Abstract
Transforming growth factor β (TGFβ)-induced epithelial-mesenchymal transition (EMT) of lens epithelial cells (LECs) plays a key role in the pathogenesis of anterior subcapsular cataract (ASC) and capsule opacification. In mouse lens, Sprouty2 (Spry2) has a negative regulatory role on TGFβ signaling. However, the regulation of Spry2 during ASC development and how Spry2 modulates TGFβ signaling pathway in human LECs have not been characterized. Here, we demonstrate that Spry2 expression level is decreased in anterior capsule LECs of ASC patients. Spry2 negatively regulates TGFβ2-induced EMT and migration of LECs through inhibition of Smad2 and ERK1/2 phosphorylation. Also, blockade of Smad2 or ERK1/2 activation suppresses EMT caused by Spry2 downregulation. Collectively, our results for the first time show in human LECs that Spry2 has an inhibitory role in TGFβ signaling pathway. Our findings in human lens tissue and epithelial cells suggest that Spry2 may become a novel therapeutic target for the prevention and treatment of ASC and capsule opacification.
Introduction
Anterior subcapsular cataract (ASC) and capsule opacification are both caused by excessive proliferation and differentiation of lens epithelial cells (LECs)[1–4]. ASC is a primary cataract characterized by star-shaped or irregular fibrotic plaques beneath the anterior capsule, resulting in dramatic visual reduction due to visual axis involvement[5]. Capsule opacification is one of the most common complications after cataract surgery. Posterior capsule opacification (PCO), also known as secondary cataract, results from proliferation and migration of residual lens epithelial cells across the posterior capsule. About 20%-40% adult patients develop PCO within 5 years after surgery, and the incidence is almost 100% in children[6–8]. On the other hand, anterior capsule opacification (ACO) occurs around capsulotomy edge and usually develops faster than PCO. Excessive ACO leads to capsule shrinkage, IOL decentration, capsule contraction syndrome and limits peripheral fundus examination[9, 10].
Proliferation and epithelial-mesenchymal transition (EMT) of LECs play key roles in the pathogenesis of ASC and capsule opacification[4, 11, 12]. During EMT, LECs undergo cytoskeletal rearrangement, lose epithelial polarity, and transdifferentiate into active fibroblast-like cells[13]. EMT is also a crucial pathologic change in various fibrotic diseases and cancer metastasis[14, 15]. Transforming growth factor β (TGFβ) is the most potent inducer of EMT[16]. Canonical TGFβ signaling requires phosphorylation of Smad2 and Smad3, which then translocate into nucleus and turn on the expression of target genes, such as α-SMA, fibronectin (Fn), vimentin (Vim), collagen I (Col I), and collagen IV (Col IV)[17]. Also, TGFβ can activate extracellular signal-regulated kinase 1/2 (ERK1/2), p38 MAPK, JNK, Rho-like GTPase and Jagged/Notch as non-canonical pathways to induce EMT[18–21]. There are three isoforms of TGFβ (TGFβ1–3) in mammals[22]. TGFβ2 is the major form in aqueous humor, and is significantly upregulated after injury or during inflammation[23–25]. Therefore, inhibition of TGFβ2-induced EMT is considered to be a promising therapeutic strategy for ASC and capsule opacification[5, 26].
Sprouty (Spry) protein family is a highly conserved group of inhibitors that suppress ERK1/2 activation in various receptor tyrosine kinase (RTK) pathways[27, 28]. It was first reported in Drosophila as an antagonistic regulator of fibroblast growth factor (FGF) and epidermal growth factor(EGF) signaling[29]. Spry is widely considered as a tumor suppressor, and downregulation of Spry has been found in prostate, breast, liver and lung cancer, especially in the metastatic stages[30]. Also, overexpression of Spry can inhibit tumorigenesis[31]. To date, four mammalian Spry members (Spry1-4) have been identified. Of them, Spry2 is the major isoform expressed in mice mature lens fiber cells[32]. During lens development, Spry2 negatively modulates ERKs to allow lens vesicle separation[33]. Conditional knockout of Spry2 in mouse lens enhances TGFβ-induced EMT, while Spry2 overexpression inhibits LEC proliferation and differentiation[34–36]. These studies highlight the importance of Spry2 in lens development and cataractogenesis in mouse. However, the role of Spry2 in human ASC and capsule opacification formation has not been studied, and the molecular mechanism of Spry2-regulated TGFβ signaling in human lens is still largely unknown.
Here we seek to address the regulatory role of Spry2 on TGFβ-induced EMT in human LECs. We compared the RNA and protein levels of Spry2 in anterior capsule LECs from ASC patients with those from age-matched controls, and measured EMT level upon Spry2 downregulation or overexpression in human LECs. Our results demonstrate that Spry2 suppresses EMT of LECs by inhibiting both the canonical Smad pathway and the non-canonical ERK1/2 pathway, suggesting that Spry2 may be potentially a potent target for modulation of TGFβ-induced EMT in human LECs.
Materials and Methods
Human anterior capsule samples collection
Anterior capsule specimens with LECs from ASC and age-matched cortical cataract patients were obtained during cataract surgery. Each capsule is about 5 mm in diameter and contains the central area. Written informed consent forms were obtained from the patients before surgery, and the tenets of the Declaration of Helsinki were followed throughout the study. In addition, age-matched postmortem anterior capsule specimens of transparent lens obtained within 6 hours from death were used as controls. Cadaver eye tissues were obtained from the eye bank of Zhongshan Ophthalmic Center. The research protocol was approved by the Institutional Review Board/Ethics Committee of the Sun Yat-sen University.
Cell culture
Human lens epithelial cell line SRA01/04 was kindly provided by Professor Fu Shang at the Laboratory for Nutrition and Vision Research (Boston, MA, USA), and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). Cells were grown in a humidified 37°C incubator with 5% CO2 and dissociated with 0.25% trypsin-0.02% ethylenediamine-tetraacetic acid (EDTA). For TGFβ2 treatment, cells were seeded in six-well plates with the density of 1×105/well, incubated in serum-free medium for 5 hours, and treated with 10 ng/mL TGFβ2 (Cell Signaling Technology, Danvers, MA, USA).
siRNA and plasmid transfection
To knockdown (KD) Spry2, Spry2 siRNA (sc-41037, Santa Cruz Biotechnology, CA, USA) or scrambled siRNA (sc-37007, Santa Cruz Biotechnology) were transfected into SRA01/04 cells using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s protocol. Cells transfected with various concentrations of FITC-conjugated siRNA (sc-36869, Santa Cruz Biotechnology) were subjected to flow cytometry to monitor transfection efficiency, and an optimal siRNA concentration of 80nM was determined. To overexpress Spry2, human Spry2 cDNA plasmid (RC204864, Origene, Rockville, MD, USA) was transfected into SRA01/04 cells using X-tremeGENE HP DNA transfection reagent (Roche, Basel, Switzerland) according to the manufacturer’s protocol. Transfection of LECs with empty pCMV6-Entry vector (PS100001, Origene) was used as a negative control.
