Abstract
Introduction
Metabolic syndrome (MetS) is a growing problem in the United Arab Emirates (UAE). Moreover, the prevalence of overweight and obesity is rapidly increasing in the UAE especially among young females. However, few studies have evaluated the prevalence of MetS among young female adults in the UAE. This study determined the prevalence of MetS in Emirati females aged 17–25 years and its relation to overweight and obesity.
Methods
In total, 555 Emirati female college students were enrolled in a cross-sectional study, conducted during 2013–2014 at United Arab Emirates University in Al Ain, UAE. Anthropometric measurements, blood pressure and biochemical measurements were collected. MetS was defined according to the harmonised International Diabetes Federation criteria.
Results
Of the 555 participants enrolled, 23.1% were overweight and 10.4% were classified as obese. The overall prevalence of MetS was 6.8%. MetS prevalence was highest among obese participants (34.5%), as compared with normal-weight (1.7%) and overweight (10.1%) participants. MetS was significantly associated with overweight (adjusted odds ratio [aOR] = 3.8, 95% confidence interval [CI]; 1.15–12.52) and obesity (aOR = 11.2, 95% CI; 3.1–40.9), as compared with normal-weight. Waist-hip ratio ≥ 0.8 (aOR = 3.04, 95% CI; 1.10–8.44) was significantly associated with MetS, as compared with waist-hip ratio <0.8. The odds of MetS were 22 fold higher in participants with glycated haemoglobin (HbA1c) ≥ 6.5% (aOR = 22.5, 95% CI; 6.37–79.42) compared to HbA1c <6.5%. This difference was 9 fold higher when HbA1c between 5.6%–6.4% was compared to HbA1c <5.6% (aOR = 8.9, 95% CI; 3.4–23.5).
Conclusion
The prevalence of MetS among obese Emirati female students was significantly higher than overweight and normal weight students. The high prevalence of MetS highlights the importance of regular screening and intervention programmes targeting weight reduction.
Introduction
Non-communicable diseases (NCDs) are the leading cause of deaths worldwide, and diabetes mellitus (DM) is the fourth major cause of NCD deaths [1].
A diagnosis of metabolic syndrome (MetS) is based on the existence of pre-diabetes combined with dyslipidaemia (elevated levels of total or low-density lipoprotein [LDL] cholesterol, or low high-density lipoprotein [HDL] cholesterol levels), elevated blood pressure and obesity [2]. Based on the International Diabetes Federation (IDF) definition for MetS; a study conducted in Emirati adults (>20 years old) by Malik and Razig in 2008 reported the total MetS prevalence was 40.5% and was higher among women (45.9%) than men (32.9%) [3]. The study by Mehairi et al. in 2013 showed an increase in the prevalence of MetS with higher body mass index (BMI) values [4].
The lifestyle of the Emirati population has changed considerably over the past 40 years due to the rapid improvement in socioeconomic status. This transition has led to less physical activity and altered eating habits. These changes, in addition to the adoption of a western lifestyle and diet, have led to the rise in the prevalence of overweight and obesity in the UAE, particularly among females [5]. There is a paucity of data available about the prevalence of MetS and its relation with overweight and obesity among young female adults in the UAE. Moreover, the population structure of UAE is mainly young and has therefore been greatly affected by the rapid socioeconomic changes.
This study aimed to determine the prevalence of MetS in Emirati females aged 17–25 years as this age range has not been studied previously, and its relation to overweight and obesity in Al Ain, UAE.
Design and Methods
Study population
A cross-sectional population-based study was conducted during the academic year 2013/ 2014 at United Arab Emirates University (UAEU) in Al Ain, UAE. The university currently enroll around 14,000 students each year.
The study population included students from all nine colleges of the university. Participants were asked to read the information sheet carefully and were given the chance to ask any question related to the study before providing written informed consent to participate. Each participant was assigned a personal identification number to maintain anonymity and data confidentiality. Ethical approval was obtained from the United Arab Emirates University Scientific Research Ethics Committee (Reference number DVCRGS/370/2014).
A stratified random sampling approach was used to select eligible participants [6]. All female students were divided into strata by college (nine strata), and then a random subsample proportional to size that consisted of 10% of the students from each college were selected. All eligible students were then contacted via e-mail to request their participation in the study. Of the total number of females registered in the university during the 2013/2014 academic year (n = 8846), a subsample of 885 students received a request to participate in the study. The enrolment process of the study participants is shown in Fig 1.
Fig 1. Study participants enrolment process.

Questionnaire
Each participant completed a self-reported questionnaire on demographic data, supplements and medication use, tobacco use, diet, physical activity, sleeping patterns, perceptions about obesity, personal history of NCDs and family history (first-degree relatives) of NCDs. Participants completed the questionnaire under the supervision of the research team to respond to any clarification needed on any aspects of the questionnaire or the study as a whole.
