Abstract
Purpose
To evaluate the effects of a palliative care intervention on clinical and family outcomes, and palliative care processes.
Methods
Prospective, before-and-after interventional study enrolling patients with high risk of mortality, morbidity, or unmet palliative care needs in a 24-bed academic intensive care unit (ICU). The intervention involved a palliative care clinician interacting with the ICU physicians on daily rounds for high-risk patients.
Results
100 patients were enrolled in the usual care phase, and 103 patients were enrolled during the intervention phase. The adjusted likelihood of a family meeting in ICU was 63% higher (RR 1.63, 95% CI 1.14 to 2.07, p=0.01), and time to family meeting was 41% shorter (95% CI 52% to 28% shorter, p<0.001). Adjusted ICU length of stay (LOS) was not significantly different between the two groups (6% shorter, 95% CI 16% shorter to 4% longer, p=0.22). Among those who died in the hospital, ICU LOS was 19% shorter in the intervention (95% CI 33% to 1% shorter, p=0.043). Adjusted hospital LOS was 26% shorter (95% CI 31% to 20% shorter, p < 0.001) with the intervention. PTSD symptoms were present in 9.1% of family respondents during the intervention versus 20.7% prior to the intervention (p=0.09). Mortality, family depressive symptoms, family satisfaction and quality of death and dying did not significantly differ between groups.
Conclusions
Proactive palliative care involvement on ICU rounds for high-risk patients was associated with more and earlier ICU family meetings and shorter hospital LOS. We did not identify differences in family satisfaction, family psychological symptoms, or family-rated quality of dying, but had limited power to detect such differences.
Keywords: End-of-life care, palliative care, ICU decision-making, family meetings, communication, family ICU syndrome
Introduction
Twenty percent of deaths in the United States occur in an ICU or shortly after an ICU stay, and an increasing proportion of older Americans spend time in an ICU during the last month of life [1, 2]. Therapies in the ICU are often accompanied by burdensome symptoms, and among survivors recovery from critical illness is often incomplete [3-7]. In addition, the family members of ICU patients are at high risk for psychological impairments following their loved one's ICU stay [8].
Guidelines recommend the incorporation of palliative care into ICU practice [9, 10]. Various strategies to improve the quality of palliative care in the ICU have been investigated in numerous studies, with mixed results on patient and family outcomes [11, 12]. Many of these studies demonstrate that palliative care in the ICU has the potential to reduce ICU and hospital length of stay, and increase frequency or quality of communication in the ICU. Patients and families in the ICU have identified timely communication as an important part of high-quality care in the ICU[13], and interdisciplinary family meetings held early during an ICU stay can shorten length of stay (LOS) [14, 15]. Despite evidence-driven recommendations, performance of this important process has been inconsistent [14, 16-18]. Studies examining triggered palliative care consultation for patients at high risk of morbidity or mortality have shown reductions in ICU and hospital length of stay [19-21], and have demonstrated that such trigger criteria can dramatically increase palliative care consultation [22, 23]. Whether or not the use of such trigger criteria can alter the behavior of the ICU physicians with regard to communication with patients and families has not been studied.
The purpose of this study was to evaluate the effects of a novel proactive palliative care intervention aimed at improving the ICU physician team's attention to palliative care needs, rather than at increasing the utilization of palliative care consultants. We hypothesized that for ICU patients with a high risk of morbidity or mortality, proactive palliative care case review and participation in bedside ICU rounds by a palliative care provider would increase the frequency of documented interdisciplinary family meetings in the ICU, reduce ICU and hospital length of stay, reduce the burden of symptoms experienced by families of patients, and increase the satisfaction with care in the ICU, thus potentially providing a less intensive and less costly alternative to interventions utilizing full palliative care consultations for such high-risk patients. Some data from this study were presented in a mini-symposium at the International Conference of the America Thoracic Society, in the session “High-Impact Trials in Critical Care” on May 19, 2015 [24].
Methods
Study Design, Population, and Eligibility Criteria
We conducted a prospective, before-and-after interventional study of patients admitted to the medical critical care service in a 24-bed ICU at a 566-bed academic medical center between June 2013 and June 2014. This time period included a ‘usual care’ phase and an intervention phase. During both phases, one of two investigators (WJE or TL) screened the ICU census on week days to identify patients with one or more of the pre-specified clinical trigger criteria indicating high risk of mortality, morbidity, or unmet palliative care needs. These criteria, informed by a prior study [21] are listed in Table 1. Patients awaiting solid organ transplantation were excluded from the study, given the unique aspects of decision-making in these patients [25]. The study protocol was determined to be exempt by the University of Wisconsin human subjects IRB.
