Abstract
Thyroid cancer is the fastest increasing cancer worldwide in all age groups. Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer in both adults and children. PTC genomic landscape has been extensively studied in adults, but information regarding sporadic pediatric patients is lacking. Although BRAF V600E mutation is highly prevalent in adults, this mutation is uncommon in pediatric cases. As adult and pediatric PTC is a mitogen‐activated protein kinase‐driven cancer, this altered pathway might be activated by different genetic events. The aim of this study was to investigate the occurrence of AGK‐BRAF fusion gene, recently described in radiation‐exposed pediatric PTC, in a cohort of exclusively sporadic pediatric PTC. The series consisted of 30 pediatric PTC younger than 18 years of age at the time of diagnosis and 15 matched lymph node metastases (LNM). Primary tumors and matched LNM were screened for the presence of the AGK‐BRAF fusion transcript by RT‐PCR. To confirm the identity of the amplified products, randomly selected samples positive for the presence of the fusion transcripts were sequenced. Moreover, BRAF dual‐color, break‐apart probes confirmed BRAF rearrangement. Overall, the AGK‐BRAF fusion gene was detected in 10% (3/30) of primary tumors. For one of these cases, paired LNM was also available, which also shows the presence of AGK‐BRAF fusion gene. This study described, for the first time, the presence of AGK‐BRAF in sporadic pediatric PTC. Understanding the molecular events underlying pediatric PTC may improve preoperative diagnosis, allow molecular prognostication and define a therapeutic approach toward sporadic PTC patients.
Keywords: AGK‐BRAF, BRAF V600E, papillary thyroid carcinoma, pediatric thyroid cancer, sporadic thyroid carcinoma
Introduction
An increasing incidence of thyroid cancer has been reported in most populations worldwide 1, 2. Thyroid cancer is the fifth most common cancer in women in the United States, accounting for approximately 5% of all cancer 3. Recently, a rise in thyroid cancer incidence rate has also been reported in pediatric patients, mainly among adolescents 3, 4. In fact, thyroid cancer is the second most prevalent cancer in females with 15–19 years of age 5. Similar to adults, the great majority of pediatric follicular cell‐derived thyroid carcinomas are papillary thyroid carcinomas (PTC), with nearly 75–90% of cases 6.
The clinical presentation and outcomes of thyroid carcinoma differ between pediatric and adult population. Although pediatric patients are more likely to present a more advanced stage of disease at diagnosis and higher risk of recurrent and persistent disease than adults, they usually have an excellent overall survival 7, 8. Furthermore, a great heterogeneity within the pediatric group has been reported. Some studies have suggested worse outcome for children compared to adolescents 9, 10.
It is still unclear whether the clinicopathological differences observed between pediatric and adult population may be due to the existence of distinct genetic alterations. In fact, the frequency and spectrum of mutations in adult PTC is markedly different than that in pediatric PTC 11, 12. Some studies have also reported a different spectrum of mutations within pediatric group. Actually, the prevalence of the BRAF V600E mutation, the most common genetic event found in adult PTC 13, is significantly lower in sporadic and radiation‐exposed pediatric PTC 14, 15, 18. On the other hand, a high prevalence of genetic rearrangements has been described in both sporadic 11, 16 and radiation‐exposed pediatric thyroid carcinomas 17, 18.
Interestingly, BRAF rearrangements, in which the BRAF kinase domain is fused to a variety of 5′ partners, have been reported in several solid tumors types 19 as well as in PTC 20. The new fusion gene (AKAP9‐BRAF) was found in radiation‐exposed PTCs and results from an in‐frame fusion of the exons 1–8 of the AKAP9 gene (A‐kinase anchor protein 9) to exons 9–18 of BRAF gene 20.
As BRAF fusion represent an alternative mechanism of BRAF activation, one could hypothesize that BRAF could be activated in pediatric PTCs through rearrangement. In fact, recently, AGK‐BRAF rearrangement was described in one case of radiation‐exposed pediatric PTC, but was not identified in pediatric cases from patients with unknown radiation exposure 18. This rearrangement was caused by an inversion of the long arm of chromosome 7, which juxtaposes the exons 1 and 2 of the AGK (acylglycerol kinase) to exons 8–18 of BRAF 18. The expression of this fusion oncogene promotes a constitutive activation of MEK and ERK phosphorylation, thus activating the mitogen‐activated protein kinase (MAPK) cascade 18. AGK‐BRAF was later described in one PTC from adult patient with apparently no history of radiation exposure 21.