Western blot
For total protein extraction, cells were lysed in RIPA buffer with protease inhibitor cocktail. After mixing with 5× SDS sample buffer, protein samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinyl difluoride (PVDF) membranes (Bio-Rad). The membrane was blocked in 5% nonfat milk and incubated with primary antibody at 4°C overnight, and washed with PBST (0.1% Tween-20 in PBS). Then the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 hour at room temperature, and washed with PBST. Protein bands were detected with chemiluminescence detection reagents. β-actin was used as a loading control. Quantitative analysis was done using Image J 1.41 (National Institutes of Health, Bethesda, MD, USA). The sources and dilutions of antibodies are: rabbit anti-Spry2 (1:200, Santa Cruz Biotechnology), rabbit anti-ERK (1:1000, Cell Signaling Technology), rabbit anti-p-ERK (1:1000, Thr 202/Tyr 204, Cell Signaling Technology), rabbit anti-Smad2 (1:1000, Cell Signaling Technology), rabbit anti-p-Smad2 (1:1000, Ser 465/467, Cell Signaling Technology), mouse anti-α-SMA (1:200, Abcam, MA, USA), rabbit anti-fibronectin (1:200, Abcam), rabbit anti-collagen type I (1:1000, Abcam), rabbit anti-collagen type IV (1:500, Abcam), rabbit anti-actin (1:3000, Abcam), horseradish peroxidase (HRP)-conjugated horse anti-mouse IgG (1:2000, Cell Signaling Technology) and HRP-conjugated goat anti-rabbit IgG (1:2000, Cell Signaling Technology).
RNA extraction and quantitative PCR
Total RNA was extracted from fresh anterior capsule tissue or LECs using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. RNA concentration was measured spectrophotometrically at 260nm. 2μg RNA was used for reverse transcription to cDNA using a reverse transcription kit (Takara, Siga, Japan). SYBR PrimeScript RT-PCR kit (Takara) was used to amplify target genes by the ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. Primer sequences were listed in Table 1. All the primers were synthesized by Beijing Genomics Institute (Beijing, China).
Table 1. Primers used for real-time quantitative PCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Spry2 | 5'-ATCCAGAGACAAGACATGTAC-3' | 5'-TTCAGATGTGTTCTAAGCC-3' |
| α-SMA | 5'-CCGACCGAATGCAGAAGGA-3' | 5'-ACAGAGTATTTGCGC-TCCGAA-3' |
| Fibronectin | 5'-GAGCTGCACATGTCTTGGGAAC-3' | 5'-GGAGCAAATGGCACCGAGATA-3' |
| Vimentin | 5'-TGAGTACCGGAGACAGGTGCAG-3' | 5'-TAGCAGCTTCAACGGCAAAGTTC-3' |
| GAPDH | 5'-GAGTCAACGGATTTG-GTCGT-3' | 5'-AATGAAGGGGTCATTGATGG-3' |
Immunofluorescence Staining
For cell staining, SRA 01/04 cells were seeded on Millicell EZ 4-well glass slides (Millipore, MA, USA). After treatment for indicated times, cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 and blocked with 1% bovine serum albumin (BSA). Cells were then incubated with primary antibodies at 4°C overnight, and incubated with secondary antibody for 1 hour at room temperature. The sources and dilutions of antibodies are: rabbit anti-Spry2 (1:50, Santa Cruz Biotechnology), rabbit anti-fibronectin (1:100, Abcam), rabbit anti-collagen type I (1:500, Abcam), rabbit anti-collagen type IV (1:200, Abcam), Alexa Fluor 488-conjugated goat anti-mouse IgG (1:1000, Cell Signaling Technology) and Alexa Fluor 555-conjugated donkey anti-rabbit IgG (1:1000, Cell Signaling Technology). Cell nuclei were stained with 50 ng/ml 4’,6-diamidino-2-phenylindole (DAPI) for 5 min. Slides were mounted with anti-fade fluorescent mounting medium (Applygen, #C1210). Images were acquired by a Zeiss LSM 510 confocal laser scanning microscope (CLSM, Carl Zeiss, Germany) and processed by Adobe Photoshop CS6.
For whole mount staining, anterior capsules were acquired from the capsulorhexis during cataract surgery. Immunofluorescence staining was performed as described above. Finally, the whole anterior capsule was placed flat on a microscope slide, and the capsule was covered with a coverslip after adding a drop of anti-fade mounting medium. The central region of each anterior capsule was scanned by a CLSM.
Scratch wound assay
Scratched wound assay was used to evaluate the effect of Spry2 on TGFβ2-induced migration of LECs. Briefly, cells were seeded on a 6-well plate with the concentration of 1.5 x 105/well, transfected with Spry2 siRNA or plasmid, and grown for 36 hours to reach 90% confluence. Cell monolayer was wounded by a 200-μl micropipette tip and washed with PBS for four times with to remove cell debris. Then the cells were incubated in 0.5% FBS medium with or without 10 ng/mL TGFβ2 for 36 hours. Migration of cells into the wound was examined by inverted microscope and photographed in a digital format (×100). Cell number was counted using Image-Pro Plus software 5.1 (Media Cybernetics, Inc. Silver Spring, MD, USA). In each group, six randomly chosen microscopic fields were analyzed, and the average cell number was calculated.
Statistical analysis
Experiments presented in the figures are representative of at least three repetitions. All data are represented as means ± S.E.M. SPSS 17.0 software package (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. One-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test was used to compare means among three or more groups. Independent samples t-test was used to compare means between two groups. All statistical tests were two tailed. Values of P <0.05 were considered statistically significant.
