Abstract
In this study, we describe the synthesis of an upright nanotubular coating with discrete, exposed nanotubes on top of superelastic Nitinol via anodization and characterization of the surface elemental composition and nickel release rates. We demonstrate, for the first time, that this coating could improve re-endothelialization by increasing the cell spreading and migration of primary human aortic endothelial cells on Nitinol. We also show the potential for reducing neointimal hyperplasia by decreasing the proliferation and expression of collagen I and MMP-2 in primary human aortic smooth muscle cells (HASMC). Furthermore, we did not observe the nanotubular surface to induce inflammation through ICAM-1 expression in HASMC as compared to the flat control. This coating could be used to improve Nitinol stents by reducing restenosis rates and, given the extensive use of Nitinol in other implantable devices, act as a generalized coating strategy for other medical devices.
Keywords: Nanotubes, Nitinol, Restenosis, Stents, Human Aortic Endothelial Cells, Human Aortic Smooth Muscle Cells
Graphical abstract

Heart disease places an enormous burden on America and is estimated to cost over $316 billion in the year 2010 alone, the majority of which is spent on coronary heart disease.1 A substantial number of these patients who received percutaneous transluminal coronary angioplasty went on to suffer recurring ischemia. It has been reported that 30% to 60% of angioplasty patients suffer from restenosis within six months of the treatment.2 Bare metal stents (BMS) helped in the prevention of early abrupt closure due to elastic recoil and generally improved patient outcomes, but in-stent restenosis (ISR) rates were still reported to be around 20% to 30%.2–5
ISR after BMS implantation has been mainly attributed to neointimal hyperplasia, which is the result of vascular smooth muscle cells (VSMC) responding to the injury caused during stenting.2–4,6 These cells respond to the implantation by migrating into the vessel lumen, where they proliferate excessively and secrete an abundance of extracellular matrix (ECM) proteins, thereby renarrowing the artery. The innovation of drug-eluting stents (DES) that release drugs that reduce the proliferation and migration of VSMC have successfully reduced ISR rates to below 10%.3 However, the antiproliferative drugs in DES are nonspecific and affect endothelial cells (EC) as well, resulting in delayed healing and an increase in the rates of late stent thrombosis.7–11 Specifically, drugs such as paclitaxel and rapamycin were known to increase the thrombogenic risk by reducing endothelial cell function, proliferation, and migration12,13
Recent studies have shown that an upright nanotubular coating on titania substrates created via an anodization process has the potential to regulate EC and VSMC in a “pro-healing” manner.14,15 When cultured on these nanotubes-coated substrates, VSMC have been shown to decrease in proliferation, motility, and expression of genes related to inflammation, whereas EC have been shown to increase in proliferation, motility, and secretion of prostaglandin I2, an antithrombogenic and antiproliferative agent for VSMC.14,15 In light of these promising results, we have turned to synthesizing a nanotubular coating for a widely used material in FDA-approved biomedical devices, Nitinol.16 Along with stainless steel and cobalt chromium, Nitinol is one of the three common materials used in stents.5 However, its material properties offer distinct advantages: Nitinol-based stents have a variety of deployment methods, while its superelasticity and kink-resistance also make it highly desirable for peripheral stents.16 Previously, Kim et al. presented a study on the synthesis of small-diameter (38 nm) NiO–TiO2 nanotubes as electrodes for supercapacitors.17 Building on that for this study, we aimed to synthesize a nanotubular coating on Nitinol with a larger nanotube diameter through the variation of the anodization parameters such as voltage, duration, and electrolyte concentration that were investigated by Kim.17 We also aimed to investigate the effects of this coating on primary human aortic endothelial cells (HAEC) and human aortic smooth muscle cells (HASMC).
Synthesis of Nanotubes-Coated Nitinol
Nitinol foils were purchased from two sources (55.85 wt % Ni, light oxide surface, NDC, U.S.A. and 55.75 wt % Ni, superelastic, pickled surface, Alfa Aesar, U.S.A.). These were cut into 1 × 1 cm pieces and cleaned sequentially using an ultrasonicator with dilute micro-90 solution (International Products Corporation), acetone, and ethanol. They were dried in nitrogen and placed in an anodization setup with the Nitinol foil as the working electrode and a platinum foil (Alfa Aesar, 0.1 mm-thick, 1.5 cm by 3 cm, 99.99%) as the counter electrode, 7 cm directly apart in a Teflon container. The optimum anodization conditions required to produce a nanotubular coating were obtained via a series of iterations beginning from two initial anodization conditions (Supporting Information, Figure 1 shows several images from the unsuccessful anodization conditions). The concentration of NH4F in the electrolyte solution, voltage, or duration of anodization were varied after each iteration based on the images examined under Scanning Electron Microscopy (Carl Zeiss Ultra 55 FE-SEM, San Francisco State University) (Supporting Information, Table 2 shows the various conditions employed). Although we were unable to produce a homogeneous, fully exposed nanotubular coating on the Nitinol foil purchased from NDC, we obtained a nanotubular coating on the Nitinol foils purchased from Alfa Aesar under the following conditions: the electrolyte solution consists of 1.48 g of NH4F (Sigma-Aldrich), 490 mL of ethylene glycol (Sigma-Aldrich), and 8.35 mL of Millipore water; the anodization voltage and duration were 85 V and 4 min, respectively.