Table 1. Trigger criteria for patients at high risk of mortality or morbidity.
| Metastatic or otherwise incurable malignancy |
| Hospital length of stay of 10 days prior to transfer to the ICU |
| Duration of ongoing invasive mechanical ventilation of 7 days or more |
| ICU length of stay of 14 days or more |
| Age 80 years old or older with 2 or more significant chronic diseases, (e.g. chronic obstructive pulmonary disease requiring home oxygen therapy, chronic kidney disease requiring renal replacement therapy); |
| Out of hospital or in-hospital cardiac arrest |
| Cerebral hemorrhage requiring mechanical ventilation |
| Admission to the ICU from a long-term acute care hospital |
Intervention
A member of the hospital's Palliative Care (PC) consult team (on most days a Palliative Care clinical nurse specialist (KFR), who has many years of experience in both hospice and palliative care practice, and on other days (<10%), a Palliative medicine fellow or faculty member) was relocated from the palliative care unit to the ICU. Every weekday morning the investigators (WJE or TL) informed the PC clinician about patients meeting trigger criteria. The PC clinician then reviewed the electronic medical record (EMR) of each of these patients, and participated in interdisciplinary morning bedside ICU rounds with the critical care medicine team. On the first day that a patient was identified, the PC clinician informed the medical team that the patient met one or more of the trigger criteria, and which trigger criteria or criterion the patient met. On subsequent days, the PC clinician would make suggestions about addressing palliative care needs, as appropriate, including recommending that interdisciplinary family meetings be held in a timely fashion. The intervention was intended to prompt the critical care team to consider patients' and families' palliative care needs, an approach that has been effective at improving other aspects of evidence based practice in the ICU [26], and to a lesser extent to provide a ‘nudge’ to more effectively meet those needs [27]. Palliative care consultation was not routinely provided, and the PC clinician neither interacted with patients or family members nor participated in interdisciplinary family meetings, unless formal palliative care consultation was requested by the medical team.
From June 2013 to January 2014 patients meeting trigger criteria were prospectively identified as described above, but the clinical treating team was not informed about this nor was the care received by these patients altered in any way. The intervention phase was implemented beginning in January 2014 and continued through June 2014.
Outcomes and Data Sources
The primary outcome was the proportion of patients for whom an interdisciplinary family meeting was documented while in the ICU. Additional outcomes of interest included time between ICU admission and the occurrence of an interdisciplinary family meeting, ICU and hospital LOS, ICU and in-hospital mortality, family satisfaction with care in the ICU, and the burden of psychological symptoms experienced by family members of those admitted to the ICU.
Data Sources
Socio-demographic data including age, sex, self-identified race and ethnicity, and marital status, and clinical data including ICU and hospital LOS, discharge diagnoses, discharge destination, and occurrence of a palliative care consultation were captured from the EMR. Documentation of an interdisciplinary family meeting was determined by searching all clinical notes during the hospitalization. A family meeting was identified as any mention within the EMR of an instance of communication between the medical team and the patient and/or family outside of routine rounding, with the explicit purpose of sharing clinical information and eliciting patient and family input for the purpose of medical decision making. For those patients discharged alive, vital status 3 months after discharge was ascertained using the EMR, obituary searches, and the Social Security death master file via a publicly available internet site [28]. Family members' satisfaction and psychological symptoms were assessed with three validated questionnaires. The Family Satisfaction in the Intensive Care Unit (FS-ICU) scale is a 24-item measure of satisfaction with overall care and decision-making in the ICU, as reflected on a 5-point Likert scale [29]. The Patient Health Questionnaire depression scale (PHQ-8) is an 8-item measure of the severity of depression symptoms, with the frequency of depression symptoms rated on a 0 (not at all) to 3 (nearly every day) scale, giving a total score range of 0-24 [30, 31]. The Post-Traumatic Stress Disorder (PTSD) Checklist-Civilian [PCL-C] is a 17-item self-report measure of PTSD symptoms experienced in the last month in response to a traumatic event (in this case, a loved one's ICU stay), with the severity rated from 1 (not at all bothersome) to 5 (extremely bothersome), giving a range of 17-85 [32]. All questionnaires were mailed to the EMR-specified family member or primary support person six weeks after the patient's discharge from the ICU. For those patients who died in the ICU, an additional single item was included in the mailed questionnaire, asking the family member to rate the overall quality of death and dying (QODD-1) on a 0 to 10 scale, with higher scores indicating better quality [33].
Statistical Analysis
Continuous variables were compared with Student's t-test for those variables whose distribution approximated normal, and with the Wilcoxon-Mann-Whitney test for LOS variables which were significantly skewed rightward. Binary and categorical variables were compared with the χ2 test. The occurrence of a documented family meeting during the ICU stay was modeled using multivariable logistic regression, adjusting for potential confounding by patient age, sex, race and ethnicity, the presence of metastatic or incurable cancer, multi-morbidity with age over 80, admission from an LTAC, and death in the ICU. Odds ratios were converted to risk ratios, since the outcome of interest did not satisfy the rare outcome assumption [34]. Time from ICU admission to the occurrence of a family meeting was modeled using multivariable Poisson regression, with the same potential confounders included in the model above. Length of stay outcomes were modeled using zero-truncated multivariable Poisson regression, given the distribution of these variables, adjusting for the same covariates as in family meeting models with the exception of death in the ICU.