Anthropometric measurements and physical examination
Height was measured using a portable stadiometer (Seca Stadiometer, Seca Ltd, Birmingham, UK), in the standing position, without shoes and recorded to the nearest millimetre [7]. Body weight (Kg) and body composition were measured using the Tanita Segmental Body Composition Analyser (Tanita BC-418, Tanita Corp., Tokyo, Japan) [8]. The World Health Organization classification of the BMI (weight / height2; (kg/m2)) was used to classify underweight, normal-weight, overweight and obesity in the studied population [9]. Waist circumference (WC) was measured in centimetres (cm) using a plastic tape, at the midway between the inferior margin of the ribs and the superior border of the iliac crest or at umbilicus level for obese participants [10]. Hip circumference (HC) was measured at the level of maximum posterior extension of the buttocks [11].Waist-hip ratio (WHR) was calculated by dividing WC by HC. Total body fat was measured from skin-fold thickness at four sites (biceps, triceps, subscapular and suprailiac) using the equation described by Durnin and Womersley in 1974 [12]. Cut-off points for body fat percentage, WHR, and anaemia were based on World Health Organization recommended values [13–15].
The anthropometric measurements were carried out by a trained anthropometrist to reduce inter-observer variations. All measurements were completed during a single 50-minute session (to eliminate missing data), with the participants reporting to the clinic having fasted for 12–14 hours prior to testing, although drinking water was allowed in moderation. Measurements were taken in the morning between 7:00–10:00 a.m. to minimize inter-day fluctuations. Participants were asked not to visit the clinic during their menstrual cycle. Participants were encouraged to rest for 15 minutes before any measures were performed to enable them to relax before performing any of the tests. Each measurement was taken three times and averaged to improve accuracy. All measuring devices were calibrated on a daily basis.
Blood pressure (BP) was measured by a registered nurse using a validated and calibrated digital automated sphygmomanometer (Omron Hem-907, Omron Healthcare, Kyoto, Japan), after the participant had rested for at least 15 minutes [16]. Two consecutive measurements were obtained 5-minutes apart and the average of the two readings recorded [17].
Laboratory measurements
A registered nurse collected a 5-ml venous blood sample from each participant after 12 hours of fasting via a vacuum system (vacuette 0.64 × 19mm, Greiner Bio-One, Kremsmünster, Austria), into a serum separator tube with clot activator (Vacutest Kima srl, Arzergrande, Italy). Blood samples were centrifuged (2,500 rpm, 15 minutes) and the serum was properly separated, identified and stored at −80°C until the time of analyses.
Samples were thawed on ice for 30 minutes with proper handling during thawing and storage [18]. The total cholesterol, triglyceride (TG), LDL-cholesterol, HDL-cholesterol (HDL-C), high-sensitivity C-reactive protein and fasting blood glucose concentrations in human serum analyses were performed using the Cobas C111 automated biochemical analyser (Roche Diagnostics, Indianapolis, IN, USA) [19]. The HemoCue Hb 201+ portable photometer system (HemoCue AB, Ängelholm, Sweden) was used for the assessment of haemoglobin concentration and the HemoCue HbA1c 501 system was used for assessing glycated haemoglobin (HbA1c) percentage in whole blood. MetS was defined according to the harmonised definition established in 2009 by the IDF and the American Heart Association/the National Heart, Lung, and Blood Institute (AHA/NHLBI) as the presence of any three of the following five factors: elevated WC ( ≥ 80 cm in women); hypertriglyceridaemia (TG ≥ 150 mg/dL or drug treatment for elevated TG); reduced HDL-C (<50 mg/dL in women or drug treatment for reduced HDL-C); elevated BP (systolic BP ≥130 mmHg and/or diastolic BP ≥85 mmHg or use of antihypertensive drugs); and elevated fasting blood glucose ≥100 mg/dL or use of hypoglycaemic medication) [20].
Sample size calculation
Sample size was calculated using the Minitab software (version 16, Minitab Inc, PA, State College, USA) and was based on an expected prevalence of 4%. At 80% power and 5% significance level, a sample size of 555 would achieve a 1.58% margin of error for the survey of the female student population.
Statistical analyses
Data analyses were carried out using Stata version 13 (Stata Corp, College Station, TX, USA). Descriptive statistics were computed and summarised; continuous variables were summarised using means and standard deviations (SD) and categorical variables using proportions. The Student’s t-test was used for continuous variables to compare mean differences between participants with and without MetS. Univariable and multivariable logistic regression analysis was used to study the association between anthropometric and chemical measures and the presence or absence of MetS as the outcome variable. To account for perfect prediction of MetS by BMI categories and the small sample size across BMI class, we applied the Firth logistic regression to obtain reasonable and robust estimates. All statistical significance was assessed at the 5% significance level.
