Abstract
A previous genome‐wide association study identified two novel esophageal squamous cell carcinoma (ESCC) susceptibility genes, ADH1B and ALDH2. We investigated the characteristics of ESCC, and the relationship between metachronous esophageal and/or pharyngeal squamous cell carcinoma (SCC) and the ADH1B & ALDH2 risk alleles. One hundred and seventeen superficial ESCC patients who underwent treatment with endoscopic submucosal dissection (ESD) were followed up using endoscopy for ≥12 months. First, we performed a replication analysis to confirm the relationship between ESCC and the ADH1B & ALDH2 risk alleles using 117 superficial ESCC cases and 1125 healthy controls. Next, we investigated the incidence and genetic/environmental factors associated with metachronous SCC development after ESD. We also analyzed the potential risk factors for metachronous SCC development using Cox's proportional hazards model. rs1229984 GG located on ADH1B and rs671 GA located on ALDH2 were significantly associated with ESCC progression (P = 7.93 × 10−4 and P = 1.04 × 10−5). Patients with rs1229984 GG, those with rs671 GA, smokers, heavy alcohol drinkers (44 g/day ethanol), and presence of multiple Lugol‐voiding lesions (LVLs) developed metachronous SCC more frequently (P = 3.20 × 10−3, 7.00 × 10−4, 4.00 × 10−4, 2.15 × 10−2, and 4.41 × 10−3, respectively), with hazard ratios were 2.84 (95% confidence interval [CI] = 1.43–5.63), 4.57 (95% CI = 1.80–15.42), 4.84 (95% CI = 1.89–16.41), and 2.34 (95% CI = 1.12–5.31), respectively. Multiple logistic regression analysis revealed that rs1229984 GG, rs671 GA, and smoking status were independently associated with the risk of developing metachronous SCCs after ESD. Moreover, we found cumulative effects of these two genetic factors (rs1229984 GG and rs671 GA) and one environmental factor (tobacco smoking) which appear to increase metachrous SCCs after ESD of ESCC risk approximately nearly 12‐fold. Our findings elucidated the crucial role of multiple genetic variations in ADH1B and ALDH2 as biomarkers of metachronous ESCC.
Keywords: ADH1B, ALDH2, endoscopy, esophageal squamous cell carcinoma, metachronous
Introduction
Esophageal cancer is the seventh most common cancer worldwide, and its incidence has increased rapidly over the past three decades 1. There is a marked geographic difference in the incidence and etiology of esophageal cancer. Although esophageal adenocarcinoma is common in Western countries, 80% of esophageal cancers that occur globally are of the squamous cell carcinoma (SCC) type. This SCC type is especially prevalent in Asian countries including China, India, and Japan 2. Most cases of esophageal SCC (ESCC) are diagnosed at advanced stages, with an overall 5‐year survival rate of 10–20%, and often involve the use of modern surgical techniques combined with various treatment modalities 3, 4. In contrast, in a previous study, patients with superficial ESCC (intramucosal or submucosal carcinoma) exhibited an overall 5‐year survival rate of >80% 5.
ESCCs are known to be associated with environmental carcinogens. Heavy alcohol consumption, tobacco smoking, advanced age, and male sex have been reported as risk factors of ESCC 6. Particularly in Japan, tobacco smoking and alcohol consumption were found to be the two major lifestyle factors related with the development of ESCC. The relative risk of developing ESCC is estimated at 3.27 for past smokers and 3.69 for current smokers, respectively, compared with nonsmokers 7. Moreover, alcohol consumption has shown to increase the risk of developing ESCC by 2–3 fold 8.
Advances in endoscopic technology have made it possible to detect ESCCs, which allows for prompt administration of further endoscopic treatment such as endoscopic mucosal resection or endoscopic submucosal dissection (ESD). However, endoscopic treatment spares a larger area of the esophageal mucosa than does surgical resection 9. Accordingly, studies claim that metachronous ESCC develops frequently in patients who have undergone endoscopic treatment 9, 10, 11. Therefore, in order to prevent death due to metachronous SCC, endoscopy‐based biomarkers and surveillance programs are necessary.
Recently, genome‐wide association studies (GWAS) have been widely used for the analyses of disease susceptibility genes 12, 13, 14, 15, 16. In 2009, Cui et al. conducted a GWAS of the Japanese population and identified a strong association between ESCC and variants of the rs1229984 and rs671 alleles on the ADH1B and ALDH2 genes 17.