Scores on items of the PHQ-8 were summed, with scores of 10-19 indicating significant depressive symptoms with scores of 20 or greater considered consistent with major depression [31]. Scores on the PCL-C were summed across items, and an algorithm consistent with the clinical diagnosis of PTSD was used to determine a positive test [35-37]. For family satisfaction, total and domain scores on the FS-ICU were transformed on a 0-100 scale, with higher scores indicating higher satisfaction [29]. Multivariable linear regression was used to evaluate for associations between the intervention and family satisfaction, family member rating of the overall quality of death and dying, and positive scores on PTSD and depression screens. These models included covariates for patient age, sex, and race, respondent sex, whether the patient and respondent were spouses, the presence of metastatic or incurable cancer, multi-morbidity with age over 80, and the occurrence of death in the ICU.
Statistical analyses were carried out with STATA version 13, StataCorp, LP, College Station, Texas.
Results
We prospectively identified 100 patients meeting trigger criteria during the usual care phase, and 103 patients during the intervention phase. While the mean age of patients during the intervention phase was 3.8 years older than those in the usual care phase, this difference was not statistically significant (Table 2). There were no significant differences in sex or race/ethnicity. A lower proportion of patients during the intervention met trigger criteria regarding ICU LOS greater than 14 days (1% versus 8%, P=0.02), but there were no other significant differences in trigger criteria between the groups.
Table 2. Demographic and clinical characteristics, by group.
| Usual Care, n=100 | Intervention, n=103 | P * | |
|---|---|---|---|
| Age, μ (σ), years | 60.6 (16.6) | 64.4 (19.0) | 0.14 |
| Women, n (%) | 41 (41) | 51 (49.5) | 0.22 |
| Race | |||
| White | 91 (91) | 88 (85) | 0.22 |
| Black | 7 (7) | 9 (9) | 0.64 |
| Asian | 0 (0) | 3 (3) | 0.09 |
| American Indian | 1 (1) | 2 (2) | 0.58 |
| Other | 0 (0) | 1 (1) | 0.98 |
| Hispanic ethnicity, n (%) | 2 (2) | 2 (2) | 0.98 |
| Trigger Criteria | |||
| Metastatic or incurable cancer | 8 (8) | 16 (16) | 0.10 |
| Cardiac arrest | 22 (22) | 19 (19) | 0.53 |
| Hospitalized ≥10 days prior to ICU | 15 (15) | 12 (12) | 0.48 |
| ICU LOS 14 days or more* | 8 (8) | 1 (1) | 0.02 |
| Ventilation for 7 or more days | 23 (23) | 29 (28) | 0.61 |
| Age over 80 with multimorbidity* | 22 (22) | 34 (33) | 0.08 |
| Cerebral hemorrhage requiring intubation | 1 (1) | 1 (1) | 0.98 |
| Admitted from LTAC | 5 (5) | 2 (2) | 0.23 |
| Family member survey returned, n (%) | 62 (62) | 58 (56) | 0.41 |
| Age of respondent, μ (σ), years | 60.2 (13.4) | 60.9 (13.9) | 0.78 |
| Women respondents, n (%) | 46 (77) | 36 (62) | 0.09 |
| Respondent lived with patient, n (%) | 34 (57) | 33 (58) | 0.89 |
| Respondent relationship | |||
| Spouse/Partner | 35 (56) | 24 (42) | 0.30 |
| Child | 11 (18) | 15 (26) | 0.26 |
| Parent | 6 (10) | 8 (14) | 0.46 |
| Sibling | 8 (13) | 4 (7) | 0.29 |
| Guardian | 2 (3) | 1 (2) | 0.61 |
| Friend | 0 (0) | 3 (5) | 0.07 |
| Other relative | 0 (0) | 1 (2) | 0.30 |
| Other | 0 (0) | 1 (2) | 0.30 |
χ2 test for binary and categorical variables, Student's t-test for continuous variables
Processes Measures
Only 35% of the subjects in the usual care period had documentation of an interdisciplinary family meeting during their ICU stay, and for those patients for whom such a meeting was documented, only 35% of these meetings took place within the first 3 days in the ICU (Table 3). In adjusted analysis, the intervention was associated with a 63% higher likelihood of a documented family meeting occurring during the ICU stay (RR 1.63, 95% Confidence Interval [CI] 1.14 to 2.07, p=0.01). The adjusted time between ICU admission and the occurrence of a family meeting in the ICU was 41% shorter in the intervention period (95% CI 52% shorter to 28% shorter, p<0.001).
