Introduction
Aspergillus spp. infect around 11,000,000 patients, resulting in about 600,000 deaths per year, but these numbers are on the rise due to the emergence of antifungal-resistant strains and a lack of sensitive diagnostic tests [1].
It is increasingly acknowledged that soluble pattern recognition receptors (PRRs), such as the complement component C1q, the collectins (MBL, SP, and CL-11), PTX3, and the ficolins (ficolin-1, 2, 3 and A), are important within anti-Aspergillus immunity [2]. Moreover, studies have highlighted that they may be used as a possible alternative to current antifungal drugs or used in combination to increase efficacy [3].
Binding of pathogen-associated molecular patterns (PAMPs) on the pathogen surface by soluble PRRs often results in opsonisation. This enhances interactions with membrane-associated PRRs on phagocytes, such as the important β-glucan receptor Dectin-1, Toll-like receptors (TLRs), complement receptors (CR1), and Fc receptors; ultimately augmenting phagocytosis, which is essential in controlling the infection [2].
Alternatively, opsonins can promote fungal damage directly or further promote opsonisation by C3b deposition via activation of the conserved complement system [4]. There are three main arms of the complement system, which are the classical, alternative, and lectin pathways. C1q primarily activates the classical antibody-mediated pathway, whereas MBL, CL-11, and the ficolins are known to activate the lectin complement pathway via activation of the mannose-binding lectin-associated serine proteases (MASPs). However, SP-A and SP-D are not involved in complement activation, and the role of CL-11 in Aspergillus immunity is yet to be explored. Furthermore, PTX3 can interact with complement activators and inhibitory components to modulate all three pathways [5]. The role of each of these PRRs in anti-Aspergillus immunity will be discussed further.
PTX3 Plays a Non-redundant Role in Aspergillus fumigatus Immunity
PTX3 is a globally expressed acute-phase protein that is synthesised locally at inflammatory sites by several cell types, particularly mononuclear phagocytes, dendritic cells (DCs), epithelial, and endothelial cells. Furthermore, PTX3 is stored within neutrophil granules containing lactoferrin and once secreted, associates with neutrophil extracellular traps (NETs), acting as a focal point for antimicrobial effector molecules [6].
PTX3 primarily functions as an opsonin in A. fumigatus immune responses, whereby it binds to galactomannan residues of dormant spores, facilitating recognition and phagocytosis [7]. PTX3 can also interact with numerous important opsonins, complement proteins, and membrane-associated PRRs to enhance antifungal immunity, including MBL, ficolin-2, C1q, Factor H, and Dectin-1, and more recently has been shown to exert its antifungal effects through TLR4/MD-2 mediated signalling [8,9]. Moreover, PTX3 can modulate all three complement pathways [5]. Current evidence indicates that PTX3 activates complement on the Aspergillus conidial surface and interacts with FcγRIIa, which mediates activation of the complement receptor CR3, leading to recognition and internalization of conidia [10].
There have been several human studies reporting single nucleotide polymorphisms (SNPs) in the PTX3 gene that are associated with susceptibility to A. fumigatus infections in haematopoietic stem cell and whole organ transplant patients [11,12].
In support of these findings, studies utilising PTX3 knockout mice have indicated a non-redundant role within immunity to A. fumigatus pulmonary infection [7]. Furthermore, PTX3 has been demonstrated to be protective against invasive aspergillosis (IA) in mice receiving allogeneic bone marrow transplants, in chronic granulomatous disease mice (p47phox-/-), and corticosteroid-treated rats [13].
Mannose-Binding Lectin (MBL) Is Essential for Defence Against A. fumigatus
MBL is one of the best characterised lectins involved in innate antifungal immunity. It is found predominantly within the serum, but, during inflammation, loss of vascular integrity can result in leakage of MBL into alveola where it can interact with A. fumigatus (Fig 1) [14]. Binding here is primarily achieved via selective and calcium-dependent binding to the carbohydrate moieties D-mannose, L-fucose, and N-acetylglucosamine (GlcNAc) in the A. fumigatus cell wall.