Results
Of the 885 students invited to participate, the response rate was 74% (n = 654). The overall prevalence of MetS was 6.8% (95% CI: 5% to 9%). The demographic and clinical characteristics of the study population by MetS status are presented as mean ± SD, in Table 1. The mean age of the study population was 20.4 ± 1.7 years. The average age of participants with MetS was not significantly different from those without MetS (20.9 vs. 20.4 years, P = 0.057). However, participants with MetS had a significantly higher weight, height, HC, BMI, body fat percentage, serum LDL (mg/dL) and HbA1c level (P< 0.01).
Table 1. Demographic and clinical characteristics by metabolic syndrome status.
| With Metabolic Syndrome (N = 38) Mean ± SD | Without Metabolic Syndrome (N = 517) Mean ± SD | Student’s t-test P-value | |
|---|---|---|---|
| Age (Year) | 20.9 ± 1.7 | 20.4 ± 1.7 | 0.057 |
| Weight (Kg) | 82.1 ± 17.1 | 58.9 ± 12.2 | < 0.001 |
| Height (cm) | 161.3 ± 5.2 | 158.9 ± 5.8 | 0.013 |
| Hip Circumference (cm) | 115.42 ± 12.35 | 98.67 ± 9.71 | <0.001 |
| Body Mass Index (Kg/m2) | 31.5 ± 6.3 | 23.2 ± 4.6 | <0.001 |
| Body Fat (%) | 40.3 ± 3.9 | 32.9 ± 5.3 | <0.001 |
| Serum Low Density Lipoprotein (mg/dL) | 102.5 ± 30.9 | 91.0 ± 24.7 | 0.006 |
| Serum Total Cholesterol (mg/dL) | 165.7 ± 37.9 | 155.6 ± 31.9 | 0.063 |
| Glycated Haemoglobin (%) | 6.3 ± 1.1 | 5.5 ± 0.8 | <0.001 |
| Haemoglobin (g/dL) | 12.00 ± 1.6 | 11.8 ± 1.5 | 0.482 |
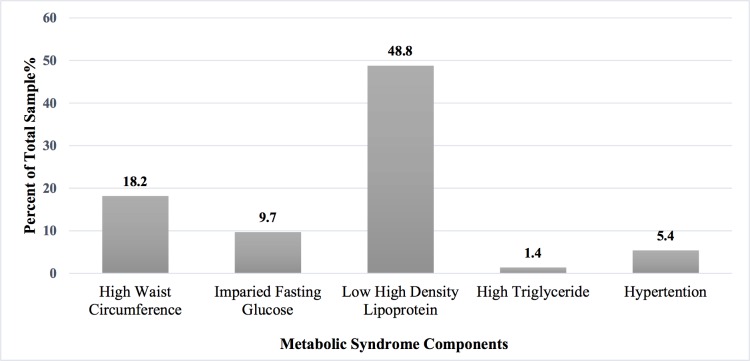
No MetS defining components were found in 242 (43.6%) participants. At least one MetS component was found in 213 participants (38.4%); two MetS components were present in 62 participants (11.2%); three MetS components were found in 27 participants (4.9%); four components of MetS were present in 10 participants (1.8%); and all five MetS components were found in only one participant (0.2%). The most frequent component of MetS was reduced HDL-C levels (48.8%), followed by central obesity (18.2%) and impaired fasting glucose (9.7%) (Fig 2).
Fig 2. Prevalence of metabolic syndrome components among UAEU young female adults 17–25 years (N = 555), Al Ain, UAE.
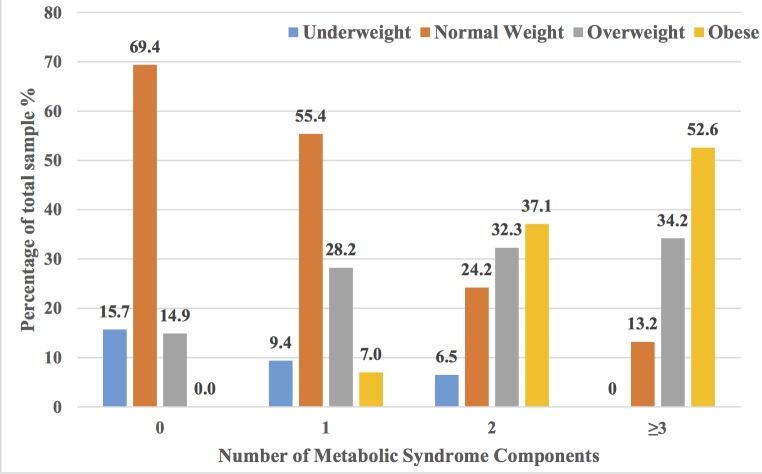
A Chi-square test of association between MetS and BMI categories among young female adults showed a statistically significant association (P < 0.001), and was particularly high among obese participants (34.5%) compared to 10.1% overweight, and 1.7% normal-weight. None of the five MetS components were observed in 69% of the normal-weight participants whereas all obese participants had at least one MetS component. Obese participants were more likely to have three or more MetS components (52.6%) than overweight (34.2%) and normal-weight (13.2%) participants. Furthermore, none of the underweight participants had three or more MetS components (Fig 3).