The nanotubular coating can be observed in the SEM images in Figure 1B and C. The nanotubes formed under such conditions have, on average, an outer diameter of about 110 nm. The substrates that were used for the subsequent experiments were all produced under this condition, whereas the control was the flat Nitinol substrate. We were also able to produce nanotubes of about 90 nm in outer diameter by decreasing the anodization voltage to 70 V, as seen in Figure 1D. In contrast, the control Nitinol substrate appears relatively free of topographical structures (Figure 1A). Besides the increase in nanotube diameter, we also observed that the nanotubes were spaced further apart than those synthesized in Kim’s study.17
Figure 1.
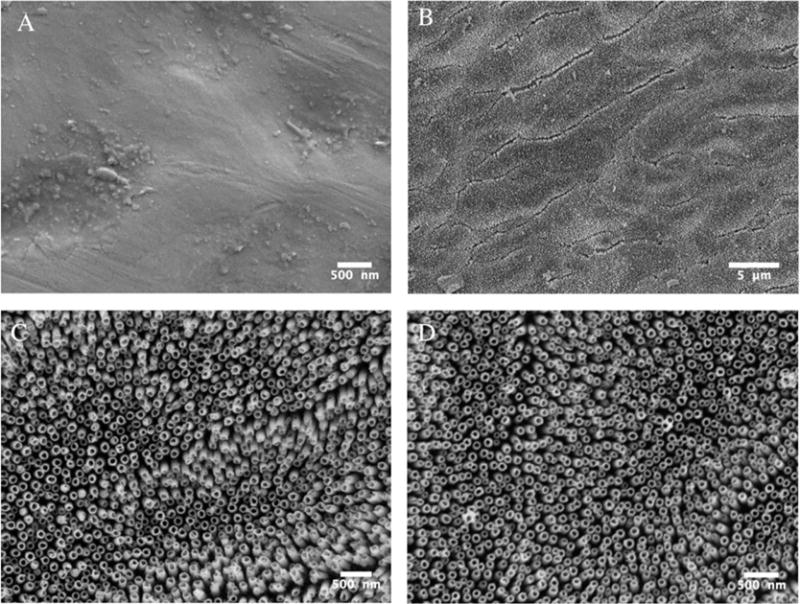
SEM images of the flat control Nitinol (A) and the upright nanotubular coating that was synthesized on top of superelastic Nitinol via an anodization process at 85 V (B and C) and 70 V (D).
Materials Characterization
We further characterized the nanotubular coating under an energy dispersive X-ray spectrometer (EDS). The EDS spectrum and elemental analysis of the region of the interest, as seen in Figure 2A, revealed the composition of the nanotubes to be a combination of NiO and TiO2. Figure 2B showed that the amount of oxide on the control Nitinol was too small to be detected by the EDS, thus corroborating the manufacturer’s claim that the surface had been “pickled”. However, Nitinol undergoes a natural process of passivation once in contact with air and we expect a thin, albeit undetectable through EDS, layer of oxide present on the control Nitinol substrates.
Figure 2.

Energy dispersive X-ray spectroscopy (EDS) data of the nanotubular coated Nitinol (A) and the flat Nitinol control (B). Surface elemental analysis data is presented in the tables and EDS spectra. The nanotubular coating was found to consist of both NiO and TiO2, whereas the control substrates had undetectable levels of a surface oxide layer.
The biocompatibility of Nitinol relies to a large extent on the presence of an oxide layer to reduce the leaching of nickel ions.16 Therefore, we conducted a nickel leaching experiment in which each 1 × 1 cm nanotubes-coated and control Nitinol substrates were submerged in 1 mL of PBS solution in a standard 24-well tissue culture plate and placed in an incubator under standard tissue culture conditions. The 1 mL of PBS samples were retrieved at specific time points and replaced with fresh PBS solution. These PBS samples were then tested using the Nickel Assay Kit (Sigma-Aldrich) following the manufacturer’s instructions (results in Figure 3). The nanotubes-coated Nitinol substrates have an average daily release rate of 37.6 ± 8.2 ng per substrate, whereas the control Nitinol substrates have a mean daily release rate of 8.8 ± 5.0 ng per substrate. There was no statistically significant difference between the amount of nickel released (mean daily or cumulative) between the nanotubular coated and flat control Nitinol. The small amount of nickel released by the control Nitinol substrates proved the presence of an oxide layer formed as discussed above. Although anodization increased the oxide layer on the Nitinol substrates, the process of nanotubes synthesis was, in essence, a generation of defects within the increased oxide layer, and could be a reason for the difference in the nickel release from the nanotubes-coated Nitinol substrates. Furthermore, the EDS surface elemental analysis in Figure 2A showed a similar atomic percentage of nickel and titanium on the nanotubular-coated substrates. Therefore, it was within expectations that there would be some nickel released. It is, however, vital to note that these release levels of nickel were well under the tolerable limit of nickel contamination in vivo. In fact, they were at least 3 orders of magnitude lower than the intravenous nickel contamination limit of 35 μg/day (for a 70 kg man).18
Figure 3.