Table 3. Palliative care process of care measures and clinical outcomes*, by group (unadjusted).
| Usual Care, n=100 | Intervention, n=103 | P | |
|---|---|---|---|
| Family meeting during ICU documented | 35 (35) | 55 (53) | 0.008 |
| Family meeting within 3 days of ICU admission documented, n (% of those with a meeting) | 14 (35) | 33 (59) | 0.02 |
| Days between ICU admission and family meeting, median (IQR) | 5 (2, 8) | 3 (1, 7) | 0.18 |
| 3 disciplines represented at meeting n, (% of those with a meeting) | 6 (17) | 33 (60) | <0.001 |
| Full palliative care consult ordered during hospitalization | 18 (18) | 20 (19) | 0.80 |
| Died in the ICU, n (%) | 28 (28) | 28 (27) | 0.90 |
| Died in the Hospital, n (%) | 35 (35) | 33 (32) | 0.66 |
| 90 day mortality, n (%) | 45 (45) | 36 (35) | 0.14 |
| Discharge destination of survivors | |||
| LTAC | 23 (35) | 19 (27) | 0.30 |
| Home | 23 (35) | 17 (24) | 0.16 |
| Inpatient Rehabilitation | 3 (5) | 5 (7) | 0.53 |
| Nursing home | 12 (18) | 19 (27) | 0.23 |
| Hospice | 2 (3) | 7 (10) | 0.11 |
| Assisted living facility | 2 (3) | 0 (0) | 0.14 |
| Another hospital | 0 (0) | 3 (4) | 0.09 |
| ICU LOS, median (iqr), days | 5 (3, 11.5) | 4 (2, 10) | 0.14 |
| Hospital length of stay, median (iqr), days | 13 (6, 26.5) | 11 (5, 20) | 0.09 |
| ICU LOS among hospital nonsurvivors, median (iqr), days | 6 (3, 12) | 4 (2, 9) | 0.15 |
| Hospital LOS among hospital nonsurvivors, median (iqr), days | 9 (4, 17) | 5 (3, 13) | 0.13 |
χ2 test for binary variables, Wilcoxon-Mann-Whitney test for time to meeting and LOS variables
The intervention was associated with a greater than threefold higher proportion of these documented meetings that included 3 or more disciplines (60% versus 17% in the usual care group, p < .001). Full palliative care consultation was ordered in less than one in five patients before or during the intervention, and this did not differ between the groups.
Outcome Measures
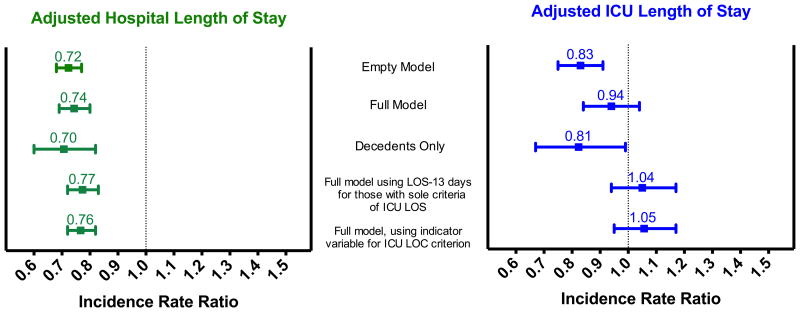
After adjusting for potential confounders in multivariable regression models, ICU LOS was not significantly different between the two groups (estimated 6% shorter in the intervention period, 95% CI 16% shorter to 4% longer, p=0.22; Fig. 1). Because earlier and improved communication might lead to earlier decisions to withdraw or limit life-sustaining therapy, we performed an exploratory analyses including only those individuals who died in the hospital; in this group the intervention was associated with a 19% reduction in ICU LOS (95% CI 33% shorter to 1% shorter, p=0.043).
Fig 1.
Association between intervention and hospital (left) and ICU (right) length of stay, with sensitivity analyses. Incidence rate ratios calculated from zero-truncated Poisson regression models are presented, with error bars representing 95% confidence intervals. The full model included the following potential confounders: patient age, sex, race and ethnicity, the presence of metastatic or incurable cancer, multimorbidity with age over 80, and admission from LTAC.
After adjusting for potential confounders, hospital LOS was significantly shorter in the intervention group, with an estimated 26% shorter hospital LOS (95% CI 31% to 20% shorter, p < 0.001). In exploratory analyses including only those individuals who died in the hospital, the intervention was associated with a 30% reduction in hospital LOS (95% CI 40% to 18% shorter, p<0.001).
Because the proportion of patients who met the trigger criterion of ICU LOS ≥ 14 days differed between the usual care and intervention groups, we performed sensitivity analyses using two different approaches. In the first approach, we ran LOS models counting ICU and hospital LOS after the date of trigger positivity for those patients whose sole trigger criterion was ICU LOS (Fig. 1). In the second approach we added an indicator variable for the ICU LOS trigger criterion. There were no significant differences in the association between the intervention and reduced hospital LOS in these analyses.
ICU mortality was nearly identical between the two groups (27% during the intervention versus 28% in the usual care group), and in-hospital mortality was similar as well (Table 3). There were no significant differences in discharge destination among those discharged alive.