To elucidate alternative mechanisms of aberrant BRAF activation, this study investigated the presence of AGK‐BRAF fusion oncogene in a cohort of predominantly sporadic pediatric PTC.
Material and Methods
Thyroid Samples
The series consists of 45 formalin‐fixed paraffin‐embedded (FFPE) sections from 30 primary PTC and 15 matched lymph node metastases (LNM) from patients who underwent thyroid surgery from 1993 through 2012 at Hospital São Paulo (Universidade Federal de São Paulo) and Hospital da Santa Casa de São Paulo. All samples were reviewed by two pathologists (RD and MTSA). As recommended by the ATA guidelines, all pediatric patients included in this study were ≤18 years of age at the time of diagnosis 22. The study was conducted under the approval of the Review Boards and Research Ethical Committees of the affiliated institutions.
RNA isolation and cDNA synthesis
Total RNA was isolated from 10‐μm thick FFPE sections using the Recover All Total Nucleic Acid isolation kit (Ambion Inc., Austin, TX). Total RNA (500 ng) was treated with DNAse and reverse‐transcribed into cDNA with both 50 μM oligo(dT)12‐18 and 50 ng random hexamers using a Superscript III transcriptase kit (Invitrogen Corp., Carlsbad, CA).
Transient transfection of AGK‐BRAF fusion gene in thyroid cells
FTC 238 thyroid carcinoma cells, established from a lung metastases of a human follicular thyroid carcinoma, purchased from the European Collection of Cell Cultures (ECACC, Health Protection Agency, Salisbury, UK) were cultured in Dulbecco's modified essential medium (DMEM):Ham's F12 (1:1) medium supplemented with 5% fetal bovine serum (FBS) (Life Technologies, Carlsbad, CA). FTC 238 cells were transiently transfected with 10 μg of pLVX‐AGK‐BRAF plasmid by electroporation using a Gene Pulser II (Bio‐Rad Laboratories Inc., Hercules, CA). The oncogene‐transfected cells were harvested, and the total RNA was isolated using TRIzol Reagents (Invitrogen Corp.) and reverse‐transcribed into cDNA, as above mentioned. The cDNA generated from cells expressing the fusion transcripts was used as a positive control. The pLVX‐AGK‐BRAF plasmid was kindly donated by Dr. James Fagin (Memorial Sloan‐Kettering Cancer Center).
Detection of AGK‐BRAF fusion transcript
All of the samples were screened for the presence of AGK‐BRAF fusion transcript by RT‐PCR, as previously described 18. Briefly, cDNA (2 μL) was subjected to PCR amplification using 1.0 unit Platinum Taq DNA Polymerase (Invitrogen Corp.) and 2 pmol of each primer, as described. The samples were incubated at 95°C for 10 min and then subjected to 40 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec, and polymerization at 72°C for 30 sec, with a 5‐min final extension at 72°C. The efficiency of cDNA synthesis was tested using RPS8 as internal control, as previously described 23. A positive and negative control was included in each real‐time PCR run. The PCR products were resolved on a 2% agarose gel and visualized on a Bio‐Rad Gel Doc EZ system (Bio‐Rad). To confirm the identity of the amplified products, positive samples were sequenced using the BigDye Terminator Cycle Sequencing Kit (PE Applied Biosystems, Foster City, CA). The primers used to detect the AGK‐BRAF fusion transcript, located in exon 2 of AGK and exon 8 of BRAF, were previously described and validated 18.
Dual‐color break‐apart fluorescence in situ hybridization (FISH)
A commercially available dual‐color, break‐apart assay was used to test possible breakage of BRAF gene resulting from structural rearrangements. The two differentially labeled probes, flanking the BRAF gene, were cohybridized in two AGK‐BRAF‐positive PTCs. Targeted tumor areas were circled, following review of the corresponding H&E slide by a pathologist (RD), prior to the FISH assay. A 3‐μm thick unstained tissue sections were deparaffinized, rehydrated, and incubated in the pretreatment solution and washed according to manufacturer's protocol (DAKO, Glostrup, Denmark). Slides were then incubated with 5 μL solution containing the labeled FISH BRAF probes and IQFISH Fast Hybridization buffer (SureFISH break‐apart probes; Agilent Technologies, Palo Alto, CA) denatured at 80°C for 10 min and hybridized overnight at 37°C. Posthybridization wash was performed in stringent wash buffer at 65°C and two nonstringent washes at room temperature. The slides were then mounted with 10 μL of Mounting Buffer with 4′,6‐diamidino‐2‐phenylindole (DAPI) as a counterstaining. The FISH results were evaluated with fluorescent microscope Zeiss (Zeiss, Oberkochen, Germany) using ISIS Karyotype Image System (Metasystems, Altlussheim, Germany). At least 100 nonoverlapping and intact nuclei were evaluated.