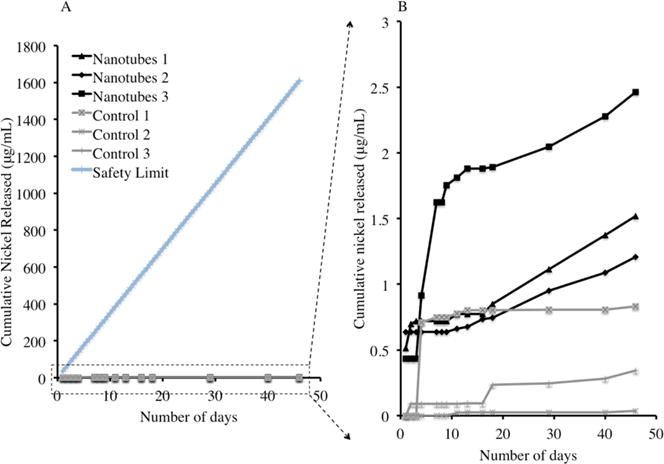
(A) Cumulative nickel released from nanotubular coated Nitinol (Nanotubes 1, 2, and 3) and flat control Nitinol (Control 1, 2, and 3) as compared to the intravenous nickel contamination limit (Safety Limit). (B) The same data enlarged, excluding the intravenous nickel contamination limit.
Cellular Experiments
Re-endothelialization is a key factor in the prevention of restenosis and thrombosis because the process of stent deployment causes denudation of the endothelial layer.13–15,19 We investigated the “pro-healing” potential of our nanotubular Nitinol coating by quantifying the migration, cell spreading, and proliferation of primary HAEC (Lonza, U.S.A.). Cells were maintained and cultured under manufacturer’s instructions and all experiments were conducted with cells between passage number 3 and 7.
We investigated the difference in the migration of HAEC onto the two types of substrates using a similar method detailed by Sprague.20 In this method, as depicted in Figure 4a, the deployment of a stent was simulated by “implantation” of the substrate of interest onto a confluent endothelial cell layer and then quantifying the number of cells that migrated onto the substrate. First, in a 12-well tissue culture plate, we seeded HAEC onto a rat tail collagen (Fisher, U.S.A.) gel with a concentration of 4 mg/mL and cultured them to confluency. Then we carefully pushed the 1 × 1 cm nanotubes-coated Nitinol and control Nitinol substrates into the gels on top of the cells. The nanotubular coating was facing up in the setup. After 4 days of culture, we carefully removed the substrates, transferred them to fresh 24-well tissue culture plates and quantified the number of cells on each substrate using the CyQuant assay (Molecular Probes, U.S.A.) according to the manufacturer’s instructions. As seen in Figure 4b, the number of HAEC on the nanotubular coated Nitinol substrates were significantly greater (paired t test, p < 0.001) than that on the control Nitinol substrates. This increase in migration corresponds to that reported by several studies on nanotubular titania surfaces.15,21,22 Because migration is the key part to wound healing, a modification of the surface of the stent to increase the migration of HAEC onto the stent surface could prove vital in the healing of the endothelium. The recovery of the endothelium would also lead to the reduction in the migration of the HASMC into the intima and the proliferation of these cells,2,14 ultimately improving the overall viability of the vessel.
Figure 4.

Nanotubular coated Nitinol increased the migration of HAEC. The schematic (A) describes the migration assay used, whereas the data (B) shows a significant increase in migration of HAEC from the collagen gel onto the nanotubular coated Nitinol as compared to flat control Nitinol. * = p < 0.001, N = 5.
In our cell spreading and proliferation experiment, 1 × 1 cm nanotubes-coated and control Nitinol substrates were sterilized with ethanol and rinsed twice with sterile PBS in 24-well tissue culture plates (VWR, U.S.A.) under sterile conditions. HAEC were seeded at a cell density of 10 000 cells/well and cultured under manufacturer’s instructions. At days 1, 4, and 7, the Nitinol substrates were rinsed in sterile PBS and transferred into fresh 24-well plates. The cells were fixed with 3.7% paraformaldehyde and blocked using a solution of 2.5% bovine serum albumin, 0.1% Triton-X and PBS. They were then actin-stained using FITC-conjugated Phalloidin and nuclei-stained using DAPI, following manufacturer’s instructions (Millipore, U.S.A.). These samples were then imaged under a Nikon C1si spectral confocal microscope (Figure 5A and B). Using ImageJ, we quantified cell spreading (Figure 5C) on these substrates by measuring the average surface area per cell by the actin staining and normalizing it to the control. The cell number was also determined with ImageJ and the results can be seen in Figure 5D.
Figure 5.