In this study, we investigated the utility of two single‐nucleotide polymorphisms (SNPs) located on ADH1B and ALDH2 as biomarkers of metachronous ESCC and/or pharyngeal SCC after ESD for ESCC.
Patients and Methods
Patients
We enrolled 217 patients with esophageal dysplasia/ESCC who consented to providing samples between December 2012 and December 2014. We excluded patients with advanced ESCC (n = 24), history of esophageal disease (n = 4), low‐grade intraepithelial neoplasia (n = 8), granular cell tumors (n = 2), and those without follow‐up for >12 months (n = 51). Furthermore, nine patients with ESCC who did not undergo ESD and two patients who underwent esophageal surgery after ESD were excluded. Finally, a total of 117 patients (101 male, 16 female; mean age: 64.7 years) were enrolled in this study, including 103 patients with superficial ESCC and 14 with high‐grade intraepithelial neoplasia (HGIN). Ninety‐five patients (81%) underwent curative resection by ESD according to the Japanese esophageal cancer treatment guidelines 18, and 10 patients underwent chemoradiotherapy (CRT) after ESD, and the remaining patients were observed without additional surgical resection. Follow‐up surveillance endoscopy was performed 12 months after the procedure and once every 12 months thereafter. The median observation period was 38.8 months (range: 13–128 months).
Metachronous tumors were defined as ≥1 primary tumors detected distant from the ESD scar. Tumors detected in close proximity to the scar were regarded as local recurrent tumors. We obtained 1125 samples, which served as healthy controls, from volunteers at the Hiroshima University, Hiroshima, Japan. We used the recommended Lugol spraying method to detect ESCCs. Because squamous dysplasia and SCCs lack glycogen, these lesions are known as Lugol‐voiding lesions (LVLs) 9, 19, 20. During endoscopic examination, Lugol's solution was sprayed via a catheter. A speckled LVL pattern was defined as much more than 10 small LVLs or numerous irregularly shaped multiform LVLs in the esophageal mucosa 21.
Evaluation of clinicopathologic features
We investigated the incidence of metachronous tumors in 117 patients using the Kaplan–Meier method and retrospectively investigated the clinicopathologic features associated with metachronous tumors, including alcohol consumption, smoking, multiple LVLs, history of CRT, rs1229984, and rs671. We also evaluated the outcomes of metachronous tumors after ESD.
In patients with synchronous multiple tumors, we chose, as the main lesion, a tumor with the highest malignant potential as determined by the presence of a malignancy, diffuse type disease, or increased tumor size or depth. Tumor location and macroscopic type were classified according to the Japanese Classification of Esophageal Carcinoma 22. The pathological diagnosis of each tumor was also determined according to the Japanese Classification of Esophageal Carcinoma criteria 22.
Two SNPs on ALDH2 and ADH1B genotyping
Genomic DNA was extracted from peripheral blood leukocytes using a standard method. We genotyped 117 cases and 1125 healthy controls by using a multiplex PCR‐based Invader assay (Third Wave Technologies).
Statistical analyses
The association between the SNPs and ESCC risk using replication analysis was tested with logistic regression analysis adjusted for age and sex by assuming allelic, dominant, recessive, and overdominant models using the JMP statistical software package (SAS Institute Inc., Cary, NC). The odds ratios (ORs) were calculated using the nonsusceptible allele as a reference, unless stated otherwise. The cumulative incidence of metachronous ESCCs/HGIN was evaluated using the Kaplan–Meier method. To analyze the potential risk factors, such as age (0 = 60 years/<60 years; 1 = >60 years), sex (0 = female, 1 = male), rs671 at ALDH2 (0 = AA+GG, 1 = GA), rs1229984 at ADH1B (0 = AA+AG, 1 = GG), alcohol consumption (0 = never or light drinker, 1 = heavy drinker), smoking (0 = never smoker, 1 = smoker), multiple LVLs (0 = none or <10 small LVLs, 1 = many more than 10 small LVLs or numerous irregularly shaped multiform LVLs 21, and CRT after ESD (0 = none, 1 = treatment); for metachronous tumors, we performed univariate analysis using the Kaplan–Meier method, log‐rank test, and Cox's proportional hazards modeling. On the basis of the median level of alcohol consumption (44 g/day) for a regular alcohol drinker, the population was categorized into two classes: nondrinkers or light drinkers (0–44 g/day) and heavy drinkers (>44 g/day). Multiple logistic regression analysis was used to assess the contributions of confounding factors with the JMP statistical software package (SAS Institute Inc), and the following explanatory variables were included in the analysis: rs671 at ALDH2, rs1229984 at ADH1B, alcohol consumption, and smoking. A P‐value of <0.05 was considered significant. Cox's proportional hazards model was used to estimate the hazard ratio and 95% confidence interval (CI).