Results
Clinical and pathological features
We systematically investigated the prevalence of AGK‐BRAF mutation in all primary tumors and matched LNM. Age ranged from 4 to 18 years old (mean = 11.36 years). Twenty‐one patients (70%) were females and 9 (30%) were males. The study included 12 classical PTC (CPTC), 12 follicular variant of PTC (FVPTC), four diffuse sclerosing variant of PTC (DSVPTC), and two other variants of PTC. The clinical and pathological features evaluated are summarized in Table 1.
Table 1.
Summary of the clinicopathological features and occurrence of AGK‐BRAF fusion oncogene in pediatric thyroid carcinoma
| Case | PTC Variant | Age (years) | Gender | Tumor Size (cm) | Multifocality | Lymph node Metastasis | Distant Metastasis | Extrathyroidal extension | TNM | Radiation Exposure | AGK‐BRAF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Classical | 7 | M | 1.4 | No | Yes | No | No | T1N1M0 | No | No |
| 2a | Follicular | 18 | F | 4.5 | No | Yes | No | NA | T3N1M0 | No | No |
| 3 | Classical | 4 | M | 1.7 | No | Yes | Yes | Yes | T4N1M1 | No | No |
| 4 | Classical | 13 | F | 3.2 | No | No | No | No | T2N0M0 | No | No |
| 5a | Diffuse Sclerosing | 17 | F | 1.5 | Yes | Yes | No | No | T1N1M0 | No | No |
| 6 | Classical | 4 | M | 0.7 | No | Yes | No | Yes | T3N1M0 | No | No |
| 7a | Classical | 18 | F | 3.5 | Yes | Yes | No | No | T2N1M0 | No | No |
| 8 | Follicular | 17 | F | 2.5 | No | No | No | No | T2N0M0 | No | No |
| 9a | Classical | 7 | F | 6 | Yes | Yes | Yes | Yes | T4N1M1 | No | No |
| 10a | Follicular | 12 | M | 3 | Yes | Yes | Yes | Yes | T4N1M1 | No | No |
| 11 | Classical | 5 | F | 3 | No | Yes | Yes | Yes | T4N1M1 | No | No |
| 12 | Diffuse Sclerosing | 13 | F | 2.5 | Yes | Yes | No | No | T2N1M0 | No | No |
| 13a | Diffuse Sclerosing | 9 | M | 1.7 | Yes | Yes | Yes | Yes | T4N1M1 | No | No |
| 14a | Classical | 18 | F | 3.5 | No | Yes | No | No | T2N1M0 | No | No |
| 15 | Follicular | 12 | F | 1.8 | No | No | No | No | T1N0M0 | No | No |
| 16 | Classical | 12 | F | NA | Yes | Yes | Yes | Yes | T4N1M1 | No | No |
| 17 | Follicular | 6 | F | NA | NA | Yes | No | NA | TxN1M0 | No | No |
| 18a | Follicular | 13 | M | 2 | Yes | Yes | No | Yes | T4N1M0 | No | No |
| 19 | Encapsulated | 10 | F | 2 | No | No | No | No | T1N0M0 | No | No |
| 20a | Classical | 15 | M | 4.5 | Yes | Yes | Yes | Yes | T4N1M1 | No | Yes |
| 21a,b | Follicular | 13 | F | 5 | Yes | Yes | Yes | Yes | T4N1M1 | No | Yes |
| 22a | Classical | 16 | F | 2 | Yes | Yes | No | No | T1N1M0 | No | No |
| 23a | Follicular | 8 | F | 2.5 | No | Yes | No | No | T2N1M0 | No | No |
| 24a | Solid | 8 | M | 0.9 | Yes | Yes | Yes | Yes | T4N1M1 | No | No |
| 25a | Diffuse Sclerosing | 9 | F | 2.1 | No | Yes | No | Yes | T4N1M0 | No | No |
| 26 | Classical | 7 | F | 1.6 | No | No | No | Yes | T3N0M0 | No | Yes |
| 27a | Follicular | 6 | F | 3.5 | Yes | Yes | Yes | Yes | T4N1M1 | No | No |
| 28 | Follicular | 14 | F | 1.7 | No | No | No | No | T1N0M0 | No | No |
| 29 | Follicular | 18 | F | 1 | No | Yes | No | No | T1N1M0 | No | No |
| 30 | Follicular | 12 | M | 2.2 | Yes | Yes | No | No | T2N1M0 | No | No |
PTC samples with matched and Lymph node metastasis.