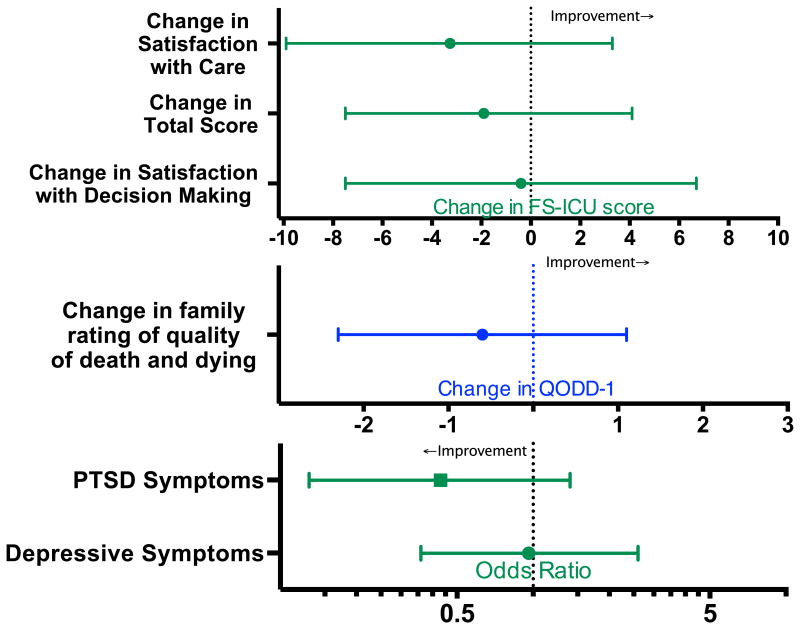
A family member returned a survey for 62% of patients in the usual care group and 56% of patients in the intervention group (Table 2). Because of missing responses, FS-ICU scores could not be calculated for 1 patient in each group, and PHQ-8 and PCL-C scores could not be calculated for 3 respondents in the usual care group and 2 individuals in the intervention groups. QODD-1 was answered for all but 1 of the 36 patients who died in the ICU and whose family member returned a survey. Total family satisfaction, satisfaction with the care their loved one received, and satisfaction with decision-making during the ICU stay were similar between the intervention and usual care groups (83.7 in usual care versus 81.7 in the intervention, p=0.52). Depressive symptoms were common in family members following their loved one's ICU stay, reported in 26% of respondents in the usual care and 22% in the intervention period (p=0.61). Total scores on the PTSD screening tool were similar between groups. Significant symptoms of PTSD were present in 20.7% of respondents in the usual care group, and 9.1% of respondents during the intervention period (p=0.09). There were no significant differences by intervention in family satisfaction scores, family member rating of the overall quality of death and dying, or the occurrence of depressive symptoms or PTSD symptoms in adjusted regression analyses (Fig. 2).
Fig 2.
Adjusted change in family satisfaction scores (by FS-ICU) (top) and adjusted change in family assessment of the quality of death and dying (QODD-1) (middle, in blue), using multivariable linear regression. Adjusted association between the intervention and psychological outcomes (bottom). All models were adjusted for patient age, sex, and race, respondent sex, whether the patient and respondent were spouses, the presence of metastatic or incurable cancer, multimorbidity with age over 80, and (except for the QODD-1 model) the occurrence of death in the ICU
Discussion
In this single-center before and after interventional study we found that straightforward trigger criteria leading to a simple intervention—a palliative care clinical nurse specialist interacting with the critical care team on rounds—was associated with earlier and more frequent interdisciplinary family meetings in the ICU. Family meeting incidence in the usual care and intervention groups both fell within the range of rates reported in the literature (15, 19). Our trigger criteria were adapted from a trial reported by Norton, et. al., in which a triggered palliative care consultation was associated with a significant overall decrease in ICU LOS. In contrast, our less intensive intervention was associated with an overall reduction in adjusted hospital but not ICU LOS. This discrepancy may be attributable to shorter baseline median ICU LOS in this study relative to the Norton study (5 days versus 12 days). Interestingly, in exploratory analyses we observed significant reductions in both hospital and ICU LOS for those individuals in the intervention group who died in the hospital. A possible explanation for this observation is that more timely family meetings in the ICU led to earlier decisions about withholding or withdrawing life sustaining treatment in patients who were unlikely to benefit from ongoing critical care therapies.
Consistent with previous reports in the literature, family member symptoms of PTSD and depression were prevalent in both cohorts [38, 39]. We did not find improvement in the burden of depressive or PTSD symptoms experienced by families of patients, or any increase in the satisfaction with care in the ICU or quality of death and dying in the ICU. Our findings are consistent with the results of a recent multi-center trial of an intervention to improve end-of-life care in the ICU that found no significant change in family reported outcomes [40]. It is notable that both trials' interventions targeted clinicians rather than patients or families, suggesting that the burden of symptoms suffered by families of ICU patients may be less affected by indirect interventions that do not directly modify communication. Direct interventions targeting improved prognostic awareness, symptom control, spiritual support, and better alignment of medical decisions with patient goals and preferences may be necessary to achieve measurable improvements in patient- and family reported outcomes. For example, interventions to standardize the structure of family meetings to improve the quality of communication might be needed to improve these family-centered measures. Another possibility is that full palliative care consultation in high-risk patients, while more resource-intensive than the intervention studied here, might provide additional patient- and family-centered benefits, and might have improved the outcomes measured in this study.