Results
In the replication analysis to confirm the relationship between superficial ESCC and the ADH1B & ALDH2 risk alleles, we genotyped 117 superficial ESCC cases and 1125 healthy controls using invader assay (Table S1). The relationship between superficial ESCC and the ADH1B rs1229984 and ALDH2 rs671 alleles are shown in Table 1. Among the superficial ESCC patients, 36 (30.8%) had the ALDH2 rs671 allele characterized by the GG genotype, 80 (68.4%) had the GA genotype, and 1 (0.9%) had the AA genotype. Furthermore, of the patients with the ADH1B rs1229984 allele, 31 (26.5%) had the GG genotype, 36 (30.8%) had the GG genotype, and 50 (42.7%) had the AA genotype. The following was observed in the healthy controls: ALDH2 rs671: GG, 642 cases (57.1%); GA, 432 cases (38.4%); AA, 51 cases (4.5%) and ADH1B rs1229984: GG, 60 cases (5.3%); GA, 389 cases (34.6%); AA, 676 cases (60.1%). Replication analysis with an Armitage‐trend model revealed that rs671 and rs1229984 were significantly associated with superficial ESCC (P = 5.62 × 10−5 and 1.65 × 10−9, OR = 1.75 and 2.43, respectively; data not shown). We found a remarkable difference between the cases and controls in terms of sex and age, and logistic regression analyses confirmed age and sex as significant covariates.
Table 1.
The effect of genetic factors on the risk of developing ESCC
| SNP | Location | Allele | ESCC patients | Healthy controls | Allelic model 1 versus 2 | Dominant model 11 + 12 versus 22 | Recessive model 11 versus 12 + 22 | Overdominant model 12 versus 11 + 22 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | 1/2 | 11 | 12 | 22 | MAF | 11 | 12 | 22 | MAF | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | |
| rs671 | 12q24 | G/A | 36 | 80 | 1 | 0.35 | 642 | 432 | 51 | 0.24 | 8.61 × 10−4 | 2.02 | 5.18 × 10−5 | 3.99 | 0.72 | 1.14 | 1.04 × 10−5 | 4.28 |
| ALDH2 | 0.31 | 0.68 | 0.01 | 0.57 | 0.38 | 0.05 | (1.3–3.14) | (2.00–7.97) | (0.14–9.04) | (2.18–8.39) | ||||||||
| rs1229984 | 4q23 | G/A | 31 | 36 | 50 | 0.42 | 60 | 389 | 676 | 0.23 | 1.35 × 10−3 | 2.05 | 2.35 × 10−2 | 1.55 | 7.93 × 10−4 | 3.94 | 0.43 | 0.83 |
| ADH1B | 0.26 | 0.31 | 0.43 | 0.05 | 0.35 | 0.60 | (1.32–3.2) | (0.8–3.03) | (1.76–8.86) | (0.57–1.23) | ||||||||
A total of 117 ESCC patients and 1125 controls were analyzed.
P ‐value was obtained using logistic regression analysis adjusted for age and sex.
ORs and CIs were calculated using the nonsusceptible alleles as a reference.
MAF, minor allele frequency; SNP, single‐nucleotide polymorphisms; ESCC, esophageal squamous cell carcinoma; OR, odds ratio; CI, confidence interval.
Futhermore, we analyzed two SNPs, rs671 on ALDH2 and rs1229984 on ADH1B, using allelic, dominant, recessive, and overdominant models adjusted for age and sex.
We found that rs1229984 on ADH1B showed the strongest association with superficial ESCC in the recessive model (allelic model: P = 1.35 × 10−3, OR = 2.05, 95% CI = 1.32–3.2; dominant model: P = 2.35 × 10−2, OR = 1.55; 95% CI = 0.8–3.03; recessive model: P = 7.93 × 10−4, OR = 3.94, 95% CI = 1.76–8.86). Consistent with previous reports 17, we found that rs671 exhibited the strongest association with development of superficial ESCC in the overdominant model (allelic model: P = 8.61 × 10−4, OR = 2.02, 95% CI = 1.32–3.14; dominant model: P = 5.18 × 10−5, OR = 3.99, 95% CI = 2.0–7.97; recessive model: P = 7.17 × 10−1, OR = 1.14, 95% CI = 0.14–9.04; overdominant model: P = 1.04 × 10−5, OR = 4.28, 95% CI = 2.18–8.39).