PTC samples with matched Lymph node metastasis which was positive for AGK‐BRAF.
Recurrent AGK‐BRAF rearrangement in sporadic pediatric PTCs
In order to optimize RT‐PCR reaction, we primarily used cDNA obtained from FTC cells transiently transfected with plasmid containing the AGK‐BRAF fusion gene. Different primer concentrations and PCR conditions were assayed. AGK‐BRAF was found in two primary tumors. Moreover, in one patient AGK‐BRAF rearrangement was identified in the LNM, while the fusion gene was not observed in the paired primary tumor. As this patient presented a multifocal PTC (case 21), additional foci were selected for further investigation. One out of five foci presented the AGK‐BRAF fusion oncogene. Overall, the AGK‐BRAF fusion gene was found in nearly 10% (3/30) of primary tumors and in about 6% (1/15) of LNMs (Table 1; Fig. 1). The presence of AGK‐BRAF fusion oncogene was confirmed by sequencing analysis (Fig. 1).
Figure 1.
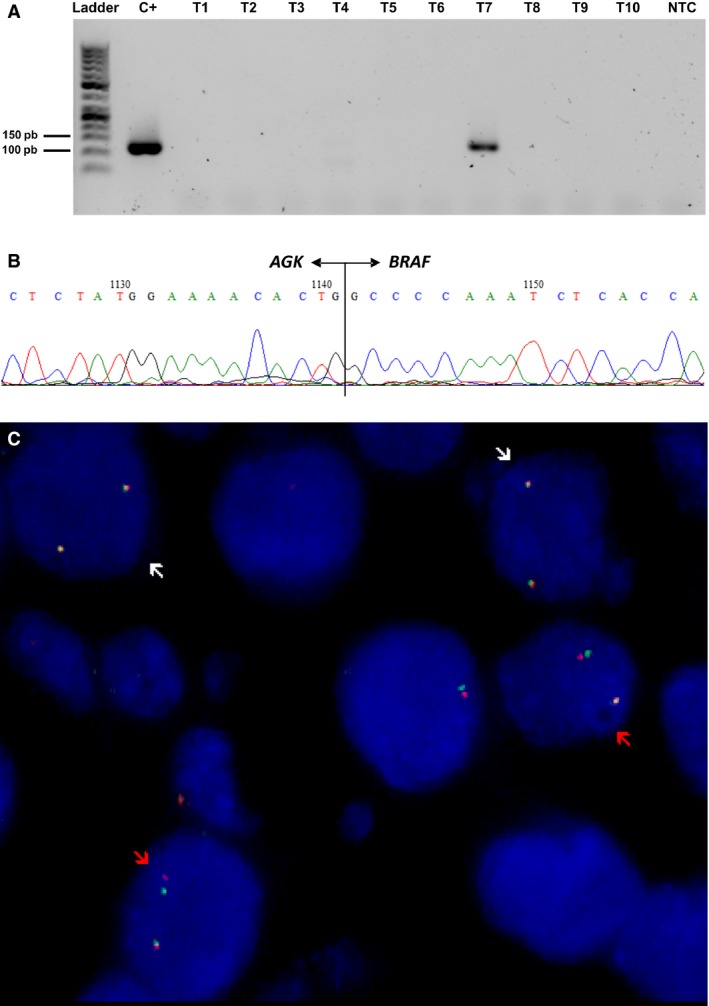
Screen for the presence of AGK‐BRAF fusion oncogene in sporadic pediatric PTC. Representative results of RT‐PCR analysis performed in sporadic pediatric PTC (T1‐T10). Positive (C+) and negative controls (NTC) were included in each run. Positive cases showed the proper size range (113 bp), as showed in C+ and case 20 (T7) (A). Sanger sequencing confirmed the presence of AGK‐BRAF fusion oncogene (B). Dual‐color, break‐apart FISH confirmed the breakage of BRAF gene resulting from structural rearrangements BRAF (C). Nuclei exhibiting rearrangement showed the presence of one red and green split signals (red arrow), in addition to the fused yellow or red‐green signal (white arrow).