Fig 3. Percentage of participants per number of metabolic syndrome components and BMI category among young female adults aged 17–25 years (n = 555), Al Ain, UAE.
Table 2 shows the univariable and multivariable logistic regression results for the odds of MetS by potential risk factors. In the univariable analyses, older participants (23–25 years) were three times more likely to have MetS (odds ratio [OR]: 2.96; 95% CI: 1.03 to 8.52) than younger participants (17–19 years) but this effect was not significant in the multivariable analyses (odds ratio [OR]: 1.14; 95% CI: 0.30 to 4.30). Participants who reported having a family history of diabetes or hypertension (n = 305) had a 2.8 times elevated risk of MetS (OR: 2.81; 95% CI: 1.31 to 6.06) compared with participants without a family history of diabetes or hypertension in the univariable analyses but not in the adjusted analyses (OR: 1.85; 95% CI: 0.73 to 4.65). Participants who were overweight or obese were, respectively, 6.4 (95% CI; 2.3 to 17.5) and 29 (95% C1; 29.2 to 79.3) times more likely to have MetS than those of normal-weight in the univariable analysis. These findings remained significant even after adjusting for other potential confounders (i.e. OR = 3.8; 95% CI: 1.15–12.52 for overweight; and OR = 11.2; 95% CI: 3.06–40.86 for obese participants). Participants with percentage body fat ≥35% (n = 227) showed a significantly higher risk for the development of MetS in the univariable analyses (OR: 14.27; 95% CI: 4.99 to 40.83; P<0.001), but the difference was not significant after adjusting for other factors (OR = 3.12; 95% CI: 0.91–10.68). A WHR of more than 0.8 was significantly associated with at least three times increased risk of MetS (P<0.001) in the adjusted analyses when compared with those with a WHR <0.8 (aOR = 3.04; 95% CI: 1.10–8.44). Elevated HbA1c (≥6.5%) showed a high significant association with the presence of MetS (OR: 14.15; 95% CI: 4.78 to 41.86; P<0.001) in univariable analyses and remained significant in the adjusted analyses (adjusted OR [aOR]: 22.49, 95% CI: 6.37 to 79.42; P<0.001). Total cholesterol ≥200 mg/dL and LDL ≥130 mg/dL conferred a greater likelihood for MetS: OR: 3.34 (95% CI: 1.48 to 7.49) and OR: 1.86 (95% CI: 0.96 to 3.63), respectively, in the univariable analyses but not in the adjusted analyses.
Table 2. Risk factors for MetS among young female adults aged 17–25 years (n = 555), Al Ain, UAE.
| Characteristics | Sample | n (%) | With MetS | |||
|---|---|---|---|---|---|---|
| Crude Odds Ratio (95%CI) | P-value | Adjusted Odds Ratio (95%CI) | P-value | |||
| Age (Year) | ||||||
| 17–19 | 194 | 8 (4.1) | Reference | Reference | ||
| 20–22 | 299 | 23 (7.7) | 1.94 (0.85, 4.42) | 0.116 | 1.89 (0.72–4.94) | 0.19 |
| 23–25 | 62 | 7 (11.3) | 2.96 (1.03, 8.52) | 0.044 | 1.14 (0.30–4.30) | 0.85 |
| Family history of diabetes or hypertension (%) | ||||||
| No | 250 | 9 (3.6) | Reference | Reference | ||
| Yes | 305 | 29 (9.5) | 2.81 (1.31, 6.06) | 0.008 | 1.85 (0.73–4.65) | 0.19 |
| Body Mass Index (Kg/m2) | ||||||
| Underweight (<18.5) | 62 | 0 (0.0) | 0.44 (0.02, 8.03) | 0.58 | 0.85 (0.04–17.26) | 0.92 |
| Normal-weight (18.5–<25) | 306 | 5 (1.6) | Reference | Reference | ||
| Overweight (25–29.9) | 129 | 13 (10.1) | 6.35 (2.30, 17.51) | <0.001 | 3.80 (1.15–12.52) | 0.028 |
| Obese (≥30.0) | 58 | 20 (34.5) | 29.19 (10.75, 79.28) | <0.001 | 11.19 (3.06–40.86) | <0.001 |
| Body Fat (%) | ||||||
| <35% | 328 | 4 (1.2) | Reference | Reference | ||
| ≥35% | 227 | 34 (14.9) | 14.27 (4.99, 40.83) | <0.001 | 3.12 (0.91–10.68) | 0.07 |
| Waist-Hip Ratio | ||||||
| <0.8 | 509 | 28 (5.5) | Reference | Reference | ||
| ≥0.8 | 46 | 10 (21.7) | 4.77 (2.15, 10.59) | <0.001 | 3.04 (1.10–8.44) | 0.033 |
| Anaemia | ||||||
| No | 271 | 22 (8.1) | Reference | Reference | ||
| Yes | 284 | 16 (5.6) | 0.67 (0.35, 1.32) | 0.249 | 1.04 (0.46–2.35) | 0.92 |
| Total Cholesterol (mg/dL) | ||||||
| < 200 | 502 | 29 (5.8) | Reference | Reference | ||
| ≥200 | 53 | 9 (16.9) | 3.34 (1.48, 7.49) | 0.004 | 1.71 (0.53–5.55) | 0.37 |
| Low Density Lipoprotein (mg/dL) | ||||||
| <130 | 380 | 21 (5.5) | Reference | Reference | ||