With only 61 surveys returned in the usual care group, and 57 in the intervention group, this study was underpowered to detect change in these family centered outcomes, and was even more underpowered to assess for change in QODD1 given only 35 surveys from decedents' families. The baseline rate of family member PTSD and depression in this study (20.7% and 25.9%) are lower than we hypothesized and quite a bit lower than levels seen in a positive interventional trial performed in France (69% and 56%), though all of the patients in that study had died [39]. This lower prevalence of PTSD and depression contributed to reduced power to detect clinically significant changes in these outcomes from this intervention. Also, the use of self-administered surveys delivered by mail to assess family outcomes may result in a less representative sample through nonresponse bias, and may limit the willingness of respondents to disclose sensitive information such as psychological symptoms[41].
There are other important limitations of this study to consider. First, the short baseline ICU LOS in our study makes small yet clinically relevant differences in ICU LOS difficult to detect. This could be addressed in subsequent trials by excluding patients with short LOS. Second, the before and after interventional study does not benefit from the minimization of confounding that randomization would offer, and this design cannot control for temporal changes in ICU care that might affect outcomes such as LOS. Third, some family meetings likely went undetected for lack of documentation, and it is possible that our study merely detected a change in documentation practices. Fourth, it is not known what aspect of the intervention may have been effective. For example, simply identifying high-risk patients through the use of trigger criteria and informing the ICU physicians about this identification may account for the findings observed in this study, independent of the presence of a clinical nurse specialist on rounds. Indeed, awareness of the ongoing study and of being observed may alone have altered ICU physicians' behavior regarding family meetings for high-risk patients. If this is the case, an even simpler and less costly intervention might have demonstrated similar effects. On the other hand, it may be that the presence of a palliative care specialist on morning rounds prompted greater consideration of patients' and families' palliative care needs and nudged critical care providers towards meeting those needs, beyond what the trigger criteria alone could have accomplished.
Conclusions
Proactive palliative care involvement on ICU rounds for high-risk patients patients to prompt ICU-physician attention to palliative care needs and to nudge the ICU physicians to better address these needs was associated with more and earlier ICU family meetings and shorter hospital LOS. We did not identify differences in family satisfaction, family psychological symptoms, or family-rated quality of dying, but had limited power to detect such differences. Given the simplicity of the intervention studied and the potentially beneficial effects observed, further study of such an intervention is warranted. A future study of this intervention should be powered to detect meaningful changes in family outcomes and include a cost-effectiveness analysis of the intervention.
Acknowledgments
Dr. Ehlenbach has received grant funding from the National Institutes of Health, National Institute of Aging (K23 AG038352), funded by The Atlantic Philanthropies, The John A. Hartford Foundation, and the Starr Foundation. This work utilized the University of Wisconsin Carbone Cancer Center (UWCCC) Survey Resource Shared Resource, which is funded in part by the UWCCC Center Support Grant (P30 CA014520) from the National Cancer Institute; the authors wish to thank Amy Godecker, PhD, and Karen Lazar, MS, for their work with the surveys for this study. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the University of Wisconsin-Madison, School of Medicine and Public Health. REDCap is supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Angus DC, Barnato AE, Linde-Zwirble WT, Weissfeld LA, Watson RS, Rickert T, Rubenfeld GD Robert Wood Johnson Foundation ICUE-O-LPG. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32:638–643. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- 2.Teno JM, Gozalo PL, Bynum JP, Leland NE, Miller SC, Morden NE, Scupp T, Goodman DC, Mor V. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309:470–477. