a −80 °C freezer for 3 h, (2) a −20 °C freezer for 3 h, or (3) snap frozen in liquid nitrogen. Freezing rate was not directly controlled. To study the effects of collagen concentration, buffered hydrogel suspensions with final collagen concentrations of 2.0 mg/mL, 4.0 mg/mL, 6.0 mg/mL, or 8.0 mg/mL were prepared using the recipe described above with suspensions of type-I bovine collagen in 0.02 acetic acid reconstituted at 3.0, 6.0, 9.0, and 12.0 mg/mL, respectively. These buffered hydrogel suspensions were self-assembled within silicone conduits, frozen at −80 °C, and lyophilized overnight. To determine the effect of a low aspect-ratio vessel on scaffold fabrication, buffered hydrogel solutions were prepared as described above, 500 µL was placed in a low aspect ratio vessel (15.6 mm diameter wells in a 24 well plate (Fisher)), self-assembled at 37 °C for 1 h, frozen at −80 °C, and lyophilized overnight. The approximate aspect ratio of these scaffolds from the low-aspect ratio vessel was 0.167. Finally, to determine if collagen self-assembly was required to generate aligned features using this process, scaffolds were fabricated from an unassembled collagen suspension. Cylindrical silicone conduits were filled with buffered collagen solution but not incubated at 37 °C. Instead, the filled conduits were immediately frozen at −80 °C and lyophilized overnight.
Scanning Electron Microscopy (SEM)
SEM imaging of freeze-dried scaffolds and rehydrated scaffolds was performed to evaluate scaffold features such as topography and alignment of surface features. Freeze-dried scaffolds were sputter coated with gold/palladium (SCD 004, Balzers Union Limited) and imaged via SEM (Amray 1830I, Amray Inc.). To observe the morphology of rehydrated scaffolds via SEM, freeze-dried scaffolds were hydrated in phosphate buffered saline (PBS) for 30 min at 37 °C. Hydrated scaffolds were then subjected to dehydration in a series of solutions with increasing acetone concentration (25%, 50%, 75%, and 95%) for 15 min each and then placed in 100% acetone overnight. These samples were then critical point dried (CPD 020, Balzers Union Limited), sputter coated with gold/palladium, and imaged via SEM.
Scaffold Diameter Measurements
To make measurements of diameter, the scaffolds were cut into 1 cm transverse sections and placed into 24-well plates. Bright field montages were obtained at 4× magnification. PBS was added to each well, and the scaffolds were allowed to hydrate for at least 30 min. A second series of bright-field montages was obtained at 4× magnification to allow a comparison of scaffold diameter between freeze-dried and hydrated states. Scaffold diameter measurements from these images were made using ImageJ (NIH). Scaffold diameter was measured as the edge-to-edge width of scaffolds.
Feature Size Measurements
The width of individual topographical features on the surface of aligned collagen scaffolds was analyzed from SEM images of the freeze-dried scaffolds. Feature size measurements were made using ImageJ.
Uniaxial Tensile Testing
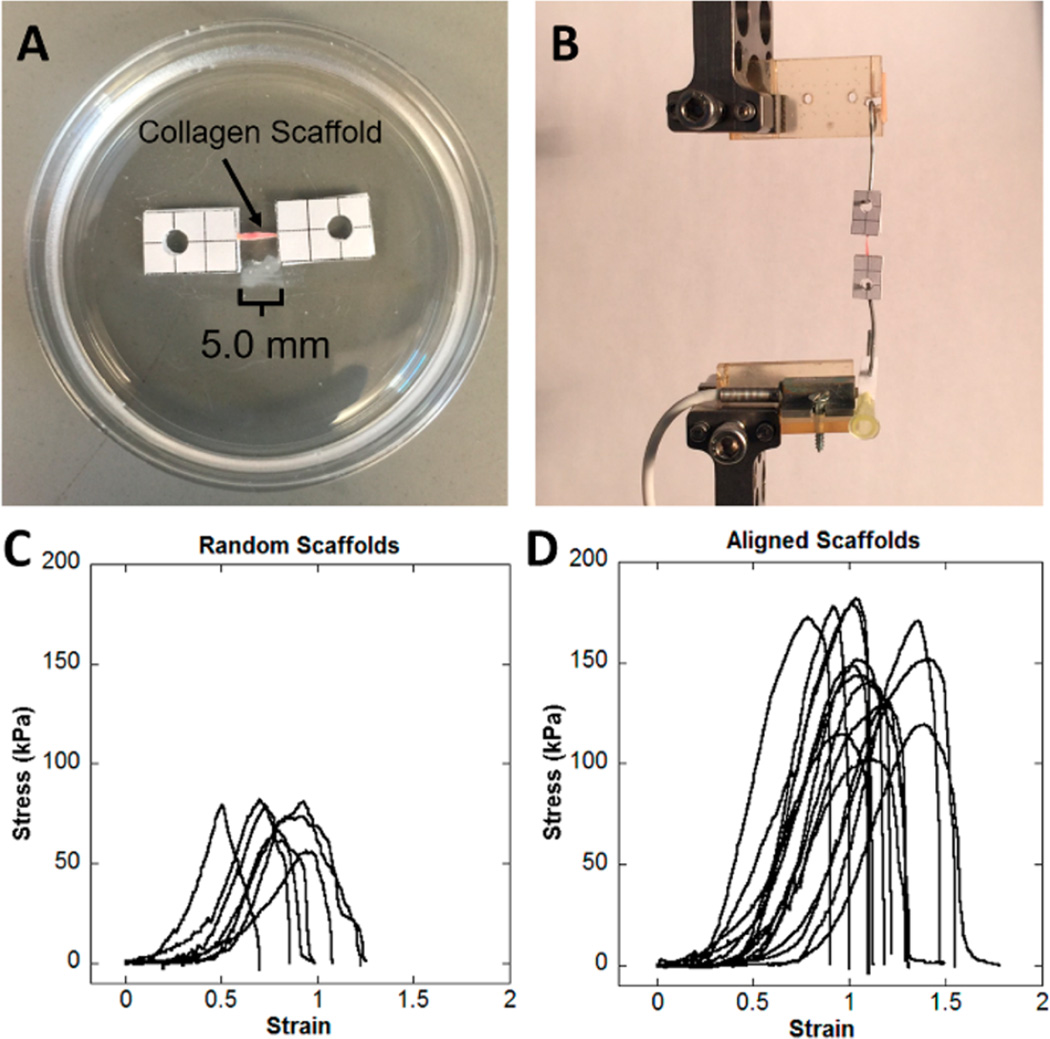
Two groups of scaffolds were tested to determine mechanical properties. Aligned scaffolds were prepared in 6 cm silicone conduits 3175 µM in diameter, self-assembled, frozen, and lyophilized as described earlier. Random scaffolds were prepared using the low aspect ratio vessel, self-assembled, frozen, and lyophilized as described in the parameter variation study. Both random and aligned scaffolds examined in tensile testing were prepared from a 2.0 mg/mL collagen hydrogel suspension and frozen at −80 °C. Aligned scaffolds were cut into 13 mm segments, and random scaffolds were cut into 13 × 2 mm segments. Rectangular pieces of polystyrene with punched holes were used as interface between the scaffolds and the mechanical testing apparatus to prevent slippage of scaffolds during testing. Ends of the scaffolds were sandwiched between two pieces of polystyrene with cyanoacrylate adhesive, leaving a consistent gauge length of 5 mm. The adhesive was allowed to dry for 1 h before the samples were rehydrated in PBS for at least 30 min. A representative image of a mounted scaffold is shown in Figure 5A.