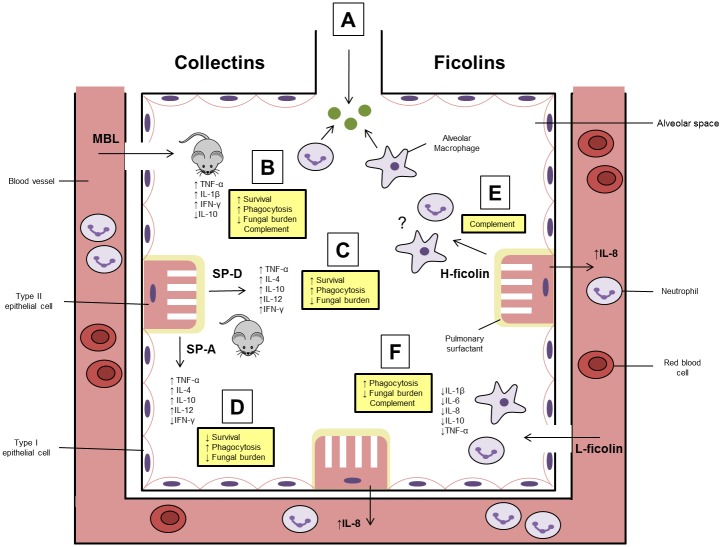
Fig 1. The role of serum lectins within the alveolar space.
(A) Upon entry into the alveolar space, resident neutrophils and macrophages are essential in the recognition and effective removal of A. fumigatus. (B) In the event that A. fumigatus evades removal, it can germinate into a filamentous hyphal form, which causes damage to the lung epithelium and vasculature. This leads to the leakage of serum mannose-binding lectin (MBL) into the alveolar space where it interacts with A. fumigatus. It has been demonstrated in vivo that MBL is capable of modulating inflammatory cytokine production, enhancing phagocytosis, fungal killing, and survival. Moreover, MBL activates the lectin complement pathway. (C and D) The surfactant proteins (SP)-A and –D are both secreted directly into the alveolar space by type II epithelial cells. SP-A can predominantly be found within the pulmonary surfactant, whereas most of the SP-D can be found within the bronchoalveolar lavage fluid (BAL). As for MBL, SP-A and SP-D have been demonstrated to modulate cytokines, increase phagocytosis, and reduce fungal burden in vivo. However, SP-A appeared to be detrimental to survival following A. fumigatus infection, and neither are capable of activating complement. (E) H-ficolin is the most abundant ficolin in the serum, but it is also produced directly into the alveolar space by type II epithelial and bronchial cells. H-ficolin is capable of activating the lectin complement pathway on A. fumigatus conidia, and H-ficolin opsonised conidia promote the secretion of IL-8 from type II epithelial cells. However, the interactions of H-ficolin opsonised A. fumigatus with other cells of the immune system (neutrophils and macrophages) or in vivo (H-ficolin is a pseudogene in rodents) is unknown. (F) L-ficolin is found circulating in the serum but can enter the alveolar space following fungal infection. L-ficolin opsonisation has been demonstrated to lead to a reduction in inflammatory cytokine production by neutrophils and macrophages, promote IL-8 production by type II epithelial cells, increase host–fungal interactions, and activate the lectin pathway of complement. However, the in vivo function of this protein is still unknown.
Neth et al. [15] were the first to show demonstrable binding of A. fumigatus by the MBL carbohydrate recognition domain (CRD). It wasn’t until much later that MBL was described to be protective against Aspergillus infection via the activation of the lectin-complement pathway on A. fumigatus conidia.
It has since been well established in humans that natural MBL deficiencies, or MBL deficiencies due to genetic polymorphisms, are significantly correlated with increased susceptibility to acute IA and chronic necrotizing pulmonary aspergillosis (CPA), respectively [16,17].
This importance has been well documented in murine models by Kaur et al., [18] in which they comprehensively demonstrated that MBL-deficiency was linked to significantly reduced phagocytosis, diminished complement activation, impaired cytokine responses, and greater mortality in a murine model of IA.
Furthermore, studies utilising serum obtained from transgenic animals have indicated that only MBL-C, and not MBL-A, can recognise A. fumigatus and is essential for complement activation [19].
Conversely, a more recent study indicated that loss of MBL in a systemic model of aspergillosis resulted in a resistant phenotype and may play a deleterious role [20], suggesting an importance within pulmonary infection rather than disseminated disease.
