Abstract
Citral is a flavor component that is commonly used in food, beverage and fragrance industries. Cronobacter sakazakii is a food-borne pathogen associated with severe illness and high mortality in neonates and infants. The objective of the present study was to evaluate antimicrobial effect of citral against C. sakazakii strains. The minimum inhibitory concentration (MIC) of citral against C. sakazakii was determined via agar dilution method, then Gompertz models were used to quantitate the effect of citral on microbial growth kinetics. Changes in intracellular pH (pHin), membrane potential, intracellular ATP concentration, and membrane integrity were measured to elucidate the possible antimicrobial mechanism. Cell morphology changes were also examined using a field emission scanning electron microscope. The MICs of citral against C. sakazakii strains ranged from 0.27 to 0.54 mg/mL, and citral resulted in a longer lag phase and lower growth rate of C. sakazakii compared to the control. Citral affected the cell membrane of C. sakazakii, as evidenced by decreased intracellular ATP concentration, reduced pHin, and cell membrane hyperpolarization. Scanning electron microscopy analysis further confirmed that C. sakazakii cell membranes were damaged by citral. These findings suggest that citral exhibits antimicrobial effect against C. sakazakii strains and could be potentially used to control C. sakazakii in foods. However, how it works in food systems where many other components may interfere with its efficacy should be tested in future research before its real application.
Introduction
Cronobacter sakazakii is a foodborne pathogen [1] that has been implicated in some severe forms of neonatal infection including bacteraemia, necrotizing enterocolitis (NEC), and infant meningitis [2]. The infant mortality rate associated with C. sakazakii infections is quite dangerously high, ranging from 50–80% [3]. In addition to those infections in neonates, diseases caused by C. sakazakii infection have been reported for almost every age group [4]. The International Commission for Microbiological Specifications for Foods (2002) ranked C. sakazakii as a “severe hazard for restricted populations, life threatening or substantial chronic sequelae of long duration” [5].
Foodborne diseases have increasingly become a worldwide public health concern. In addition to different food processing for reducing or eliminating spoilage or pathogenic microorganisms in food, synthetic preservatives are extensively used in food industry to increase shelf-life or enhance food safety [6]. But there is currently enthusiasm for search of natural antimicrobials for use in food industry due to many reasons. For instance, imprudent use of antibiotics in husbandry has led to increasing resistance in foodborne pathogens [7]; And consumers prefer to foods with less synthetic preservatives for fear of their potential negative health effects [6]. All of these have stimulate research on natural antimicrobials with an intention to reduce the usage of antibiotic and synthetic preservatives. Plant-derived compounds have been considered to be important alternative, consumer accepted and widely available sources of natural antimicrobials [8].
Citral (C10H16O) is one of the most common flavor compounds found in citrus oils, which has been already widely used in foods and beverages (e.g., soft drinks and desserts) [9]. Citral is a monoterpenoid aldehyde [10] often present in the form of stereoisomers geranial and neral [11] that has been identified in the leaves and fruits of several plant species including myrtle trees, basil, lemon, lime, lemongrass, orange, and bergamot [10,12]. A number of experimental and clinical studies have shown that citral has favorable anti-inflammatory [13] and anti-corrosive [14] effects, and there is increasing evidence that citral acts as a fungicidal and bactericidal agent [15].
Although citral has been reported to be effective against a variety of microbial species, there have been no reports on its antimicrobial activity against C. sakazakii and possible mode of action. To fill this gap, the aim of the present study was to determine antimicrobial effect of citral against C. sakazakii. We also investigated possible antimicrobial mechanisms by measuring changes in intracellular ATP concentrations, membrane potential, intracellular pH (pHin), and membrane integrity, as well as changes in cell membrane microstructures.
Materials and Methods
Reagents
Citral (CAS:5392-40-5) was obtained from Chengdu Must Bio-technology Co., Ltd (Chengdu, Sichuan, China) at a HPLC purity of at least 99.2%. Sample solution was prepared in ultrapure sterilized water and sterilized by filtration immediately before use to minimize any oxidation of the compound. All other chemicals were of analytical grade and used as-received.
Bacterial strains and culture conditions
C. sakazakii strains ATCC 29544 and ATCC 29004 were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Three other C. sakazakii strains (CS 1, CS 2, CS 3) were taken from our laboratory strain collection, which were originally isolated from infant formula and infant rice cereal collected from supermarkets in China. All isolates were used in minimum inhibitory concentration assay and only C. sakazakii ATCC 29544 was used for antimicrobial mechanism analysis. All strains were stored in tryptone soya broth (TSB) with 20% glycerol (v/v) at -80°C. Before each experiment, stock cultures were streaked on tryptone soya agar (TSA) and grew at 37°C for 18 h. Then a loopful of each strain was inoculated into 30 mL TSB and incubated for 18 h at 37°C.