Figure 5.
Representative images of mechanical testing setup and resulting stress–strain curves. (A) Photograph of aligned collagen scaffold mounted to polystyrene rectangles. (B) Photograph of aligned collagen scaffolds loaded on BOSE Enduratec for tensile testing. (C) Stress–strain curves observed in tensile testing of random scaffolds prepared in low aspect ratio vessels. (D) Stress–strain curves for aligned scaffolds fabricated in high aspect ratio conduits. Both scaffold groups displayed nonlinear, strain-stiffening behavior.
Samples were strained in uniaxial tension on a BOSE Enduratec ELF 3200 (BOSE Corporation, Eden Prairie, MN, USA), and load was measured using a 1N cantilever load cell (Measurement Specialties, Hampton, VA, USA). Hooks were formed from 20G steel wire and used to apply strain to the samples via the polystyrene. Samples were strained at 0.5 mm/s (10% s−1) until failure. Load and displacement were recorded at 24.4 Hz. Load was converted to nominal stress by dividing the load by the cross-sectional area. For aligned scaffolds, cross-sectional area was assumed to be that of a solid cylinder with diameter equal to that of the scaffold. For random scaffolds, cross sectional area was assumed rectangular and equal to the width times the thickness of the scaffold. The thickness of the random scaffolds was determined using a Kinexus Ultra rotational rheometer (Malvern Instruments). Samples were laid flat on the bottom plate of the rheometer stage, and the cylindrical upper top plate was lowered until 1.5 mm above the bottom of the stage. The top plate was then lowered until a 100 mN normal force was developed, and the thickness of the random scaffold was taken to be the distance from the bottom plate to the top plate.
Ultimate tensile strength (UTS) and strain at UTS were determined. Elastic modulus (E) was taken to be the slope of the linear region of the stress–strain curves, determined using linear regression in MATLAB.
Rat Dermal Fibroblast Culture
Rat dermal fibroblasts (RDFs) that constitutively expressed green fluorescent protein (GFP) were isolated from a transgenic animal (a gift from the W.M. Keck Center for Collaborative Neuroscience). RDFs were cultured in DMEM containing 10% FBS, 1% l-glutamine, and 1% penicillin/streptomycin. RDFs were seeded directly onto hydrated aligned scaffolds and hydrated random scaffolds formed in low aspect ratio vessels, at a density of ~80 000 cells/cm2, and maintained in culture for 2 days prior to epifluorescent imaging on an Olympus IX81. The alignment of cells was measured by finding the cosine of the angle between the major axis of the cell and the prevailing orientation of the scaffold features. The major axis of the cells was determined using the entire cell as visualized by the epifluorecent images. These values were squared to produce an alignment index, Φ = cos2 θ, for each cell, where Φ = 1 indicated alignment parallel to the features and Φ = 0 indicated alignment perpendicular to the features.39
Neural Explant Culture
Dorsal root ganglia (DRG) were isolated from pathogen-free chick eggs at embryonic day 8 (Charles River Laboratories, Cambridge, MA) and cultured in DMEM containing 10% fetal bovine serum (FBS), 1% l-glutamine, and 1% penicillin/streptomycin supplemented with 100 ng/mL nerve growth factor. DRGs were plated onto hydrated aligned collagen scaffolds as well as hydrated random collagen scaffolds and maintained in culture for 3 days. DRG cultures were fixed in 4% paraformaldehyde solution and then stained immunohistochemically with mouse antineurofilament 200 followed by goat-antimouse AlexaFluor 568 secondary antibody (Life Technologies). Epifluorescent images were taken on an Olympus IX81.