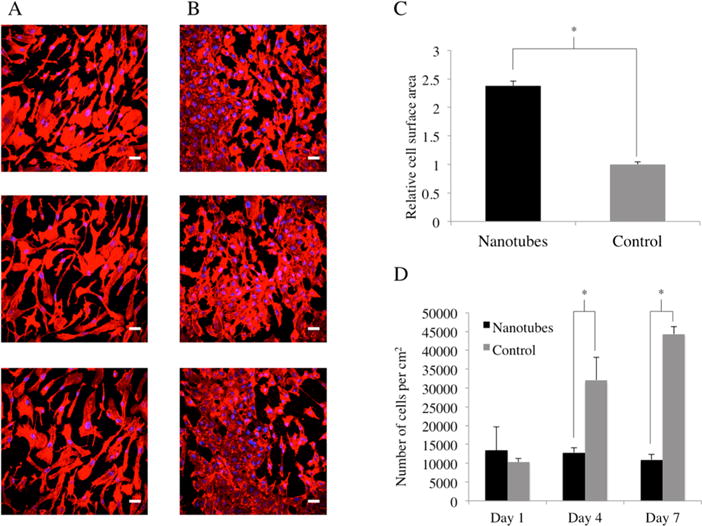
Fluorescence microscopy images of HAEC on nanotubular coated Nitinol (A) and flat control Nitinol (B) after 7 days of culture. FITC-Phalloidin staining of F-actin is shown in red, whereas DAPI staining of cell nuclei is shown in blue. Scale bars are 50 μm. Cell spreading is represented by the average cell surface area (C) normalized to that of the control, while HAEC growth (D) is represented in number of cells per cm2 over a period of 7 days. * = p < 0.01, N = 5.
We observed a morphological difference between the HAEC cultured on the nanotubular coated Nitinol and the flat controls (Figure 5A and 5B). HAEC cultured on the nanotubes-coated Nitinol appeared to be individually larger and more spread out than those cultured on the flat control. They also displayed a more elongated and extended morphology. Here, we have shown that by creating a nanotubular coating, we could increase the cell surface area of HAEC cultured on Nitinol by about 2-fold (Figure 5C). This substantiates previous literature that showed that mechanical factors, chemical factors, or surface topography can be utilized to control cell morphology.14,23,24 As seen in Figure 5D, the number of HAEC on nanotubular coated Nitinol was similar over the course of 7 days in culture, whereas the number of HAEC on the control was greater by days 4 and 7. This showed that proliferation of HAEC was not increased on the nanotubular coating. However, previous studies on TiO2 nanotubes have shown that endothelial cells proliferated significantly faster on nanotubes with smaller diameters (15 nm as compared to 100 nm).25 Therefore, we encourage further studies in the future on optimizing nanotube diameter of our coating for increasing the proliferation of HAEC.
We proceeded to investigate the effects of the nanotubular Nitinol coatings on primary HASMC (Lonza, U.S.A.) between passage number 3 and 7. As mentioned earlier, in the process of restenosis, HASMC migrate through the vessel walls, begin proliferating excessively and release ECM proteins such as collagen,2 resulting in restenosis. We studied the effect of the nanotubular Nitinol coating on HASMC proliferation by the same methods as previously mentioned for HAEC proliferation. The nanotubes-coated Nitinol and control Nitinol were similarly prepared and the HASMC were seeded at a density of 10 000 cells/well in 24-well tissue culture plates. At days 1, 4, and 7, the substrates were rinsed with PBS, transferred to fresh 24-well tissue culture plates, and actin, and nuclei stained with DAPI and FITC-Phalloidin, respectively. The cells were imaged and counted in ImageJ, and the results are shown in Figure 6C.
Figure 6.
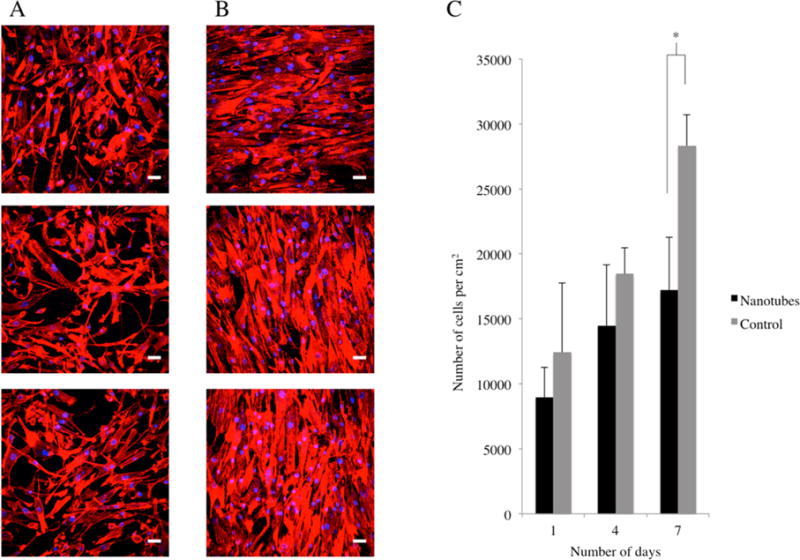
Fluorescence microscopy images of HASMC on nanotubular coated Nitinol (A) and flat control Nitinol (B) after 7 days of culture. FITC-Phalloidin staining of F-actin is shown in red, whereas DAPI staining of cell nuclei is shown in blue. Scale bars are 50 μm. HASMC growth (C) is represented in number of cells per cm2 over a period of 7 days. * = p < 0.01, N = 5.