| ≥ 130 | 173 | 17 (9.8) | 1.86 (0.96, 3.63) | 0.067 | 0.92 (0.37–2.32) | 0.86 |
| Glycated Haemoglobin (%) | ||||||
| < 5.6 | 374 | 6 (1.6) | Reference | Reference | ||
| 5.6–6.4 | 133 | 23 (17.3) | 12.82 (5.09, 32.29) | <0.001 | 8.92 (3.39–23.48) | <0.001 |
| ≥ 6.5 | 48 | 9 (18.8) | 14.15 (4.78, 41.86) | <0.001 | 22.49 (6.37–79.42) | <0.001 |
A subgroup analysis was conducted for the study population excluding all females with HbA1c > = 6.5 resulting in a total sample size of 507 participants. Results of the subgroup analysis remain largely unchanged based on the magnitude of the effect sizes, direction of significance and overall conclusions. However, in the multivariate analysis, only waist hip-ratio was no longer significant in the subgroup analysis (OR = 2.12; 95% CI: 0.65–6.87; P = 0.211)
Discussion
In the United Arab Emirates (UAE) rapid socioeconomic growth has resulted in profound lifestyle changes including sedentary behaviours, westernized diets and increased energy intake [5]. The prevalence of MetS among Emirati females has been reported to be higher than that for Emirati males in the adult population (32.9% among men, 45.9% among women) [3]. This research highlights the importance of investigating MetS among young female adults, to facilitate understanding of the prevalence and risk factors of MetS. There is paucity of data on the prevalence of MetS among Emirati females aged 17–25 years. The current study reveals a MetS prevalence of 6.8% among young female Emirati adults aged 17–25 years, and 34.5% among young obese female Emirati adults.
The results of the current study are in line with findings among college students (18–26 years) in Saudi Arabia [21], where the overall MetS prevalence was 7.8%, and 26.4% in obese students. In Kuwait, the prevalence of MetS was even higher among female adolescents (10–19 years) at 9.1% and 14.8% according to ATP III and IDF criteria, respectively [22]. These numbers are certainly close to those described in the UAE, which is not surprising, considering the relatively similar rapid increase in obesity and diabetes rates throughout the Gulf region [23, 24]. These trends are likely to be a result of the sedentary and westernised lifestyle [25–28], and could also be partially explained by the “thrifty genes” hypothesis [29], which suggests that the genotype of mankind existed as hunter-gatherers can efficiently store food in the adipose tissue during periods of food abundance, to compensate for periods of food shortage.
Worldwide, the prevalence of MetS among young female adults in the USA (18–21 years) was 4.7% [30], in Brazilian college students it was 1.7% [31], in Chinese female adolescents (14–16 years) it was 2.5% [32], in Spanish female adolescents (10–15 years) it was 3.85% [33], in Tunisian female adolescents (10–19 years) it was 2.4% [34], and 11.7% among Indian female adolescents (10–19 years) [35]. Clearly, the prevalence of MetS could differ between countries depending on the MetS defining criteria used, study method and target population. We have used the IDF and AHA/NHLBI joint statement, as it was an international attempt to harmonise the definition of MetS; central obesity is not an obligatory component of this definition and it is ethnic specific.
The most frequent component of MetS in this study was reduced HDL-C levels, which was also reported in female Emirati adolescents [4], and female Kuwaiti adolescents [22]. Reduced HDL-C accompanied by elevated triglyceride levels indicates dyslipidaemia, which is highly prevalent among the UAE population [36]. Insufficient physical activity and poor dietary habits are associated with low HDL-C levels [37–39]. Elmagd et. al. [40] reported low physical activity (defined as less than 150 minutes/week) in 60% of Emirati college students. The consumption of high caloric diet was also reported in 33.5% of female Emirati adolescents [5]. These findings could explain the high prevalence of low-HDL-C levels observed in our study. Regular checks and screening in this age group could be helpful in identifying participants at an increased risk of developing MetS.