Statistical Analysis
Data were analyzed with a two-way ANOVA followed by posthoc pairwise comparisons with Tukey’s All Pairs Comparisons. Differences were considered significant at P < 0.05.
RESULTS
Scanning Electron Microscopy
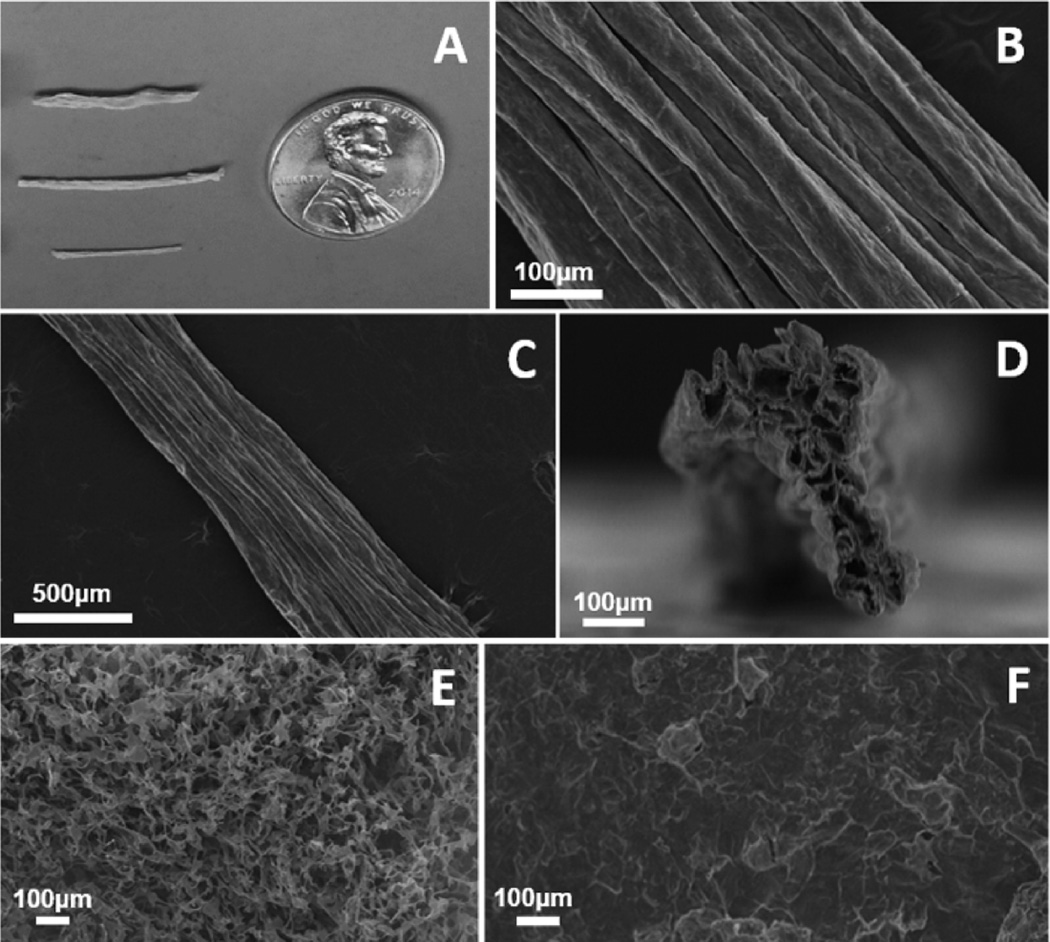
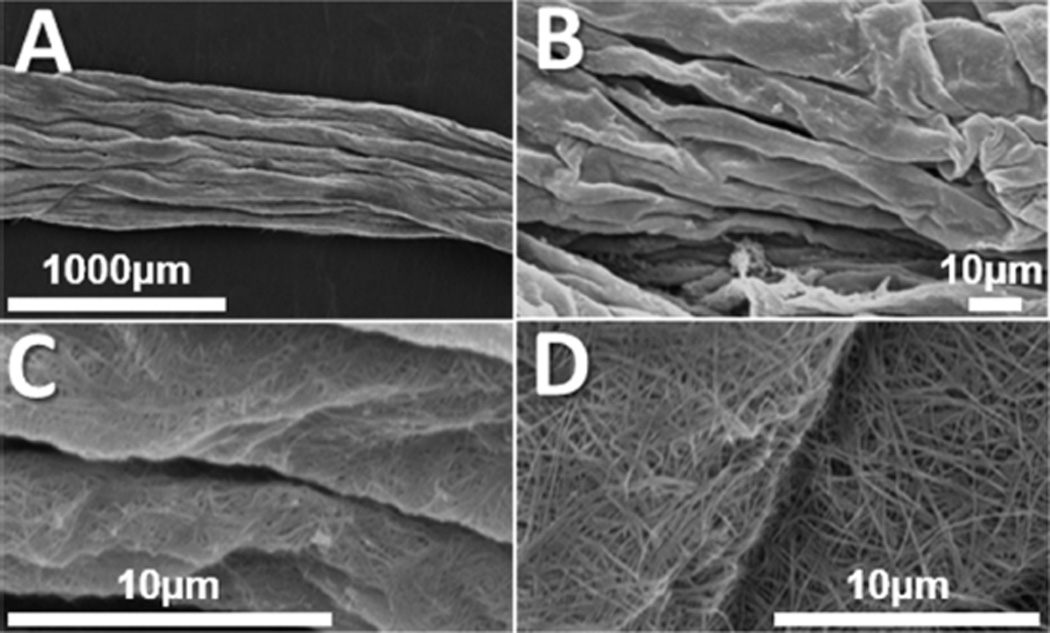
SEM revealed a highly aligned, topographical architecture with long-range uniformity on the collagen scaffolds. The exterior of the scaffolds appeared as a cable-like group of aligned cylindrical structures (Figure 1B), which was preserved along the length of the aligned collagen scaffolds (Figure 1C). Cross sections revealed a highly porous interior, which may have given rise to folds and ridges on the exterior surface as the scaffolds contracted during lyophilization (Figure 1D). Scaffolds fabricated from unassembled collagen suspensions in high aspect ratio cylindrical conduits (Figure 1E) showed a highly porous topography, but failed to show any alignment of topographical features. Scaffolds fabricated from self-assembled collagen gels in a low aspect ratio vessel (Figure 1F) similarly did not exhibit any preferential alignment of topographical features.
Figure 1.
Example images of collagen scaffold morphology. (A) Optical image of scaffolds fabricated of various diameters (top to bottom 6350 µm, 4762 µm, and 3175 µm). (B) SEM revealed highly aligned topographical features along the surface of collagen scaffolds. (C) Aligned features were maintained uniformly along the length of collagen scaffolds. (D) Cross sectional SEM images revealed a highly porous interior within the scaffolds. (E) Scaffolds fabricated from unassembled collagen suspensions and (F) scaffolds fabricated from fibrillar collagen in low aspect ratio vessels showed no preferential orientation of topographical features. All samples featured here were fabricated at a collagen concentration of 2.0 mg/mL and a freezing temperature of −80 °C and were imaged in the dry, as-fabricated state.
Scaffold Diameter Measurements
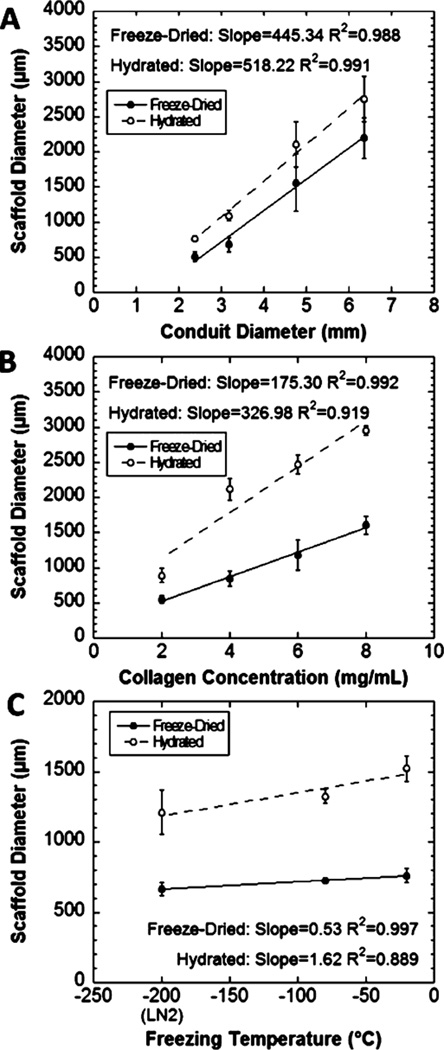
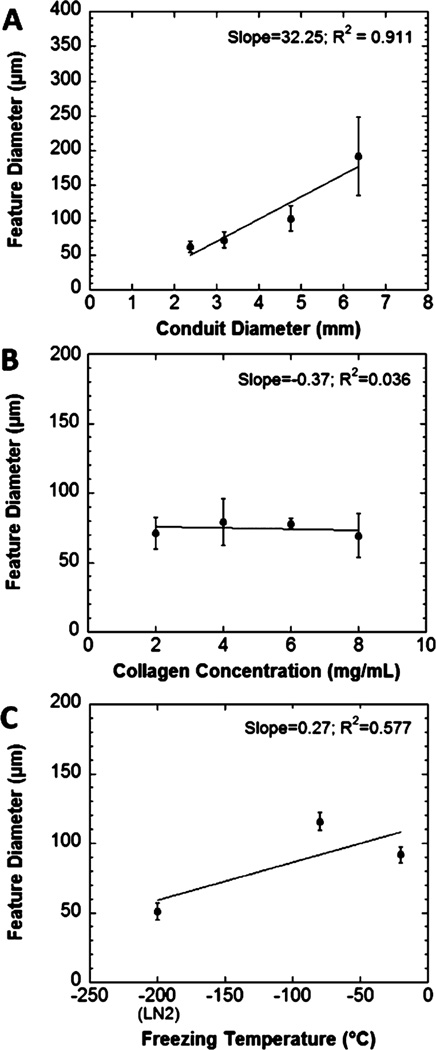
For scaffolds prepared in the same length of tubing, scaffold diameter after freezing and sublimation was significantly related to the initial diameter for both dry and hydrated scaffolds (P < 0.0001; Figure 2A). Posthoc pairwise comparisons indicated that all scaffold diameters were significantly different from one another (P < 0.0001) except for the comparison between scaffolds fabricated in the two smallest conduits (P = 0.676). The collagen concentration also significantly influenced the resulting scaffold diameter for both dry and hydrated scaffolds (P < 0.0001) (Figure 2B). The effect of freezing temperature on scaffold diameter was also examined (Figure 2C). Significant differences were observed between scaffolds frozen at −20 °C and snap frozen in liquid nitrogen in both dry (P = 0.0195) and hydrated states (P = 0.0074). Despite this, freezing temperature was not identified as an important factor for determining scaffold diameter, based on the minimal increase in scaffold diameter for a corresponding increase in temperature (freeze-dried, 0.53 µm/°C; hydrated, 1.62 µm/°C). Hydration significantly increased the diameter of scaffolds in collagen concentration and freezing temperature studies (P < 0.0001). Hydrating the scaffolds generally increased the diameter of scaffolds in the conduit diameter study, but there was high variability in the final diameter with larger scaffolds, and the increase was not statistically significant (P = 0.059).