By day 7, we observed a significant difference (paired t test, p < 0.01) in HASMC numbers between nanotubes-coated Nitinol and control Nitinol substrates. Furthermore, by day 7, the cells on control Nitinol have begun to reach confluency, as seen in the images in Figure 6B. In the confluent cell clusters, the HASMC also begin to exhibit alignment on the control Nitinol surfaces. In contrast, the HASMC on nanotubes-coated Nitinol (Figure 6A) are less confluent and not as aligned. We can anticipate, in a stent scenario, that the HASMC that have migrated to the lumen and come into contact with the nanotubular coating will exhibit less proliferation. Because vascular smooth muscle cell proliferation is observed in neointimal hyperplasia,2,3 this nanotubular coating strategy can be particularly useful in Nitinol stents.
Another major component of restenosis is the release of ECM proteins by the activated HASMC at the lesion site. We investigated the protein expression of collagen I in HASMC using immunofluorescent staining. The HASMC were seeded on 1 × 1 cm nanotubes-coated and control Nitinol substrates I 24-well tissue culture plates at a seeding density of 30 000 cells/well. After 48 h, the cells were fixed and blocked as mentioned previously. They were then stained with mouse anticollagen I primary antibody (Abcam) and Alexa Fluor 488 goat antimouse secondary antibody (Molecular Probes). Images were taken with the Nikon C1si spectral confocal microscope at the same laser intensity and processed with the same color balance (Figure 7A and B). Using quantitative PCR, we investigated the effects of the nanotubular Nitinol coating on the relative mRNA expression levels of the two major ECM proteins—collagen I and collagen III. We also looked at the relative mRNA expression levels of matrix metalloproteinase 2, which has been shown to be related to the migration of HASMC.26 For this experiment, HASMC were seeded on 1 × 1 cm nanotubes-coated and control Nitinol substrates in 24-well tissue culture plates at a seeding density of 30 000 cells/well. After 48 h, the substrates were transferred into fresh 24-well tissue culture plates and 0.5% EDTA was used to life the cells from each substrate. After 6 min of exposure to EDTA, the cell suspension was transferred to an Eppendorf tube and centrifuged before aspirating the solution. mRNA isolation was performed on the cells using the Qiagen RNeasy Mini Kit (Qiagen, U.S.A.) and TRIzol Reagent (Ambion) following manufacturer’s instructions. mRNA concentration and purity were determined using the Nano Drop ND-1000 Spectrophotometer (Thermo Scientific, U.S.A.). iScript cDNA Synthesis Kit (Bio-Rad, U.S.A.) was used to synthesize cDNA. Then, forward and reverse primers and Fast SYBR Green Master Mix (Applied Biosystems, U.S.A.) were used to amplify the cDNA of interest in an Applied Biosystems Viia7 real-time polymerase chain reaction system. Biological triplicates were performed for each substrate and the results are presented as fold changes relative to the control using delta–delta CT values. A housekeeping gene, GAPDH, was used as the reference gene.
Figure 7.
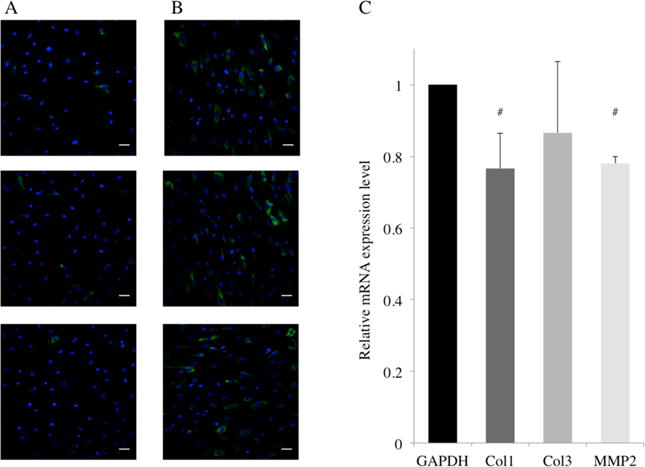
Immunofluorescent staining of Col1A (green) and DAPI staining of nuclei (blue) in HASMC grown on nanotubes-coated Nitinol (A) and the control (B). Relative mRNA expression levels of Collagen 1 (Col1), Collagen 3 (Col3) and Matrix Metalloproteinase 2 (MMP2) genes in HASMC (C) is presented as fold change using delta–delta CT values with GAPDH as the reference gene. # = p < 0.05, N = 5.