Additionally, two cases that were positive for AGK‐BRAF by RT‐PCR were selected to test possible breakage of the BRAF gene and, therefore, to confirm the BRAF rearrangement. In addition to the fused yellow or red‐green signal, the selected fields showed the presence of red and green split signals (Fig. 1). The split‐apart BRAF signal was identified in 30% and 36% of cells. Nearly 70% of cells exhibited only two fused yellow or red‐green signals, confirming tumor heterogeneity.
AGK‐BRAF fusion oncogene and clinical‐pathological features of sporadic pediatric PTC
Among three patients positive for AGK‐BRAF, the mean age at diagnosis was 11.66 years (range 7–15 years). Two tumors with AGK‐BRAF fusion were of classical histology and one of follicular variant. Extrathyroidal extension was observed in all patients with AGK‐BRAF rearrangement. The prevalence of patients with distant metastasis at diagnosis and multifocality was higher in the AGK‐BRAF‐positive groups than in AGK‐BRAF‐negative groups (Table 2).
Table 2.
Pediatric PTC characteristics according to the prevalence of AGK‐BRAF
| Total No.(n = 30) | AGK‐BRAF Negative No (%)(n = 27) | AGK‐BRAF Positive No (%)(n = 3) | |
|---|---|---|---|
| Age ± SD (mean/years) | 11.36 | 11.33 | 11.66 |
| Tumor size ± SD (mean/cm) | 2.55 | 2.41 | 3.7 |
| Gender | |||
| Female | 21 | 19 (70) | 2 (66) |
| Male | 9 | 8 (30) | 1 (34) |
| Extrathyroidal extension | 14 | 11 (40) | 3 (100) |
| Multifocal disease | 14 | 12 (44) | 2 (66) |
| LN metastases | 24 | 22 (81) | 2 (66) |
| Distant metastases | 10 | 8 (29) | 2 (66) |
Discussion
The Cancer Genome Atlas (TCGA) Research Network, using different platforms combined with clinicopathological data, characterized the genomic landscape of nearly 500 PTCs of adults. The study confirmed that PTC is a MAPK‐driven cancer, identified new cancer‐causing gene mutations, as well as new fusion transcripts and somatic copy number alteration. These findings reduced the so‐called “dark matter” of the PTC. Importantly, the large collection of genetic alterations, combined with a comprehensive transcriptomic and proteomic analysis, exposed fundamental biological variances between PTCs. This increased knowledge helped to stratify PTC into subgroups, which eventually will improve preoperative diagnosis of thyroid nodules, prognosis, and treatment of adult PTC 21.
Despite intensive efforts, much less is known about the genetic alterations that are, in fact, “driver genes” in pediatric PTC. Although pediatric PTCs are also a MAPK‐driven cancer, the spectrum of mutations differs between adults and pediatric tumors. Furthermore, radiation‐exposed and sporadic pediatric PTCs likely have different genetic landscapes. In fact, Nikiforov et al. 17, provided the first evidence that they have different molecular signature. The authors reported that the prevalence of RET/PTC rearrangements is markedly different between sporadic and radiation‐exposed pediatric PTC. Not only the overall prevalence of RET/PTC diverges between sporadic and radiation‐exposed PTC but also the prevalence of RET/PTC3 isoform was higher than RET/PTC1 in radiation‐exposed cases 12, 17.
Recently, another group described that the proportion of samples harboring fusion oncogenes in radiation‐exposed pediatric PTC is markedly higher (85%) from that seen in nonradiation‐exposed group (33%), while point mutations have been mainly found in nonradiation‐exposed patients than in radiation‐exposed PTC patients 18
Most of the efforts to determine the landscape of pediatric cases have been concentrated in radiation‐exposed pediatric thyroid cancer, while in the routine most cases of thyroid cancer are actually sporadic cases.
The molecular differences between adult and pediatric PTC and the fact that fewer genetic events were described in pediatric PTC may impact on the utility of molecular testing for diagnosis of thyroid nodules in children. In fact, the ATA Guidelines for Children with thyroid nodules and differentiated thyroid cancer suggested that, although in adults molecular testing aids in the management of thyroid nodules with indeterminate cytopathology, insufficient data exist in children to rely on negative genetic studies. Therefore, the test cannot be recommended in routine clinical on pediatric patients practice until further studies are conducted 22.