The current study found strong correlations between BMI, body fat, HbA1c and the prevalence of MetS in Emirati female students. The relationship between overweight and obesity and MetS has been supported by many other studies [41–43]. The association between HbA1c and the prevalence of MetS has not been previously reported; nevertheless, insulin resistance is a major underlying mechanism accountable for the prevalence of the MetS [44]. Interestingly, the prevalence of diabetes (8.5%) was also high in the study population. Future studies need to explore this finding more closely.
The strengths of this study include a trained researcher who obtained all measurements in the study and each measurement was repeated three times and the average used in the analyses. Anthropometric measures and blood withdrawal were conducted during one 50-minute morning session after assurance of a 12-hour overnight fast. Furthermore, to the best of our knowledge, no other studies exploring MetS prevalence in college students have been conducted in the UAE. UAEU is the main university in the UAE and it enrols students from all seven emirates. However, restricting the study to college students makes it not representative of all the Emirati females in this age group. Moreover, the cross-sectional design is another limitation of this study, as causal inference cannot be drawn. Participants were voluntarily enrolled in the study, which could have caused selection bias (overweight and obese individuals might avoid anthropometric measurements). In addition, studying female students only does not allow for examination of gender differences or generalisability of results to all young adults. Therefore, future prospective studies are needed to confirm the prevalence of MetS and its relation to overweight and obesity in Emirati young adults. Additionally, it was challenging to clearly define the “young adult” age group. Some studies reported the MetS prevalence in adolescents and included ages 12–18 years [4] or 10–19 years old [22]. Other studies defined young adults as 18–24 years [30], 17–37 years [45] or college students aged 17–25 years [46]. Having one international definition for the “young adult” age group would be helpful for future data comparisons.
Conclusions
In summary, we have shown that the prevalence of MetS is high among UAEU female young adults aged 17–25 years (6.8%). Identification and possible intervention programmes maybe useful for this age group in order to improve their future health. In addition, reduced HDL-C levels followed by central obesity were the most frequent components of MetS. BMI, body fat percentage and HbA1c were significantly associated with MetS.
Acknowledgments
This study was supported by a research start-up grant from the United Arab Emirates University (CFA-31F038). The authors wish to thank Ms. Rahla Daneshi, Mr. Noura Alneyadi, Ms. Sara Alyousefi, Ms. Abeer Alwahedi, Ms. Moza Alkaabi, Ms. Haneen Ateya and Mr. Asma Numan for their help in recruiting study participants. We thank all UAEU female students who participated in this study.
Data Availability
The United Arab Emirates University ethical restrictions prohibit the authors from making the dataset publicly available. However, the data can be provided upon request to all interested researchers by contacting the lead author Ayesha Salem Al Dhaheri, email: Ayesha_aldhaheri@uaeu.ac.ae.
Funding Statement
This study was supported by a research start-up grant from the United Arab Emirates University (CFA-31F038) and was given to ASA. The funding agent had no role with the study design, data collection and analysis, decision to publish, or preparation of the manuscript. http://www.uaeu.ac.ae/en/
References
- 1.WHO. Global status report on noncommunicable diseases 2014 Geneva: World Health Organization; 2014. 298 p. [Google Scholar]
- 2.Alberti K, Zimmet P, Shaw J. Metabolic syndrome—a new world‐wide definition. A consensus statement from the international diabetes federation. Diabetic Medicine. 2006;23(5):469–80. [DOI] [PubMed] [Google Scholar]
- 3.Malik M, Razig SA. The prevalence of the metabolic syndrome among the multiethnic population of the United Arab Emirates: a report of a national survey. Metabolic syndrome and related disorders. 2008;6(3):177–86. Epub 2008/08/14. 10.1089/met.2008.0006 . [DOI] [PubMed] [Google Scholar]
- 4.Mehairi AE, Khouri AA, Naqbi MM, Muhairi SJ, Maskari FA, Nagelkerke N, et al. Metabolic syndrome among Emirati adolescents: a school-based study. PLoS One. 2013;8(2):e56159 10.1371/journal.pone.0056159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng SW, Zaghloul S, Ali H, Harrison G, Yeatts K, El Sadig M, et al. Nutrition transition in the United Arab Emirates. European journal of clinical nutrition. 2011;65(12):1328–37. 10.1038/ejcn.2011.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries PG. Stratified random sampling Sampling Theory for Forest Inventory: Springer; 1986. p. 31–55. [Google Scholar]