In Figure 7A, the immunofluorescent staining of Col1A (green) showed a lower protein expression in HASMC cultured on nanotubes-coated Nitinol as compared to those grown on the control (Figure 7B). The staining in the control group was less extensive and lower in intensity. This implied that fewer cells expressed detectable levels of the protein, and among the cells that did, most of the individual levels of expression were lower. Furthermore, in Figure 7C, relative to GAPDH, the mRNA expression levels of collagen I was significantly downregulated on nanotubes-coated Nitinol as compared to control Nitinol. Collagen expression is an important factor because collagen is a major component of the neointima.2,27 In the scenario of stent deployment, a nanotubular coating could reduce restenosis by directly affecting the formation of the neointima by downregulating the mRNA expression levels of collagen I in HASMC that are in contact with the surface. Furthermore, the downregulation of the mRNA expression of MMP-2 is equally important in this scenario. HASMC are known to produce MMP-2 to degrade the vessel basement membrane in order to migrate into the intima.28 Thus, the downregulation in the mRNA expression levels of MMP-2 in HASMC might be a sign of the reduction in the migration rates of the HASMC on the surface. If the HASMC in contact with the coated stent surfaces secrete less MMP-2, our surface could potentially contribute in the reduction of the migration of other HASMC traveling into the intima.
Finally, we wanted to characterize the functional activation of HASMC on our nanotubes-coated Nitinol substrates. Intercellular adhesion molecule-1 (ICAM-1) has been known to play an important role in the inflammatory response of vascular cells.29–32 In HASMC, ICAM-1 induction in response to tumor necrosis factor-alpha (TNF-alpha) stimulation is a known inflammatory response that can be monitored by flow cytometery.32 We seeded HASMC on nanotubes-coated and control Nitinol substrates for 1 day and identified the endogenous and TNF-alpha induced levels of ICAM-1 expression on the cell-surface using a FACSCalibur II Flow Cytometer operated by FACSQuest Software. To lift the cells prior to sorting, four technical replicates of the nanotubular or control surfaces were rinsed with PBS (calcium- and magnesium-free) with 0.04% EDTA and then incubated with PBS (calcium- and magnesium-free) with 0.04% EDTA at 37 °C until the cells had a round morphology, with a few already detached from the substrate. The PBS (calcium- and magnesium-free) with 0.04% EDTA was then transferred to a collection tube and replaced with 0.2 mL of 0.05% trypsin for 30–60 s at 37 °C. The flask was tapped sharply to dislodge the remaining cells and the cells were collected in medium. Cells were counted and pelleted at 160g for 4 min. Pelleted cells were resuspended in 100ul of DMEM/F12 medium at a concentration of 107/mL and fluorescently tagged for ICAM-1 (BioLegend 353107, Clone HA58) for 30 min on ice, as specified by the manufacturer. Finally, labeled cells were washed twice with PBS to remove any unbound antibodies and resuspended in DMEM/F12.
As shown in Figure 8, both the nanotubular and flat Nitinol surfaces had similar levels of endogenous ICAM-1 on the cell surface (plots labeled “No TNF”). Furthermore, with 2 ng/mL and 10 ng/mL TNF-alpha, we noticed no measurable differences in ICAM-1 expression between HASMC on nanotubular or flat control surfaces. Taken together, these data suggest that the nanotubular Nitinol surfaces do not induce inflammation when compared to the flat control Nitinol surface.
Figure 8.
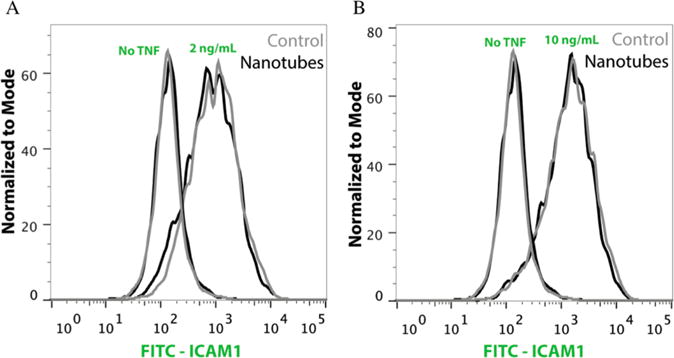
Flow cytometry histogram plots of HASMC stained for ICAM-1 and cultured on nanotubes-coated (Nanotubes) or flat control Nitinol (Control) surfaces. ICAM-1 induction with 2 ng/mL (A) and 10 ng/mL (B) of TNF-alpha is shown as a comparison to the endogenous level of the HASMC cultured on both surfaces (No TNF).
Considering all this, our study has shown that with the appropriate anodization conditions, we could synthesize a nanotubular coating on top of superelastic Nitinol substrates with a nickel release rate that is well within defined safety limits. We have also shown that this coating can contribute to the reduction of restenosis by reducing the proliferation, ECM production, and migration of HASMC. The nanotubes-coated substrates also provided an equally noninflammatory surface, in terms of ICAM-1 activation in HASMC, as compared to flat control substrates. Furthermore, we have demonstrated the “pro-healing” properties of our nanotubular coating via the significant increase in cell spreading and migration of HAEC onto our nanotubes-coated Nitinol substrates, a key factor in the recovery of the endothelium poststenting. There is, however, room for optimization on the nanotube diameter of the coating so as to improve HAEC proliferation. Overall, this study presents an exciting prospect for the improvement of not only Nitinol stents but also other Nitinol biomedical devices through the effects of the nanotubular coating on other cell types.