Surfactant Protein-D Is an Important Initiator of the Fungal Immune Response to A. fumigatus
The roles of SP-A and SP-D in Aspergillus defence have been extensively studied, with SP-D exhibiting particular importance. SP-D is found in alveolar lung lining and primarily binds β-1,6-glucan in the A. fumigatus cell wall. Interestingly, SP-D can also bind A. fumigatus hyphae in a calcineurin-sensitive manner, hinting at an additional role in the later stages of infection [21].
Recognition by SP-D has been observed to augment the immune response to Aspergillus in vitro and in vivo. In particular, SP-D is essential in vivo, whereby it has been observed that administration of SP-D can protect immunosuppressed mice against an otherwise fatal dose of Aspergillus, and SP-D–deficient mice are highly susceptible to IA [22,23]. Conversely, SP-A–deficient mice become more resistant to invasive infection, indicating SP-A may even facilitate pathology [23].
However, it appears that surfactant proteins may play a greater role within allergic bronchopulmonary aspergillosis (ABPA) rather than IA. Human studies have indicated a polymorphism in the collagen region of SP-A (SP-A2) that is correlated with increased risk of ABPA and increased allergic responses, but no SNPs have so far been shown to enhance susceptibility to IA [24].
Ficolins: The Emergence of a Novel Participant in the Host Fungal Response
We and others have recently implicated ficolins within fungal host–microbe interactions. L-ficolin and H-ficolin, in addition to rodent ficolin-A, bind avidly to A. fumigatus via a range of carbohydrate moieties, including GlcNAc, N-aceytlgalactosamine, D-mannose, and L-fucose [19,25–28]. Furthermore, ficolin-A also recognises the resting, swollen, and germinating morphotypes of A. fumigatus, in addition to the less pathogenic species: A. flavus, A. terreus, and A. niger [19].
Following binding to A. fumigatus, both L- and H-ficolin activate the lectin-complement pathway on A. fumigatus conidia, whereas ficolin-A was shown to play a redundant role to MBL-C [19,26,27]. Consequently, opsonisation by L-ficolin, ficolin-A, and H-ficolin has been demonstrated to enhance the phagocytosis of conidia by primary macrophages, neutrophils, and the type II epithelial cell line (A549), but it is only following interaction with the macrophages and neutrophils where significant fungal killing is observed [25,26,29].
Furthermore the inflammatory response elicited by ficolin-opsonised conidia is dependent upon the cell type involved. Following cell challenge with ficolin-opsonised conidia, a MAPK-dependent increase in IL-8 production was observed from epithelial cells, whereas down-regulation of IL-1β, IL-6, IL-8, IL-10, and TNF-α production was observed from macrophages and neutrophils via currently uncharacterized mechanisms [25,26,29]. These observations have raised some interesting questions; however, the implications of ficolins in disease models have yet to be elucidated, and our understanding of the role of ficolins in antifungal immunity are in their infant stages.
Diagnostic and Therapeutic Potential of Soluble PRRs
Antifungal drug resistance and a lack of conclusive diagnostics are two of the major challenges limiting the cure of aspergillosis, and many opsonins demonstrate therapeutic potential.
It has been demonstrated that administration of recombinant MBL is protective in a murine model of invasive A. fumigatus infection and can significantly reduce mortality [18]. The therapeutic potential of SP-D has also been explored in mice, and administration of native and recombinant SP-D is associated with decreased fungal burden in the lungs and increased levels of antifungal IFN-γ in IA [30].
Administration of recombinant PTX3 can ameliorate infection and increase survival in a pulmonary model of A. fumigatus infection in mice [13]. An interesting caveat of PTX3 is its ability to have an additive effect on the efficacy of commonly used antifungals such as ambisome and voriconazole [3]. Importantly, in combination with PTX3, the antifungal dose could be lowered whilst maintaining efficacy, which could lead to the reduced risk of drug-related side effects. This would be especially beneficial for severely immunocompromised patients [3].
Unlike MBL, PTX3, and SP-D, the therapeutic potential of ficolins is yet to be explored. Unsurprisingly, H-ficolin BAL concentrations are increased during A. fumigatus infection, but as L-ficolin is not produced directly in the lung, it was hypothesised that it enters the alveolar space following vascular damage and may be useful as a diagnostic marker in combination with other fungal specific markers such as galactomannan [25,26]. As ficolins have been observed to dampen pro-inflammatory cytokine production by phagocytic cells, it could be hypothesised that they may have the potential to be exploited therapeutically [25,29].