Minimum inhibitory concentration (MIC) determinations
The MIC of citral was determined by agar dilution method as described by the European Committee for Antimicrobial Susceptibility Testing [16] with some modifications. Escherichia coli ATCC 25922 was used as a quality control. While determining the antimicrobial activity of citral, ampicillin was used as a positive reference standard for all test strains. The stock antibiotic solutions were prepared in sterile water, then sterilized through a 0.22 μm Acrodisc filter (Gelman, USA). Then TSA was aseptically transferred into sterile 24-well plates containing either citral or the antibiotic. The content (final volume 500 μL) of each well was gently mixed. The final concentrations of citral were 0, 0.07, 0.135, 0.27, 0.41, 0.54, 0.81, and 1.08 mg/mL, and that of ampicillin was 0.1 mg/mL. After hardening, the TSA was spotted with 2 μL (approximately 104 CFU) of the tested bacterium. The spots were left to dry, then the plates were incubated at 37°C for 24 h. The lowest concentration of citral at which no visible growth of test organisms occurs was defined as the MIC.
Growth curves and kinetic parameters
The growth curves in TSB at 37°C were determined as previously described [17]. C. sakazakii strain ATCC 29544 was grown to an OD600 value of 0.1 in TSB, then 125 μL of the culture was transferred into each well of a 96-well microtiter plate (Nunc, Copenhagen, Denmark). Citral was added to the cultures to obtain final concentrations of 1/16MIC, 1/8MIC, 1/4MIC, 1/2MIC, and MIC, and TSB was used as a negative control. Bacteria were further cultured at 37°C, and cell growth was monitored at 600 nm using a multimode plate reader (Tecan, Infinite™ M200 PRO, Männedorf, Switzerland).
The model used to fit growth curves to the data as-obtained was comprised of a modified Gompertz equation as follows:
where ODt is optical density at 600 nm (OD600) at time t, t is the time (in hours) that had elapsed since incubation, B is the maximum OD600, A is the initial OD600, M is the time (in hours) of the inflexion point in the exponential phase of model function, μ is the relative growth rate at time M, λis the lag time (i.e., the time until the lag period ended,) and μmax is the maximum growth rate achieved (ΔOD600 per hour.) The model was evaluated for goodness of fit according to the coefficient of determination R2.
Intracellular ATP concentrations
The method described by Sanchez et al. [18] was followed (with slight modification) to determine intracellular ATP concentration. Briefly, the overnight culture of C. sakazakii strain ATCC 29544 was centrifuged for 5 min at 5000 × g and the supernatant was removed, the cell pellets were washed three times with 0.1 mol/L of phosphate buffered saline (PBS, pH 7.0), then the cells were collected by centrifugation under identical conditions. A cell suspension (OD600 = 0.5) made with 50 mL of PBS and 2 mL of the cell suspension was placed into a 2.5 mL Eppendorf tube for citral treatment. Citral was added to each tube to achieve final concentrations of 0 (control), MIC, and 2MIC, respectively. Then the samples were maintained at 37°C for 30 min. To extract the ATP, cells were lysed on ice by ultrasound, and centrifuged at 5000 × g for 5 min. The top layer was retrieved and stored on ice to prevent ATP loss until measurement. Intracellular ATP was measured with an ATP assay kit (Beyotime Bioengineering Institute, Shanghai, China). After adding 125 μL of ATP assay mix to 125 μL of supernatant in white, opaque, 96-well microtiter plates, the supernatant ATP concentrations were measured with a microplate reader (Tecan, Infinite™ M200 PRO, Männedorf, Switzerland) and recorded as the intracellular ATP concentration.
Intracellular pHin measurements
Intracellular pH was determined according to Breeuwer et al. with slight modification [19]. To load a fluorescent probe in the sample cells, 250 μL of overnight cultures of C. sakazakii strain ATCC 29544 was transferred into TSB (30 mL) and incubated at 37°C for 8 h (approximately OD = 0.6 at 600 nm). Cells were then harvested by centrifugation (5000 × g, 10 min) and washed twice with 50 mM HEPES buffer (containing 5 mM EDTA, pH 8.0). Then cell pellets were re-suspended in 20 mL of the same buffer. Next, 3.0 μM of the probe, carboxyfluorescein diacetate succinimidyl ester (cFDA-SE; Molecular Probes, Sigma, Louis, USA), was added. Then cells were incubated for 20 min at 37°C, washed once in 50 mM potassium phosphate buffer with 10 mM MgCl2 (pH 7.0), and re-suspended in 10 mL of buffer. To eliminate non-conjugated cFDA-SE, glucose (10 mM, final concentration) was added and the cells were incubated for an additional 30 min at 37°C. Finally, cells were washed twice, re-suspended in 50 mM PBS (pH 7.0), and stored on ice.