Next, we investigated the incidence of metachronous SCC after ESD in 117 patients with esophageal tumors using the Kaplan–Meier method (Fig. S1–8). Sample characteristics are showed in Table 2. Thirty‐four patients developed metachronous SCC (15 SCCs and 19 HGINs), and the median period until discovery after initial ESD was 39.9 months (range: 12–101 months). According to the investigation of initial/metachronous tumors, three patients had HGINs/HGINs, 19 had SCCs/HGINs, and 12 had SCCs/SCCs, respectively. The cumulative incidence curve of metachronous esophageal tumors revealed a gradual increase and an incidence of 10.4% per year.
Table 2.
Characteristics of samples obtained from 117 patients after ESD
| ESCC (metachronous/without metachronous) | 34/83 |
|---|---|
| Sex (Male/Female) | 101/16 |
| Multiple LVLs (+/−) | 99/18 |
| Heavy alcohol consumption (+/−) | 63/54 |
| Smoking (+/−) | 83/34 |
| CRT (+/−) | 10/107 |
| rs671 at ALDH2 (GA/GG+AA) | 80/37 |
| rs1229984 at ADH1B (GG/GA+AA) | 31/86 |
LVLs, Lugol‐voiding lesions; ESCC, esophageal squamous cell carcinoma; CRT, chemoradiotherapy.
To further validate the incidence of metachronous SCC development after ESD in association with genetic/environmental factors (age, sex, presence of multiple LVLs, alcohol consumption, smoking status, history of CRT for ESCCs treated with ESD, ALDH2 rs671, ADH1B rs1229984; Table S2). Presence of multiple LVLs, heavy alcohol consumption, smoking, rs671 GA, and rs1229984 GG significantly affected the incidence of metachronous tumors on the basis of the univariate analysis performed using the Kaplan–Meier method and log‐rank test (P = 3.58 × 10−1, 2.46 × 10−2, 1.20 × 10−3, 1.70 × 10−3, and 1.72 × 10−3, respectively; Fig. S1–8). These associations persisted after adjustment using the Cox proportional hazards model. The hazard ratios were as follows: heavy alcohol consumption, 2.34 (95% CI = 1.12–5.31); smoking, 4.84 (95% CI = 1.89–16.41); ALDH2 rs671 GA, 4.57 (95% CI = 1.80–15.42); and ADH1B rs1229984 GG, 2.84 (95% CI = 1.43–5.63; Table S2). Multivariate analysis revealed that ADH1B rs1229984 GG, ALDH2 rs671 GA, and smoking status were independently associated with the risk of developing metachronous SCCs after ESD (Table 3).
Table 3.
Multiple Cox's proportional hazards analysis for the risk factors of metachronous SCC after ESD
| Risk factor | Metachronous cases | Without metachronous cases | Hazard ratios | 95% CI | P‐value |
|---|---|---|---|---|---|
| Multiple LVLs | 34 | 65 | 0.09 | ||
| Heavy alcohol consumption | 25 | 38 | 1.35 | 0.64–3.13 | 0.45 |
| Smoking | 30 | 53 | 3.38 | 1.28–11.68 | 1.19 × 10−2 |
| ALDH2; rs671 GA | 30 | 50 | 3.28 | 1.28–11.15 | 1.08 × 10−2 |
| ADH1B; rs1229984 GG | 17 | 14 | 2.21 | 1.10–4.45 | 2.59 × 10−2 |
Herein, 34 patients with metachronous SCC and 83 patients without metachronous SCC after endoscopic resection were analyzed.
All patients with metachronous SCC had multiple LVLs.
Hazard ratios and CIs were calculated using the nonrisk environmental factors and nonsusceptible allele as a reference.
LVLs, Lugol‐voiding lesions; CI, confidence interval; CRT, chemoradiotherapy; SCC, squamous cell carcinoma.
We also examined the additive effect of two SNPs and smoking (Table 4 and Fig. 1) on the development of metachronous SCCs after ESD. We found that the risk of metachronous SCC after ESD increased 4.56‐fold (95% CI = 1.66–15.9) in patients with two risk factors and 11.95‐fold (95% CI = 4.21–42.69) in patients with three risk factors, which was higher than that in patients with none or one risk factors; these findings therefore indicate the cumulative effects of these variants on metachronous SCC susceptibility.
Table 4.