To date, there has been no indication that soluble PRRs can be exploited for their diagnostic potential, albeit the presence of PRRs such as L-ficolin and MBL in the lung during inflammation and infection highlights the necessity to investigate soluble PRRs as potential diagnostic tools. Moreover, further larger-scale clinical trials are needed to assess the full diagnostic potential of ficolins and other PRRs in combination with current fungal and host biomarkers in order to evaluate their role in diagnostics and possible impact on patient outcomes.
Acknowledgments
We would like to thank the laboratories of Dr. Darren Sexton, Dr. Darius Armstrong-James, Prof. Russell Wallis, Prof. Wilhelm Schwaeble, the Imperial College Healthcare Biomedical Research Centre, and the Royal Brompton and Harefield Respiratory Biomedical Research Unit for their involvement in our ficolin studies.
Funding Statement
This work was supported by a Faculty of Health, University of East Anglia, UK PhD studentship FMH 04.4.66 C4. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schelenz S, Barnes RA, Barton RC, Cleverley JR, Lucas SB, et al. (2015) British Society for Medical Mycology best practice recommendations for the diagnosis of serious fungal diseases. Lancet Infect Dis 15: 461–474. 10.1016/S1473-3099(15)70006-X [DOI] [PubMed] [Google Scholar]
- 2. Margalit A, Kavanagh K (2015) The innate immune response to Aspergillus fumigatus at the alveolar surface. FEMS Microbiol Rev 39: 670–687. 10.1093/femsre/fuv018 [DOI] [PubMed] [Google Scholar]
- 3. Gaziano R, Bozza S, Bellocchio S, Perruccio K, Montagnoli C, et al. (2004) Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob Agents Chemother 48: 4414–4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Speth C, Rambach G, Wurzner R, Lass-Florl C (2008) Complement and fungal pathogens: an update. Mycoses 51: 477–496. 10.1111/j.1439-0507.2008.01597.x [DOI] [PubMed] [Google Scholar]
- 5. Doni A, Garlanda C, Bottazzi B, Meri S, Garred P, et al. (2012) Interactions of the humoral pattern recognition molecule PTX3 with the complement system. Immunobiology 217: 1122–1128. 10.1016/j.imbio.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 6. Jaillon S, Peri G, Delneste Y, Fremaux I, Doni A, et al. (2007) The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med 204: 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, et al. (2002) Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 420: 182–186. [DOI] [PubMed] [Google Scholar]
- 8. Inforzato A, Reading PC, Barbati E, Bottazzi B, Garlanda C, et al. (2012) The "sweet" side of a long pentraxin: how glycosylation affects PTX3 functions in innate immunity and inflammation. Front Immunol 3: 407 10.3389/fimmu.2012.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bozza S, Campo S, Arseni B, Inforzato A, Ragnar L, et al. (2014) PTX3 binds MD-2 and promotes TRIF-dependent immune protection in aspergillosis. J Immunol 193: 2340–2348. 10.4049/jimmunol.1400814 [DOI] [PubMed] [Google Scholar]
- 10. Moalli F, Doni A, Deban L, Zelante T, Zagarella S, et al. (2010) Role of complement and Fc{gamma} receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus. Blood 116: 5170–5180. 10.1182/blood-2009-12-258376 [DOI] [PubMed] [Google Scholar]
- 11. Cunha C, Aversa F, Lacerda JF, Busca A, Kurzai O, et al. (2014) Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation. N Engl J Med 370: 421–432. 10.1056/NEJMoa1211161 [DOI] [PubMed] [Google Scholar]
- 12. Wojtowicz A, Lecompte TD, Bibert S, Manuel O, Rueger S, et al. (2015) PTX3 Polymorphisms and Invasive Mold Infections After Solid Organ Transplant. Clin Infect Dis 61: 619–622. 10.1093/cid/civ386 [DOI] [PubMed] [Google Scholar]
- 13. Salvatori G, Campo S (2012) Current understanding of PTX3 protective activity on Aspergillus fumigatus infection. Med Mycol 50: 225–233. 10.3109/13693786.2011.648215 [DOI] [PubMed] [Google Scholar]
- 14. Summerfield JA (1993) The role of mannose-binding protein in host defence. Biochem Soc Trans 21: 473–477. [DOI] [PubMed] [Google Scholar]