An aliquot of cell suspension labeled by fluorescence was dispensed into tubes with citral at three different concentrations (0, MIC, 2MIC), then transferred into black, opaque, 96-well microtiter plates. After treatment for 20 min, fluorescence intensities were measured under two excitation wavelengths, 440 nm and 490 nm, while the monochromator was rapidly alternated between the wavelengths. The emission was collected at 520 nm, where excitation and emission slit widths were 9 and 20 nm, respectively. The pHin of the bacteria was determined according to the ratio of the fluorescence signal at the pH-sensitive wavelength (490 nm) and the fluorescence signal at the pH-insensitive wavelength (440 nm). All measurements were carried out on a microplate reader (Tecan, Infinite™ M200 PRO, Männedorf, Switzerland). During the assay, the system was maintained at 25°C. The fluorescence of the cell-free controls was deducted from the values of the treated samples.
Calibration curves were determined for cFDA-SE loaded cells with buffers of different pH. Buffers consisted of glycine (50 mM), citric acid (50 mM), Na2HPO4•2H2O (50 mM), and KCl (50 mM). pH of buffers were adjusted with either NaOH or HCl to various values (3, 4, 5, 6, 7, 8, 9, and 10). After equilibration of the pHin and pHout by adding valinomycin (10 μM) and nigericin (10 μM), fluorescence intensity was measured at 25°C.
Membrane potentials
The methods described by Sanchez et al. was used with slight modification to examine membrane potentials [18]. Briefly, cells were grown in 30 mL of TSB at 37°C to an optical density of 0.5 at 600 nm, then harvested by centrifugation (5000 × g, 5 min) and washed twice with PBS (pH 7.0). Next, 125 μL of cell suspensions were placed in black, opaque, 96-well microtiter plates for 30 min at 37°C, then 1 μM of the membrane potential-sensitive fluorescent probe bis-(1, 3-dibutylbarbituric acid) trimethine oxonol (DiBAC4 (3); Molecular Probes, Sigma, Louis, USA) was added and incubated for 30 min at 37°C, followed by addition of citral at three concentrations (0, MIC, 2MIC). Fluorescence was then measured on a fluorescence microplate reader (Tecan, Infinite™ M200 PRO, Männedorf, Switzerland) at excitation and emission wavelengths 492 and 515 nm, respectively. The excitation and emission slit widths were 3 and 5 nm, respectively. Background fluorescence resulting from the medium was determined and the results corrected as necessary.
Bacterial membrane integrity
Cell membrane integrity was determined according to the method described by Alakomi et al. with slight modification [20], using the LIVE/DEAD® BacLight™ Bacterial Viability Kit (Molecular Probes, Eugene, OR). The overnight culture of C. sakazakii strain ATCC 29544 was harvested by centrifugation (10,000 × g, 15 min), then the supernatant was removed and pellets re-suspended in 2 mL of 0.85% NaCl. To acquire viable and non-viable cells, 1 mL of the suspension was added to each of two 50 mL centrifuge tubes containing either 20 mL of 0.85% NaCl (live bacteria) or 20 mL of 70% isopropyl alcohol (dead bacteria). After incubating both samples at room temperature for 1 h (while mixing every 15 min), both samples were pelleted by centrifugation twice at 10,000 × g for 10 minutes, then optical density was adjusted at 600 nm to 0.5 and five viable cells’ proportions (0, 10%, 50%, 90%, and 100%) were mixed to obtain standard samples. A 2X working stain solution was prepared by mixing equal volumes of SYTO 9 and propidium iodide (PI) and adding the mixture into 2 mL of filter-sterilized dH2O.
Cells treated with citral (0, MIC, 2MIC) at 37°C for 15 min were quickly centrifuged (11,000 × g, 1 min), then 100 μL of samples were pipetted in three respective parallel samples into black, opaque, 96-well microtiter plates. Aliquots of 100 μL of the 2X staining solution were then added to each well and mixed completely. The plate was then incubated at 25°C in the dark for 15 min before measurement of the fluorescence of bacterial suspensions with a fluorescence microplate reader (Tecan, Infinite™ M200 PRO, Männedorf, Switzerland). The excitation/emission maxima for the dyes were 485/542 nm for SYTO 9 and 485/610 nm for PI. Cell suspension without citral treatment served as the control.
Field emission scanning electron microscopy (FESEM)
The FESEM assay was carried out accordingly to a previously published method with slight modification [21]. Cells (OD600 = 0.5) were treated with citral at 0, MIC, and 2MIC, then after being incubated at 37°C for 2 h, the cells were harvested by centrifugation for 10 min at 5000 × g, washed twice with PBS (pH 7.0), and re-suspended in water containing 2.5% glutaraldeyde then kept at 4°C for 12 h to fix the cells. After centrifugation, the cells were dehydrated in water-alcohol solutions with different concentrations of alcohol (30%, 50%, 70%, 80%, 90%, 100%) for 10 min each. The samples were finally fixed on FESEM supports, sputter-coated with gold under vacuum conditions, and examined under a scanning electron microscope (S-4800, Hitachi, Tokyo, Japan).
Statistical analysis
All experiments were performed in triplicate. Statistical analyses were performed in SPSS software (Version 19.0; SPSS, Inc., Chicago, IL). The data are presented as the mean ± SD (n = 3). Differences between means were tested by Student’s t-test. Differences were defined as significant at P ≤ 0.05.