Figure 2.
Final scaffold diameter in dry and hydrated states. The diameter changed with (A) the initial scaffold diameter, which is the inner diameter of the tube in which the gel is cast, and (B) the collagen concentration of the hydrogel solution (P < 0.0001). (C) The scaffold diameter varied significantly with freezing temperature in both dry (P = 0.0233) and hydrated states (P = 0.0088), with posthoc comparisons revealing only the differences between scaffolds frozen at −20 °C and snap frozen in liquid nitrogen were significant (P = 0.0195 for freeze-dried scaffolds, P = 0.0074 for hydrated scaffolds).
Feature Size Measurements
The diameter of individual topographical features on the surface of aligned collagen scaffolds was significantly related to the diameter of cylindrical conduits used for fabrication (Figure 3A; P < 0.0001). However, posthoc pairwise comparisons indicated that only features from scaffolds made in largest diameter conduits were significantly larger than features from the other conduits (P < 0.0001). No other comparisons were significant (min P = 0.1868). The freezing temperature at which scaffolds were fabricated also significantly affected the diameter of these individual topographical features (P < 0.0001). However, these factors were poorly correlated (R2 = 0.577). Snap-freezing in liquid nitrogen resulted in smaller diameter features than freezing at −80 °C or −20 °C (Figure 3B). The concentration of collagen in the initial hydrogel suspension did not have a significant effect on the feature diameter (P = 0.5439). SEM images of the scaffolds fabricated from collagen assembled in low aspect ratio vessels demonstrated no preferentially aligned or oriented features at any magnification (Figure 1F).
Figure 3.
(A) The diameter of individual topographical features on the surface of the aligned collagen scaffolds. The diameter was significantly affected by the initial conduit diameter with posthoc tests revealing that only the largest conduits produced a significant difference (P < 0.0001). (B) The collagen concentration of the hydrogel suspension did not significantly affect the diameter of topographical features (P = 0.5439). (C) Freezing temperature significantly affected the diameter of individual fiber-like features (P < 0.0001).
Hydrated Scaffold Morphology
Critical point drying of hydrated aligned collagen scaffolds was performed to obtain SEM images of aligned collagen scaffolds in the hydrated state. Critical point drying is commonly utilized to dehydrate biological samples for SEM observation without disrupting the morphology or structure of the sample.40 The SEM of hydrated scaffolds revealed that the aligned topographical features observed in the freeze-dried scaffolds were preserved after scaffold swelling (Figure 4A). At higher magnification, folds and ridges of individual topographical features could be seen that were aligned in the same direction as the larger aligned topographical features (Figure 4B). At greater than 6500× magnification, randomly aligned collagen fibers were observed, which collectively comprise the larger aligned topographical features (Figure 4C and D).
Figure 4.
Representative SEM images of aligned collagen scaffolds critical point dried in the hydrated state. (A) Aligned topographical features are preserved in the hydrated state observed at 50×. (B) Folds and ridges of individual features are aligned in the same direction as other scaffold features observed at 1300×. (C) The surface of collagen scaffolds shows evidence of a randomly oriented, fibrillar topography at high magnification. Panels B and C are high magnification frames of panel A. (D) Representative image showing randomly oriented, fibrillar topography on the surface of a separate collagen scaffold.
Uniaxial Tensile Testing
When strained in tension, the scaffolds displayed nonlinear strain-stiffening behavior. A region of nearly elastic deformation is visible on the stress–strain curves after the toe region and before achieving the maximum stress (Figure 5). Significant differences were observed in scaffold mechanical properties (Table 1). Aligned scaffolds fabricated in high aspect ratio conduits demonstrated a significantly greater ultimate tensile strength than random scaffolds fabricated in low aspect ratio vessels, which displayed no preferential orientation (P < 0.0001). The strain at the ultimate tensile strength was also significantly greater in aligned scaffolds than in random scaffolds (P = 0.0005). Differences in elastic modulus were also observed, with aligned scaffolds having significantly greater E than random scaffolds (P = 0.0008).
Table 1.
Ultimate Tensile Strength (UTS), Young’s Modulus, and Strain at UTS for Aligned Scaffolds, Fabricated in High Aspect Ratio Conduits, and for Random Scaffolds, Fabricated in Low Aspect Ratio Vesselsa
| sample | ultimate tensile strength (kPa) |
Young’s modulus (kPa) |
strain at UTS |
|---|---|---|---|
| aligned scaffolds |
147 ± 25.2 | 567 ± 134 | 1.10 ± 0.177 |
| random scaffolds |
73.4 ± 9.91 | 349 ± 85.5 | 0.784 ± 0.157 |
Results are reported as average +/− standard deviation.