Results
Expression level of Spry2 is downregulated in the anterior capsule LECs of ASC patients
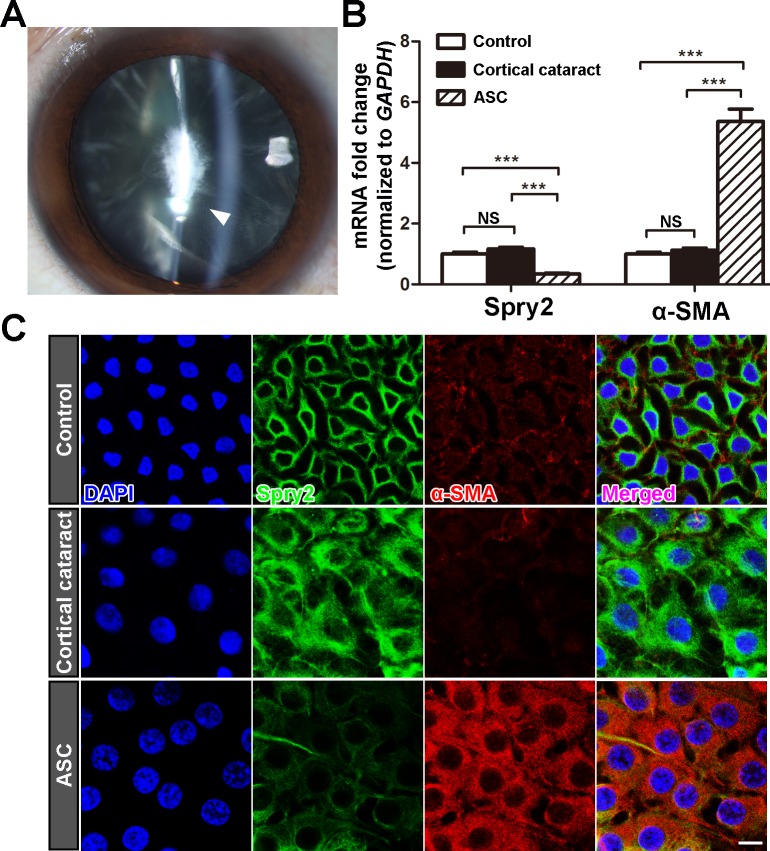
It has been reported that Spry2 is expressed in whole lens during embryogenesis, and then becomes restrictively expressed in LECs[32]. Spatial regulation of Spry2 expression indicates its key regulatory role in LEC proliferation and differentiation in response to growth factors. Downregulation of Spry2 in mouse lens promoted TGFβ-induced EMT and formation of anterior subcapsular plaques[35]. In order to know whether Spry2 is deregulated in the development of ASC in human, we compared the expression level of Spry2 from anterior capsule LECs of ASC patients, cortical cataract patients and those from LECs of age-matched transparent lens (control). A representative slit-lamp microscope photo of an ASC patient showed a focal irregular opacity was formed beneath the anterior capsule (Fig 1A). Spry2 mRNA expression in LECs from ASC patients was decreased to approximately 1/3 of that from the human transparent lens, while the mRNA level of α-SMA was significantly increased (Fig 1B). Consistently, immunofluorescent staining of lens anterior capsule from ASC patients revealed decreased expression of Spry2 and increased expression level of α-SMA (Fig 1C). We also noticed that LECs from cortical cataract and ASC patients exhibited enlarged nuclei and abundant cytoplasm compared to those from transparent lens. However, Spry2 and α-SMA expression levels were changed only in LECs from ASC patients, but not in LECs from cortical cataract patients. The distinct expression patterns of Spry2 and α-SMA suggested that these two types of cataracts underwent different pathological changes. In ASC, LECs tend to transdifferentiate into myofibroblasts directly beneath the lens capsule, so a reduced expression level of Spry2 and a high level of EMT could be detected in these anterior capsule samples. Collectively, these data show that Spry2 is downregulated during human ASC formation, and negatively correlated with α-SMA upregulation.
Fig 1. Spry2 expression level is decreased in anterior capsule LECs of ASC patients.
(A) Representative slit-lamp microscope photo of an ASC patient (60 years old, Female). The white arrow indicates the irregular fibrotic opacity beneath the anterior capsule. (B) Total RNA was extracted from the anterior capsules of ASC patients, age-matched cortical cataract patients and postmortem human lens (control). The mRNA level of Spry2 was determined using real-time PCR and normalized to GAPDH. ***P<0.001, NS: not significant, n = 6. Fold change relative to the level of the control groups is displayed. (C) Lens anterior capsule whole-mounts from ASC patients, age-matched cortical cataract patients, and postmortem human lens (control) were probed for Spry2 (green), α-SMA (red) and DAPI (blue). Images were acquired from the central area of each sample. Scale bar: 10μm.
Spry2 negatively regulates TGFβ2-induced EMT in LECs
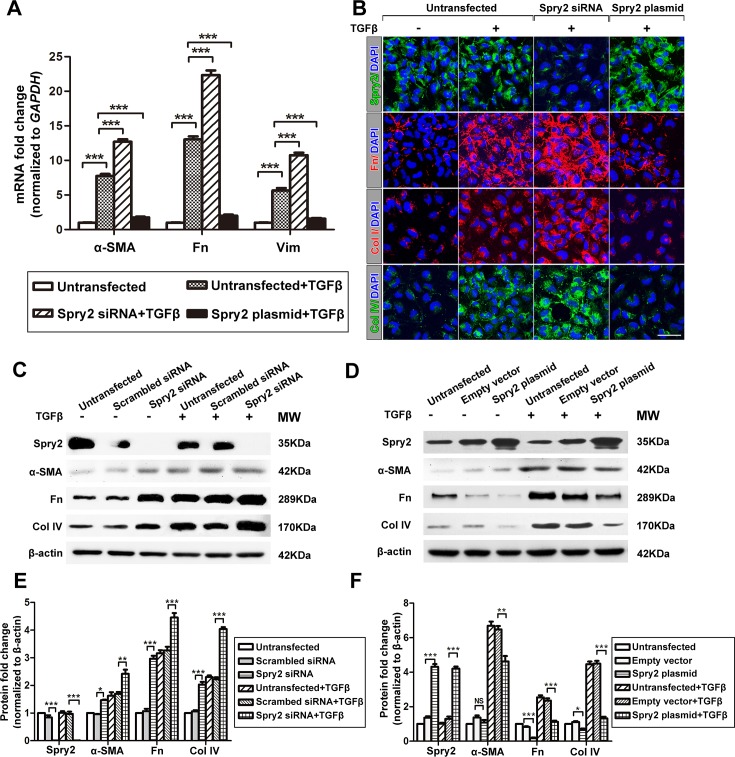
Since TGFβ2 plays a pivotal role in EMT and ASC development, it is likely that Spry2 downregulation will result in enhancement of TGFβ2-induced EMT. It has been reported in LECs that TGFβ2-induced EMT can be detected by upregulation of various mesenchymal cell markers, including α-SMA, Fn, Vim, Col I, and Col IV[12, 37]. Consistently, when we treated LECs by TGFβ2 for 48h, we found that the mRNA expression levels of α-SMA, Fn, and Vim were significantly increased (Fig 2A). Also, the protein expression levels of α-SMA, Fn, Col I, and Col IV were significantly increased (Fig 2B–2F), suggesting that LECs underwent EMT. To further determine the regulatory role of Spry2 in TGFβ2-induced EMT, we downregulated or overexpressed Spry2 in LECs during TGFβ2 treatment. When Spry2 was downregulated, the mRNA and protein expression levels of α-SMA, Fn, Col I, Col IV or Vim were significantly increased compared to those in LECs treated by TGFβ2 alone (Fig 2A, 2B, 2C and 2E). When Spry2 was overexpressed, the mRNA and protein expression levels of these EMT markers were significantly decreased compared to TGFβ2 treatment alone (Fig 2A, 2B, 2D and 2F). Collectively, these data indicate that Spry2 inhibits TGFβ2-induced EMT in LECs.
Fig 2. Spry2 inhibits TGFβ-induced EMT in human lens epithelial cells.
(A) Cultured human lens epithelial cells were transfected with Spry2 siRNA or Spry2 plasmid and treated with or without TGFβ for 48h. The mRNA levels of α-SMA, Fn and Vim were determined by real-time PCR and normalized to GAPDH. Fold change relative to the level of the untransfected groups is displayed. **P<0.01, ***P<0.001, n = 3 (B) Cells were transfected with Spry2 siRNA or Spry2 plasmid and treated with or without TGFβ for 48h. Immunofluorescence was performed by probing Spry2, Fn, Col I, Col IV and DAPI. Scale bar: 50μm (C-D) Cells were transfected with Spry2 siRNA or Spry2 plasmid and treated with or without TGFβ for 48h. Untransfected cells, cells transfected with scrambled siRNA or empty vector were used as controls. Proteins were extracted and probed for Spry2, α-SMA, Fn, Col IV. β-actin was used as a loading control. (E-F) Quantification of the protein expression levels in C and D, respectively. Fold change relative to the level of the untransfected groups is displayed. *P<0.05, **P<0.01, ***P<0.001, NS: not significant, n = 3.