Supplementary Material
Acknowledgments
Funding for this work was provided by The Alfred E. Mann Institute for Biomedical Engineering. P.L. was supported by the Agency for Science, Technology and Research, Singapore, through the National Science Scholarship. A.C. was supported by the U. S. Department of Defense through the NDSEG Fellowship. We gratefully acknowledge use of the Carl Zeiss Ultra 55 FE-SEM and supporting equipment at SF State. The FE-SEM and supporting facilities were obtained under NSF-MRI award #0821619 and NSF-EAR award #0949176, respectively. Spectral confocal microscopy was conducted at the Nikon Imaging Center, UCSF.
Footnotes
Supporting Information
SEM images of unsuccessful anodization attempts and a list of anodization conditions that was generated from the iterative process. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Notes
The authors declare no competing financial interest.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart Disease and Stroke Statistics—2012 Update: A Report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajagopal V. Coronary Restenosis: A Review of Mechanisms and Management. Am J Med. 2003;115:547–553. doi: 10.1016/s0002-9343(03)00477-7. [DOI] [PubMed] [Google Scholar]
- 3.Lüscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, Virmani R. Drug-Eluting Stent and Coronary Thrombosis: Biological Mechanisms and Clinical Implications. Circulation. 2007;115:1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 4.Khan W, Farah S, Domb AJ. Drug Eluting Stents: Developments and Current Status. J Controlled Release. 2012:703–712. doi: 10.1016/j.jconrel.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Mani G, Feldman MD, Patel D, Agrawal CM. Coronary Stents: A Materials Perspective. Biomaterials. 2007;28:1689–1710. doi: 10.1016/j.biomaterials.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular Mechanisms of Decreased Smooth Muscle Differentiation Marker Expression after Vascular Injury. J Clin Invest. 2000;106:1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of Drug-Eluting Stents in Humans: Delayed Healing and Late Thrombotic Risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular Responses to Drug Eluting Stents: Importance of Delayed Healing. Arterioscler, Thromb, Vasc Biol. 2007;27:1500–1510. doi: 10.1161/ATVBAHA.107.144220. [DOI] [PubMed] [Google Scholar]
- 9.Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Jüni P, Sianos G, Hellige G, et al. Early and Late Coronary Stent Thrombosis of Sirolimus-Eluting and Paclitaxel-Eluting Stents in Routine Clinical Practice: Data from a Large Two-Institutional Cohort Study. Lancet. 2007;369:667–678. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 10.Wenaweser P, Daemen J, Zwahlen M, van Domburg R, Jüni P, Vaina S, Hellige G, Tsuchida K, Morger C, Boersma E, et al. Incidence and Correlates of Drug-Eluting Stent Thrombosis in Routine Clinical Practice. 4-Year Results from a Large 2-Institutional Cohort Study. J Am Coll Cardiol. 2008;52:1134–1140. doi: 10.1016/j.jacc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 11.McFadden EP, Stabile E, Regar E, Cheneau E, Ong ATL, Kinnaird T, Suddath WO, Weissman NJ, Torguson R, Kent KM, et al. Late Thrombosis in Drug-Eluting Coronary Stents after Discontinuation of Antiplatelet Therapy. Lancet. 2004;364:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- 12.Steffel J, Latini Ra, Akhmedov A, Zimmermann D, Zimmerling P, Lüscher TF, Tanner FC. Rapamycin, but Not FK-506, Increases Endothelial Tissue Factor Expression: Implications for Drug-Eluting Stent Design. Circulation. 2005;112:2002–2011. doi: 10.1161/CIRCULATIONAHA.105.569129. [DOI] [PubMed] [Google Scholar]
- 13.Steffel J, Tanner FC. Biological Effects of Drug-Eluting Stents in the Coronary Circulation. Herz. 2007;32:268–273. doi: 10.1007/s00059-007-3000-5. [DOI] [PubMed] [Google Scholar]
- 14.Peng L, Eltgroth ML, LaTempa TJ, Grimes Ca, Desai Ta. The Effect of TiO2 Nanotubes on Endothelial Function and Smooth Muscle Proliferation. Biomaterials. 2009;30:1268–1272. doi: 10.1016/j.biomaterials.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Peng L, Barczak AJ, Barbeau Ra, Xiao Y, LaTempa TJ, Grimes Ca, Desai Ta. Whole Genome Expression Analysis Reveals Differential Effects of TiO2 Nanotubes on Vascular Cells. Nano Lett. 2010;10:143–148. doi: 10.1021/nl903043z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duerig T, Pelton A, Sto D. An Overview of Nitinol Medical Applications. Mater Sci Eng. 1999;275:149–160. [Google Scholar]