Cellular Response to Aligned Scaffolds
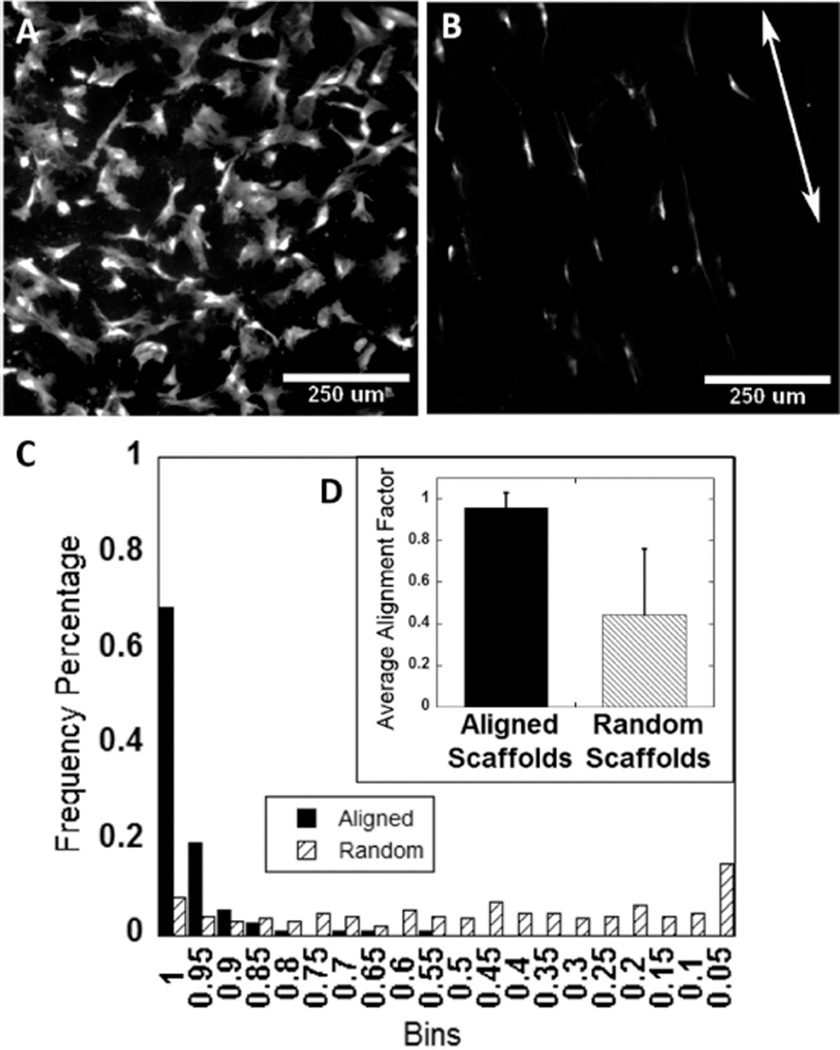
Green fluorescent protein-expressing rat dermal fibroblasts were seeded directly onto aligned and random, hydrated collagen scaffolds. While the seeding density was the same for both, the different geometries caused differences in cell density observed after 48 h. The geometry of the low aspect ratio vessel results in a flat disc-like scaffold that the cells were easily able to settle onto during seeding. In comparison, the aligned collagen scaffolds retained a basic cylindrical geometry. As a result of the curvature, cells were less able to settle on top of the scaffolds, and many cells settled on the bottom of the well plate instead, resulting in an apparent lower cell density on the aligned collagen scaffolds as compared to the random scaffolds prepared in the low-aspect ratio vessel. After 48 h in culture, those fibroblast cells which did seed to the surface of the aligned collagen scaffolds were oriented and extended along the same axis as the aligned topography (Figure 6B). In contrast, cells on scaffolds prepared in the low aspect ratio vessel, which demonstrated random orientation of microfeatures, did not demonstrate any preferential alignment or direction of growth (Figure 6A). An alignment index was generated for each scaffold, where an average value of 1 indicates alignment parallel to the axis of scaffold orientation, an average value of 0 indicates alignment perpendicular to the axis, and an average value of 0.5 indicates random alignment. Cells on aligned scaffolds had an alignment parameter near 1 (0.957 ± 0.073), which was significantly greater than the alignment parameter for cells on randomly oriented scaffolds (0.445 ± 0.317; P < 0.0001; Figure 6D). Alignment was exponentially distributed on aligned scaffolds but uniformly distributed on random scaffolds (Figure 6C).
Figure 6.
(A) GFP-expressing rat dermal fibroblasts cultured on random collagen scaffolds fabricated in low aspect ratio vessels showing no preferential alignment. (B) However, GFP expressing rat dermal fibroblasts cultured on aligned collagen scaffolds elongate and orient parallel to the features on the aligned collagen scaffolds (arrow indicates direction of aligned scaffold features). (C and D) Alignment of GFP expressing rat dermal fibroblasts on aligned collagen scaffolds was quantified, and the average alignment factor was found to be close to 1 on aligned collagen scaffolds.
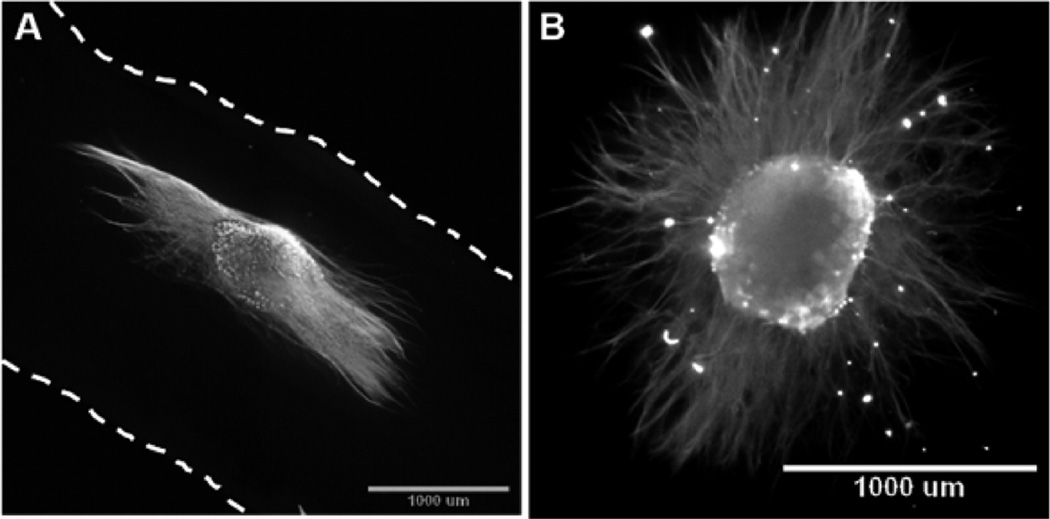
Chick DRG explants cultured on aligned collagen scaffolds extended processes along the length of the aligned collagen scaffolds (Figure 7A). DRG explants cultured on randomly oriented collagen scaffolds demonstrated no preferentially oriented outgrowth (Figure 7B).
Figure 7.
Chick dorsal root ganglia explants cultured on both aligned and randomly oriented collagen scaffolds and immunolabeled with antineurofilament M. (A) Epifluorescence image of DRG extending axons along the length of aligned collagen scaffold (dashed lines indicate the edge of scaffold). (B) Epifluoresence image of DRG explant cultured on a randomly oriented collagen scaffold fabricated in a low aspect ratio vessel, which demonstrated no preferentially oriented outgrowth.