- 7.Gibson RS. Principles of Nutritional Assessment. Oxford University Press; 2005. p. 233–402. [Google Scholar]
- 8.Subhedar R, Subhedar V, Dave P, Mishra P, Kaur A. Bioelectrical impedance technology for evaluating human body composition parameters:“an advanced diagnostic technology for body composition and physical fitness analysis”. Int J Physiother Res. 2014;2(6):824–30. [Google Scholar]
- 9.WHO. Obesity: preventing and managing the global epidemic Geneva: World Health Organization, 2000. [PubMed] [Google Scholar]
- 10.Van der Kooy K, Seidell JC. Techniques for the measurement of visceral fat: a practical guide. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 1993;17(4):187–96. [PubMed] [Google Scholar]
- 11.Lohman TG. Skinfolds and body density and their relation to body fatness: a review. Human Biology. 1981:181–225. [PubMed] [Google Scholar]
- 12.Durnin J, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. British Journal of Nutrition. 1974;32(01):77–97. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Physical Status: The Use and Interpretation of Anthropometry. Geneva: World Health Organization, 1995. Contract No.: 854. [PubMed] [Google Scholar]
- 14.WHO. Waist circumference and waist-hip ratio: Report of a WHO expert consultation, Geneva, 8–11 December 2008: World Health Organization; 2011. [Google Scholar]
- 15.WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity Vitamin and Mineral Nutrition Information System (WHO/NMH/NHD/MNM/11.1). Geneva: World Health Organization; 2011. [Google Scholar]
- 16.El Assaad MA, Topouchian JA, Darne BM, Asmar RG. Validation of the Omron HEM-907 device for blood pressure measurement. American Journal of Hypertension. 2002;15(S3):87A–A. [DOI] [PubMed] [Google Scholar]
- 17.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142–61. [DOI] [PubMed] [Google Scholar]
- 18.Dimagno, E. P., CORLE, D., O'BRIEN, J. F., MASNYK, I. J., GO, V. L., & AAMODT, R. (1989, October). Effect of long-term freezer storage, thawing, and refreezing on selected constituents of serum. In Mayo Clinic Proceedings (Vol. 64, No. 10, pp. 1226–1234). Elsevier. [DOI] [PubMed]
- 19.Bowling JL, Katayev A. An Evaluation of the Roche Cobas c 111. Lab Medicine. 2010;41(7):398–402. [Google Scholar]
- 20.Alberti K, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the Metabolic Syndrome A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 21.Abolfotouh MA, Al-Alwan IA, Al-Rowaily MA. Prevalence of metabolic abnormalities and association with obesity among Saudi college students. International journal of hypertension. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Isa A, Akanji AO, Thalib L. Prevalence of the metabolic syndrome among female Kuwaiti adolescents using two different criteria. British journal of nutrition. 2010;103(01):77–81. [DOI] [PubMed] [Google Scholar]
- 23.ALNohair S. Obesity in gulf countries. International journal of health sciences. 2014;8(1):79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alharbi NS, Almutari R, Jones S, Al-Daghri N, Khunti K, de Lusignan S. Trends in the prevalence of type 2 diabetes mellitus and obesity in the Arabian Gulf States: Systematic review and meta-analysis. Diabetes research and clinical practice. 2014;106(2):e30–e3. 10.1016/j.diabres.2014.08.019 [DOI] [PubMed] [Google Scholar]
- 25.Musaiger AO, Al-Roomi K, Bader Z. Social, dietary and lifestyle factors associated with obesity among Bahraini adolescents. Appetite. 2014;73:197–204. 10.1016/j.appet.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 26.Selmi S, Daghash G, Mathis S, Nahas K, Wilbur K. A program for obese youth at-risk for diabetes in Qatar. Avicenna. 2015;1:1–13. [Google Scholar]
- 27.Musaiger AO, Al-Kandari FI, Al-Mannai M, Al-Faraj AM, Bouriki FA, Shehab FS, et al. Perceived barriers to weight maintenance among university students in Kuwait: the role of gender and obesity. Environmental health and preventive medicine. 2014;19(3):207–14. 10.1007/s12199-013-0377-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Kaabi J, Al-Maskari F, Afandi B, Parkar H, Nagelkerke N. Physical activity and reported barriers to activity among type 2 diabetic patients in the United Arab Emirates. The review of diabetic studies: RDS. 2009;6(4):271 10.1900/RDS.2009.6.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eapen V, Mabrouk A, Yousef S. Metabolic syndrome among the young obese in the United Arab Emirates. Journal of tropical pediatrics. 2010;56(5):325–8. Epub 2009/12/25. 10.1093/tropej/fmp128 [DOI] [PubMed] [Google Scholar]