Results
MIC of citral against C. sakazakii strains
Citral showed inhibitory effects against all five C. sakazakii strains tested (Table 1). The MICs of citral against ATCC 29544, ATCC 29004 and CS 1 were 0.54 mg/mL, and the MICs against isolates CS 2 and CS 3 were 0.27 mg/mL. And only C. sakazakii ATCC 29544 was selected for further study.
Table 1. Minimum inhibitory concentrations of citral against different strains of C. sakazakii.
| Strain | Origin | MIC (mg/mL) |
|---|---|---|
| ATCC 29544 | Child's throat | 0.54 |
| ATCC 29004 | Infant formula | 0.54 |
| CS1 | Infant formula | 0.54 |
| CS2 | Infant rice cereal | 0.27 |
| CS3 | Infant rice cereal | 0.27 |
Effect of citral on C. sakazakii growth
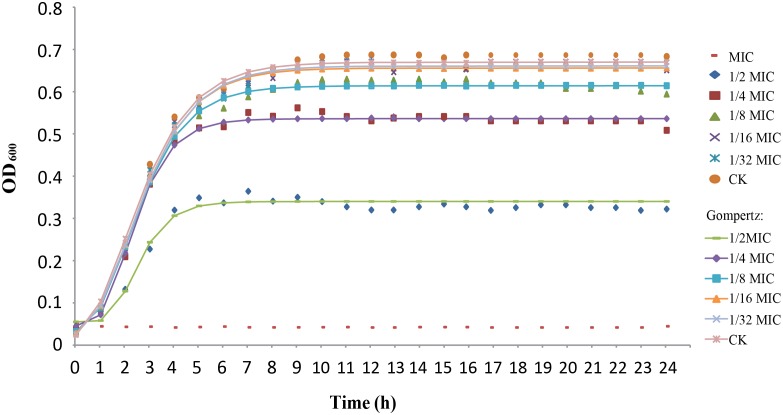
C. sakazakii growth in TSB was fitted to the modified Gompertz equation (Fig 1), where high R2 values (> 0.996) indicate good fitness of the model. By comparison of different growth curves, it was demonstrated that higher concentrations of citral resulted in longer lag phase and lower growth rate compared to lower citral concentrations (Table 2).
Fig 1. Growth curves of C. sakazakii ATCC 29544 cultured in TSB with various concentrations of citral.
The lines represent the fit of the experimental data to the modified Gompertz model. Bars represent the standard deviation (n = 3).
Table 2. Kinetic parameters of C. sakazakii cells grown in TSB with different concentration of citral.
| Concentration of citral | λ±SE | μmax±SE | ODmax |
|---|---|---|---|
| 1/2 MIC | 1.424±0.006A | 0.125±0.001A | 0.340±0.001A |
| 1/4 MIC | 0.814±0.002B | 0.147±0.002B | 0.536±0.002B |
| 1/8 MIC | 0.745±0.003C | 0.164±0.003C | 0.614±0.001C |
| 1/16 MIC | 0.660±0.002D | 0.163±0.003C | 0.656±0.001D |
| 1/32 MIC | 0.631±0.002E | 0.161±0.003C | 0.661±0.001E |
| 0 (CK) | 0.498±0.001F | 0.162±0.001C | 0.670±0.001F |
λ, lag phase (in hours); μmax, maximum growth rate (in OD per hour); ODmax, maximum optical density (determined at 600 nm).
Mean values in the same column followed by different letters are statistically different (P ≤ 0.05) (n = 6).
Effect of citral on intracellular ATP concentrations
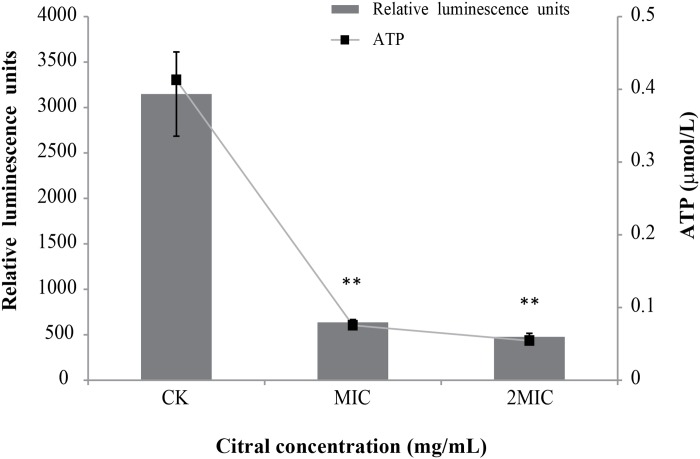
There was a good linearity between relative luminescence units and ATP concentration (y = 7445.5x + 74.167; R2 = 0.999). The level of intracellular ATP of C. sakazakii decreased significantly (P ≤ 0.01) as citral concentration increased (Fig 2). The original ATP concentration of C. sakazakii ATCC 29544 was 0.41 μmol/L. Addition of citral at MIC and 2MIC caused ATP concentrations reduced to 0.075 and 0.054 μmol/L respectively.