Cox's proportional hazards analysis for the risk of metachronous SCCs after ESD according to the number of risk factors
| Number of risk factors | Metachronous cases | Without metachronous cases | Hazard ratios | 95% CI | P ‐value |
|---|---|---|---|---|---|
| 0–1 | 4 | 42 | 1 | ||
| 2 | 16 | 34 | 4.56 | 1.66–15.9 | 2.3 × 10−3 |
| 3 | 14 | 7 | 11.95 | 4.21–42.69 | <1.0 × 10−3 |
Herein, 34 patients with metachronous SCC and 83 patients without metachronous SCC after endoscopic resection were analyzed.
The three risk factors are ADH1B rs129984GG allele, ALDH2 rs671 GA allele, and smoking.
CI, confidence interval; SCC, squamous cell carcinoma, ESD, endoscopic submucosal dissection
Figure 1.
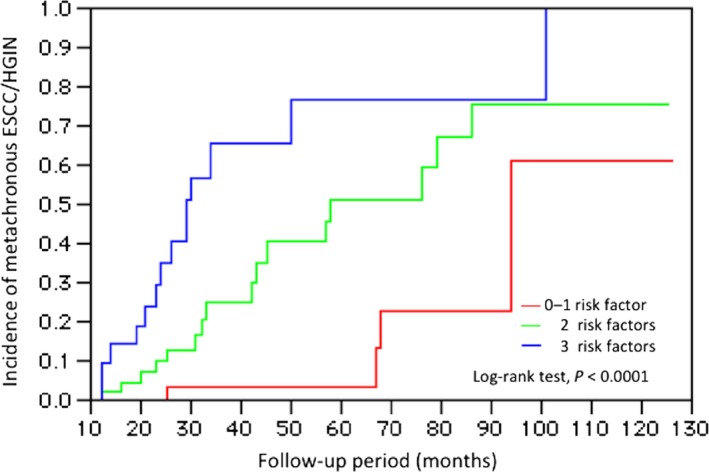
The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with ESD, according to the number of independent risk factors (ADH1B rs1229984 GG,ALDH2 rs671 GA, and smoking) ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia.
Discussion
Alcohol consumption has shown to increase the risk of developing various types of cancer 8, but pure ethanol was not found to act as a carcinogen in animal studies 23. Acetaldehyde, a primary metabolite of ethanol, is considered to be a plausible candidate with carcinogenic effects; in fact, acetaldehyde inhalation was shown to induce various types of tumors, particularly adenocarcinoma and SCC of the nasal mucosa, in animal models 24, 25. The ethanol in alcohol is metabolized to acetaldehyde by alcohol dehydrogenase‐1B (ADH1B), and the acetaldehyde is metabolized to acetate by aldehyde dehydrogenase‐2 (ALDH2). ADH1B is located at 4q23, which encodes the beta subunit of class I alcohol dehydrogenase (ADH), an enzyme that catalyzes the rate‐limiting step for ethanol metabolism: the oxidation of alcohol to acetaldehyde. ALDH2 is located at 12q24.2, which encodes a member of the alcohol dehydrogenase family. Members of this enzyme family metabolize a wide variety of substrates, including ethanol, retinol, other aliphatic alcohols, hydroxysteroids, and lipid peroxidation products. This encoded protein, consisting of several homodimers and heterodimers of alpha, beta, and gamma subunits, exhibits high activity for ethanol oxidation and plays a major role in ethanol catabolism. The ADH1B and ALDH2 genes contain SNPs that modulate enzymatic activity. A previous GWAS identified two novel ESCC susceptibility genes: ADH1B (rs1229984) and ALDH2 (rs671) 17, 26. A nonsynonymous SNP (rs1229984) generates two allelic variants: ADH1B*1 (Arg48, G213) and ADH1B*2 (His48, A213). ADH1B*2 (A) was reported to exhibit 30–40‐fold higher enzymatic activity for ethanol oxidation than ADH1B*1 (G) 27. A nonsynonymous SNP (rs671) also generates two allelic variants: ALDH2*1 (Glu504, G1951) and ALDH2*2 (Lys504 A1951). ALDH2*2 (A) allele encodes a catalytically inactive subunit. ADH1B*1 (G) and ALDH2*2 (A) are prevalent genotypes found in approximately 90% and 50% of populations in East Asian countries such as Japan, China, and Korea 28. In this study, we selected superficial esophageal SCC cases treated with ESD, and performed a replication study to confirm the relationship between ESCC and the ADH1B & ALDH2 risk alleles by using an Invader assay. ADH1B rs1229984 GG allele and ALDH2 rs671 GA allele were found to be risk factors of ESCC.