- 30.Fernandes J, Lofgren IE. Prevalence of metabolic syndrome and individual criteria in college students. Journal of American College Health. 2011;59(4):313–21. 10.1080/07448481.2010.508084 [DOI] [PubMed] [Google Scholar]
- 31.F de Freitas RW Jr, M de Araújo MF, P Marinho NB, A de Vasconcelos HC, S Lima AC, R Pereira DC, et al. Prevalence of the metabolic syndrome and its individual components in brazilian college students. Journal of clinical nursing. 2013;22(9–10):1291–8. 10.1111/jocn.12015 [DOI] [PubMed] [Google Scholar]
- 32.Yi‐Qun X, Cheng‐Ye J. Prevalence of the metabolic syndrome in secondary school adolescents in Beijing, China. Acta Paediatrica. 2008;97(3):348–53. 10.1111/j.1651-2227.2008.00665.x [DOI] [PubMed] [Google Scholar]
- 33.González-Jiménez E, Montero-Alonso MA, Schmidt-RioValle J, García-García CJ, Padez C. Metabolic syndrome in Spanish adolescents and its association with birth weight, breastfeeding duration, maternal smoking, and maternal obesity: a cross-sectional study. European journal of nutrition. 2014;54(4):589–97. 10.1007/s00394-014-0740-x [DOI] [PubMed] [Google Scholar]
- 34.Aounallah-Skhiri H, El Ati J, Traissac P, Gartner A, Ben Gharbia H, Hsairi M, et al. Prevalence of metabolic syndrome and associated behavioural factors in Tunisian adolescents. Obesity Facts. 2015;8(Suppl. 1). [Google Scholar]
- 35.Bhalavi V, Deshmukh PR, Goswami K, Garg N. Prevalence and correlates of metabolic syndrome in the adolescents of Rural Wardha. Indian journal of community medicine: official publication of Indian Association of Preventive & Social Medicine. 2015;40(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smitha F, Meenakshi J, Padma R, Multani S. Survey and evaluation of various epidemiological factors in a multiethnic Diabetic Population in Ras Al-Khaimah, UAE. Indian Journal of Pharmacy Practice. 2011;4(1). [Google Scholar]
- 37.Hamer M, Stamatakis E, Steptoe A. Effects of substituting sedentary time with physical activity on metabolic risk. 2014; 46(10): 1946–1950. 10.1249/MSS.0000000000000317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed HM, Blaha MJ, Nasir K, Rivera JJ, Blumenthal RS. Effects of physical activity on cardiovascular disease. The American journal of cardiology. 2012;109(2):288–95. 10.1016/j.amjcard.2011.08.042 [DOI] [PubMed] [Google Scholar]
- 39.Kant A, Whitley M, Graubard B. Away from home meals: associations with biomarkers of chronic disease and dietary intake in American adults, NHANES 2005–2010. International journal of obesity. 2015;39(5):820–7. 10.1038/ijo.2014.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elmagd MA, Mossa AH, Sami MM, El-Marsafawy TS, Al Jadaan O, Eldin MS. The Impact of Physical Activity on the Academic Performance among Medical and Health Sciences Students: A Cross Sectional Study from RAKMHSU-Ras Alkhaimah-UAE. International Journal of Physical Education, Sports and Health 2015; 2(1): 92–95. [Google Scholar]
- 41.Al-Daghri NM. Extremely high prevalence of metabolic syndrome manifestations among Arab youth: a call for early intervention. European journal of clinical investigation. 2010;40(12):1063–6. Epub 2010/07/14. 10.1111/j.1365-2362.2010.02341.x . [DOI] [PubMed] [Google Scholar]
- 42.Al-Daghri NM, Al-Attas OS, Alokail MS, Alkharfy KM, Sabico SL, Chrousos GP. Decreasing prevalence of the full metabolic syndrome but a persistently high prevalence of dyslipidemia among adult Arabs. PLoS One. 2010;5(8):e12159 Epub 2010/08/24. 10.1371/journal.pone.0012159 ; PubMed Central PMCID: PMCPmc2921394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mabry RM, Reeves MM, Eakin EG, Owen N. Gender differences in prevalence of the metabolic syndrome in Gulf Cooperation Council Countries: a systematic review. Diabetic medicine: a journal of the British Diabetic Association. 2010;27(5):593–7. Epub 2010/06/12. 10.1111/j.1464-5491.2010.02998.x . [DOI] [PubMed] [Google Scholar]
- 44.Guo S. Insulin signaling, resistance, and metabolic syndrome: insights from mouse models into disease mechanisms. Journal of Endocrinology. 2014;220(2):T1–T23. 10.1530/JOE-13-0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jain J, Rao T, Desmukh P, Banait S. Prevalence and Correlates of Metabolic Syndrome in Young Population: A Cross Sectional Study. J Diabetes Metab. 2015;6(503):2. [Google Scholar]
- 46.Kim D-i, Kim JY, Lee MK, Lee H-D, Lee J-W, Jeon JY. The relationship between fitness, BMI and risk factors of metabolic syndrome among university students in Korea. The Korean Journal of Obesity. 2012;21(2):99–107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The United Arab Emirates University ethical restrictions prohibit the authors from making the dataset publicly available. However, the data can be provided upon request to all interested researchers by contacting the lead author Ayesha Salem Al Dhaheri, email: Ayesha_aldhaheri@uaeu.ac.ae.