Spry2 inhibits TGFβ2-induced LEC migration
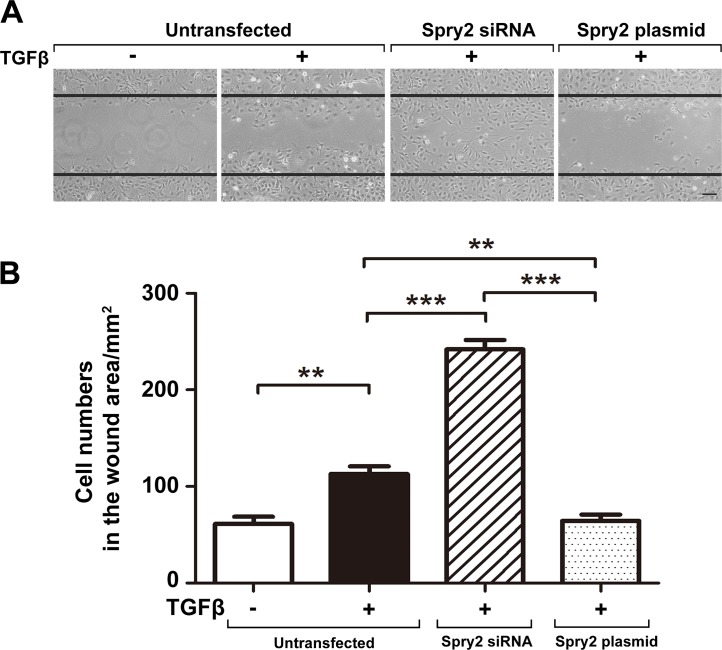
EMT facilitates cell migration by degradation of the underlying basement membrane[38]. After cataract surgery, TGFβ in the aqueous humor facilities the migration of LECs to the posterior capsule, and blockade of TGFβ pathway inhibits LECs migration[12, 26]. To assess whether Spry2 also negatively regulates TGFβ2-induced LECs migration, we performed wound scratch assays. Consistent with previous studies[39, 40], the number of LECs in the wound area was significantly increased after treatment by TGFβ2 for 36 hours. Cell migration was enhanced upon Spry2 downregulation and suppressed upon Spry2 overexpression (Fig 3A and 3B). These findings demonstrate that Spry2 negatively regulates TGFβ2-induced LECs migration, which is a crucial stage in the pathogenesis of PCO.
Fig 3. Spry2 prevents TGFβ-induced migration of human lens epithelial cells.
(A) Cultured human lens epithelial cells were transfected with Spry2 siRNA or Spry2 plasmid and treated with or without TGFβ for 36h. Cell migration was observed by an inverted phase contrast microscope. Straight black lines indicate the wound edges. Scale bar: 100μm. (B) Quantification of the number of cells that migrated into the wound area. **P<0.01, ***P<0.001, n = 6.
Spry2 suppresses TGFβ2 signaling through inhibition of Smad2 and ERK1/2 phosphorylation
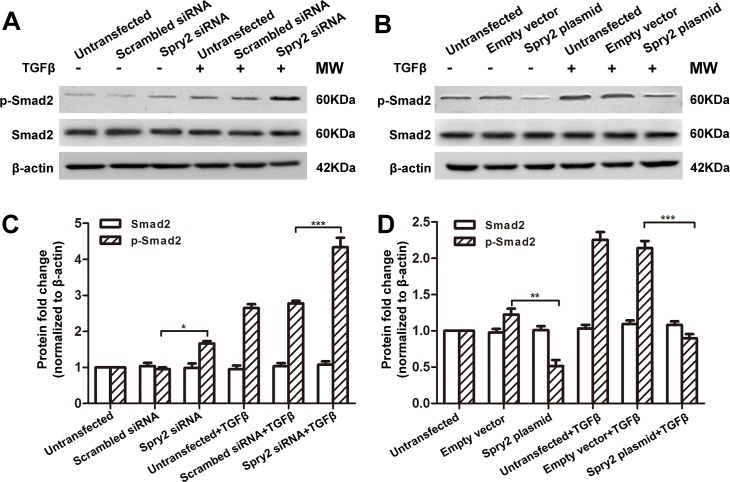
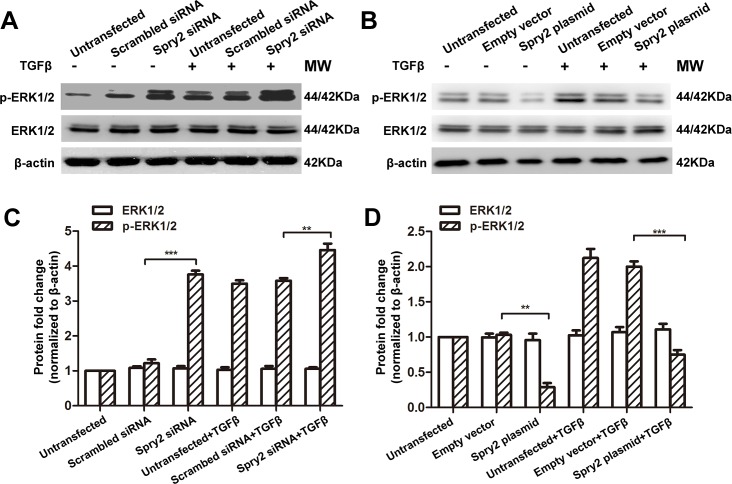
The negative regulatory role of Spry2 in of TGFβ2-induced EMT and migration suggest that Spry2 is an inhibitor of TGFβ signaling. To further dissect the mechanism of how Spry2 regulates TGFβ2-induced LECs EMT and migration, we measured the phosphorylation levels of Smad2 and ERK1/2, which were involved in the activation of canonical and non-canonical TGFβ signaling pathways, respectively[17, 18]. Consistent with our previous study[41], TGFβ treatment for 48 hours significantly increased the phosphorylation level of Smad2 and ERK1/2 in LECs, while the total expression level of Smad2 and ERK1/2 remained constant (Figs 4 and 5). Phosphorylation levels of Smad2 and ERK1/2 in LECs were significantly increased when Spry2 was downregulated, compared to those in LECs treated with TGFβ2 alone (Figs 4A, 4C, 5A and 5C). Also, phosphorylation levels of Smad2 and ERK1/2 levels were significantly decreased when Spry2 was overexpressed (Figs 4B, 4D, 5B and 5D). These data suggest that Spry2 is a potent negative regulator of TGFβ2 signaling through inhibition of both canonical and non-canonical TGFβ2 signaling pathways.
Fig 4. Spry2 suppresses Smad2 phosphorylation in human lens epithelial cells.