DISCUSSION
We have presented a simple means of generating aligned collagen scaffolds by freezing and sublimation. We hypothesized that constraining the shape of the hydrogels to a vessel with high aspect ratio would induce alignment through anisotropic shrinkage of the gel during lyophilization. We are specifically achieving greater contraction in the radial direction than the axial direction of our cylindrical collagen gels. Although contraction in all three dimensions was not analyzed or quantified as a part of this work, we expect this introduced strain may be transversely isotropic; however, we will refer to this as anisotropic strain (or nonisotropic). The introduction of anisotropic strain to a fibrillar hydrogel induces alignment in the same plane as the tensile strain and/or orthogonal to the direction of compression.37 Because of the geometry of the cylindrical conduit and extra mass along the length, there is greater resistance to dehydration-induced compaction in the axial direction than the radial direction. This method resulted in collagen scaffolds with highly aligned features on the scaffold surface (Figure 1B and C). The formation of these aligned features was dependent on the initial presence of an assembled fibrillar network within the hydrogel and the high aspect ratio vessel.
Collagen sponges have long been fabricated for a variety of medical applications, and a number of commercially available products are currently FDA-approved for human use.23,25,41 Sponges of this type have been traditionally fabricated from a suspension of collagen, rather than a self-assembled, fibrillar hydrogel, which is then frozen and lyophilized to form a porous network that supports cellular infiltration and growth but generally lacks any preferential alignment. When we fabricated collagen scaffolds from an unassembled collagen suspension in a high-aspect ratio tube, we obtained a porous collagen network that displayed no preferentially aligned topographical features on the surface of scaffolds (Figure 1E). Fibrillar gels that were cast in low aspect ratio vessels also did not have aligned features (Figure 1F). Other groups have modified the approach to prepare collagen sponges to generate scaffolds with aligned pores from collagen suspensions in high aspect ratio conduits. However, the characterization of these scaffolds was largely limited to the interior pore structure, and little evidence was presented to characterize the scaffold surface.32–36 In our approach, the highly aligned surface topography is dependent on the continuous fibrillar hydrogel network prior to freezing and the high aspect ratio conduit used for fabrication. SEM images of cross sections revealed that the aligned scaffolds fabricated from self-assembled gels cast in cylindrical conduits had a highly porous interior. Instead of appearing as individual, hollow fibers, the topographical features on the scaffold surface may be folds or ridges that arise as the scaffold contracts during lyophilization. It is not yet known if the porous interior is continuous.
Collagen scaffolds demonstrated nonlinear strain-stiffening behavior characteristic of collagenous soft tissues (Figure 5).42,43 Two groups of collagen scaffolds were prepared for mechanical testing. Aligned scaffolds were fabricated in high aspect ratio conduits and displayed preferentially aligned microfeatures on the scaffold surface (Figure 1B and C). Random scaffolds were fabricated in low aspect ratio vessels and displayed no preferentially aligned microfeatures (Figure 1F). Both groups were made at the same collagen concentration and freezing temperature. Aligned collagen scaffolds displayed a greater elastic modulus, ultimate tensile strength, and strain at ultimate tensile strength than random collagen scaffolds (Table 1). Similar to native tissues and other scaffolds with oriented microstructure, the alignment of collagen features induced stronger and stiffer mechanical behavior.
Several fabrication parameters were easily varied to control features of the scaffold. For example, casting the initial collagen gel in conduits of different diameter allowed control over the final diameter of the aligned scaffold as well as the size of the aligned features of the scaffold. Changing the concentration of the collagen also allowed control of the scaffold diameter, but without changing the size of the individual features. Finally, changing the freezing temperature altered the feature size without changing the scaffold diameter. Freeze-dried collagen scaffolds swell when hydrated, as measured by the increase in scaffold diameter (Figure 2). Swelling occurred in a uniform fashion across all scaffold conditions, preserving the linear trends measured in freeze-dried states. The size of the individual, aligned features was also sensitive to hydration. Thus, these three parameters provide a broad design space for scaffold and feature geometry, which increases the versatility of this simple approach for a variety of applications in tissue engineering.
The freeze-dried scaffold state is preferable for storage and handling, but to seed and support cells, the scaffolds must be presented in a hydrated state. SEM imaging revealed that the aligned topographical features observed in the freeze-dried state were maintained in the hydrated state (Figure 4A). Further examination revealed individual ridges on the surface of aligned features that were on the order of 10 µm (Figure 4B). Randomly oriented collagen fibers were visible at 6500× magnification (Figure 4C,D). These fibers were approximately 100 nm in diameter and comprised the larger aligned topographical features on the scaffold surface. This interesting result raised the question of which topographical cues would guide cell behavior. Several studies have investigated the influence of feature size on cell alignment.44–49 Many groups have identified nanoscale features as a critical determinant for guiding cell alignment, which led us to believe that the randomly oriented nanotopography would direct cell alignment in our scaffolds instead of the 10–100 µM microfeatures.45–47 We found that both chick DRG explants and rat dermal fibroblasts could sense and respond to the microscale aligned topographical cues on the scaffold surface. Individual axons extending from sensory neurons in the DRG explants aligned and extended in the direction of the larger, aligned scaffold features rather than the finer random, nanoscale features (Figure 7A). Individual rat dermal fibroblasts demonstrated similar behavior. We did not attempt to control the orientation of the nanoscale features, nor did we study alternate conditions with aligned nanofeatures on an otherwise random surface or scaffold. A well designed study conducted by Moffa et al. examined the effects of substrates that combined nanofiber topographies with aligned microgrooves on cellular alignment and orientation.48 They found that substrates with randomly oriented nanofibers and aligned microgrooves showed significantly higher cellular alignment and orientation as compared to substrates with random nanofibers and no microgrooves. They examined samples with grooves spaced 15, 50, and 100 µm apart, distances which compare well with the size of topographical features in our aligned collagen scaffolds. Our results would seem to provide further evidence that aligned microfeatures in combination randomly oriented nanofibers are capable of inducing cellular alignment. It is clear from our results that cells and cellular projections sense and respond to the microscale alignment, and that this function is not overridden by nanoscale features.
This method represents a simple alternative to traditional fabrication of collagen sponges that allows for the introduction of aligned topography. Previously, generating aligned collagen from collagen gels required complex equipment, whereas our method only requires an incubator, a freezer, and a lyophilizer, which are commonly found in most laboratory settings. Our technique provides another option for preparing aligned collagen scaffolds toward tissue engineered solutions where structural anisotropy is a desirable feature. Skeletal muscle, tendon and ligament, peripheral nerve and spinal cord, and the circumferentially aligned lining of blood vessels have all presented significant challenges to the field of tissue engineering. This simple method holds the potential to serve as a building block for new strategies which address the need presented by replacement of these tissues.