Fig 2. Effects of citral on intracellular ATP production by C. sakazakii ATCC 29544.
Values represent the means of triplicate measurements. Bars represent the standard deviation (n = 3). **P ≤ 0.01.
Effect of citral on pHin
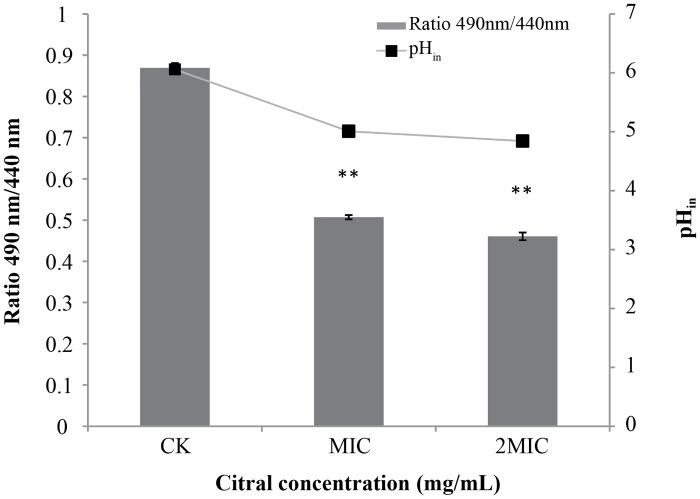
A clear change in intracellular pH was found after citral addition (Fig 3). The pHin of C. sakazakii ATCC 29544 was 6.06 ± 0.03. The addition of citral at MIC caused a significant (P ≤ 0.01) decrease in pHin of C. sakazakii, from 6.06 ± 0.03 to 5.01 ± 0.02. The addition of citral at 2MIC further decreased (P ≤ 0.01 compared to control) the pHin to 4.84 ± 0.03.
Fig 3. Effects of citral on the intracellular pH of C. sakazakii ATCC 29544.
Values represent the means of triplicate measurements. Bars represent the standard deviation (n = 3). **P ≤ 0.01
Effect of citral on membrane potential
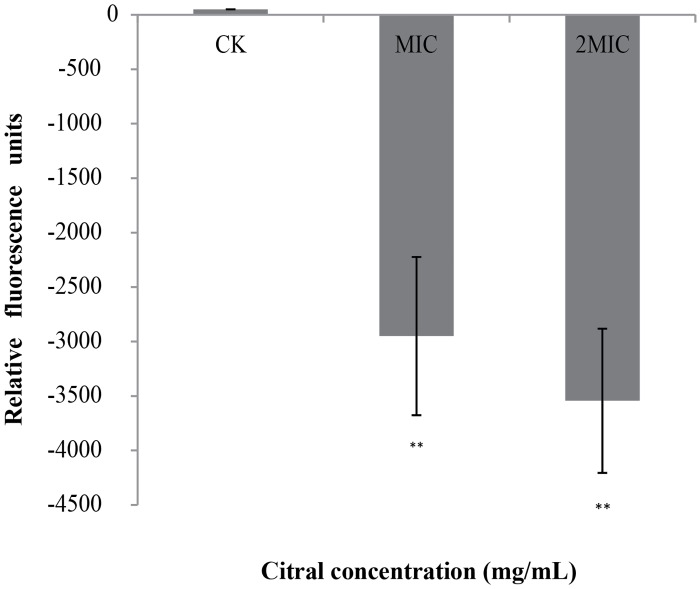
Cells treated with citral showed hyperpolarized cell membranes, as evidenced by decreases (i.e., negative values) in fluorescence (Fig 4). We observed more decreases in fluorescence as citral concentration increased from MIC to 2MIC.
Fig 4. Effects of citral on the membrane potentials of C. sakazakii ATCC 29544.
Negative values indicate hyperpolarization. Values represent the means of triplicate measurements. Bars represent the standard deviation (n = 3). **P ≤ 0.01.
Fluorimetric detection of cell membrane injury
There was a good linearity between green fluorescent intensity and the percentage of viable bacteria (y = 26564x + 14473; R2 = 0.9931). Both tested concentrations of citral caused a significant reduction in viable cell fluorescence. Citral at MIC caused a 56% reduction in cell fluorescence, and citral at 2MIC caused a 85% reduction (Table 3).
Table 3. Reduction of viable cell fluorescence of C. sakazakii ATCC 29544 treated with citral at various concentrations.
| Concentration of citral | Green fluorescence units | Percent viable cell fluorescence | Percent reduction of viable cell fluorescence |
|---|---|---|---|
| 0 (CK) | 40356 | 97% | 3% |
| MIC | 26299 | 44% | 56% |
| 2MIC | 13381 | 15% | 85% |
FESEM observations
Cell morphology changes were examined by FESEM. Untreated C. sakazakii cells are shown in Fig 5A, and there were significant morphological differences between treated cells (Fig 5B and 5C) and the control. Untreated C. sakazakii cells exhibited a structure typical of gram-negative bacilli, with an undulating appearance, while the outer membrane of cells exposed to citral at MIC possessed a more wrinkled surface (Fig 5B). Those cells treated with citral at 2MIC showed substantial surface collapse of obvious cell wall damage due to disruption of cell wall permeability. The number of damaged cells and degree of damage increased as citral concentration increased.