The incidence of metachronous SCC after ESD was estimated at 29% in this study, which is higher than that previously reported (12–15%) 10, 29. However, a study did claim that metachronous ESCC occurred in 35% of alcoholic patients after endoscopic resection. In that report, ALDH2*1/*2 was found to be the risk factor of metachronous ESCC 11. In this study, the proportion of ESCC patients with the ALDH2 GA allele was 68.4%, which may explain the high incidence of metachronous ESCC. The median interval to the detection of a second tumor after the initial ESD was 39.9 months (range: 12–101 months), and the Kaplan–Meier curve seemed to reach a plateau after 100 months.
Despite some previous studies wherein metachronous ESCC occurred more frequently in patients with the speckled LVL pattern in the background mucosa compared with patients without LVLs9, 30, LVLs was not found to be a risk factor for metachronous SCC in this study. This may be attributable to the advances in endoscopic technology such as magnifying endoscopy. We can detect early‐stage lesions more easily by using white light endoscopy and narrow band imaging before using the Lugol spraying method.
ADH1B rs1229984 GG, ALDH2 rs671 GA, and smoking status, but not heavy alcohol consumption, were independently associated with the risk of developing metachronous SCCs after ESD in this study. We believe that this is due to the genetic risk factors: rs1229984 GG allele and rs671 GA allele had a stronger influence and closer association, which would have diminished the effects of alcohol consumption. However, this study included only a few patients with metachronous SCC, which made a detailed analysis difficult. Further studies involving more patients are needed. Moreover, the study was retrospective in nature and conducted at only a single center. Future studies should be aimed at a prospective analysis in multiple centers.
Several previous GWAS have reported SNPs associated with disease incidence; however, most SNPs are not used in clinical pathology. This study elucidated the crucial role of two SNPs, identified using a GWAS in the ADH1B & ALDH2 genes, as biomarkers of metachronous SCC after ESD in superficial ESCC. ESD for superficial ESCC successfully improved the survival rate. However, metachronous SCCs occurred highly frequently after ESD. Therefore, the estimation of metachronous SCC risk after ESD for superficial ESCC would be essential to guide personalized treatment and achieve optimal results. In this study, we developed a risk model for metachronous SCCs using genetic and environmental factors. We found that individuals in the highest risk category have a nearly 12‐fold higher risk of developing metachronous SCCs than those in the lowest risk category. We are confident that our findings will greatly contribute to the establishment of personalized surveillance for superficial ESCCs after ESD. Our findings elucidated the crucial role of multiple genetic variations in ADH1B and ALDH2 as biomarkers of metachronous ESCC.
Conflict of Interest
The authors declare no conflict of interest associated with this manuscript.
Supporting information
Figure S1. The cumulative incidence of metachronous ESCC/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to age. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia.
Figure S2. The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to sex. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia.
Figure S3.The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to the presence of multiple LVLs. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia; LVLs, Lugol‐voiding lesions.
Figure S4.The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to alcohol consumption. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia.
Figure S5.The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to smoking status. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia.
Figure S6.The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to history of CRT. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia; CRT, chemoradiotherapy.
Figure S7.The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to the presence of the ALDH2 rs671 genotype. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia.
Figure S8.The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to the presence of the ADH1B rs1229984 genotype. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia.
Table S1. Characteristics of samples and methods used in this study.
Table S2. Cox's proportional hazards analysis for the risk factors of metachronous SCCs after ESD.
Acknowledgments
We thank all the patients and members of Hiroshima Liver Study Group in Japan, who donated their DNA for this study. We also thank Sakamiya and Izumoto, RIKEN, and the technical staff members of the Laboratory for Genotyping Development, Center for Genomic Medicine, RIKEN for their technical support.