(A-B) Cultured human lens epithelial cells were transfected with Spry2 siRNA or Spry2 plasmid and treated with or without TGFβ for 48h. Untransfected cells, cells transfected with scrambled siRNA or empty vector were used as controls. Proteins were extracted and probed for pSmad2 and total Smad. β-actin was used as a loading control. (C-D) Quantification of the pSmad2 and total Smad expression levels in A and B, respectively. Fold change relative to the level of untransfected groups is displayed. *P<0.05, **P<0.01, ***P<0.001, n = 3.
Fig 5. Spry2 suppresses ERK1/2 phosphorylation in human lens epithelial cells.
(A-B) Cultured human lens epithelial cells were transfected with Spry2 siRNA or Spry2 plasmid and treated with or without TGFβ for 48h. Untransfected cells, cells transfected with scrambled siRNA or empty vector were used as controls. Proteins were extracted and probed for pERK1/2 and total ERK1/2. β-actin was used as a loading control. (C-D) Quantification of the pERK1/2 and total ERK1/2 expression levels in A and B, respectively. Fold change relative to the level of untransfected groups is displayed. **P<0.01, ***P<0.001, n = 3.
Inhibition of Smad2 or ERK1/2 suppresses Spry2 KD-induced EMT
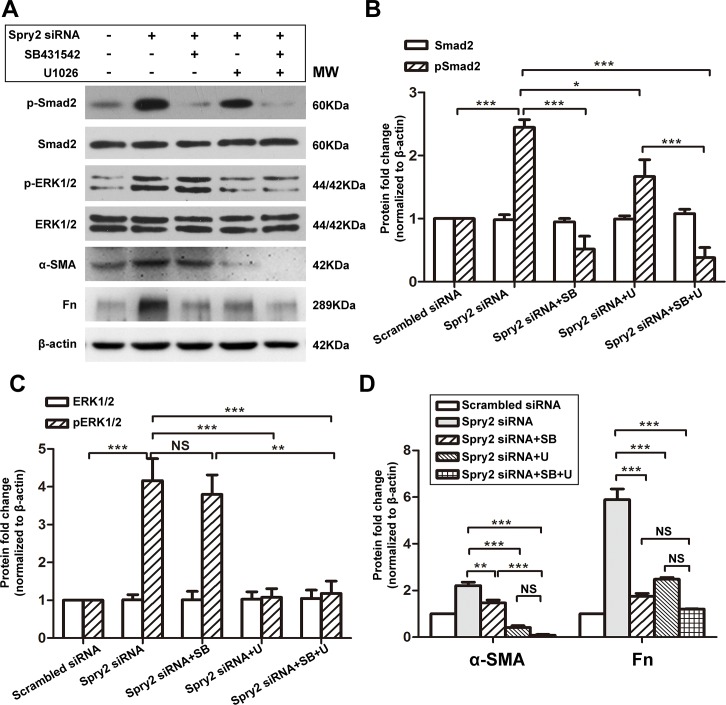
Interestingly, we noticed that even in conditions without TGFβ2 treatment, the expression levels EMT markers α-SMA, Fn, and Col IV were increased upon Spry2 downregulation and were decreased upon Spry2 overexpression (Fig 2C–2F). Also, without TGFβ2 treatment, there was a significant increase of p-Smad2 and p-ERK1/2 upon Spry2 downregulation and a significant decrease of p-Smad2 and p-ERK1/2 upon Spry2 overexpression (Figs 4 and 5). This can be explained by the basal activity of TGFβ and some other growth factors or cytokines that were contained in FBS[42] or secreted by LECs[43]. Also, these results suggested that Spry2 was a downstream regulator in TGFβ pathway. To further investigate how Spry2 inhibits TGFβ2 signaling, we treated Spry2 KD LECs with kinase inhibitors SB431542 and U0126, respectively. SB431542 inhibits TGFβ2 signaling by abrogating Smad2 phosphorylation and nuclear translocation[44]. U0126 is a specific inhibitor for ERK1/2 phosphorylation[45]. We found that SB431542 significantly suppressed upregulation of pSmad2 due to Spry2 downregulation, but did not affect pERK1/2 level. However, U1026 significantly suppressed upregulation of both pERK1/2 and pSmad2 due to Spry2 downregulation (Fig 6A–6C). These data confirmed our previous finding that canonical Smad signaling can be at least partially induced by noncanonical ERK1/2 signaling[41, 46]. We also found that SB431542 or U0126 treatment for 48 hours significantly suppressed upregulation of α-SMA and Fn due to Spry2 downregulation in LECs (Fig 6A and 6D). Moreover, we observed an enhanced suppression of α-SMA by combined treatment with both SB431542 and U1026 compared to treatment with SB431542 alone. Collectively, these results further supported that Spry2 suppressed TGFβ signaling by inhibiting Smad2 and ERK1/2 phosphorylation.
Fig 6. EMT induced by Spry2 downregulation was inhibited by Smad and ERK1/2 specific inhibitors.
(A) Cultured human lens epithelial cells were transfected with scrambled siRNA or Spry2 siRNA and treated with Smad inhibitor SB431542 (10.0μM) or ERK1/2 inhibitor U1026 (10.0μM) or both for 24h. Proteins were extracted and probed for pSmad, total Smad, pERK1/2, total ERK1/2, α-SMA and Fn. β-actin was used as a loading control. (B-D) Quantification of the protein expression levels in A. Fold change relative to the level of the scrambled siRNA transfected group is displayed. *P<0.05, **P<0.01, ***P<0.001, NS: not significant, n = 3.
Discussion
Various growth factors, including fibroblast growth factor (FGF), hepatocyte growth factor (HGF), epidermal growth factor (EGF), and TGF, contribute to the development of ASC and capsule opacification[47, 48]. Inhibition of specific growth factor signaling pathways, such as TGF[12, 35] or EGF[49], could prevent formation of ASC or PCO. Ideally, one effective approach for ASC or PCO prevention is to target a common signal protein in multiple growth factor pathways. Growth factors mainly signal through activation of RTK. Intriguingly, Spry2 is a general inhibitor in RTK signaling, such as FGF, EGF and TGF[29, 35]. In this study, we demonstrated for the first time that Spry2 is expressed in human LECs, and that Spry2 is downregulated in the anterior capsule of ASC patients. Also, in human LECs, Spry2 negatively regulates TGFβ2-induced EMT and migration through inhibition of both Smad2 and ERK1/2 phosphorylation. These data combine to suggest a critical role of Spry2 in TGFβ2-induced EMT of LECs and human ASC formation (Fig 7). Although the regulatory role of Spry2 on other growth factor signaling pathways in LECs remains to be investigated, inhibition of ERK1/2 by Spry2 suggests that Spry2 may also negatively regulate FGF and EGF signaling in human LECs. Therefore, targeting Spry2 may be a potent strategy for controlling ASC and capsule opacification.