Fig 5. Scanning electron micrographs of C. sakazakii ATCC 29544 untreated (A), treated with citral at MIC for 2 h (B), and treated with citral at 2MIC for 2 h (C).
Discussion
Natural antimicrobials have gained more and more attention because of their effectiveness as well as growing demand for preservative-free food products [22]. Plant-derived essential oils including citral have been traditionally used to preserve foods and enhance food flavor [23]. Citral is currently a natural flavoring approved by the U.S. Food and Drug Administration (FDA) that is generally recognized as safe (GRAS 182.10). Citral has been reported to exhibit antimicrobial activity against pathogenic and food-spoilage bacteria such as E. coli O157:H7, Salmonella Typhimurium, Listeria monocytogenes, and Staphylococcus aureus [24,25]. However, its activity against C. sakazakii and how they exert their antibacterial activity have not yet been completely understood [17]. In this study, we investigated the effect of citral against C. sakazakii and determine citral’s mode of action by measuring changes in intracellular ATP concentration, membrane potential, intracellular pH (pHin), membrane integrity, and cell morphology.
Citral was shown be effective against five C. sakazakii strains either from clinical or food source, which may suggest its general effectiveness against C. sakazakii strains. Due to the limited number of strains tested in this study, more strains from clinical or environmental sources should be tested in the future study. Several reports have determined the antimicrobial effect of plant-derived compounds against C. sakazakii strains. The MICs of carvacrol and thymol were 0.19 mg/mL, and the MIC of cinnamic acid was higher than 0.8 mg/mL [26]. And it was previously shown that the MICs of lipoic acid against C. sakazakii strains ranged from 2.5 to 5.0 mg/mL [27]. In addition, tea polyphenols at 5 mg/mL could eliminate approximately 7.0 log CFU/mL of C. sakazakii within 1 h [28]. Comparing with most of these plant-derived compounds, citral exhibited a stronger antimicrobial activity against C. sakazakii.
ATP, one of the most important small molecules in living organisms, plays a crucial role in many cellular functions that are necessary for growth, replication, and survival. Intracellular ATP is necessary for storing and supplying metabolic energy, as well as for enzymatic reactions and signaling functions [29]. Citral significantly reduced the intracellular ATP of C. sakazakii (Fig 2). In a similar study, chlorogenic acid was shown to decrease the level of intracellular ATP in Staphylococcus aureus cells [21]. The reduction of intracellular ATP of C. sakazakii may be attributable to an increased rate of ATP hydrolysis inside the cells [18], or to increased membrane permeability which can cause intracellular ATP leakage through defective cell membranes [30].
Resting membrane potential is one of the most important parameters of living cells [31], as it relates closely to cell antibiotic uptake and bactericidal action [32]. We investigated the effect of citral on C. sakazakii membrane potential by applying DiBAC4 (3), which is an anionic fluorescent membrane potential dye that indicates changes in membrane polarization [33]. Upon cell depolarization, the dye enters the cells and fluorescence emitted by the dye is thus enhanced by bonding activation [34]. We found that C. sakazakii displayed rapid cell membrane hyperpolarization after exposure to citral. Sanchez et al. reported that basil, white sagebrush, and sweet acacia extracts caused a hyperpolarization of bacterial cellular membranes [18]. Studies have suggested that hyperpolarization occurs first due to change in pH, and second due to homeostasis of the cell membrane when K+ diffuses externally through K+ channels to balance the conductivity of superficial charges [31].
Many cellular processes are dependent on pHin, including DNA transcription, protein synthesis, and enzyme activities [35]. The cFDA-SE technique for measuring the pHin of bacteria is based on identifying the intracellular conjugation of the cFSE succinimidyl group via the aliphatic amines of intracellular proteins, followed by the elimination of the free probe after a short incubation in the presence of glucose [36]. We found that citral induced changes in pHin of C. sakazakii strains. Turgis et al. similarly demonstrated that the addition of mustard essential oil significantly lowers pHin from 6.23 to 5.20 in Escherichia coli O157:H7 and from 6.59 to 5.44 in Salmonella typhi [37]. And reduced pHin is reported be indicative of membrane damage [18].
Cell membrane structures are examined by FESEM and we found that citral caused severe morphological alterations in the cell membrane and cell wall. Previously such morphological alterations have been observed for various kinds of microbial pathogens. Di Pasqua et al. used SEM and found that eugenol is able to disrupt the E. coli O157:H7 membrane [38], allowing the leakage of intracellular constituents, while other compounds (carvacrol, cinnamaldehyde, limonene, and thymol) cause just structural alteration of the outer membrane. Rhayour et al. found that the effects of clove essential oils on membrane morphology vary among different strains [39]. In E. coli cells, holes are observed in the cell envelope, while in Bacillus subtilis cells, only cells with deformed shape is observed. In this study, C. sakazakii treated with citral exhibited large surface collapse without cell disintegration. This suggest that citral may bind to the cell surface and then penetrate to the target sites and interact with cell target molecules [30].