This study identified the presence of AGK‐BRAF fusion gene in sporadic pediatric PTC. Although BRAF V600E mutation is uncommon in both radiation‐exposed and sporadic pediatric PTC 12, 15, 24, our findings reveal that BRAF fusion might be an alternative mechanism of MAPK pathway activation. It has been previously demonstrated that expression of AGK‐BRAF in NIH3T3 and COS‐7 cells promotes constitutive activation of MAPK signaling pathway and induces NIH3T3 cell growth and colony formation 18.
The frequency of BRAF fusion in this cohort of sporadic cases, one of the largest of literature, was validated using different approaches. FISH analysis allowed us to detect clonal rearrangements and to ratify tumor heterogeneity. Finally, dual‐color, break‐apart BRAF probe will help us to detect the presence of any fusion within the BRAF gene in sporadic pediatric cancer.
It has been suggested that biological differences may explain the clinical and pathological features differences between pediatric and adult patients. It still remains unclear whether AGK‐BRAF correlates with clinicopathological parameter in PTC such as age, presence of metastases, histological subtypes, and advanced clinical stages. In our study, AGK‐BRAF fusion appears to be related to a more aggressive biological behavior, as extrathyroidal extension was seen in all patients with AGK‐BRAF rearrangement. Additionally, multifocality, lymph node, and distant metastasis at diagnosis were observed in two patients out of three patients with AGK‐BRAF rearrangement. Unfortunately, no clinical information is available for the previously described AGK‐BRAF‐positive radiation‐exposed PTC 18. Nevertheless, further analysis, ideally multicenter studies, is needed to confirm this hypothesis and to better elucidate the biological behavior of sporadic pediatric PTC with AGK‐BRAF fusion gene.
In summary, our findings provide additional insight to our current understanding of tumor biology of sporadic pediatric PTC. Further efforts should be undertaken to define the genomic landscape of sporadic pediatric PTC. The knowledge of the molecular events underlying this group of patients would be extremely useful to improve the accurate diagnosis of thyroid nodules and prevent unnecessary thyroid surgeries, allow molecular prognostication and define a therapeutic approach toward sporadic PTC patients.
Conflict of Interest Statement
The authors have reported no conflicts of interest.
Cancer Medicine 2016; 5(7):1535–1541
References
- 1. Lise, M. , Franceschi S., Buzzoni C., Zambon P., F. Falcini , Crocetti E., et al. 2012. Changes in the incidence of thyroid cancer between 1991 and 2005 in Italy: a geographical analysis. Thyroid 22:27–34. [DOI] [PubMed] [Google Scholar]
- 2. Davies, L. , and Welch H. G.. 2006. Increasing incidence of thyroid cancer in the United States, 1973‐2002. JAMA 295:2164–2167. [DOI] [PubMed] [Google Scholar]
- 3. Siegel, R. , Ma J., Zou Z., and Jemal A.. 2014. Cancer statistics, 2014. CA Cancer J. Clin. 64:9–29. [DOI] [PubMed] [Google Scholar]
- 4. Vergamini, L. B. , Frazier A. L., Abrantes F. L., Ribeiro K. B., and Rodriguez‐Galindo C.. 2014. Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population‐based study. J. Pediatr. 164:1481–1485. [DOI] [PubMed] [Google Scholar]
- 5. Wu, X. C. , Chen V. W., Steele B., Roffers S., Klotz J. B., Correa C. N., et al. 2003. Cancer incidence in adolescents and young adults in the United States, 1992‐1997. J. Adolesc. Health 32:405–415. [DOI] [PubMed] [Google Scholar]
- 6. Hogan, A. R. , Zhuge Y., Perez E. A., Koniaris L. G., Lew J. I., and Sola J. E.. 2009. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J. Surg. Res. 156:167–172. [DOI] [PubMed] [Google Scholar]
- 7. Zimmerman, D. , Hay I. D., Gough I. R., Goellner J. R., Ryan J. J., Grant C. S., et al. 1988. Papillary thyroid carcinoma in children and adults: long‐term follow‐up of 1039 patients conservatively treated at one institution during three decades. Surgery 104:1157–1166. [PubMed] [Google Scholar]