- 17.Kim J-H, Zhu K, Yan Y, Perkins CL, Frank AJ. Microstructure and Pseudocapacitive Properties of Electrodes Constructed of Oriented NiO-TiO2 Nanotube Arrays. Nano Lett. 2010;10:4099–4104. doi: 10.1021/nl102203s. [DOI] [PubMed] [Google Scholar]
- 18.Sunderman FJ. Potential Toxicity from Nickel Contamination of Intravenous Fluids. Ann Clin Lab Sci. 1983;13:1–4. [PubMed] [Google Scholar]
- 19.Chan JM, Rhee J-W, Drum CL, Bronson RT, Golomb G, Langer R, Farokhzad OC. In Vivo Prevention of Arterial Restenosis with Paclitaxel-Encapsulated Targeted Lipid-Polymeric Nanoparticles. Proc Natl Acad Sci U S A. 2011;108:19347–19352. doi: 10.1073/pnas.1115945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sprague EA, Luo J, Palmaz JC. Human Aortic Endothelial Cell Migration onto Stent Surfaces under Static and Flow Conditions. J Vasc Interventional Radiol. 1997;8:83–92. doi: 10.1016/s1051-0443(97)70521-9. [DOI] [PubMed] [Google Scholar]
- 21.Brammer K, Oh S, Gallagher J. Enhanced Cellular Mobility Guided by TiO2 Nanotube Surfaces. Nano Lett. 2008;8:786–793. doi: 10.1021/nl072572o. [DOI] [PubMed] [Google Scholar]
- 22.Park J, Bauer S, von der Mark K, Schmuki P. Nanosize and Vitality: TiO2 Nanotube Diameter Directs Cell Fate. Nano Lett. 2007;7:1686–1691. doi: 10.1021/nl070678d. [DOI] [PubMed] [Google Scholar]
- 23.Ma Z, He W, Yong T, Ramakrishna S. Grafting of Gelatin on Electrospun Poly(caprolactone) Nanofibers to Improve Endothelial Cell Spreading and Proliferation and to Control Cell Orientation. Tissue Eng. 2005;11:1149–1158. doi: 10.1089/ten.2005.11.1149. [DOI] [PubMed] [Google Scholar]
- 24.Levesque MJ, Nerem RM. The Elongation and Orientation of Cultured Endothelial Cells in Response to Shear Stress. J Biomech Eng. 1985;107:341–347. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- 25.Park J, Bauer S, Schmuki P, von der Mark K. Narrow Window in Nanoscale Dependent Activation of Endothelial Cell Growth and Differentiation on TiO2 Nanotube Surfaces. Nano Lett. 2009;9:3157–3164. doi: 10.1021/nl9013502. [DOI] [PubMed] [Google Scholar]
- 26.Pauly RR, Passaniti a, Bilato C, Monticone R, Cheng L, Papadopoulos N, Gluzband Ya, Smith L, Weinstein C, Lakatta EG. Migration of Cultured Vascular Smooth Muscle Cells through a Basement Membrane Barrier Requires Type IV Collagenase Activity and Is Inhibited by Cellular Differentiation. Circ Res. 1994;75:41–54. doi: 10.1161/01.res.75.1.41. [DOI] [PubMed] [Google Scholar]
- 27.Nagler A, Miao H-Q, Aingorn H, Pines M, Genina O, Vlodavsky I. Inhibition of Collagen Synthesis, Smooth Muscle Cell Proliferation, and Injury-Induced Intimal Hyperplasia by Halofuginone. Arterioscler, Thromb, Vasc Biol. 1997;17:194–202. doi: 10.1161/01.atv.17.1.194. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Han Y, Lin C, Zhen Y, Song X, Teng S, Chen C, Chen Y, Zhang Y, Hui R. PDGF-D Contributes to Neointimal Hyperplasia in Rat Model of Vessel Injury. Biochem Biophys Res Commun. 2005;329:976–983. doi: 10.1016/j.bbrc.2005.02.062. [DOI] [PubMed] [Google Scholar]
- 29.Braun M, Pietsch P, Felix SB, Baumann G. Modulation of Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1 on Human Coronary Smooth Muscle Cells by Cytokines. J Mol Cell Cardiol. 1995;2579:2571–2579. doi: 10.1006/jmcc.1995.0044. [DOI] [PubMed] [Google Scholar]
- 30.Braun M, Pietsch P, Schror K, Baumann G, Felix SB. Cellular Adhesion Molecules on Vascular Smooth Muscle Cells. Cardiovasc Res. 1999;41:395–401. doi: 10.1016/s0008-6363(98)00302-2. [DOI] [PubMed] [Google Scholar]
- 31.Wright PS, Cooper JR, Kropp KE, Busch SJ, Elisa C. Induction of Vascular Cell Adhesion Molecule-1 Expression by IL-4 in Human Aortic Smooth Muscle Cells Is Not Associated With Increased Nuclear NF-κB Levels. J Cell Physiol. 1999;389:381–389. doi: 10.1002/(SICI)1097-4652(199909)180:3<381::AID-JCP9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Couffinhal T, Duplaa C, Labat L, Lamaziere JM, Moreau C, Printseva O, Bonnet J. Tumor Necrosis Factor-Alpha Stimulates ICAM-1 Expression in Human Vascular Smooth Muscle Cells. Arterioscler, Thromb, Vasc Biol. 1993;13:407–414. doi: 10.1161/01.atv.13.3.407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