In summary, citral has antimicrobial activity against C. sakazakii and it exerts its effect by inducing changes in ATP concentration, cell membrane hyperpolarization, and reduction in cytoplasmic pH. These findings indicate the potential application of citral to control C. sakazakii in foods. More research on the interference of food components with citral and citral’s influence on organoleptic properties of food are among those issues that need to be resolved before its application in food systems, either alone or combined with other preservation technologies.
Acknowledgments
We thank Xiaoli Peng, Baowei Yang and Guoyun Zhang for technical assistance.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported in part by New Century Excellent Talent Support Plan (NCET-13-0488), the Twelve-five Science and Technology Support Program (No.2015BAD16B08), National Natural Science Foundation of China (31301498), International Collaboration Partner Plan (A213021203) in Northwest A&F University and Special Fund for Sino-US Joint Research Center for Food Safety (A200021501).
References
- 1.Iversen C, Waddington M, On SL, Forsythe S. Identification and phylogeny of Enterobacter sakazakii relative to Enterobacter and Citrobacter Species. J Clin Microbiol. 2004;42: 5368–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muytjens HL, Kollee LA. Enterobacter sakazakii meningitis in neonates: causative role of formula? Pediatr Infect Dis J. 1990;9: 372–373. [DOI] [PubMed] [Google Scholar]
- 3.Healy B, Cooney S, O'Brien S, Iversen C, Whyte P, Nally J, et al. Cronobacter (Enterobacter sakazakii): an opportunistic foodborne pathogen. Foodborne Pathog Dis. 2010;7: 339–350. 10.1089/fpd.2009.0379 [DOI] [PubMed] [Google Scholar]
- 4.Hayes M, Barrett E, Ross RP, Fitzgerald GF, Hill C, Stanton C. Evaluation of an antimicrobial ingredient prepared from a Lactobacillus acidophilus casein fermentate against Enterobacter sakazakii. J Food Prot. 2009;72: 340–346. [DOI] [PubMed] [Google Scholar]
- 5.Iversen C, Forsythe S. Risk profile of Enterobacter sakazakii, an emergent pathogen associated with infant milk formula. Trends Food Sci Tech. 2003;14: 443–454. [Google Scholar]
- 6.Gyawali R, Ibrahim SA. Natural products as antimicrobial agents. Food Control. 2014;46: 412–429. [Google Scholar]
- 7.Dikici A. Antimicrobial resistance of emerging foodborne pathogens: status quo and global trends. Crit Rev Microbiol. 2013;39: 57–69. 10.3109/1040841X.2012.691458 [DOI] [PubMed] [Google Scholar]
- 8.Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Control. 2010;21: 1199–1218. [Google Scholar]
- 9.Choi SJ, Decker EA, Henson L, Popplewell LM, McClements DJ. Inhibition of citral degradation in model beverage emulsions using micelles and reverse micelles. Food Chem. 2010;122: 111–116. [Google Scholar]
- 10.Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol. 2012;3: 12 10.3389/fmicb.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benvenuti F, Gironi F, Lamberti L. Supercritical deterpenation of lemon essential oil, experimental data and simulation of the semicontinuous extraction process. J Supercrit Fluid. 2001;20: 29–44. [Google Scholar]
- 12.Fisher K, Phillips CA. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J Appl Microbiol. 2006;101: 1232–1240. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz MI, Gonzalez-Garcia MP, Ponce-Monter HA, Castaneda-Hernandez G, Aguilar-Robles P. Synergistic effect of the interaction between naproxen and citral on inflammation in rats. Phytomedicine. 2010;18: 74–79. 10.1016/j.phymed.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 14.Korenblum E, de Vasconcelos Goulart FR, de Almeida Rodrigues I, Abreu F, Lins U, Alves PB, et al. Antimicrobial action and anti-corrosion effect against sulfate reducing bacteria by lemongrass (Cymbopogon citratus) essential oil and its major component, the citral. AMB express. 2013;3: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva CD, Guterres SS, Weisheimer V, Schapoval EES. Antifungal activity of the lemongrass oil and citral against Candida spp. Braz J Infect Dis. 2008;12: 63–66. [DOI] [PubMed] [Google Scholar]
- 16.European Committee for Antimicrobial Susceptibility Testing of the European Society of Clinical M, Infectious D. EUCAST Definitive Document E.DEF 3.1, June 2000: Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin Microbiol Infect. 2000;6: 509–515. [DOI] [PubMed] [Google Scholar]