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox CE, Martinu T, Sathy SJ, Clay AS, Chia J, Gray AL, Olsen MK, Govert JA, Carson SS, Tulsky JA. Expectations and outcomes of prolonged mechanical ventilation. Crit Care Med. 2009;37:2888–2894. doi: 10.1097/CCM.0b013e3181ab86ed. quiz 2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox CE, Carson SS, Lindquist JH, Olsen MK, Govert JA, Chelluri L Quality of Life After Mechanical Ventilation in the Aged I. Differences in one-year health outcomes and resource utilization by definition of prolonged mechanical ventilation: a prospective cohort study. Crit Care. 2007;11:R9. doi: 10.1186/cc5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 6.Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, Larson EB. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, Zawistowski C, Bemis-Dougherty A, Berney SC, Bienvenu OJ, Brady SL, Brodsky MB, Denehy L, Elliott D, Flatley C, Harabin AL, Jones C, Louis D, Meltzer W, Muldoon SR, Palmer JB, Perme C, Robinson M, Schmidt DM, Scruth E, Spill GR, Storey CP, Render M, Votto J, Harvey MA. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 9.Truog RD, Campbell ML, Curtis JR, Haas CE, Luce JM, Rubenfeld GD, Rushton CH, Kaufman DC American Academy of Critical Care M. Recommendations for end-of-life care in the intensive care unit: a consensus statement by the American College [corrected] of Critical Care Medicine. Crit Care Med. 2008;36:953–963. doi: 10.1097/CCM.0B013E3181659096. [DOI] [PubMed] [Google Scholar]
- 10.Lanken PN, Terry PB, Delisser HM, Fahy BF, Hansen-Flaschen J, Heffner JE, Levy M, Mularski RA, Osborne ML, Prendergast TJ, Rocker G, Sibbald WJ, Wilfond B, Yankaskas JR Force ATSE-o-LCT. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177:912–927. doi: 10.1164/rccm.200605-587ST. [DOI] [PubMed] [Google Scholar]
- 11.Aslakson R, Cheng J, Vollenweider D, Galusca D, Smith TJ, Pronovost PJ. Evidence-based palliative care in the intensive care unit: a systematic review of interventions. J Palliat Med. 2014;17:219–235. doi: 10.1089/jpm.2013.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau BD, Aslakson RA, Wilson RF, Fawole OA, Apostol CC, Martinez KA, Vollenweider D, Bass EB, Dy SE. Methods for improving the quality of palliative care delivery: a systematic review. Am J Hosp Palliat Care. 2014;31:202–210. doi: 10.1177/1049909113482039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson JE, Puntillo KA, Pronovost PJ, Walker AS, McAdam JL, Ilaoa D, Penrod J. In their own words: patients and families define high-quality palliative care in the intensive care unit. Crit Care Med. 2010;38:808–818. doi: 10.1097/ccm.0b013e3181c5887c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lilly CM, De Meo DL, Sonna LA, Haley KJ, Massaro AF, Wallace RF, Cody S. An intensive communication intervention for the critically ill. Am J Med. 2000;109:469–475. doi: 10.1016/s0002-9343(00)00524-6. [DOI] [PubMed] [Google Scholar]
- 15.Lilly CM, Sonna LA, Haley KJ, Massaro AF. Intensive communication: four-year follow-up from a clinical practice study. Crit Care Med. 2003;31:S394–399. doi: 10.1097/01.CCM.0000065279.77449.B4. [DOI] [PubMed] [Google Scholar]
- 16.Azoulay E, Chevret S, Leleu G, Pochard F, Barboteu M, Adrie C, Canoui P, Le Gall JR, Schlemmer B. Half the families of intensive care unit patients experience inadequate communication with physicians. Crit Care Med. 2000;28:3044–3049. doi: 10.1097/00003246-200008000-00061. [DOI] [PubMed] [Google Scholar]
- 17.Nelson JE, Angus DC, Weissfeld LA, Puntillo KA, Danis M, Deal D, Levy MM, Cook DJ Critical Care Peer Workgroup of the Promoting Excellence in End-of-Life Care P. End-of-life care for the critically ill: A national intensive care unit survey. Crit Care Med. 2006;34:2547–2553. doi: 10.1097/01.CCM.0000239233.63425.1D. [DOI] [PubMed] [Google Scholar]
- 18.Penrod JD, Pronovost PJ, Livote EE, Puntillo KA, Walker AS, Wallenstein S, Mercado AF, Swoboda SM, Ilaoa D, Thompson DA, Nelson JE. Meeting standards of high-quality intensive care unit palliative care: clinical performance and predictors. Crit Care Med. 2012;40:1105–1112. doi: 10.1097/CCM.0b013e3182374a50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell ML, Guzman JA. Impact of a proactive approach to improve end-of-life care in a medical ICU. Chest. 2003;123:266–271. doi: 10.1378/chest.123.1.266. [DOI] [PubMed] [Google Scholar]
- 20.Campbell ML, Guzman JA. A proactive approach to improve end-of-life care in a medical intensive care unit for patients with terminal dementia. Crit Care Med. 2004;32:1839–1843. doi: 10.1097/01.ccm.0000138560.56577.88. [DOI] [PubMed] [Google Scholar]
- 21.Norton SA, Hogan LA, Holloway RG, Temkin-Greener H, Buckley MJ, Quill TE. Proactive palliative care in the medical intensive care unit: effects on length of stay for selected high-risk patients. Crit Care Med. 2007;35:1530–1535. doi: 10.1097/01.CCM.0000266533.06543.0C. [DOI] [PubMed] [Google Scholar]