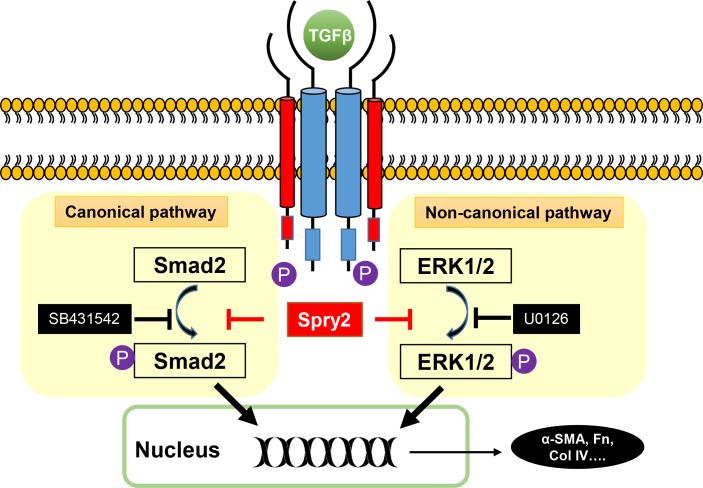
Fig 7. A working model for Spry2 in regulation of TGFβ signaling pathway.
TGFβ signals through canonical pathway and non-canonical pathway. The canonical pathway needs phosphorylation of Smad2, and can be blocked by SB431542. The non-canonical pathway needs phosphorylation of ERK1/2, and can be inhibited by U0126. Spry2 can block both canonical and non-canonical pathways, thus suppress TGFβ-induced EMT.
It has been reported that in mouse, conditional KD of Spry2 in lens elevates RTK-mediated ERK1/2 activation and facilitates TGF-induced EMT[35]. Also, overexpression of Spry2 in the mouse lens suppresses LECs proliferation and differentiation by inhibiting RTK signaling[34]. Following these studies, several questions need to be addressed. First, whether Spry2 is expressed in human lens and how it is regulated during human ASC and capsule opacification developments need to be characterized. Second, growth factor signaling pathways between mouse and human, such as FGF and TGF, are not completely identical[50, 51], and the expression profiles of crystalline and phospholipids between mouse and human lens are different[52]. These findings highlight that an evolutionary diversity may exist between species in the context of lens diseases. Moreover, recent findings suggest that Spry2 performs atypical roles in EMT in different cell types. Spry2 downregulation in colorectal epithelium inhibits EMT and serves as a biomarker for poor prognosis of colorectal cancer [53]. Conversely, Spry2 downregulation in breast epithelial cells induces EMT and migration[54]. Different responses to Spry2 alteration between different cell types may be caused by cell-type specific Spry targets[53, 55].Therefore, it is of great significance to characterize the regulatory role of Spry2 in human lens tissues and human LECs. Our study for the first time demonstrated the expression of Spry2 in human lens, and proved that it has an inhibitory role in TGFβ2-induced EMT in human LECs.
TGFβ signals through phosphorylation of Smad2/3 and ERK1/2. Although Spry family is known as an inhibitor of ERK1/2 phosphorylation, we demonstrated in human LECs that Spry2 can also inhibit activation of Smad2, which is consistent as previously reported in mouse[35]. Spry2 contains a canonical Casitas B-lineage lymphoma tyrosine kinase-binding motif(c-Cbl TKB): N-X-Y(p)-S/T-X-X-P with a key tyrosine residue (Y55)[56]. Binding to the c-Cbl TKB to protein phosphatase 2A(PP2A) exposes its C-terminal cryptic SH3-binding motif, and promotes the interactions between Spry2 with adaptor protein GRB2 and other SH3-containing targets, thus leading to inhibition of the downstream effector ERK1/2[57]. Since current data and our previous findings all suggest that Smad2 phosphorylation can be at least partially induced by ERK1/2 phosphorylation[41, 46], and ERK can stabilize pSmad2[58], it is likely that downregulation of pSmad2 by Spry2 overexpression is a consequence of ERK1/2 inhibition. Whether Spry2 can directly interact with Smad2 needs further investigation. But according to our data, combined treatment with SB431542 and U1026 exerted more suppression on α-SMA compared to SB431542 treatment alone, suggesting that EMT caused by Smad2 and ERK1/2 activation are not fully in one tandem pathway, and that Spry2 may have a negative regulatory role on both.
Phosphorylation of Smad2 can occur at Ser 465/467 or Ser 245/250/255. Phosphorylation of Smad2 at Ser 465/467 by the TGFß receptor positively regulates TGFß signaling, while phosphorylation of Smad2 at Ser 245/250/255 by MAP kinase negatively regulates TGFß signaling[59]. In this study, we demonstrated that Spry2 suppressed TGFß-induced Smad2 phosphorylation at Ser 465/467. However, upon TGFβ2 treatment, phosphorylation of Smad2 can also occur at Ser 245/250/255 in lens epithelial cells[60]. Whether Spry2 also affects Smad2 phosphorylation at Ser 245/250/255 remains to be investigated.
Spry2 inhibits TGFβ signaling, but the expression of Spry2 can also be regulated by TGFβ through inhibition of Spry2 mRNA transcription or promoting Spry2 protein degradation[61]. Also, repressed expression of Spry2 can be caused by downregulation of miR-181a in LECs from PCO patients[62, 63]. Although we did not observe a downregulation of Spry2 in the presence of TGFβ in cultured human LECs, we found that Spry2 was significantly downregulated in anterior capsule samples from ASC patients. This result suggested that the downregulation of Spry2 may be a trigger in the development of ASC and capsule opacification. Taken together, our work facilitates the understanding of fundamental biology by defining the role of Spry2 in human LECs transdifferentiation, and provides clinical evidence from human patients for the prevention of ASC and PCO development by using Spry2 as a potential therapeutic target.