- 17.Silva-Angulo AB, Zanini SF, Rosenthal A, Rodrigo D, Klein G, Martinez A. Comparative study of the effects of citral on the growth and injury of Listeria innocua and Listeria monocytogenes Cells. Plos One. 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez E, Garcia S, Heredia N. Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl Environ Microbiol. 2010;76: 6888–6894. 10.1128/AEM.03052-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breeuwer P, Drocourt J, Rombouts FM, Abee T. A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6-)-carboxyfluorescein succinimidyl ester. Appl Environ Microbiol. 1996;62: 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi C, Jia Z, Chen Y, Yang M, Liu X, Sun Y, et al. Inactivation of Cronobacter sakazakii in reconstituted infant formula by combination of thymoquinone and mild heat. J Appl Microbiol. 2015;119: 1700–1706. 10.1111/jam.12964 [DOI] [PubMed] [Google Scholar]
- 21.Li GH, Wang X, Xu YF, Zhang BG, Xia XD. Antimicrobial effect and mode of action of chlorogenic acid on Staphylococcus aureus. Eur Food Res Technol. 2014;238: 589–596. [Google Scholar]
- 22.Nair MK, Joy J, Venkitanarayanan KS. Inactivation of Enterobacter sakazakii in reconstituted infant formula by monocaprylin. J Food Prot. 2004;67: 2815–2819. [DOI] [PubMed] [Google Scholar]
- 23.Holley RA, Patel D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005;22: 273–292. [Google Scholar]
- 24.Somolinos M, Garcia D, Manas P, Condon S, Pagan R. Effect of environmental factors and cell physiological state on Pulsed Electric Fields resistance and repair capacity of various strains of Escherichia coli. Int J Food Microbiol. 2008;124: 260–267. 10.1016/j.ijfoodmicro.2008.03.021 [DOI] [PubMed] [Google Scholar]
- 25.Fisher K, Phillips C. Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci Tech. 2008;19: 156–164. [Google Scholar]
- 26.Lee SY, Jin HH. Inhibitory activity of natural antimicrobial compounds alone or in combination with nisin against Enterobacter sakazakii. Lett Appl Microbiol. 2008;47: 315–321. [DOI] [PubMed] [Google Scholar]
- 27.Shi C, Sun Y, Zhang XR, Zheng ZW, Yang MC, Ben H, et al. Antimicrobial effect of lipoic acid against Cronobacter sakazakii. Food Control. 2016;59: 352–358. [Google Scholar]
- 28.Li R, Fei P, Man CX, Lou BB, Niu JT, Feng J, et al. Tea polyphenols inactivate Cronobacter sakazakii isolated from powdered infant formula. J Dairy Sci. 2015;99: 1019–1028. 10.3168/jds.2015-10039 [DOI] [PubMed] [Google Scholar]
- 29.Mempin R, Tran H, Chen CN, Gong H, Ho KK, Lu SW. Release of extracellular ATP by bacteria during growth. Bmc Microbiol. 2013;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bajpai VK, Sharma A, Baek KH. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control. 2013;32: 582–590. [Google Scholar]
- 31.Bot C, Prodan C. Probing the membrane potential of living cells by dielectric spectroscopy. Eur Biophys J Biophy. 2009;38: 1049–1059. [DOI] [PubMed] [Google Scholar]
- 32.Mates SM, Eisenberg ES, Mandel LJ, Patel L, Kaback HR, Miller MH. Membrane potential and gentamicin uptake in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1982;79: 6693–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki H, Wang ZY, Yamakoshi M, Kobayashi M, Nozawa T. Probing the transmembrane potential of bacterial cells by voltage-sensitive dyes. Anal Sci. 2003;19: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 34.Whiteaker KL, Gopalakrishnan SM, Groebe D, Shieh CC, Warrior U, Burns DJ, et al. Validation of FLIPR membrane potential dye for high throughput screening of potassium channel modulators. J Biomol Screen. 2001;6: 305–312. [DOI] [PubMed] [Google Scholar]
- 35.Breeuwer P, Drocourt J, Rombouts FM, Abee T. A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6-)-carboxyfluorescein succinimidyl ester. Appl Environ Microbiol. 1996;62: 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holyoak CD, Stratford M, McMullin Z, Cole MB, Crimmins K, Brown AJ, et al. Activity of the plasma membrane H(+)-ATPase and optimal glycolytic flux are required for rapid adaptation and growth of Saccharomyces cerevisiae in the presence of the weak-acid preservative sorbic acid. Appl Environ Microbiol. 1996;62: 3158–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turgis M, Han J, Caillet S, Lacroix M. Antimicrobial activity of mustard essential oil against Escherichia coli O157:H7 and Salmonella typhi. Food Control. 2009;20: 1073–1079. [Google Scholar]
- 38.Di Pasqua R, Betts G, Hoskins N, Edwards M, Ercolini D, Mauriello G. Membrane toxicity of antimicrobial compounds from essential oils. J Agr Food Chem. 2007;55: 4863–4870. [DOI] [PubMed] [Google Scholar]
- 39.Rhayour K, Bouchikhi T, Tantaoui-Elaraki A, Sendide K, Remmal A. The mechanism of bactericidal action of oregano and clove essential oils and of their phenolic major components on Escherichia coli and Bacillus subtilis. J Essent Oil Res. 2003;15: 356–362. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.