- 22.Villarreal D, Restrepo MI, Healy J, Howard B, Tidwell J, Ross J, Hartronft S, Jawad M, Sanchez-Reilly S, Reed K, Espinoza SE. A model for increasing palliative care in the intensive care unit: enhancing interprofessional consultation rates and communication. J Pain Symptom Manage. 2011;42:676–679. doi: 10.1016/j.jpainsymman.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Sihra L, Harris M, O'Reardon C. Using the improving palliative care in the intensive care unit (IPAL-ICU) project to promote palliative care consultation. J Pain Symptom Manage. 2011;42:672–675. doi: 10.1016/j.jpainsymman.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Braus N, Ferguson S, Campbell TC, Kehl K, Krupp A, Kwekkeboom K, Roberts KF, Ehlenbach WJ. Effects Of Proactive Palliative Care On Processes And Outcomes In A Medical ICU (abstract) Am J Respir Crit Care Med. 2015;191:A5127. [Google Scholar]
- 25.DiMartini A, Crone C, Fireman M, Dew MA. Psychiatric aspects of organ transplantation in critical care. Crit Care Clin. 2008;24:949–981. x. doi: 10.1016/j.ccc.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss CH, Moazed F, McEvoy CA, Singer BD, Szleifer I, Amaral LA, Kwasny M, Watts CM, Persell SD, Baker DW, Sznajder JI, Wunderink RG. Prompting physicians to address a daily checklist and process of care and clinical outcomes: a single-site study. Am J Respir Crit Care Med. 2011;184:680–686. doi: 10.1164/rccm.201101-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thaler RH, Sunstein CR. Nudge: Improving decisions about health, wealth, and happiness. Yale University Press; New Haven, CT: 2008. [Google Scholar]
- 28.Taichman DB, Christie J, Biester R, Mortensen J, White J, Kaplan S, Hansen-Flaschen J, Palevsky HI, Elliott CG, Hopkins RO. Validation of a brief telephone battery for neurocognitive assessment of patients with pulmonary arterial hypertension. Respir Res. 2005;6:39. doi: 10.1186/1465-9921-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wall RJ, Engelberg RA, Downey L, Heyland DK, Curtis JR. Refinement, scoring, and validation of the Family Satisfaction in the Intensive Care Unit (FS-ICU) survey. Crit Care Med. 2007;35:271–279. doi: 10.1097/01.CCM.0000251122.15053.50. [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatric Annals. 2002;32:509–521. [Google Scholar]
- 31.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Ruggiero KJ, Del Ben K, Scotti JR, Rabalais AE. Psychometric properties of the PTSD Checklist-Civilian Version. J Trauma Stress. 2003;16:495–502. doi: 10.1023/A:1025714729117. [DOI] [PubMed] [Google Scholar]
- 33.Glavan BJ, Engelberg RA, Downey L, Curtis JR. Using the medical record to evaluate the quality of end-of-life care in the intensive care unit. Crit Care Med. 2008;36:1138–1146. doi: 10.1097/CCM.0b013e318168f301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 35.Diagnostic and statistical manual of mental disorders. 4th. American Psychiatric Association; Washington, DC: 2000. text rev. [Google Scholar]
- 36.Weathers F, Ford J. Psychometric review of PTSD checklist AND (PCL-C, PCL-S, PCL-M, PCL-PR) In: Stamm B, editor. Measurement of Stress, Trauma and Adaptation. Sidran Press; Lutherville, MD: 1996. pp. 250–251. [Google Scholar]
- 37.Kross EK, Engelberg RA, Gries CJ, Nielsen EL, Zatzick D, Curtis JR. ICU care associated with symptoms of depression and posttraumatic stress disorder among family members of patients who die in the ICU. Chest. 2011;139:795–801. doi: 10.1378/chest.10-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azoulay E, Pochard F, Kentish-Barnes N, Chevret S, Aboab J, Adrie C, Annane D, Bleichner G, Bollaert PE, Darmon M, Fassier T, Galliot R, Garrouste-Orgeas M, Goulenok C, Goldgran-Toledano D, Hayon J, Jourdain M, Kaidomar M, Laplace C, Larche J, Liotier J, Papazian L, Poisson C, Reignier J, Saidi F, Schlemmer B, Group FS. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171:987–994. doi: 10.1164/rccm.200409-1295OC. [DOI] [PubMed] [Google Scholar]
- 39.Lautrette A, Darmon M, Megarbane B, Joly LM, Chevret S, Adrie C, Barnoud D, Bleichner G, Bruel C, Choukroun G, Curtis JR, Fieux F, Galliot R, Garrouste-Orgeas M, Georges H, Goldgran-Toledano D, Jourdain M, Loubert G, Reignier J, Saidi F, Souweine B, Vincent F, Barnes NK, Pochard F, Schlemmer B, Azoulay E. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007;356:469–478. doi: 10.1056/NEJMoa063446. [DOI] [PubMed] [Google Scholar]
- 40.Curtis JR, Nielsen EL, Treece PD, Downey L, Dotolo D, Shannon SE, Back AL, Rubenfeld GD, Engelberg RA. Effect of a quality-improvement intervention on end-of-life care in the intensive care unit: a randomized trial. Am J Respir Crit Care Med. 2011;183:348–355. doi: 10.1164/rccm.201006-1004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowling A. Mode of questionnaire administration can have serious effects on data quality. J Public Health (Oxf) 2005;27:281–291. doi: 10.1093/pubmed/fdi031. [DOI] [PubMed] [Google Scholar]