- 15. Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ, et al. (2000) Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun 68: 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lambourne J, Agranoff D, Herbrecht R, Troke PF, Buchbinder A, et al. (2009) Association of mannose-binding lectin deficiency with acute invasive aspergillosis in immunocompromised patients. Clin Infect Dis 49: 1486–1491. 10.1086/644619 [DOI] [PubMed] [Google Scholar]
- 17. Crosdale DJ, Poulton KV, Ollier WE, Thomson W, Denning DW (2001) Mannose-binding lectin gene polymorphisms as a susceptibility factor for chronic necrotizing pulmonary aspergillosis. J Infect Dis 184: 653–656. [DOI] [PubMed] [Google Scholar]
- 18. Kaur S, Gupta VK, Thiel S, Sarma PU, Madan T (2007) Protective role of mannan-binding lectin in a murine model of invasive pulmonary aspergillosis. Clin Exp Immunol 148: 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bidula S, Kenawy H, Ali Y, Sexton D, Schwaeble W, et al. (2013) Role of ficolin-A and lectin complement pathway in the innate defense against pathogenic Aspergillus species. Infect Immun 81: 1730–1740. 10.1128/IAI.00032-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clemons KV, Martinez M, Tong AJ, Stevens DA (2010) Resistance of MBL gene-knockout mice to experimental systemic aspergillosis. Immunol Lett 128: 105–107. 10.1016/j.imlet.2009.12.021 [DOI] [PubMed] [Google Scholar]
- 21. Geunes-Boyer S, Heitman J, Wright JR, Steinbach WJ (2010) Surfactant protein D binding to Aspergillus fumigatus hyphae is calcineurin-sensitive. Med Mycol 48: 580–588. 10.3109/13693780903401682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Madan T, Kishore U, Singh M, Strong P, Hussain EM, et al. (2001) Protective role of lung surfactant protein D in a murine model of invasive pulmonary aspergillosis. Infect Immun 69: 2728–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Madan T, Reid KB, Clark H, Singh M, Nayak A, et al. (2010) Susceptibility of mice genetically deficient in SP-A or SP-D gene to invasive pulmonary aspergillosis. Mol Immunol 47: 1923–1930. 10.1016/j.molimm.2010.02.027 [DOI] [PubMed] [Google Scholar]
- 24. Vaid M, Kaur S, Sambatakou H, Madan T, Denning DW, et al. (2007) Distinct alleles of mannose-binding lectin (MBL) and surfactant proteins A (SP-A) in patients with chronic cavitary pulmonary aspergillosis and allergic bronchopulmonary aspergillosis. Clin Chem Lab Med 45: 183–186. [DOI] [PubMed] [Google Scholar]
- 25. Bidula S, Sexton DW, Abdolrasouli A, Shah A, Reed A, et al. (2015) The serum opsonin L-ficolin is detected in lungs of human transplant recipients following fungal infections and modulates inflammation and killing of Aspergillus fumigatus. J Infect Dis 212: 234–246. 10.1093/infdis/jiv027 [DOI] [PubMed] [Google Scholar]
- 26. Bidula S, Sexton DW, Yates M, Abdolrasouli A, Shah A, et al. (2015) H-ficolin binds Aspergillus fumigatus leading to activation of the lectin complement pathway and modulation of lung epithelial immune responses. Immunology 146: 281–291. 10.1111/imm.12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma YJ, Doni A, Hummelshøj T, Honore C, Bastone A, et al. (2009) Synergy between ficolin-2 and pentraxin 3 boosts innate immune recognition and complement deposition. J Biol Chem 284: 28263–28275. 10.1074/jbc.M109.009225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hummelshøj T, Ma YJ, Munthe-Fog L, Bjarnsholt T, Moser C, et al. (2012) The interaction pattern of murine serum ficolin-A with microorganisms. PLoS ONE 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bidula S, Sexton DW, Schelenz S (2015) Serum opsonin ficolin-A enhances host-fungal interactions and modulates cytokine expression from human monocyte-derived macrophages and neutrophils following Aspergillus fumigatus challenge. Med Microbiol Immunol. [DOI] [PubMed] [Google Scholar]
- 30. Strong P, Reid KB, Clark H (2002) Intranasal delivery of a truncated recombinant human SP-D is effective at down-regulating allergic hypersensitivity in mice sensitized to allergens of Aspergillus fumigatus. Clin Exp Immunol 130: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]