Cancer Medicine 2016; 5(7):1397–1404
References
- 1. Holmes, R. S. , and Vaughan T. L.. 2007. Epidemiology and pathogenesis of esophageal cancer. Semin. Radiat. Oncol. 17:2–9. [DOI] [PubMed] [Google Scholar]
- 2. Xing, D. , Tan W., and Lin D.. 2003. Genetic polymorphisms and susceptibility to esophageal cancer among Chinese population (review). Oncol. Rep. 10:1615–1623. [PubMed] [Google Scholar]
- 3. Tamoto, E. , Tada M., Murakawa K., Takada M., Shindo G., and Teramoto K.. 2004. Gene‐expression profile changes correlated with tumor progression and lymph node metastasis in esophageal cancer. Clin. Cancer Res. 10:3629–3638. [DOI] [PubMed] [Google Scholar]
- 4. Coleman, M. P. , Gatta G., Verdecchia A., Estève J., Sant M., and Storm H.. 2003. EUROCARE‐3 summary: cancer survival in Europe at the end of the 20th century. Ann. Oncol. 14(Suppl 5):v128–v149. [DOI] [PubMed] [Google Scholar]
- 5. Kim, J. H. , Chung H. S., Youn Y. H., Park S. W., Song S. Y., and Chung J. B.. 2007. Treatment outcomes of 70 cases of early esophageal carcinoma: 12 years of experience. Dis. Esophagus 20:297–300. [DOI] [PubMed] [Google Scholar]
- 6. Hiyama, T. , Yoshihara M., Tanaka S., and Chayama K.. 2007. Genetic polymorphisms and esophageal cancer risk. Int. J. Cancer 121:1643–1658. [DOI] [PubMed] [Google Scholar]
- 7. Ishiguro, S. , Sasazuki S., Inoue M., Kurahashi N., Iwasaki M., and Tsugane S.. 2009. Effect of alcohol consumption, cigarette smoking and flushing response on esophageal cancer risk: a population‐based cohort study (JPHC study). Cancer Lett. 275:240–246. [DOI] [PubMed] [Google Scholar]
- 8. Seitz, H. K. , and Stickel F.. 2007. Molecular mechanisms of alcohol‐mediated carcinogenesis. Nat. Rev. Cancer 7:599–612. [DOI] [PubMed] [Google Scholar]
- 9. Urabe, Y. , Hiyama T., Tanaka S., Oka S., Yoshihara M., and Arihiro K.. 2009. Metachronous multiple esophageal squamous cell carcinomas and Lugol‐voiding lesions after endoscopic mucosal resection. Endoscopy 41:304–309. [DOI] [PubMed] [Google Scholar]
- 10. Shimizu, Y. , Tukagoshi H., Fujita M., Hosokawa M., Kato M., and Asaka M.. 2001. Metachronous squamous cell carcinoma of the esophagus arising after endoscopic mucosal resection. Gastrointest. Endosc. 54:190–194. [DOI] [PubMed] [Google Scholar]
- 11. Yokoyama, A. , Omori T., Yokoyama T., Sato Y., Kawakubo H., and Maruyama K.. 2008. Risk of metachronous squamous cell carcinoma in the upper aerodigestive tract of Japanese alcoholic men with esophageal squamous cell carcinoma: a long‐term endoscopic follow‐up study. Cancer Sci. 99:1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cui, R. , Okada Y., Jang S. G., Ku J. L., Park J. G., and Kamatani Y.. 2011. Common variant in 6q26‐q27 is associated with distal colon cancer in an Asian population. Gut 60:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar, V. , Matsuo K., Takahashi A., Hosono N., Tsunoda T., and Kamatani N.. 2011. Common variants on 14q32 and 13q12 are associated with DLBCL susceptibility. J. Hum. Genet. 56:436–439. [DOI] [PubMed] [Google Scholar]
- 14. Kumar, V. , Kato N., Urabe Y., Takahashi A., Muroyama R., and Hosono N.. 2011. Genome‐wide association study identifies a susceptibility locus for HCV‐induced hepatocellular carcinoma. Nat. Genet. 43:455–458. [DOI] [PubMed] [Google Scholar]
- 15. Tanikawa, C. , Urabe Y., Matsuo K., Kubo M., Takahashi A., and Ito H.. 2012. A genome‐wide association study identifies two susceptibility loci for duodenal ulcer in the Japanese population. Nat. Genet. 44:430–434. [DOI] [PubMed] [Google Scholar]
- 16. Urabe, Y. , Tanikawa C., Takahashi A., Okada Y., Morizono T., and Tsunoda T.. 2012. A genome‐wide association study of nephrolithiasis in the Japanese population identifies novel susceptible Loci at 5q35.3, 7p14.3, and 13q14.1. PLoS Genet. 8:e1002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cui, R. , Kamatani Y., Takahashi A., Usami M., Hosono N., and Kawaguchi T.. 2009. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology 137:1768–1775. [DOI] [PubMed] [Google Scholar]
- 18. Japanese Society for Cancer of the Esophagus . 2012. General rules for clinical and pathological studies on cancer of esophagus, 3rd ed Kanehara & Co. Ltd, Tokyo. [Google Scholar]
- 19. Shimizu, Y. , Tukagoshi H., Fujita M., Hosokawa M., Kato M., and Asaka M.. 2001. Endoscopic screening for early esophageal cancer by iodine staining in patients with other current or prior primary cancers. Gastrointest. Endosc. 53:1–5. [DOI] [PubMed] [Google Scholar]