CONCLUSIONS
We have developed a rapid and simple method for fabricating collagen scaffolds with highly aligned topographical features, which does not require the need for specialized equipment. This method is dependent on both the high aspect ratio vessels used for fabrication and the fibrillar network within a self-assembled collagen hydrogel. These aligned collagen scaffolds are tunable in both total scaffold diameter and the size of individual, microscale topography. SEM imaging also revealed the presence of a randomly oriented, nanoscale fibrillar topography, consistent with the size of collagen fibrils, that comprises the surface of the larger aligned microfeatures. The aligned collagen scaffolds supported the growth and alignment of axons from chick DRG explants and rat dermal fibroblasts. Overall, this simple method provides another option to enhance regenerative medicine strategies across a variety of tissue types where tissue anisotropy is critical to functional outcome, such as nerve tissue grafts, engineered tendons and ligaments, or smooth muscle constructs.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (NIH-NINDS 1R01NS078385), a Graduate Fellowship from the New Jersey Commission on Brain Injury Research (CBIR14FEL004), the National Science Foundation REU in Cellular Bioengineering: From Biomaterials to Stem Cells (NSF EEC 1262924), and the Aresty Research Center for Undergraduates.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
REFERENCES
- 1.Li M, Dickinson CE, Finkelstein EB, Neville CM, Sundback CA. The role of fibroblasts in self-assembled skeletal muscle. Tissue Eng., Part A. 2011;17(21–22):2641–2650. doi: 10.1089/ten.TEA.2010.0700. [DOI] [PubMed] [Google Scholar]
- 2.Khan M, Xu Y, Hua S, Johnson J, Belevych A, Janssen PM, Gyorke S, Guan J, Angelos MG. Evaluation of Changes in Morphology and Function of Human Induced Pluripotent Stem Cell Derived Cardiomyocytes (HiPSC-CMs) Cultured on an Aligned-Nanofiber Cardiac Patch. PLoS One. 2015;10(5):e0126338. doi: 10.1371/journal.pone.0126338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Lu J, Li H, Wei J, Li X. Engineering blood vessels through micropatterned co-culture of vascular endothelial and smooth muscle cells on bilayered electrospun fibrous mats with pDNA inoculation. Acta Biomater. 2015;11:114–125. doi: 10.1016/j.actbio.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi M, Lei NY, Wang Q, Wu BM, Dunn JC. Orthogonally oriented scaffolds with aligned fibers for engineering intestinal smooth muscle. Biomaterials. 2015;61:75–84. doi: 10.1016/j.biomaterials.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Snedeker JG. Wired silk architectures provide a biomimetic ACL tissue engineering scaffold. Journal of the mechanical behavior of biomedical materials. 2013;22:30–40. doi: 10.1016/j.jmbbm.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Cai C, Chen C, Chen G, Wang F, Guo L, Yin L, Feng D, Yang L. Type I collagen and polyvinyl alcohol blend fiber scaffold for anterior cruciate ligament reconstruction. Biomed. Mater. 2013;8(3):035001. doi: 10.1088/1748-6041/8/3/035001. [DOI] [PubMed] [Google Scholar]
- 7.Beason DP, Connizzo BK, Dourte LM, Mauck RL, Soslowsky LJ, Steinberg DR, Bernstein J. Fiber-aligned polymer scaffolds for rotator cuff repair in a rat model. Journal of shoulder and elbow surgery. 2012;21(2):245–250. doi: 10.1016/j.jse.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Breidenbach AP, Gilday SD, Lalley AL, Dyment NA, Gooch C, Shearn JT, Butler DL. Functional tissue engineering of tendon: Establishing biological success criteria for improving tendon repair. J. Biomech. 2014;47(9):1941–1948. doi: 10.1016/j.jbiomech.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikkhah M, Eshak N, Zorlutuna P, Annabi N, Castello M, Kim K, Dolatshahi-Pirouz A, Edalat F, Bae H, Yang Y, Khademhosseini A. Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials. 2012;33(35):9009–9018. doi: 10.1016/j.biomaterials.2012.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornwell KG, Pins GD. Enhanced proliferation and migration of fibroblasts on the surface of fibroblast growth factor-2-loaded fibrin microthreads. Tissue Eng., Part A. 2010;16(12):3669–3677. doi: 10.1089/ten.TEA.2009.0600. [DOI] [PubMed] [Google Scholar]
- 11.Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, Phillips J, Lambrichts I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014;28(4):1634–1643. doi: 10.1096/fj.13-243980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgiou M, Bunting SC, Davies HA, Loughlin AJ, Golding JP, Phillips JB. Engineered neural tissue for peripheral nerve repair. Biomaterials. 2013;34(30):7335–7343. doi: 10.1016/j.biomaterials.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Bettinger CJ, Langer R, Borenstein JT. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew. Chem., Int. Ed. 2009;48(30):5406–5415. doi: 10.1002/anie.200805179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlin RL, Kasper FK, Mikos AG. Polymeric nanofibers in tissue engineering. Tissue Eng., Part B. 2011;17(5):349–364. doi: 10.1089/ten.teb.2011.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Mondrinos MJ, Gandhi MR, Ko FK, Weiss AS, Lelkes PI. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 2005;26(30):5999–6008. doi: 10.1016/j.biomaterials.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of Nanofiber Matrix as Tissue-Engineering Scaffolds. Tissue Eng. 2005;11(1–2):101–109. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Yao M, Zhou J, Zheng W, Zhou C, Dong D, Liu Y, Teng Z, Jiang Y, Wei G, Cui X. The promotion of neural progenitor cells proliferation by aligned and randomly oriented collagen nanofibers through beta1 integrin/MAPK signaling pathway. Biomaterials. 2011;32(28):6737–6744. doi: 10.1016/j.biomaterials.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 18.Ricard-Blum S. The collagen family. Cold Spring Harbor Perspect. Biol. 2011;3(1):a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antoine EE, Vlachos PP, Rylander MN. Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue Eng., Part B. 2014;20(6):683–696. doi: 10.1089/ten.teb.2014.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sell SA, McClure MJ, Garg K, Wolfe PS, Bowlin GL. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv. Drug Delivery Rev. 2009;61(12):1007–1019. doi: 10.1016/j.addr.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Kolacna L, Bakesova J, Varga F, Kostakova E, Planka L, Necas A, Lukas D, Amler E, Pelouch V. Biochemical and Biophysical Aspects of Collagen Nanostructure in the Extracellular Matrix. Physiol. Res. 2007;56(S1):S51–S60. doi: 10.33549/physiolres.931302. [DOI] [PubMed] [Google Scholar]