Acknowledgments
We thank Professor Fu Shang for kindly providing the human lens epithelium cell line SRA01/04 for this study.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by the International Cooperation and Communication Project of the National Natural Science Foundation of China (No. 81320108008), the National Natural Science Foundation of China (No. 81270981) and the Science and Technology Program of Guangdong Province (No. 2013B051000029). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Banh A, Deschamps PA, Gauldie J, Overbeek PA, Sivak JG, West-Mays JA. Lens-specific expression of TGF-beta induces anterior subcapsular cataract formation in the absence of Smad3. Invest Ophth Vis Sci. 2006;47:3450–60. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lovicu FJ, Schulz MW, Hales AM, Vincent LN, Overbeek PA, Chamberlain CG, et al. TGF beta induces morphological and molecular changes similar to human anterior subcapsular cataract. Brit J Ophthalmol. 2002;86:220–6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirai K, Saika S, Tanaka T, Okada Y, Flanders KC, Ooshima A, et al. A new model of anterior subcapsular cataract: involvement of TGFbeta/Smad signaling. Molecular vision. 2006;12:681–91. . [PubMed] [Google Scholar]
- 4.Saika S. Relationship between posterior capsule opacification and intraocular lens biocompatibility. Progress in retinal and eye research. 2004;23:283–305. 10.1016/j.preteyeres.2004.02.004 . [DOI] [PubMed] [Google Scholar]
- 5.Lovicu FJ, Schulz MW, Hales AM, Vincent LN, Overbeek PA, Chamberlain CG, et al. TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. The British journal of ophthalmology. 2002;86:220–6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apple DJ, Solomon KD, Tetz MR, Assia EI, Holland EY, Legler UF, et al. Posterior capsule opacification. Survey of ophthalmology. 1992;37:73–116. . [DOI] [PubMed] [Google Scholar]
- 7.Hodge WG. Posterior capsule opacification after cataract surgery. Ophthalmology. 1998;105:943–4. 10.1016/S0161-6420(98)96040-7 . [DOI] [PubMed] [Google Scholar]
- 8.Petric I, Lacmanovic Loncar V. Surgical technique and postoperative complications in pediatric cataract surgery: retrospective analysis of 21 cases. Croat Med J. 2004;45:287–91. . [PubMed] [Google Scholar]
- 9.Macky TA, Pandey SK, Werner L, Trivedi RH, Izak AM, Apple DJ. Anterior capsule opacification. International ophthalmology clinics. 2001;41:17–31. . [DOI] [PubMed] [Google Scholar]
- 10.Werner L, Pandey SK, Apple DJ, Escobar-Gomez M, McLendon L, Macky TA. Anterior capsule opacification: correlation of pathologic findings with clinical sequelae. Ophthalmology. 2001;108:1675–81. . [DOI] [PubMed] [Google Scholar]
- 11.Xiao W, Chen X, Li W, Ye S, Wang W, Luo L, et al. Quantitative analysis of injury-induced anterior subcapsular cataract in the mouse: a model of lens epithelial cells proliferation and epithelial-mesenchymal transition. Scientific reports. 2015;5:8362 10.1038/srep08362 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng D, Song T, Zhongliu X, Wu M, Liang J, Liu Y. Downregulation of transforming growth factor-beta type II receptor prohibit epithelial-to-mesenchymal transition in lens epithelium. Molecular vision. 2012;18:1238–46. . [PMC free article] [PubMed] [Google Scholar]
- 13.Nalluri SM, O'Connor JW, Gomez EW. Cytoskeletal signaling in TGFbeta-induced epithelial-mesenchymal transition. Cytoskeleton. 2015. 10.1002/cm.21263 . [DOI] [PubMed] [Google Scholar]
- 14.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. The Journal of clinical investigation. 2003;112:1776–84. 10.1172/JCI20530 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bill R, Christofori G. The relevance of EMT in breast cancer metastasis: Correlation or causality? FEBS letters. 2015;589:1577–87. 10.1016/j.febslet.2015.05.002 . [DOI] [PubMed] [Google Scholar]
- 16.Dawes LJ, Sleeman MA, Anderson IK, Reddan JR, Wormstone IM. TGFbeta/Smad4-dependent and -independent regulation of human lens epithelial cells. Investigative ophthalmology & visual science. 2009;50:5318–27. 10.1167/iovs.08-3223 . [DOI] [PubMed] [Google Scholar]
- 17.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. 10.1038/nature02006 . [DOI] [PubMed] [Google Scholar]
- 18.Akhurst RJ. The paradoxical TGF-beta vasculopathies. Nat Genet. 2012;44:838–9. 10.1038/ng.2366 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen XF, Zhang HJ, Wang HB, Zhu J, Zhou WY, Zhang H, et al. Transforming growth factor-beta1 induces epithelial-to-mesenchymal transition in human lung cancer cells via PI3K/Akt and MEK/Erk1/2 signaling pathways. Molecular biology reports. 2012;39:3549–56. 10.1007/s11033-011-1128-0 . [DOI] [PubMed] [Google Scholar]
- 20.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell research. 2009;19:128–39. 10.1038/cr.2008.328 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–74. 10.1038/sj.onc.1208927 . [DOI] [PubMed] [Google Scholar]
- 22.Saika S. TGFbeta pathobiology in the eye. Laboratory investigation; a journal of technical methods and pathology. 2006;86:106–15. 10.1038/labinvest.3700375 . [DOI] [PubMed] [Google Scholar]
- 23.Jampel HD, Roche N, Stark WJ, Roberts AB. Transforming growth factor-beta in human aqueous humor. Current eye research. 1990;9:963–9. . [DOI] [PubMed] [Google Scholar]
- 24.Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2001;239:109–13. . [DOI] [PubMed] [Google Scholar]
- 25.Min SH, Lee TI, Chung YS, Kim HK. Transforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uveitic eyes. Korean journal of ophthalmology: KJO. 2006;20:162–5. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun CB, Teng WQ, Cui JT, Huang XJ, Yao K. The effect of anti-TGF-beta2 antibody functionalized intraocular lens on lens epithelial cell migration and epithelial-mesenchymal transition. Colloids and surfaces B, Biointerfaces. 2014;113:33–42. 10.1016/j.colsurfb.2013.08.024 . [DOI] [PubMed] [Google Scholar]
- 27.Cabrita MA, Christofori G. Sprouty proteins, masterminds of receptor tyrosine kinase signaling. Angiogenesis. 2008;11:53–62. 10.1007/s10456-008-9089-1 . [DOI] [PubMed] [Google Scholar]
- 28.Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends in cell biology. 2006;16:45–54. 10.1016/j.tcb.2005.11.004 . [DOI] [PubMed] [Google Scholar]
- 29.Kramer S, Okabe M, Hacohen N, Krasnow MA, Hiromi Y. Sprouty: a common antagonist of FGF and EGF signaling pathways in Drosophila. Development. 1999;126:2515–25. . [DOI] [PubMed] [Google Scholar]
- 30.Masoumi-Moghaddam S, Amini A, Morris DL. The developing story of Sprouty and cancer. Cancer Metastasis Rev. 2014;33:695–720. 10.1007/s10555-014-9497-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minowada G, Miller YE. Overexpression of Sprouty 2 in mouse lung epithelium inhibits urethane-induced tumorigenesis. Am J Respir Cell Mol Biol. 2009;40:31–7. 10.1165/rcmb.2008-0147OC . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boros J, Newitt P, Wang Q, McAvoy JW, Lovicu FJ. Sef and Sprouty expression in the developing ocular lens: implications for regulating lens cell proliferation and differentiation. Seminars in cell & developmental biology. 2006;17:741–52. 10.1016/j.semcdb.2006.10.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuracha MR, Burgess D, Siefker E, Cooper JT, Licht JD, Robinson ML, et al. Spry1 and Spry2 are necessary for lens vesicle separation and corneal differentiation. Invest Ophthalmol Vis Sci. 2011;52:6887–97. 10.1167/iovs.11-7531 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin EH, Zhao G, Wang Q, Lovicu FJ. Sprouty gain of function disrupts lens cellular processes and growth by restricting RTK signaling. Developmental biology. 2015;406:129–46. 10.1016/j.ydbio.2015.09.005 . [DOI] [PubMed] [Google Scholar]