- 20. Dubuc, J. , Legoux J. L., Winnock M., Seyring J‐., Barrioz T., and Laugier R.. 2006. Endoscopic screening for esophageal squamous‐cell carcinoma in high‐risk patients: a prospective study conducted in 62 French endoscopy centers. Endoscopy 38:690–695. [DOI] [PubMed] [Google Scholar]
- 21. Muto, M. , Hitomi Y., Ohtsu A., Ebihara S., Yoshida S., and Esumi H.. 2000. Association of aldehyde dehydrogenase 2 gene polymorphism with multiple oesophageal dysplasia in head and neck cancer patients. Gut 47:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shimoda, T. 2011. Japanese classification of esophageal cancer, the 10th edition‐pathological part. Nihon Rinsho 69(Suppl 6):109–120. [PubMed] [Google Scholar]
- 23. Boyle, P. , Autier P., Bartelink H., Baselga J., Boffetta P., and Burn J.. 2003. European code against cancer and scientific justification: third version (2003). Ann. Oncol. 14:973–1005. [DOI] [PubMed] [Google Scholar]
- 24. Woutersen, R. A. , Appelman L. M., Van Garderen‐Hoetmer A., and Feron V. J.. 1986. Inhalation toxicity of acetaldehyde in rats III. Carcinogenicity study. Toxicology 41:213–231. [DOI] [PubMed] [Google Scholar]
- 25. Feron, V. J. , Kruysse A., and Woutersen R. A.. 1982. Respiratory tract tumours in hamsters exposed to acetaldehyde vapour alone or simultaneously to benzo(a)pyrene or diethylnitrosamine. Eur. J. Cancer Clin. Oncol. 18:13–31. [DOI] [PubMed] [Google Scholar]
- 26. Tanaka, F. , Yamamoto K., Sizuki S., Inoue H., Tsurumaru M., and Kajiyama Y.. 2010. Strong interaction between the effects of alcohol consumption and smoking on oesophageal squamous cell carcionoma among indivisuals with ADH1B and/or ALDH2 risk alleles. Gut 59:1457–1464. [DOI] [PubMed] [Google Scholar]
- 27. Yin, S. J. , Bosron W. F., Li T. K., Ohnishi K., Okuda K., and Ishii H.. 1984. Polymorphism of human liver alcohol dehydrogenase: identification of ADH2 2‐1 and ADH2 2‐2 phenotypes in the Japanese by isoelectric focusing. Biochem. Genet. 22:169–180. [DOI] [PubMed] [Google Scholar]
- 28. Higuchi, S. , Matsushita S., Murayama M., Takagi S., and Hayashida M.. 1995. Alcohol and aldehyde dehydrogenase polymorphisms and the risk for alcoholism. Am. J. Psychiatry 152:1219–1221. [DOI] [PubMed] [Google Scholar]
- 29. Katada, C. , Muto M., Tanabe S., Higuchi K., Sasaki T., and Azuma M.. 2013. Surveillance after endoscopic mucosal resection or endoscopic submucosal dissection for esophageal squamous cell carcinoma. Dig. Endosc. 25(Suppl 1):39–43. [DOI] [PubMed] [Google Scholar]
- 30. Muto, M. , Hirohata S., Nakane M., Boku N., Ohtsu A., and Yoshida S.. 2002. Association of multiple Lugol‐voiding lesions with synchronous and metachronous esophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest. Endosc. 56:517–521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The cumulative incidence of metachronous ESCC/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to age. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia.
Figure S2. The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to sex. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia.
Figure S3.The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to the presence of multiple LVLs. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia; LVLs, Lugol‐voiding lesions.
Figure S4.The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to alcohol consumption. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia.
Figure S5.The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to smoking status. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia.
Figure S6.The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to history of CRT. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia; CRT, chemoradiotherapy.
Figure S7.The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to the presence of the ALDH2 rs671 genotype. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia.
Figure S8.The cumulative incidence of metachronous ESCCs/HGIN in 117 patients with ESCC who underwent treatment with endoscopic submucosal dissection, according to the presence of the ADH1B rs1229984 genotype. ESCC, esophageal squamous cell carcinoma; HGIN, high‐grade intraepithelial neoplasia.
Table S1. Characteristics of samples and methods used in this study.
Table S2. Cox's proportional hazards analysis for the risk factors of metachronous SCCs after ESD.