- 22.Cheung H-Y, Lau K-T, Lu T-P, Hui D. A critical review on polymer-based bio-engineered materials for scaffold development. Composites, Part B. 2007;38(3):291–300. [Google Scholar]
- 23.Glowacki J, Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89(5):338–344. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices. 2011;8(5):607–626. doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abou Neel EA, Bozec L, Knowles JC, Syed O, Mudera V, Day R, Hyun JK. Collagen–emerging collagen based therapies hit the patient. Adv. Drug Delivery Rev. 2013;65(4):429–456. doi: 10.1016/j.addr.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Lee C, Singla A, Lee Y. Biomedical applications of collagen. Int. J. Pharm. 2001;221:1–22. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 27.Dong B, Arnoult O, Smith ME, Wnek GE. Electrospinning of collagen nanofiber scaffolds from benign solvents. Macromol. Rapid Commun. 2009;30(7):539–542. doi: 10.1002/marc.200800634. [DOI] [PubMed] [Google Scholar]
- 28.Cheng X, Gurkan UA, Dehen CJ, Tate MP, Hillhouse HW, Simpson GJ, Akkus O. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials. 2008;29(22):3278–3288. doi: 10.1016/j.biomaterials.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 29.Girton TS, Dubey N, Tranquillo RT. Magnetic-Induced Alignment of Collagen Fibrils in Tissue Equivalents. Tissue Engineering. 1999;18:67–73. doi: 10.1385/0-89603-516-6:67. [DOI] [PubMed] [Google Scholar]
- 30.Wray LS, Rnjak-Kovacina J, Mandal BB, Schmidt DF, Gil ES, Kaplan DL. A silk-based scaffold platform with tunable architecture for engineering critically-sized tissue constructs. Biomaterials. 2012;33(36):9214–9224. doi: 10.1016/j.biomaterials.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoppel WL, Hu D, Domian IJ, Kaplan DL, Black LD., 3rd Anisotropic silk biomaterials containing cardiac extracellular matrix for cardiac tissue engineering. Biomed Mater. 2015;10(3):034105. doi: 10.1088/1748-6041/10/3/034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chamberlain LJ, Yannas IV, Arrizabalaga A, Hsu H-P, Norregaard TV, Spector M. Early peripheral nerve healing in collagen and silicone tube implants: Myofibroblasts and the cellular response. Biomaterials. 1998;19:1393–1403. doi: 10.1016/s0142-9612(98)00018-0. [DOI] [PubMed] [Google Scholar]
- 33.Chamberlain LJ, Yannas IV, Hsu H-P, Strichartz G, Spector M. Collagen-GAG Substrate Enhances the Quality of Nerve Regeneration through Collagen Tubes up to Level of Autograft. Exp. Neurol. 1998;154:315–329. doi: 10.1006/exnr.1998.6955. [DOI] [PubMed] [Google Scholar]
- 34.Chamberlain LJ, Yannas IV, Hsu H-P, Spector M. Connective Tissue Response to Tubular Implants for Peripheral Nerve Regeneration: The Role of Myofibroblasts. J. Comp. Neurol. 2000;417:415–430. doi: 10.1002/(sici)1096-9861(20000221)417:4<415::aid-cne3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Chamberlain LJ, Yannas IV, Hsu H-P, Strichartz G, Spector M. Near-Terminus Axonal Structure and Function Following Rat Sciatic Nerve Regeneration Through a Collagen-GAG Matrix in a Ten-Millimeter Gap. J. Neurosci. Res. 2000;60:666–677. doi: 10.1002/(SICI)1097-4547(20000601)60:5<666::AID-JNR12>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Madaghiele M, Sannino A, Yannas IV, Spector M. Collagen-based matrices with axially oriented pores. J. Biomed. Mater. Res., Part A. 2008;85(3):757–767. doi: 10.1002/jbm.a.31517. [DOI] [PubMed] [Google Scholar]
- 37.Vader D, Kabla A, Weitz D, Mahadevan L. Strain-Induced Alignement in Collagen Gels. PLoS One. 2009;4(6):e5902. doi: 10.1371/journal.pone.0005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girton TS, Barocas VH, Tranquillo RT. Confined Compression of a Tissue-Equivalent: Collagen Fibril and Cell Alignment in Response to Anisotropic Strain. J. Biomech. Eng. 2002;124(5):568. doi: 10.1115/1.1504099. [DOI] [PubMed] [Google Scholar]
- 39.Knapp DM, Helou EF, Tranquillo RT. A Fibrin or Collagen Gel Assay for Tissue Cell Chemotaxis: Assesment of Fibroblast Chemotaxis to GRGDSP. Exp. Cell Res. 1999;243(2):543–553. doi: 10.1006/excr.1998.4364. [DOI] [PubMed] [Google Scholar]
- 40.Bray DF, Bagu J, Koegler P. Comparison of hexamethyldisilazane (HDMS), Peldri II, critical-point drying methods for scanning electron microscopy of biological specimins. Microsc. Res. Tech. 1993;26(6):489–495. doi: 10.1002/jemt.1070260603. [DOI] [PubMed] [Google Scholar]
- 41.Mohd Hilmi AB, Halim AS. Vital roles of stem cells and biomaterials in skin tissue engineering. World journal of stem cells. 2015;7(2):428–436. doi: 10.4252/wjsc.v7.i2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA. Nonlinear elasticity in biological gels. Nature. 2005;435:191–194. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 43.Fung YC. A First Course In Continuum Mechanics. 2nd. Englewood Cliffs, NJ: Prentice-Hall, Inc.; 1977. p. 340. [Google Scholar]
- 44.Fozdar DY, Lee JY, Schmidt CE, Chen S. Selective axonal growth of embryonic hippocampal neurons according to topographic features of various sizes and shapes. Int. J. Nanomed. 2011;6:45–57. doi: 10.2147/IJN.S12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jana S, Leung M, Chang J, Zhang M. Effect of nano- and micro-scale topological features on alignment of muscle cells and commitment of myogenic differentiation. Biofabrication. 2014;6(3):035012. doi: 10.1088/1758-5082/6/3/035012. [DOI] [PubMed] [Google Scholar]
- 46.Li JY, Ho YC, Chung YC, Lin FC, Liao WL, Tsai WB. Preparation of micron/submicron hybrid patterns via a two-stage UV-imprint technique and their dimensional effects on cell adhesion and alignment. Biofabrication. 2013;5(3):035003. doi: 10.1088/1758-5082/5/3/035003. [DOI] [PubMed] [Google Scholar]
- 47.Loesberg WA, te Riet J, van Delft FC, Schon P, Figdor CG, Speller S, van Loon JJ, Walboomers XF, Jansen JA. The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials. 2007;28(27):3944–3951. doi: 10.1016/j.biomaterials.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 48.Moffa M, Sciancalepore AG, Passione LG, Pisignano D. Combined nano- and micro-scale topographic cues for engineered vascular constructs by electrospinning and imprinted micro-patterns. Small. 2014;10(12):2439–2450. doi: 10.1002/smll.201303179. [DOI] [PubMed] [Google Scholar]
- 49.Seunarine K, Curtis ASG, Meredith DO, Wilkinson CDW, Riehle MO, Gadegaard N. A Hierarchical Response of Cells to Perpendicular Micro- and Nanometric Textural Cues. IEEE Transactions on Nanobioscience. 2009;8(3):219–225. doi: 10.1109/TNB.2009.2016477. [DOI] [PubMed] [Google Scholar]