- 8. Alzahrani, A. S. , Alkhafaji D., Tuli M., Al‐Hindi H., Bin Sadiq B.. 2015. Comparison of differentiated thyroid cancer in children and adolescents (</= 20 years) with young adults. Clin. Endocrinol. (Oxf) 84:571–577. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9. Lazar, L. , Lebenthal Y., Steinmetz A., Yackobovitch‐Gavan M., and Phillip M.. 2009. Differentiated thyroid carcinoma in pediatric patients: comparison of presentation and course between pre‐pubertal children and adolescents. J. Pediatr. 154:708–714. [DOI] [PubMed] [Google Scholar]
- 10. Grigsby, P. W. , Gal‐or A., Michalski J. M., and Doherty G. M.. 2002. Childhood and adolescent thyroid carcinoma. Cancer 95:724–729. [DOI] [PubMed] [Google Scholar]
- 11. Sassolas, G. , Hafdi‐Nejjari Z., Ferraro A., Decaussin‐Petrucci M., Rousset B., Borson‐Chazot F., et al. 2012. Oncogenic alterations in papillary thyroid cancers of young patients. Thyroid 22:17–26. [DOI] [PubMed] [Google Scholar]
- 12. Cordioli, M. I. , Moraes L., Cury A. N., and Cerutti J. M.. 2015. Are we really at the dawn of understanding sporadic pediatric thyroid carcinoma? Endocr. Relat. Cancer 22:R311–R324. [DOI] [PubMed] [Google Scholar]
- 13. Xing, M. 2007. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr. Rev. 28:742–762. [DOI] [PubMed] [Google Scholar]
- 14. Penko, K. , Livezey J., Fenton C., Patel A., Nicholson D., Flora M., et al. 2005. BRAF mutations are uncommon in papillary thyroid cancer of young patients. Thyroid 15:320–325. [DOI] [PubMed] [Google Scholar]
- 15. Lima, J. , Trovisco V., Soares P., Maximo V., Magalhaes J., Salvatore G., et al. 2004. BRAF mutations are not a major event in post‐Chernobyl childhood thyroid carcinomas. J. Clin. Endocrinol. Metab. 89:4267–4271. [DOI] [PubMed] [Google Scholar]
- 16. Fenton, C. L. , Lukes Y., Nicholson D., Dinauer C. A., Francis G. L., and Tuttle R. M.. 2000. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J. Clin. Endocrinol. Metab. 85:1170–1175. [DOI] [PubMed] [Google Scholar]
- 17. Nikiforov, Y. E. , Rowland J. M., Bove K. E., H. Monforte‐Munoz , and Fagin J. A.. 1997. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation‐induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 57:1690–1694. [PubMed] [Google Scholar]
- 18. Ricarte‐Filho, J. C. , Li S., Garcia‐Rendueles M. E., Montero‐Conde C., Voza F., Knauf J. A., et al. 2013. Identification of kinase fusion oncogenes in post‐Chernobyl radiation‐induced thyroid cancers. J. Clin. Invest. 123:4935–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ross, J. S. , Wang K., Chmielecki J., Gay L., Johnson A., Chudnovsky J., et al. 2015. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int. J. Cancer 138:881–890 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ciampi, R. , Knauf J. A., Kerler R., Gandhi M., Zhu Z., Nikiforova M. N., et al. 2005. Oncogenic AKAP9‐BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J. Clin. Invest. 115:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cancer Genome Atlas Research N . 2014. Integrated genomic characterization of papillary thyroid carcinoma. Cell 159:676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Francis, G. , Waguespack S. G., Bauer A. J., Angelos P., Benvenga S., Cerutti J., et al. 2015. Management guidelines for children with thyroid nodules and differentiated thyroid cancer the American Thyroid Association Guidelines Task Force on pediatric thyroid cancer. Thyroid 25:716–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cerutti, J. M. , Delcelo R., Amadei M. J., Nakabashi C., Maciel R. M., Peterson B., et al. 2004. A preoperative diagnostic test that distinguishes benign from malignant thyroid carcinoma based on gene expression. J. Clin. Invest. 113:1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumagai, A. , Namba H., Saenko V. A., Ashizawa K., Ohtsuru A., Ito M., et al. 2004. Low frequency of BRAFT1796A mutations in childhood thyroid carcinomas. J. Clin. Endocrinol. Metab. 89:4280–4284. [DOI] [PubMed] [Google Scholar]