- 35.Shin EHH, Basson MA, Robinson ML, McAvoy JW, Lovicu FJ. Sprouty Is a Negative Regulator of Transforming Growth Factor beta-Induced Epithelial-to-Mesenchymal Transition and Cataract. Mol Med. 2012;18:861–73. 10.2119/molmed.2012.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao G, Wojciechowski MC, Jee S, Boros J, McAvoy JW, Lovicu FJ. Negative regulation of TGFbeta-induced lens epithelial to mesenchymal transition (EMT) by RTK antagonists. Experimental eye research. 2015;132:9–16. 10.1016/j.exer.2015.01.001 . [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Ye Y, Lin X, Wu K, Yu M. Inhibition of pirfenidone on TGF-beta2 induced proliferation, migration and epithlial-mesenchymal transition of human lens epithelial cells line SRA01/04. PloS one. 2013;8:e56837 10.1371/journal.pone.0056837 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. 10.1038/nrm3758 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang KC, Petrash JM. Aldose Reductase Mediates Transforming Growth Factor beta2 (TGF-beta2)-Induced Migration and Epithelial-To-Mesenchymal Transition of Lens-Derived Epithelial Cells. Invest Ophthalmol Vis Sci. 2015;56:4198–210. 10.1167/iovs.15-16557 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao J, Yang W, Liu Y, Sun YX, Jiang Q. Dexamethasone inhibits TGF-beta2-induced migration of human lens epithelial cells: implications for posterior capsule opacification prevention. Mol Med Rep. 2012;5:1509–13. 10.3892/mmr.2012.827 . [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Ye S, Xiao W, Wang W, Luo L, Liu Y. ERK1/2 pathway mediates epithelial-mesenchymal transition by cross-interacting with TGFbeta/Smad and Jagged/Notch signaling pathways in lens epithelial cells. International journal of molecular medicine. 2014;33:1664–70. 10.3892/ijmm.2014.1723 . [DOI] [PubMed] [Google Scholar]
- 42.Oida T, Weiner HL. Depletion of TGF-beta from fetal bovine serum. J Immunol Methods. 2010;362:195–8. 10.1016/j.jim.2010.09.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen JB, Davidson MG, Nasisse MP, Fleisher LN, McGahan MC. The lens influences aqueous humor levels of transforming growth factor-beta 2. Graefes Arch Clin Exp Ophthalmol. 1998;236:305–11. . [DOI] [PubMed] [Google Scholar]
- 44.Hjelmeland MD, Hjelmeland AB, Sathornsumetee S, Reese ED, Herbstreith MH, Laping NJ, et al. SB-431542, a small molecule transforming growth factor-beta-receptor antagonist, inhibits human glioma cell line proliferation and motility. Mol Cancer Ther. 2004;3:737–45. . [PubMed] [Google Scholar]
- 45.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. The Journal of biological chemistry. 1998;273:18623–32. . [DOI] [PubMed] [Google Scholar]
- 46.Chen XY, Xiao W, Wang WC, Luo LX, Ye SB, Liu YZ. The Complex Interplay between ERK1/2, TGF beta/Smad, and Jagged/Notch Signaling Pathways in the Regulation of Epithelial-Mesenchymal Transition in Retinal Pigment Epithelium Cells. Plos One. 2014;9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishida I, Saika S, Okada Y, Ohnishi Y. Growth factor deposition in anterior subcapsular cataract. Journal of cataract and refractive surgery. 2005;31:1219–25. 10.1016/j.jcrs.2004.11.039 . [DOI] [PubMed] [Google Scholar]
- 48.Sinha R, Shekhar H, Sharma N, Titiyal JS, Vajpayee RB. Posterior capsular opacification: A review. Indian J Ophthalmol. 2013;61:371–6. . [Google Scholar]
- 49.Wertheimer C, Siedlecki J, Kook D, Mayer WJ, Wolf A, Klingenstein A, et al. EGFR inhibitor Gefitinib attenuates posterior capsule opacification in vitro and in the ex vivo human capsular bag model. Graefes Arch Clin Exp Ophthalmol. 2015;253:409–17. 10.1007/s00417-014-2875-0 . [DOI] [PubMed] [Google Scholar]
- 50.Kunath T, Yamanaka Y, Detmar J, MacPhee D, Caniggia I, Rossant J, et al. Developmental differences in the expression of FGF receptors between human and mouse embryos. Placenta. 2014;35:1079–88. 10.1016/j.placenta.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 51.Abnaof K, Mallela N, Walenda G, Meurer SK, Sere K, Lin Q, et al. TGF-beta stimulation in human and murine cells reveals commonly affected biological processes and pathways at transcription level. Bmc Syst Biol. 2014;8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwata JL, Bardygula-Nonn LG, Glonek T, Greiner JV. Interspecies comparisons of lens phospholipids. Curr Eye Res. 1995;14:937–41. . [DOI] [PubMed] [Google Scholar]
- 53.Zhang Q, Wei T, Shim K, Wright K, Xu K, Palka-Hamblin HL, et al. Atypical role of sprouty in colorectal cancer: sprouty repression inhibits epithelial-mesenchymal transition. Oncogene. 2015. 10.1038/onc.2015.365 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sigurdsson V, Ingthorsson S, Hilmarsdottir B, Gustafsdottir SM, Franzdottir SR, Arason AJ, et al. Expression and functional role of sprouty-2 in breast morphogenesis. PLoS One. 2013;8:e60798 10.1371/journal.pone.0060798 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7. 10.1038/nature11003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lupher ML Jr, Songyang Z, Shoelson SE, Cantley LC, Band H. The Cbl phosphotyrosine-binding domain selects a D(N/D)XpY motif and binds to the Tyr292 negative regulatory phosphorylation site of ZAP-70. J Biol Chem. 1997;272:33140–4. . [DOI] [PubMed] [Google Scholar]
- 57.Guy GR, Jackson RA, Yusoff P, Chow SY. Sprouty proteins: modified modulators, matchmakers or missing links? The Journal of endocrinology. 2009;203:191–202. 10.1677/JOE-09-0110 . [DOI] [PubMed] [Google Scholar]
- 58.Hough C, Radu M, Dore JJE. TGF-Beta Induced Erk Phosphorylation of Smad Linker Region Regulates Smad Signaling. Plos One. 2012;7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gadir N, Lee E, Garcia A, Toschi A, Foster DA. Suppression of TGF-beta signaling by phospholipase D. Cell Cycle. 2007;6:2840–5. 10.4161/cc.6.22.4921 . [DOI] [PubMed] [Google Scholar]
- 60.Nahomi RB, Pantcheva MB, Nagaraj RH. alphaB-crystallin is essential for the TGF-beta2-mediated epithelial to mesenchymal transition of lens epithelial cells. Biochem J. 2016;473:1455–69. 10.1042/BCJ20160128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding W, Shi W, Bellusci S, John G, Heisterkamp N, Minoo P, et al. Sprouty2 downregulation plays a pivotal role in mediating crosstalk between TGF-beta 1 signaling and EGF as well as FGF receptor tyrosine kinase-ERK pathways in mesenchymal cells. J Cell Physiol. 2007;212:796–806. . [DOI] [PubMed] [Google Scholar]
- 62.Dong N, Tang X, Xu B. miRNA-181a inhibits the proliferation, migration, and epithelial-mesenchymal transition of lens epithelial cells. Invest Ophthalmol Vis Sci. 2015;56:993–1001. 10.1167/iovs.14-15860 . [DOI] [PubMed] [Google Scholar]
- 63.Huang ZP, Chen JF, Regan JN, Maguire CT, Tang RH, Dong XR, et al. Loss of MicroRNAs in Neural Crest Leads to Cardiovascular Syndromes Resembling Human Congenital Heart Defects. Arterioscl Throm Vas. 2010;30:2575–U485. . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.